Sensitivity Validation of EWOD Devices for Diagnosis of Early Mortality Syndrome (EMS) in Shrimp Using Colorimetric LAMP–XO Technique
Abstract
:1. Introduction
2. Sample Preparation
3. Electrowetting-on-Dielectric (EWOD) Platform Fabrication
3.1. Simple Fabrication Method of EWOD Lab-on-a-Chip (LOC) Components
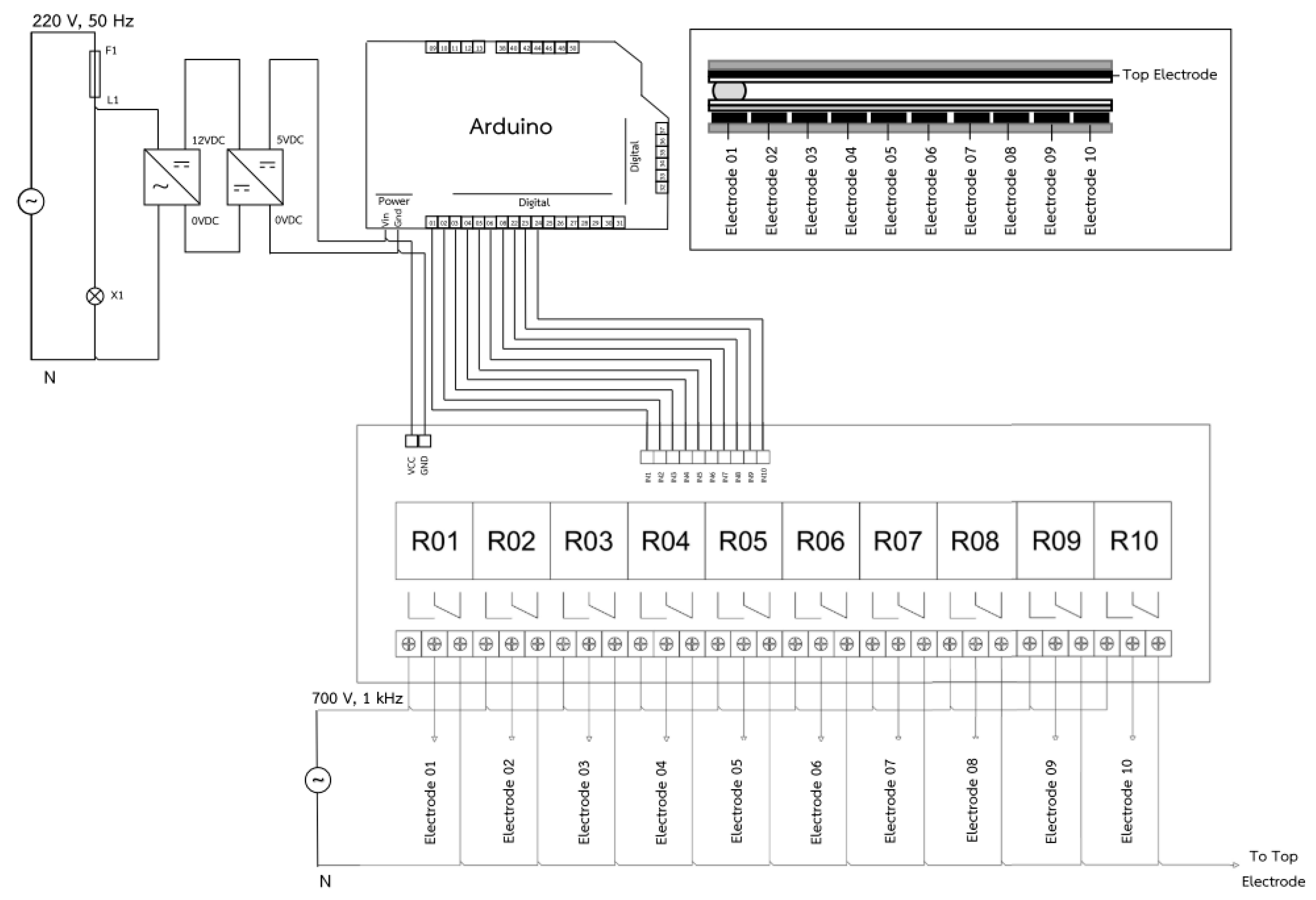
3.1.1. Electrode Design
Electrode Sizing
Electrode Pattern Design
3.1.2. Plate-through-Hole Print Circuit Board (PTH-PCB) Fabrication of Electrode Layout on the Bottom Substrate
3.1.3. Fabrication of the Dielectric and Bottom Hydrophobic Layers
3.1.4. Spacer Layer Set-Up
3.1.5. Top Plate Fabrication
3.2. Control Elements of LAMP–LOC Platform
3.2.1. Isothermal Heating and Temperature Control Elements
3.2.2. Droplet Controller
4. Qualitative Sensitivity Validation of LAMP–XO EWOD Platforms
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Donald, V.L.; Redman, R.M.; Pantoja, C.R.; Noble, B.L.; Tran, L. Early mortality syndrome affects shrimp in Asia. Advocate 2012, 15, 40. [Google Scholar]
- Zorriehzahra, J.M.; Banaederakhshan, R. Early Mortality Syndrome (EMS) as new Emerging Threat in Shrimp Industry. Adv. Anim. Vet. Sci. 2015, 3, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Sahul Hameed, A.S.; Parameswaran, V.; Syed Musthaq, S.; Sudhakaran, R.; Balasubramanian, G.; Yoganandhan, K. A simple PCR procedure to detect white spot syndrome virus (WSSV) of shrimp, Penaeus monodon (Fabricious). Aquac. Int. 2005, 13, 441–450. [Google Scholar] [CrossRef]
- Han, J.E.; Tang, K.F.J.; Pantoja, C.R.; White, B.L.; Lightner, D.V. qPCR assay for detecting and quantifying a virulence plasmid in acute hepatopancreatic necrosis disease (AHPND) due to pathogenic Vibrio parahaemolyticus. Aquac. Int. 2015, 442, 12–15. [Google Scholar] [CrossRef]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino NHase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Njiru, K.Z. Loop-Mediated Isothermal Amplification Technology: Towards Point of Care Diagnostics. PLoS Negl. Trop. Dis. 2012, 6, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milita, K.; Meada, T.; Yoshikawa, S.; Ono, T.; Miyahira, Y.; Kawana, A. The Direct Boil-LAMP method: A simple and rapid diagnostic method for cutaneous leishmaniasis. Parasitol. Int. 2014, 63, 785–789. [Google Scholar]
- Zaczek-Moczydlowska, A.M.; Mohamed-Smith, L.; Toldra, A.; Hooper, C.; Campas, M.; Furones, M.D.; Bean, P.T.; Campbell, K. A Single-Tube HNB-Based Loop-Mediated Isothermal Amplification for the Robust Detection of theOstreid herpesvirus 1. Int. J. Mol. Sci. 2020, 21, 6605. [Google Scholar] [CrossRef]
