1. Introduction
Quantitative elastography is a method to evaluate the stiffness of tissue using various imaging techniques. The initial proposal for quantitative ultrasound elastography was published by Ophir et al. [
1]. The techniques and algorithms underwent improvements and new methods were developed: the quasi-static approach proposed by Ophir et al. was joined by algorithms such as shear wave elastography, methods using crawling waves and single tracking, among others [
2,
3]. Additionally, further imaging techniques such as X-ray and computed tomographic elastography were utilized to estimate the elastic properties of tissue. Clinical applications of elastography kept the pace of technical improvements and more and more organs were examined [
3] (pp. 7–11).
The basic idea of quasi-static elastography is to apply a small deformation to a sample or the tissue by an ultrasound transducer and record this compression. The estimated displacement can be computed to a strain field in the compressed specimen. This step results in strain images, often synonymously used for elastography [
4]. Further, with reasonable stress assumptions, the elastic properties of the evaluated tissue or sample can be computed [
5] (p. 62ff). The calculation of quantitative results, e.g., the Young’s modulus, of the tissue needs further assumptions, such as a simplified or a continuum mechanical model [
6]. Copious models and applications for different organ systems, such as liver, breast, thyroid, kidney, were developed and used clinically [
2]. This algorithm was developed for measuring the elastic properties of vocal folds [
7].
Quantitative solutions for the quasi-static elastography can be analytically solved by using the proposals of Sumi et al. [
8], Barbone et al. [
9], Fehrenbach et al. [
10], etc. Iterative methods have been published, such as those by Doyley et al. [
11], Oberai et al. [
12], Smyl et al. [
13], Mohammadi et al. [
14], to name just a few. Additionally, deep learning approaches have been proposed [
15]. Recent reviews give an overview of the current state of research, such as those by Parker et al. [
16], Doyley [
6], Sigrist et al. [
2], and Alam and Garra [
17].
In this work, the compression of homogeneous and non-homogeneous gelatin blocks will be evaluated by a novel quasi-static elastography algorithm. The blocks were compressed with an ultrasound transducer and the compressing force was measured by a load cell. The resulting data were computed with an image registration algorithm using DeepFlow [
18]. Further, the strain was calculated by a Savitzky–Golay differentiator [
19]. Using this approach, the axial strain component can be computed separately due to the two-dimensional image registration approach. The axial strains are a marker for the degree of bonding between stiffer regions and the surrounding material [
4]. This information can, for example, help to distinguish between malignant and benign changes in the tissue [
4,
20,
21]. The lateral strains, perpendicular to the compression direction, are computed as well, enabling the user to gain further information on the lateral tissue movement [
22]. The whole registration and strain calculation process is evaluated by a performance descriptor, which automatically chooses the representative frames for further processing [
23]. The plane–strain and plane–stress assumptions are only valid when the measurement is performed using very controlled experimental setups, which guarantee well-defined boundary conditions [
6]. Finally, the Young’s modulus is calculated by the quasi-elastic approach, using the stress assumption that Love [
24] proposed.
The algorithm enables the user to measure the elastic properties of the specimens without access to the radio frequency (RF) signal of the ultrasound device. Therefore, usual CINE frames, which are basically ultrasound video sequences, of the compression process can be used.
The present paper will first introduce the used methods: starting with the governing equations of elastography, the proposed algorithm is explained. Further, the production of the testing specimens and their mechanical measurements as reference for the elastography results is described. Following the Methods section, the results are presented. Conclusively, the results are discussed and compared to other algorithms.
2. Methods
The elastic modulus of the specimens was measured mechanically and by the elastography algorithm, while the mechanical measurements were used as reference values. This section is structured as follows: first, a brief introduction to the theory of linear materials will be given. Secondly, the measurement setup will be presented. Further, the production of the gelatin specimens and, conclusively, the measurement methods and evaluation algorithms will be explained. Two types of mechanical measurements, compression and indentation, were carried out to estimate the elastic modulus of the specimens. The results were analyzed with a finite element (FE) model. At the end of this section, the elastography algorithm will be presented.
