A Comparison of Inertial Measurement Unit and Motion Capture Measurements of Tibiofemoral Kinematics during Simulated Pivot Landings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimen Procurement and Preparation
2.2. Testing Apparatus
2.3. Data Analysis
2.3.1. Knee Joint Angle Calculations
2.3.2. Comparison of the Knee Angles between the Two Measurement Methods
2.3.3. Peak Tibial and Femoral Angular Velocity Change Calculations
2.3.4. Comparison of the Peak Tibial and Femoral Angular Velocity Changes Using the Two Measurement Systems
3. Results
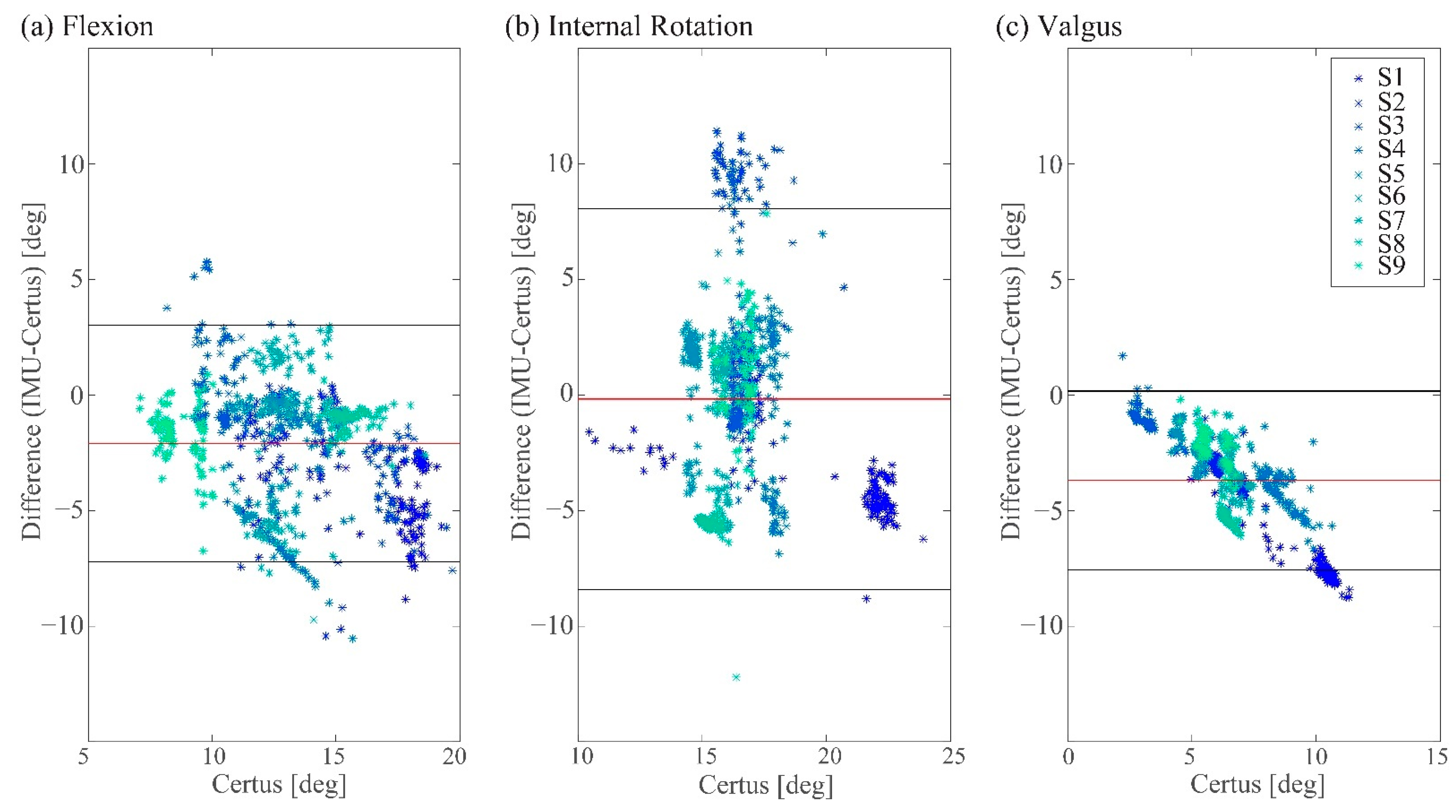
3.1. Measured Peak Knee Angle Changes
3.2. Comparison of the Tibial and Femoral Angular Velocity Changes Calculated from Certus System Data with IMU Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Musahl, V.; Karlsson, J. Anterior cruciate ligament tear. N. Engl. J. Med. 2019, 380, 2341–2348. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.K.; Lipps, D.B.; Ashton-Miller, J.A.; Wojtys, E.M. What strains the anterior cruciate ligament during a pivot landing? Am. J. Sports Med. 2012, 40, 574–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipps, D.B.; Wojtys, E.M.; Ashton-Miller, J.A. Anterior cruciate ligament fatigue failures in knees subjected to repeated simulated pivot landings. Am. J. Sports Med. 2013, 41, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, M.L.; Ashton-Miller, J.A.; Wojtys, E.M. Loading mechanisms of the anterior cruciate ligament. Sport. Biomech. 2021, 1–29. [Google Scholar] [CrossRef]
- Kaeding, C.C.; Léger-St-Jean, B.; Magnussen, R.A. Epidemiology and diagnosis of anterior cruciate ligament injuries. Clin. Sports Med. 2017, 36, 1–8. [Google Scholar] [CrossRef]
- Kim, H.Y.; Ahn, J.; Park, K.Y.; Kim, K.; Cha, Y.; Lee, K.; Kim, H. Translational movement of the tibiofemoral joint in normal gait: 3-Dimensional measurement. J. Korean Orthop. Res. Soc. 2008, 11, 37–43. [Google Scholar]
- Wojtys, E.M.; Beaulieu, M.L.; Ashton-Miller, J.A. New perspectives on ACL injury: On the role of repetitive sub-maximal knee loading in causing ACL fatigue failure. J. Orthop. Res. 2016, 34, 2059–2068. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, T.; Saito, H. Tests of wireless wearable sensor system in joint angle measurement of lower limbs. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 5469–5472. [Google Scholar] [CrossRef]