- Puthawibool, T.; Senapin, S.; Flegel, W.T.; Kiatpathomchai, W. Rapid and sensitive detection of Macrobrachium rosenbergii nodavirus in giant freshwater prawns by reverse transcription loop-mediated isothermal amplification combined with a lateral flow dipstick. Mol. Cell. Probes 2010, 24, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Kanchanaphum, P. Identification of human DNA by loop-mediated isothermal amplification (LAMP) technique combined with white ring precipitation of Cu(OH)2. Songklanakarin Thai J. Sci. Technol. 2018, 40, 738–742. [Google Scholar]
- Seetang-Nun, Y.; Jaroenrama, W.; Sriurairatana, S.; Suebsing, R.; Kiatpathomchai, W. Visual detection of white spot syndrome virus using DNA-functionalized gold nanoparticles as probes combined with loop-mediated isothermal amplification. Mol. Cell. Probes 2013, 27, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Wangta, J.; Bhoopat, T.; Bhoopat, L. Efficiency and Sensitivity of Loop Mediated Isothermal Amplification (LAMP) Method for Sex Determination from Human Blood Samples. Forensic Med. J. 2012, 4, 234–243. [Google Scholar]
- Dangtip, S.; Kampeera, J.; Suvannakad, R.; Khumwan, P.; Jaroenram, W.; Sonthi, M.; Senapin, S.; Kiatpathomchai, W. Colorimetric detection of scale drop disease virus in Asian sea bass using loop-mediated isothermal amplification with xylenol orange. Aquaculture 2019, 510, 386–391. [Google Scholar] [CrossRef]
- Karuwan, C.; Sukthang, K.; Patthanasettakul, V.; Wisitsoraat, A.; Tuantranont, A. Droplet-Based Electrochemical Detection on Electrowetting-on-Dielectric Microfluidic Chip. Talanta 2010, 84, 1384–1389. [Google Scholar] [CrossRef]
- Grant, N. Automated Sample Preparation Using Adaptive Digital Microfluidics for Lab-on-Chip Devices. Master’s Thesis, Colorado State University, Fort Collins, CO, USA, 2018. [Google Scholar]
- Karan, V.I.; Kaler, S.; Prakash, R. Droplet Microfluidics for Chip-Based Diagnostics. Sensors 2014, 14, 23283–23306. [Google Scholar]
- Dimov, N.; McDonnell, B.M.; Munro, I.; Coudron, L. Electrowetting-based Digital Microfluidics Platform for Automated Enzymelinked Immunosorbent Assay. J. Vis. Exp. 2020, 156. [Google Scholar] [CrossRef]
- Taylor, J.B.; Howell, A.; Yanow, K.S. A lab-on-chip for malaria diagnosis and surveillance. Malaria J. 2014, 13, 179. [Google Scholar] [CrossRef] [Green Version]
- Rival, A.; Jary, D.; Delattre, C.; Fouillet, Y.; Castellan, G.; Bellemin-Comte, A.; Gidrol, X. An EWOD-based microfluidic chip for single-cell isolation, mRNA purification and subsequent multiplex qPCR. Lab Chip 2014, 14, 3739–3749. [Google Scholar] [CrossRef]