2.1. Linear Continuous Materials
We will use different models to describe the behavior of the gelatin block, but all models are based on the same assumptions. We assume that gelatin is an isotropic, nearly incompressible, linear–elastic, and locally homogeneous material [
2]. The contact surfaces are considered to be a non-slip boundary [
25]. The linear formulation of Hooke’s Law with strains
and stresses
is
with the 4th-order stiffness tensor
C, which describes the material’s reaction to stress [
26,
27]. For orthotropic, (transversal) isotropic materials,
C simplifies drastically. All elements of
C except
are zero. Therefore, the stiffness tensor is defined by two values, namely the Poisson’s ratio
and the shear modulus
G. The nonzero entries of
C are consequently defined by
and
The Poisson’s ratio
is calculated through the ratio of compressing strain to the expansion in the perpendicular direction, and hence it is defined by
The Young’s modulus is defined as follows:
and therefore a Hookean material is fully described by the Young’s modulus
E and the Poisson’s ratio
[
26].
The material can be described as linear–elastic, when only considering small deformations [
6]. It is further assumed that the stress has no perpendicular components (
x and
y-direction) to the axial compressing stress in the z-direction. The underlying idea is that the tissue expands in perpendicular directions, preserving the volume of the tissue [
5]. Therefore, we can define the axial stress
with
when all other components of the stress are zero (
). Hooke’s Law (Equation (
1)) can be written, using the axial strain
, as
Conclusively, we can compute the elastic modulus
E with
In non-linear materials, the measured strain depends on the applied stress or deformation. Therefore, a strain-dependent elastic modulus can be modeled by a Veronda–Westmann material [
5]. In the special case of small strains and uniaxial stress, their relation can be described by
The non-linearity parameter
of the apparent Young’s modulus is a tissue-dependent material parameter [
5]. Using this, we can define a strain-dependent Young’s modulus
The values chosen in this paper are
and
, which have yielded good results [
28,
29].
2.2. Setup