- Bell, K.M.; Onyeukwu, C.; McClincy, M.P.; Allen, M.; Bechard, L.; Mukherjee, A.; Hartman, R.A.; Smith, C.; Lynch, A.D.; Irrgang, J.J. Verification of a portable motion tracking system for remote management of physical rehabilitation of the knee. Sensors 2019, 19, 1021. [Google Scholar] [CrossRef] [Green Version]
- Dahl, K.D.; Dunford, K.M.; Wilson, S.A.; Turnbull, T.L.; Tashman, S. Wearable sensor validation of sports-related movements for the lower extremity and trunk. Med. Eng. Phys. 2020, 84, 144–150. [Google Scholar] [CrossRef]
- Roell, M.; Mahler, H.; Lienhard, J.; Gehring, D.; Gollhofer, A.; Roecker, K. Validation of wearable sensors during team sport-specific movements in indoor environments. Sensors 2019, 19, 3458. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, M.; Jaitner, T.; Nolte, K.; Rheinländer, C.; Wille, S.; Wehn, N. A wearable inertial sensor unit for jump diagnosis in multiple athletes. In Proceedings of the 2nd International Congresson Sports Sciences Research and Technology Support (icSPORTS), Rome, Italy, 24–26 October 2014; pp. 216–220. [Google Scholar] [CrossRef] [Green Version]
- Zügner, R.; Tranberg, R.; Timperley, J.; Hodgins, D.; Mohaddes, M.; Kärrholm, J. Validation of inertial measurement units with optical tracking system in patients operated with total hip arthroplasty. BMC Musculoskelet. Disord. 2019, 20, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maletsky, L.P.; Sun, J.; Morton, N.A. Accuracy of an optical active-marker system to track the relative motion of rigid bodies. J. Biomech. 2007, 40, 682–685. [Google Scholar] [CrossRef] [PubMed]
- Garling, E.H.; Kaptein, B.L.; Mertens, B.; Barendregt, W.; Veeger, H.E.J.; Nelissen, R.G.H.H.; Valstar, E.R. Soft-tissue artefact assessment during step-up using fluoroscopy and skin-mounted markers. J. Biomech. 2007, 40, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Akbarshahi, M.; Schache, A.G.; Fernandez, J.W.; Baker, R.; Banks, S.; Pandy, M.G. Non-invasive assessment of soft-tissue artifact and its effect on knee joint kinematics during functional activity. J. Biomech. 