- Fan, S.K.; Huang, P.W.; Wang, T.T.; Peng, Y.H. Cross-scale electric manipulations of cells and droplets by frequency-modulated dielectrophoresis and electrowetting. Lab Chip 2008, 8, 1325–1331. [Google Scholar] [CrossRef]
- Shah, P.; Zhu, X.; Chen, C.; Hu, Y.; Li, C.Z. Lab-on-chip device for single cell trapping and analysis. Biomed. Microdevices 2014, 16, 35–41. [Google Scholar] [CrossRef]
- Jebrail, M.J.; Yang, H.; Mudrik, J.M.; Lafreniere, N.M.; McRoberts, C. A digital microfluidic method for dried blood spot analysis. Lab Chip 2011, 11, 3218–3224. [Google Scholar] [CrossRef]
- Barbulovic-Nad, I.; Yang, H.; Park, P.S.; Wheeler, A.R. Digital microfluidics for cell-based assays. Lab Chip 2008, 8, 519–526. [Google Scholar] [CrossRef]
- Fouillet, Y.; Jary, D.; Brachet, A.G.; Berthier, J.; Blervaque, R.; Davous, L.; Roux, J.M.; Achard, J.L.; Peponnet, C. Ewod digital microfluidics for lab on a chip. In Proceedings of the 4th ASME ICNMM 2006, Limerick, Ireland, 19–21 June 2006. [Google Scholar]
- Beatriz, C.; Bruno, V.; Pedro, V.B. Digital Microfluidics for Nucleic Acid Amplification. Sensors 2017, 17, 1495. [Google Scholar] [CrossRef] [Green Version]
- Fair, R.B. Digital microfluidics: Is a true lab-on-a-chip possible? Microfluid Nanofluid 2007, 3, 245–281. [Google Scholar] [CrossRef]
- Walker, W.S.; Shapiro, B. Modeling the Fluid Dynamics of Electrowetting on Dielectric (EWOD). J. Microelectromech. Syst. 2006, 15, 986–1000. [Google Scholar] [CrossRef]
- Yuhua, Y.; Jianfeng, C.; Jia, Z. Parallel-plate lab-on-a-chip based on digital microfluidics for on-chip electrochemical analysis. J. Micromech. Microeng. 2014, 24, 1–7. [Google Scholar]
- Arunrut, N.; Kampeera, J.; Sirithammajak, S.; Sanguanrut, P.; Proespraiwong, P.; Suebsing, R.; Kiatpathomchai, W. Sensitive Visual Detection of AHPND Bacteria Using Loop-Mediated Isothermal Amplification Combined with DNA Functionalized Gold Nanoparticles as Probes. PLoS ONE 2016, 11, 1–18. [Google Scholar] [CrossRef]
- Malk, R.; Fouillet, Y.; Davoust, L. Rotating flow within a droplet actuated with AC EWOD. Sens. Actuators B Chem. 2011, 154, 191–198. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Chen, T.; Dong, C.; Jia, Y.; Mak, P.; Vai, M.; Martins, R.P. Adhesion promoter for a multi-dielectric-layer on a digital microfluidic chip. RSC Adv. 2015, 5, 48626–48630. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Zhang, K.; Wang, X.; Zhang, J.; Liu, H.; Zhou, J. Feedback control system for large scale 2 digital microfluidic platforms. Sens. Actuators B Chem. 2018, 255, 3616–3622. [Google Scholar] [CrossRef]