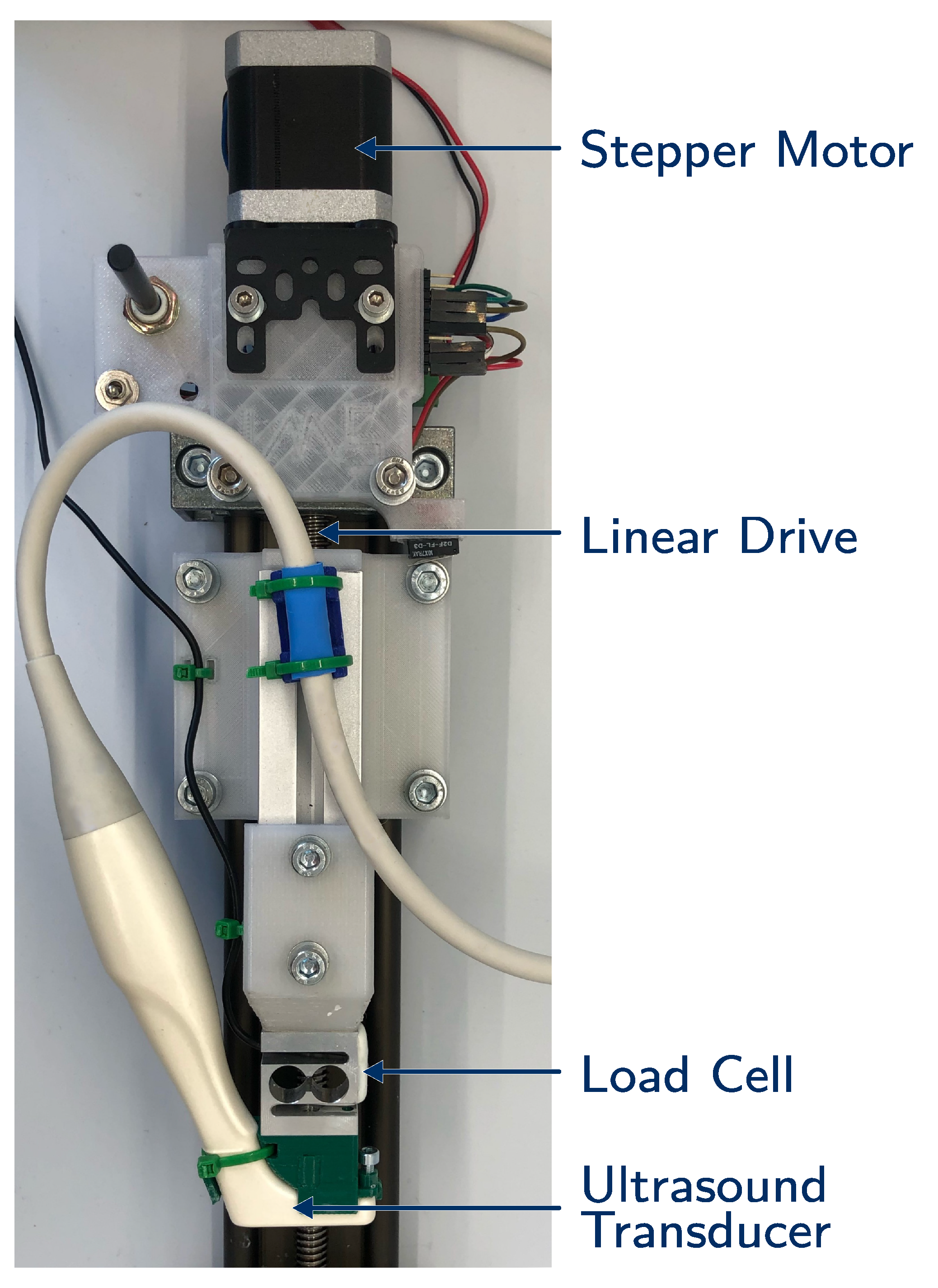
The measurement setup consists of three basic components, which are displayed in
Figure 1: a linear drive powered by a stepper motor, a load cell (KD23s-2N, ME-Messsysteme, Hennigsdorf, Germany) and the ultrasound transducer (IO 8-17, Alpinion, Anyang, Korea) connected to the ultrasound device (E-Cube 15EX, Alpinion, Anyang, Korea). The ultrasound transducer has a frequency range of 8
to 17
.
The CINE frames were recorded at a central ultrasound frequency of 15 with a lateral and axial pixel size of . The frame rate was frames/, whereas the exact frame rate depends on the ultrasound device and cannot be influenced directly.
The specimen was placed on a solid baseplate and compressed by the transducer (elastography and indentation measurements) or a stiff plate (compression measurement). The baseplate was coated with sandpaper (320 grid) to ensure the non-slip boundaries. In the case of the compression measurements, the top plate was coated in the same way. The whole measurement process was controlled and monitored by Matlab (2020a, MathWorks, Natick, MA USA). The stepper motor was driven by a micro controller (Tic500, Pololu, Las Vegas, NV, USA) with a serial interface, which also stored the covered steps. During the compression, the load cell measured the force and the data were recorded by an analog input module (NI-9219, National Instruments, Austin, TX, USA). Simultaneously, the start of the CINE sequence was triggered.
2.3. Specimen Preparation
Extensive research has been done on tissue-mimicking phantoms for ultrasound elastography using different materials [
30]. Water-based phantoms are made of gelatin and/or agar [
31,
32] as natural matrix materials, whereas also synthetic polymers have been used [
30]. To achieve better imaging quality and contrast, solid scatter particles are added. Their size must be chosen carefully according to the ultrasound frequency. Mostly, particles with a diameter of 0.5
–50
are used [
30,
31,
32]. For this purpose, glass beads [
31], silica [
32], talc powder [
33] etc., have been successfully tested. Further additives can be used to avoid bacterial colonization or to increase the cross-link between the gelatin molecules [
31].