2010, 43, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- Feuvrier, F.; Sijobert, B.; Azevedo, C.; Griffiths, K.; Alonso, S.; Dupeyron, A.; Laffont, I.; Froger, J. Inertial measurement unit compared to an optical motion capturing system in post-stroke individuals with foot-drop syndrome. Ann. Phys. Rehabil. Med. 2020, 63, 195–201. [Google Scholar] [CrossRef]
- Sangeux, M.; Marin, F.; Charleux, F.; Dürselen, L.; Ho Ba Tho, M.C. Quantification of the 3D relative movement of external marker sets vs. bones based on magnetic resonance imaging. Clin. Biomech. 2006, 21, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Vitali, R.V.; Cain, S.M.; McGinnis, R.S.; Zaferiou, A.M.; Ojeda, L.V.; Davidson, S.P.; Perkins, N.C. Method for estimating three-dimensional knee rotations using two inertial measurement units: Validation with a coordinate measurement machine. Sensors 2017, 17, 1970. [Google Scholar] [CrossRef] [Green Version]
- Oh, Y.K.; Kreinbrink, J.L.; Ashton-Miller, J.A.; Wojtys, E.M. Effect of ACL transection on internal tibial rotation in an in vitro simulated pivot landing. J. Bone Jt. Surg.—Ser. A 2011, 93, 372–380. [Google Scholar] [CrossRef] [Green Version]
- Withrow, T.J.; Huston, L.J.; Wojtys, E.M.; Ashton-Miller, J.A. The relatioship between quadriceps muscle force, knee flexion, and anterior cruciate ligament strain in an in vitro simulated jump landing. Am. J. Sports Med. 2006, 34, 269–274. [Google Scholar] [CrossRef]
- Withrow, T.J.; Huston, L.J.; Wojtys, E.M.; Ashton-Miller, J.A. Effect of varying hamstring tension on anterior cruciate ligament strain during in vitro impulsive knee flexion and compression loading. J. Bone Jt. Surg. 2008, 90, 815–823. [Google Scholar] [CrossRef] [Green Version]
- Thompson, S.; Penney, G.; Dasgupta, P.; Hawkes, D. Improved modelling of tool tracking errors by modelling dependent marker errors. IEEE Trans. Med. Imaging 2013, 32, 165–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richards, J.G. The measurement of human motion: A comparison of commercially available systems. Hum. Mov. Sci. 1999, 18, 589–602. [Google Scholar] [CrossRef]