- Vandana, J.; Pravin, R.; Raghvendra, D.; Rajendra, P. Design, fabrication and characterization of low cost printed circuit board based EWOD device for digital microfluidics applications. Microsyst. Technol. 2015, 21, 11. [Google Scholar] [CrossRef]
- Yafia, M.; Naffaran, H. Electrowetting on screen printed paper based substrate. Nanotechnology 2014, 2, 125–128. [Google Scholar]
- Gong, J.; Kim, C.J. Direct-referencing Two-dimensional-array Digital Microfluidics Using Multi-layer Printed Circuit Board. J. Microelectromech. Syst. 2008, 17, 257–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thitamadee, S.; Prachumwat, A.; Srisala, J.; Jaroenlak, P.; Salachan, P.V.; Sritunyalucksana, K.; Flegel, T.W.; Itsathitphaisarn, O. Review of current disease threats for cultivated penaeid shrimp in Asia. Aquaculture 2016, 452, 69–87. [Google Scholar] [CrossRef]















| Amplification Technique | Detection Technique | Chemical Liquid for DNA Amplification | |
|---|---|---|---|
| Substance | Volume [µL] | ||
| Reverse transcription loop-mediated isothermal amplification (RT-LAMP) [9] | Gel Electrophoresis | LAMP reaction mixture | 25 |
| RNA Template | 2 | ||
| Loop-mediated isothermal amplification (LAMP) and polymerase chain reaction (PCR) [10] | Gel Electrophoresis and Visual Inspection | LAMP reaction mixture | 25 |
| Reaction products | 10 | ||
| Loop-mediated isothermal amplification combined with DNA functionalized gold nanoparticles as probes (LAMP–AuNP) [11] | Colorimetric Detection | LAMP reaction mixture | 25 |
| Product Volume | 12 | ||
| DNA-functionalized AuNPs | 5 | ||
| 0.18 M MgSO4 | 2 | ||
| Loop-mediated isothermal amplification (LAMP) [12] | Agarose Gel | Primer Mix | 25 |
| Product | 5 | ||
| Loading dye | 1 | ||
| Colorimetric loop-mediated isothermal amplification method with pH-sensitive xylenol orange (LAMP–XO) [13] | Colorimetric Detection | RNA Template | 2 |
| LAMP reaction mixture | 23 | ||
| Reference | Application | Procedure | Details of Droplet | |
|---|---|---|---|---|
| Substance | Volume [µL] | |||
| Karuwan, C. et al. [14] | The electrowetting-on-dielectric (EWOD) electrochemical system for iodide droplet detection | Electrochemical detection | Buffer solution (i.e., hexahydroxy, HCL, etc.) | 16 |
| Grant, N. [15] | Electrowetting-on-dielectric (EWOD) for the extraction and isolation of DNA | Droplet preparation | DNA sample (blood) | 0.26 |
| PNI buffer | 1.3 | |||
| PE buffer | 1.3 | |||
| Elution buffer | 0.78 | |||
| Karan V. et al. [16] | Real-time quantitative PCR microchip for influenza virus detection in human samples (nasopharyngeal swabs; throat swabs) | RT-PCR | PCR reagent mixture | 5 |
| RNA | 5 | |||