The gelatin blocks were cast in a mold, which measured
; see
Figure 2. One softer and one harder mixture was produced (
Table 1). The gelatin concentration can be assumed to be proportional to the square root of the Young’s modulus of the resulting specimen [
11]. Three different kinds of specimens were produced: soft, hard and with inclusion, which consisted of both mixtures. The non-homogeneous specimens had a cylindrical inclusion with a diameter of 8
. The soft samples are called
, the stiffer ones
and samples with inclusion
. Furthermore, the different measurements were numbered chronologically M
.
The production process was the same for all samples, which followed Yengul et al. [
33]. First, gelatin from porcine skin (SKU G2500 300 Bloom Type A, Sigma-Aldrich, Vienna, Austria) was hydrated with distilled water and heated up to 45
in a water bath. After the clarification of the solution, the glass beads (SiLibeads SOLID 0-50 my, Sigmund Lindner GmbH, Warmensteinach, Germany) were added. The solution was stirred with a magnetic stirrer for an additional five minutes. Thereafter, the gelatin was cast in the prepared mold, which was lubed with petroleum jelly (TRVAS90, Silikonfabrik, Ahrensburg, Germany). The casts were rotated by a stepper motor with 5 rotations/
, in order to avoid sinking of the glass beads.
The specimens were stored at 4
overnight. After 24
in the fridge, the placeholder for the inclusion was removed. Next, the harder mixture was prepared according to the explained recipe. It was cast into the space for the inclusion and in an additional mold for a homogeneous specimen. Before measurement, the specimens were stored for 24
in the fridge. The storage time was chosen to be shorter than in Yengul et al. [
33], due to the swelling of stiffer inclusions over time [
34].
The measurement protocol for all specimens was identical. After resting in the fridge, the specimens were stored at room temperature ( 23 ) for 5 . The first measurements were five iterations of the compression measurement. After this, five iterations of elastography measurements were performed, which were followed by the indentation measurements. Therefore, for every sample, 15 measurements () were carried out. The measurement duration for the stepwise indentation and compression measurements was around 10 each and the elastography measurement took around 2 .
2.4. Mechanical Reference Measurements
The mechanical measurements were carried out to obtain reference data for the elastography results. In this section, the different evaluation methods for the mechanical measurements are presented—first, the compression measurement, followed by the indentation measurements.
2.4.1. Compression Test
The compression measurements were carried out with a stiff plate larger than the top surface of the specimen. These measurements are used to evaluate the compression modulus—Equation (
11)—of the homogeneous gelatin blocks. The specimen was compressed step-wise, in which every step took 1
, with a step width of
. The displacement was estimated by the steps of the stepper motor driving the linear drive and the compression force
F was measured after every single step. Around 20 data points were recorded; therefore, the overall compression process per iteration took around 20
per iteration. Exemplary results can be found in
Figure 3.
The Young’s modulus
E can be determined by the relation for the compressing stress
Here,
Z is a shape factor, which is related to the ratio
S of one bonded to the free surface, by
. Furthermore,
is the ratio of strained to unstrained height of the specimen [
35] (p. 155f). The samples were compressed up to a strain rate of 15%. Above this strain rate, the expanding part of the sidewalls could touch the compressing plate and thus the compressing area enlarged [
36]. Although the strain rate is close to that value, in our measurement, such behavior was not visible.
2.4.2. Indentation Test
For the indentation measurements, the homogeneous specimen was compressed step-wise by the ultrasound transducer. The data acquisition was done in the same way as in the compression measurements. The Bulychev–Alekhin–Shorshorov (BASh) relation—Equation (
12)—was used to evaluate the indentation modulus of the specimens [
26] (p. 33).