- Small, C.F.; Bryant, J.T.; Dwosh, I.L.; Griffiths, P.M.; Pichora, D.R.; Zee, B. Validation of a 3D optoelectronic motion analysis system for the wrist joint. Clin. Biomech. 1996, 11, 481–483. [Google Scholar] [CrossRef]
- Washabaugh, E.P.; Kalyanaraman, T.; Adamczyk, P.G.; Claflin, E.S.; Krishnan, C. Validity and repeatability of inertial measurement units for measuring gait parameters. Gait Posture 2017, 55, 87–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grood, E.S.; Suntay, W.J. A joint coordinate system for the clinical description of three-dimensional motions: Application to the knee. J. Biomech. Eng. 1983, 105, 136–144. [Google Scholar] [CrossRef]
- Ajdaroski, M.; Tadakala, R.; Nichols, L.; Esquivel, A. Validation of a device to measure knee joint angles for a dynamic movement. Sensors 2020, 20, 1747. [Google Scholar] [CrossRef] [Green Version]
- Winter, D.A. Biomechanics and Motor Control of Human Movement, 4th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, US, 2009; ISBN 9780470398180. [Google Scholar]
- Cong, Y.; Lam, W.K. Effects of shear reduction shoes on joint loading, ground reaction force and free moment across different cutting angles. J. Sports Sci. 2021, 39, 1386–1394. [Google Scholar] [CrossRef]
- DeGoede, K.M.; Ashton-Miller, J.A. Fall arrest strategy affects peak hand impact force in a forward fall. J. Biomech. 2002, 35, 843–848. [Google Scholar] [CrossRef]
- Altman, D.G.; Bland, T.M. Measurement in medicine: The analysis of method comparison studies. Statistician 1983, 32, 307–317. [Google Scholar] [CrossRef]
- Giavarina, D. Understanding Bland Altman analysis. Biochem. Medica 2015, 25, 141–151. [Google Scholar] [CrossRef] [Green Version]
- Olofsen, E.; Dahan, A.; Borsboom, G.; Drummond, G. Improvements in the application and reporting of advanced Bland–Altman methods of comparison. J. Clin. Monit. Comput. 2014, 29, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Ashton-Miller, J.A. Age and gender effects on the proximal propagation of an impulsive force along the adult human upper extremity. Ann. Biomed. Eng. 2014, 42, 25–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ajdaroski, M.; Ashton-Miller, J.A.; Baek, S.Y.; Shahshahani, P.M.; Esquivel, A.O. Testing a quaternion conversion method to determine human three-dimensional tibiofemoral angles during an in vitro simulated jump landing. J. Biomech. Eng. 2022, 144, 041002. [Google Scholar] [CrossRef] [PubMed]