| Dimov, N. et al. [17] | Droplet microfluidics (DMF) immunoassay platform for direct detection of pathogens | Real-time chemiluminescent measurements | Running buffer | 2.5 |
| Pathogen antigens | 2.5 | |||
| Pathogen samples | 2.5 | |||
| Taylor, J. B. et al. [18] | Hydrogel wax chip for malaria diagnosis | RT-PCR | Mastermixes | 13 |
| DNA Template | 8.5 | |||
| Rival, A. et al. [19] | The isolation of a single cell, mRNA purification and gene expression analysis on EWOD platforms | mRNA extraction | qRT-PCR kit (i.e., buffer A, buffer B, elution buffer, etc.) | 0.256–0.036 |
| Fan, S. K. et al. [20] | Dielectrophoresis (DEP) and electrowetting-on-dielectric (EWOD) for concentration improvement of droplets containing neuroblastoma cells | Manipulated concentration of cell droplets | Neuroblastoma cells and polystyrene beads | 0.45 |
| Shah, P. et al. [21] | Single-cell nanotoxicity analysis using pDEP technique | Electrochemical detection | Trypan blue solution, PBS buffer, etc. | 10 |
| Jebrail, M. J. et al. [22] | Microfluidic system for quantification of amino acids in dried blood spots | DBS sample analysis | Blood samples | 5 |
| Solvent | 10 | |||
| Barbulovic-Nad, I. et al. [23] | A cytotoxicity assay using Jurkat T-cells on a digital microfluidic platform | Cell-based assays | Jurkat T-cells (i.e., Tween 20, dyes, etc.) | 0.15 |
| Component | Final Concentration | Standard Laboratory Volume [μL] | LAMP–LOC Test Volume [μL] |
|---|---|---|---|
| 100 μM FIP (EMS) | 2 μM | 0.5 | 0.5 |
| 100 μM BIP (EMS) | 2 μM | 0.5 | 0.5 |
| 10 μM F3 (EMS) | 0.2 μM | 0.5 | 0.5 |
| 10 μM B3 (EMS) | 0.2 μM | 0.5 | 0.5 |
| 100 μM LF (EMS)) | 2 μM | 0.5 | 0.5 |
| 100 μM LB (EMS) | 2 μM | 0.5 | 0.5 |
| 10× low buffer for LAMP dye (pH 8.5) | 1× | 2.5 | 2.5 |
| 100 mM MgSO4 | 6 mM | 1.5 | 1.5 |
| 10 mM dNTPs mix | 1.2 mM | 3 | 3 |
| 5 M Betaine (Sigma) | 0.4 M | 2 | 2 |
| 5 mM pH-sensitive dye (xylenol orange; XO) | 0.12 mM | 0.6 | 0.6 |
| Sterile distilled water | 9.4 | 9.4 | |
| 8 U/µL Bst 2.0 WarmStart® DNA Polymerase | 8 U | 1.0 | 1.0 |
| EMS DNA plasmid | 2 | 3 | |
| Total volume | 25 | 26 | |
| Primers | Sequence (5′-3′) | Length (bp) |
|---|---|---|
| FIP-EMS | CGTTTGGTTCGACAGTCCAATTTTTATGAGTAACAATATAAAACATGA | 48 |
| BIP-EMS | GAGGCGGTCACAGAACTAGACATTTTCCCGTATTCTCAATGTCTACAC | 47 |
| F3-EMS | GTGCAATTTAATAGGAGAACATC | 23 |
| B3-EMS | GATTGGTAAGCTCCCCAC | 18 |
| LF-EMS | CGTGAGAATAGTCAGTT | 17 |
| LB-EMS | ACATACACCTATCATCCCGGAAG | 23 |
| Manufacturing Technology | Fabrication Method | Electrical Compatibility | Typical Feature Size | Typical Device Size | Array Electrode Design | Typical Fabrication Cost |