The experimental estimation of
E was done by an indentation test with a rectangular indenter, namely the ultrasound transducer. Therefore, the generalized BASh relation
was used to estimate the indentation modulus
, in which
F is the compression force,
the displacement of the indenter,
the contact area shape factor and
A the contact area [
26] (p. 33).
The contact area shape factor
defines the difference from a circular indenter and can be derived analytically [
26] (p. 34). The value for rectangular intenders varies between
[
37] and
[
38]. The ultrasound transducer is not an exact rectangle and it deforms itself. Nevertheless, a value of
fits the results estimated by a finite element simulation.
Conclusively, the Young’s modulus
E can be calculated with
2.4.3. Finite Element Model
In this section, we will examine finite element modeling of homogeneous gelatin blocks under compression. The blocks were compressed either by the ultrasound transducer or by a large compressor. The finite element models (
Figure 3) of the samples and both compression processes were designed with the GIBBON toolbox [
39] (v3.5) for Matlab and the FE model was solved by FEBio [
40] (v3.0.1) and its optimization module. This method is understood as the most exact, due to the complex boundary conditions, which are not considered to full extent in the simpler models described before [
25,
36].
The FEBio material type
Neo-Hookean was chosen, which describes non-linear stress–strain behavior with two material constants—the Young’s modulus
E and the Poisson’s ratio
. In the case of small strains, as in ours, this model reduces to the classical linear elasticity [
41]. The governing equation, describing the hyperelastic strain energy function
W, is
where
and
are the first and second Lamé parameters, respectively.
J indicates the determinant and
the first invariant of the right Cauchy–Green deformation tensor [
40,
41].
The defined boundaries of the model describing the compression with the ultrasound transducer are shown in
Figure 3. Regarding the compression test, the whole top surface was chosen as the displacement boundary. The measured displacement defines the movement of the boundary nodes shown in green in
Figure 3. The nodes indicated in red are the fixed bottom of the model. The model is well defined for a forward simulation with these boundaries (
Figure 4). To estimate the elastic modulus of the gelatin block, an inverse approach was applied. The built-in optimization module of FEBio was used, which minimizes the function
The optimization module seeks the minimum of
, in which the data points
are user-defined and
are calculated by the forward model [
40]. The Levenberg–Marquardt method is utilized to find this minimum [
42,
43]. In our case, the reaction force at the transducer gelatin interface is used as the optimization input and the elastic modulus of the gelatin is the optimization target
.
2.5. Elastography
The proposed elastography workflow to calculate the Young’s modulus of the specimen is displayed in
Figure 5. The specimens were compressed by the ultrasound transducer up to a compression depth of 3
measured from the top surface. The compression should last around one quarter up to one second [
5] (p. 62ff). Due to the limited frame rate of the ultrasound machine, the compressing speed was set to 1
/
.
The evaluation algorithm consists of four basic steps: the displacement (1) between the single frames needs to be estimated; the strain (2) is computed, which is combined with a reasonable stress assumption (3); to conclusively calculate the Young’s modulus (4) with Equation (
8). These steps will be presented in the following sections.
2.5.1. Displacement Estimation
Most elastography algorithms work with the RF signal and are able to detect movements up to a few micrometers. In our case, we use an image registration algorithm to detect the movements [
44]. The intrinsic assumption is that the acoustic properties of the probe are constant over the whole measurement process [
5,
45].
The optical flow algorithm DeepFlow [
18] was used to estimate the displacement
between the ultrasound frames. The images were preprocessed using a Gaussian Blur, because this is assumed by the DeepFlow algorithm [
18]. The displacement field describes the movement of every tissue part between the unloaded and loaded state. Working with image data and a dense optical flow algorithm, the tissue is discretized by the pixels of the ultrasound frames. Since the displacement between the unloaded frame and every flowing frame needs to be calculated, large displacements could occur. To avoid the failure of the algorithm, the performance after every frame is evaluated by correlation of the reference frame and the warped target frame, using the estimated displacement. The correlation coefficient is calculated by
in which
A and
B are the ultrasound frames
and
k, respectively, and
is the mean of
A [
46]. The
frame is warped with the estimated displacement field
; hence,
is an indicator of the quality of the optical flow field. If
falls below a predefined value, the current frame is used as the new reference frame and the displacement calculated so far is added to all further displacement fields; see
Figure 6 [
47]. The minimum correlation coefficient is chosen
.
2.5.2. Strain Estimation
The strain in the tissue can be calculated by deriving the displacement field
where
and
are the
x- and
z-components of the displacement field [
5]. Therefore, two consecutive displacement fields are computed, and this process is illustrated in
Figure 7.
The displacement field is noisy, due to random changes in the image data. Moreover, the derivation during strain computation magnifies the primary error. Thus, the displacement field needs to be smoothed or filtered [
48,
49,
50]. The strain can be directly derived, with the mentioned shortcomings, or filters can be used, such as the Least-Squares strain estimator [
51]. We use a Savitzky–Golay differentiator to compute the strain, which smooths the displacement field additionally [
19]. The 2D Savitzky–Golay differentiator is given by
The applied filter width
M determines the degree of smoothing and
indicates the grid step, which is defined by the pixel width of the ultrasound images. Further, the strain
at the point
can be calculated by
The estimated strain is used to calculate the Young’s modulus and the Poisson’s ratio, where the latter is computed by Equation (
4).
2.5.3. Stress Distribution
There is no measurement method known that is able to quantify the internal stress distribution. Therefore, the stress distribution is usually assumed constant [
6]. Love proposed an analytic solution to calculate the stress distribution in a semi-infinite, isotropic and homogeneous specimen loaded with a rectangular compressor [
24,
52,
53].
Here, we present only the solution, as the complete derivation is cumbersome and can be found in the work of Love [
24]. The stress
in compression direction
z can be written as
where
V is the Newtonian potential. The derivatives can be explicitly solved, depending on the compressing pressure
p and the geometric dimensions of the compressor
and
; see
Figure 8. The first partial derivative
can be written as
with
In this,
x,
y and
z are the coordinates of the current observation point. The second partial derivative of
V results in
with
,
,
and
indicating the distance of the observation point to the corners
A,
B,
C and
D, respectively. The pressure
p is calculated by dividing the applied force
F by the compressed area
A.
We consider two compressors at the top (transducer) and at the bottom (floor) of the phantoms. Due to this, the effect of both can be superposed with
where
is the surface of the compressing transducer and
the base area of the specimen [
54,
55,
56].
In
Figure 9, Love’s solution (
Figure 9a) is compared to a finite element simulation of a homogeneous sample (
Figure 9b). It can be seen that the stress field is quite similar. However, the relative error is still high (
Figure 9c), but lower compared to the plane-stress assumption (
Figure 9d).
The stress estimation with Love’s solution is valid for homogeneous elastic materials. Nevertheless, tissue and phantoms could and will have inclusions or stiffer parts. Therefore, we will compare the prediction of Love’s solution with the finite element simulation of a specimen with a stiffer inclusion in
Figure 10. The stress estimations with Love and the FE model are shown in Panel (a) and (b), respectively. The relative error between the FEBio result and the stress estimations is indicated in Panels (c) and (d). The difference between the two assumptions shows that, especially in the inclusion’s case, Love’s solution fits better.
Figure 11 shows the mean Young’s modulus of a homogeneous specimen calculated by the presented algorithm, in which
Figure 11a shows the results of the plane stress assumption and
Figure 11b the one of Love’s assumption. It can be clearly seen that the estimated elastic modulus is much more homogeneous with the assumptions of Love.