|---|---|---|---|---|---|---|
| IC industry * | Thin-film planar and photolithography | 2–10 layers | 0.13–1 µm | 1–100 mm | No | USD 31/cm2 |
| PTH-PCB * | Electroplating and multi-layer lamination | 2–30 layers | 75–250 µm | 1–100 mm | Yes | USD 0.021/cm2 |
| PCB | Photolithography | 1 layer | 250 µm–10 mm | 2–100 mm | No | USD 0.018/cm2 |
| Screen-printing | Screen-printing | 1 layer | 350 µm–10 mm | 2–100 mm | No | USD 0.0036/cm2 |
| Polydimethylsiloxane (PDMS) molding * | Molding and soft lithography | 1 layer | 10 µm–10 mm | 1–10 mm | No | Not sold commercially |
| In-house cleanroom * | Thin-film planar and photolithography | 1–3 layer | 2–100 µm | 1–100 mm | No | USD 2/cm2 |
| Sample Type | LAMP Amplification and AGE Detection [29] | LAMP–AuNP [29] | LAMP–XO on Thermal Cycler | LAMP–XO on LAMP–LOC Platform | |
|---|---|---|---|---|---|
| Results | Repeatability | ||||
| Negative control (sterile distilled water) | Negative | Negative | Negative | Negative | 5/5 (100%) |
| EMS DNA plasmid; 102 copies (LOD—limit of detection) | Positive | Positive | Positive | Positive | 5/5 (100%) |
| EMS DNA plasmid; 103 copies | Positive | Positive | Positive | Positive | 5/5 (100%) |
| Positive control (EMS DNA plasmid; 108 copies) | N/A | N/A | Positive | Positive | 5/5 (100%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sukthang, K.; Kampeera, J.; Sriprachuabwong, C.; Kiatpathomchai, W.; Pengwang, E.; Tuantranont, A.; Wechsatol, W. Sensitivity Validation of EWOD Devices for Diagnosis of Early Mortality Syndrome (EMS) in Shrimp Using Colorimetric LAMP–XO Technique. Sensors 2021, 21, 3126. https://doi.org/10.3390/s21093126
Sukthang K, Kampeera J, Sriprachuabwong C, Kiatpathomchai W, Pengwang E, Tuantranont A, Wechsatol W. Sensitivity Validation of EWOD Devices for Diagnosis of Early Mortality Syndrome (EMS) in Shrimp Using Colorimetric LAMP–XO Technique. Sensors. 2021; 21(9):3126. https://doi.org/10.3390/s21093126
Chicago/Turabian StyleSukthang, Kreeta, Jantana Kampeera, Chakrit Sriprachuabwong, Wansika Kiatpathomchai, Eakkachai Pengwang, Adisorn Tuantranont, and Wishsanuruk Wechsatol. 2021. "Sensitivity Validation of EWOD Devices for Diagnosis of Early Mortality Syndrome (EMS) in Shrimp Using Colorimetric LAMP–XO Technique" Sensors 21, no. 9: 3126. https://doi.org/10.3390/s21093126
APA StyleSukthang, K., Kampeera, J., Sriprachuabwong, C., Kiatpathomchai, W., Pengwang, E., Tuantranont, A., & Wechsatol, W. (2021). Sensitivity Validation of EWOD Devices for Diagnosis of Early Mortality Syndrome (EMS) in Shrimp Using Colorimetric LAMP–XO Technique. Sensors, 21(9), 3126. https://doi.org/10.3390/s21093126