2.5.4. Performance Descriptor
Different approaches to dynamically choose the frames, which are taken into account for further processing, were proposed [
4,
23,
57]. To evaluate the reliability of the strain field and the elastography data, a performance descriptor, inspired by Jiang et al. [
23], was used. Jiang et al. proposed to measure the strain performance
with
where
are the normalized correlation coefficients—see Equation (
16)—between the
and
frame of the RF and the strain data, respectively. In order to achieve a meaningful correlation, the strain and RF data of the
frame were warped using the estimated displacement field. The performance indicator
varies between 0 and 1, whereat a minimum value of
was considered as trustworthy. In our case,
was replaced with
, using the image data, which represent the computed RF data. After the strain estimation, the reliability of the results was evaluated with Equation (
25). The strain frames which could not fulfill the minimum performance requirement
were discarded, as illustrated in
Figure 12.
Extending the descriptor of Jiang et al. [
23], we used a similar descriptor for the elastography algorithm. The reliability of the elastography data was computed in the same way as
for the strain data, and the resulting descriptor was called
. Therefore, the overall performance
is given by
2.5.5. Poisson’s Ratio and Young’s Modulus
The presented algorithm calculates the strain in all four directions. Therefore, using the axial and lateral components, the spatial distribution of the Poisson’s ratio can be computed [
58]. It is calculated by Equation (
4), whereby Fehrenbach [
10] showed that the two-dimensional Poisson’s ratio, now indicated as
, is related to the three-dimensional Poisson’s ratio by
At the end, the Young’s modulus
E of the sample can be calculated. This is done by using Equation (
8) in a framewise manner. The strain
is estimated by the Savitzky–Golay Differentiator Equation (
19), whereas only frames which fulfill the performance requirements of Equation (
25) are considered. The stress
is computed by Equation (
24). The force
F was continuously measured by the load cell and the compressing areas
were measured beforehand.
The results of the elastography algorithm were evaluated by the overall performance
using Equation (
26). Conclusively, the mean Young’s modulus over all frames was calculated. Therefore, the framewise results are warped on the original configuration using the estimated displacement field. In this process, all frames which did not meet the minimum performance were discarded.
2.5.6. Data Visualization
The results of the elastography were compared to the mechanical results. Hence, regions of interest were chosen and the mean value of the estimated Young’s modulus in these regions was calculated [
4]. The number of regions depends on the nature of the specimen: for homogeneous samples, one region and for specimens with inclusion three regions were defined. The selected regions of interest are shown in
Figure 13.
4. Discussion
In this paper, we present an elastography algorithm that can be easily implemented without accessing the raw RF data of the imaging device. This makes the algorithm more widely applicable because it is not limited to research systems. The use of a CINE sequence in any compression process enables the user to display strain images, often vaguely referred to as (strain) elastography. The additional force measurement allowed quantitative results. Quantitative algorithms for ultrasound elastography have been presented before, starting from the first proposals by Ophir et al. [
1] to recent works [
60]. The quantitative algorithms require either a stress indicator or a force measurement.
The underlying material model relies on assumptions, which possibly do not reflect the real material. The Young’s modulus is not constant over strain rates of 15%; in our case, we do not exceed this limit so linear approaches are sufficient [
61]. The linearity of the examined gelatin samples was experimentally confirmed by the mechanical measurements, where the samples showed linear behavior up to a 15% strain rate. Nevertheless, we take non-linear behavior into account with an approximation of a non-linear material model; see Equation (
10). The non-linearity parameter was chosen to be
, as reported in the associated literature [
5]. The method of Goenezen et al. [
28] could be applied to estimate
for gelatin and other materials or tissue. Further experiments could also validate the assumption of near incompressibility, by measuring the Poisson’s ratio, although, as Fehrenbach [
10] pointed out, the exact value of the Poisson’s ratio has little influence on the resulting Young’s modulus. The isotropy and local homogeneity are properties which were not further evaluated in this study.
The elastography results show good agreement with the mechanically estimated Young’s modulus. Comparing the median values from
Table 2 and
Table 3 for the non-homogeneous specimen, the relative error for the inclusion and the body is 34% and 24%, respectively. Taking a closer look at
Figure 21, the Young’s modulus of the inclusion seems to be drastically underestimated. This is in contrast to the expected hardening of the inclusion over time, due to the diffusion of water. Further, it would be expected that the inclusion would appear harder because of target hardening artifacts [
62]. Therefore, the possible reason for this artifact could be the temporal course of the measurement, in which the elastography measurement was always the last one. The drying of the specimen increases its hardness, whereas the inclusion was protected from this process.
Although elastic behavior is a reasonable assumption for quasi-static processes, the relatively fast compression of 1
/
during the elastography measurement could push the assumption to its limits. The viscoelastic properties, which imply that the strain and stress are out-of-phase, may become relevant. In particular, in the first few seconds after the compression, the force and therefore the stress decrease rapidly, which can be traced back to a creep in the material [
63]. During the mechanical reference measurements, the specimen had more time to find a stable state, whereas during the elastography measurement, the force was measured immediately. Further, the inertia of the specimen might play a role [
64]. Therefore, the viscoelastic nature of gelatin and tissue will be taken into account for further algorithms.
The stress indicator is determined to obtain a reference stress value or Young’s modulus to transform the strain ratios into quantitative results [
1,
8]. In this proposal, an additional load cell was used, which could be replaced by a stress indicator, e.g., an elastic reference layer between the tissue and the transducer. This would make the algorithm more widely applicable.
The extensive assumptions of Love’s stress estimation lead to shortcomings in the approach. First, the finite specimen contradicts the assumption of an infinite elastic domain. This assumption is valid for specimens which are at least four times larger than the compressor [
65]. Second, the material is considered relatively homogeneous; this assumption is valid for modulus contrasts of
[
54]. For larger contrasts, the stress distribution is not properly described by Love’s solution. A solution could be contrast-transfer efficiency, proposed by Ponnekanti et al. [
66]. Third, we assume that the compressor is uniformly loaded, whereas in reality, the surface is uniformly displaced, which corresponds to nonuniform loading [
54,
67]. The largest error due to this is caused close to the compressed surface [
24,
54,
65].
In
Figure 9 and
Figure 10, the results for
with Love’s approach are shown. For comparison, the results of FEBio for the same boundary conditions are indicated in the same figures. Some of the assumptions in Love’s approach are not met, e.g., the dimensions of the specimen are not four times the transducer size. Due to this, the match of the estimated stress fields is not perfect and the finite element approach may map the real stress distribution better.
The stress assumptions avoid the limitation of uniform stress, the so-called target hardening, by computing non-uniform stress with the stress solution of Love [
24,
56,
65]. Therefore, the stress is realistically adopted and maps the Young’s modulus with high precision. The modulus contrast could have also been calculated by the function for the modulus contrast for cylindrical inclusions, which Kallel et al. [
68] proposed and was widely used [
34,
54,
60]. In order to make the algorithm more universal and applicable to non-cylindrical inclusions, we waived this approach. Unfortunately, the stress assumption that we used does not take into account the fact that the specimen has a varying Young’s modulus. Hence, artifacts such as shadowing artifacts occur and are visible in
Figure 19 [
69]. Additionally, the stiffer specimen could itself shadow the ultrasound image. Using our mixture, this artifact was not visible.
Various algorithms for a performance evaluation of strain data were proposed [
4,
23,
57,
70]. The presented descriptor is quick to compute and can be easily extended to the description of the elastography data performance. Hence, it is an easy-to-use indicator for the user and for further computation. The indicator and therefore the trustworthiness of the result are highlighted for the user.
The presented algorithm was able to detect the inclusion and measure its Young’s modulus with a precision of a few Kilopascal. The whole algorithm is based on very few data; therefore, comprehensive assumptions were made. Nevertheless, the results support these assumptions. Using this algorithm in vivo, some adaptions may be necessary, due to the changed boundary conditions in the tissue, e.g., a non-axial compression would alter the results and violate the assumptions made. The proposed algorithm will be evaluated on tissue in the near future to test its applicability and accuracy.