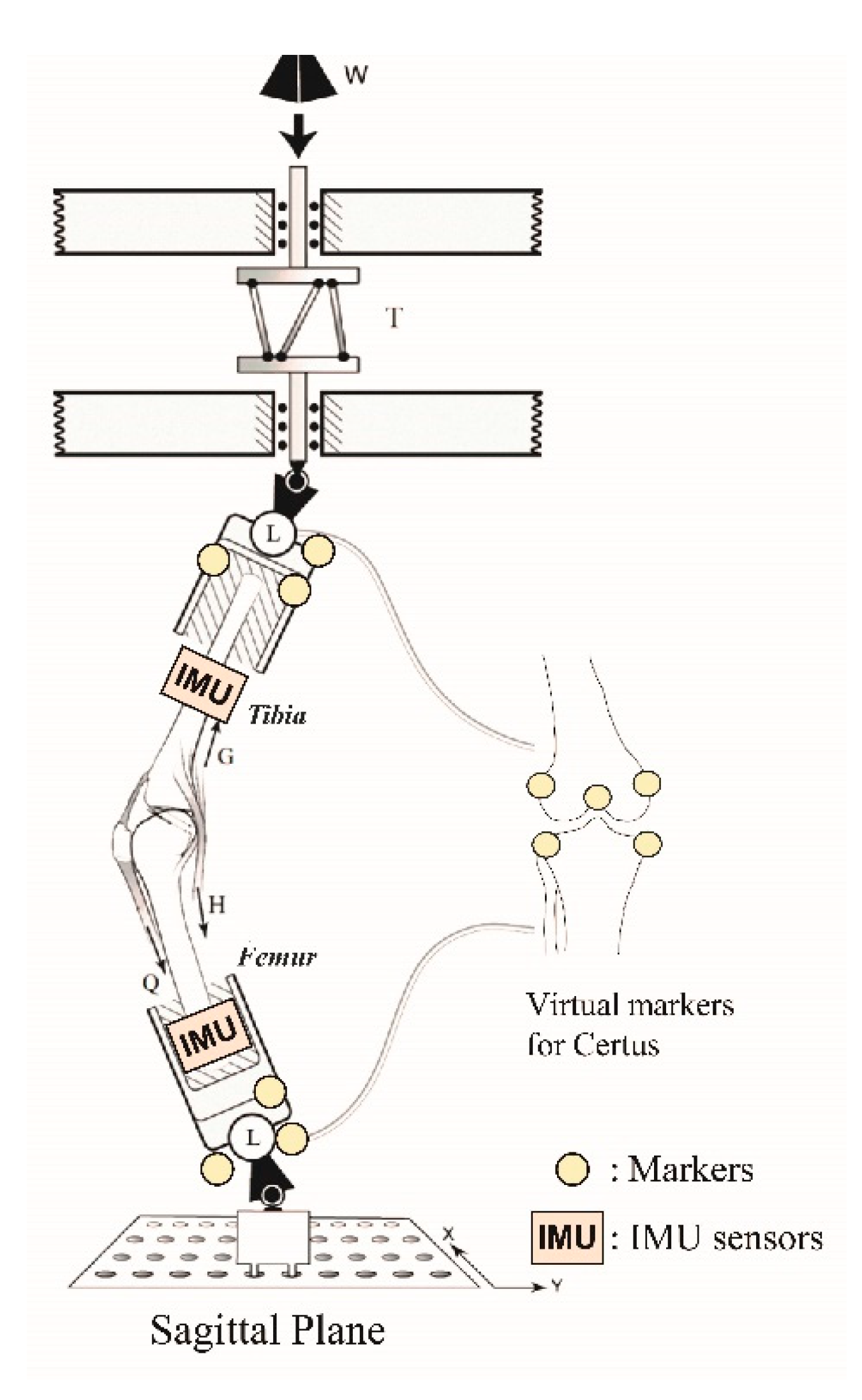


| Specimen No. | Gender | Side | Age [years] | Weight [kg] | Testing Condition |
|---|---|---|---|---|---|
| S1 | F | L | 20 | 86.6 | 3 BW 100 trials |
| S2 | F | R | 28 | 63.5 | 4 BW 100 trials |
| S3 | F | R | 30 | 82.1 | 3 BW 52 trials * |
| S4 | M | L | 39 | 54.4 | 4 BW 100 trials |
| S5 | M | R | 32 | 68.0 | 4 BW 100 trials |
| S6 | M | R | 32 | 88.5 | 3 BW 100 trials |
| S7 | M | R | 25 | 86.2 | 3 BW 100 trials |
| S8 | M | R | 31 | 92.5 | 3 BW 100 trials |
| S9 | M | R | 33 | 49.9 | 4 BW 100 trials |
| Total | 852 trials | ||||
| Flexion | Internal Rotation | Valgus | |
|---|---|---|---|
| Certus | 13.3 (2.9)° | 16.7 (2.1)° | 6.6 (2.1)° |
| IMU | 11.2 (3.2)° | 16.6 (4.3)° | 2.9 (1.1)° |
| Bias | −2.1° | −0.2° | −3.0° |
| LoA | (−7.2°, 3.0°) | (−8.4°, 8.1°) | (−7.6°, 0.2°) |
| Diff [%] | −15.3% (19.8%) | −0.6% (25.4%) | −52.8% (18.3%) |
| Agreement Range [%] | ±38.8% | ±49.8% | ±35.9% |
| Certus [°/s] | IMU [°/s] | Certus-IMU [°/s] | LoA [°/s] | Diff [%] | |
|---|---|---|---|---|---|
| Sagittal plane | |||||
| Tibia | 182.3 (32.6) | 109.7 (31.5) | 72.7 (28.0) | (17.8, 127.5) | 75.4% (38.6%) |
| Femur | 159.7 (33.0) | 195.5 (41.7) | −35.8 (16.6) | (−68.3, −3.3) | −17.9% (6.5%) |
| Transverse plane | |||||
| Tibia | 673.4 (103.2) | 832.5 (120.2) | −159.1 (75.9) | (−308.0, −10.3) | −18.9% (7.3%) |
| Femur | 188.8 (45.3) | 300.8 (131.6) | −112.0 (97.1) | (−302.3, 78.2) | −31.8% (17.4%) |
| Frontal plane | |||||
| Tibia | 684.9 (117.5) | 148.6 (38.4) | 536.3 (95.8) | (348.5, 724.1) | 384.4% (121.6%) |
| Femur | 191.3 (65.1) | 90.9 (19.2) | 100.4 (60.2) | (−17.6, 218.4) | 115.5% (75.3%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, S.Y.; Ajdaroski, M.; Shahshahani, P.M.; Beaulieu, M.L.; Esquivel, A.O.; Ashton-Miller, J.A. A Comparison of Inertial Measurement Unit and Motion Capture Measurements of Tibiofemoral Kinematics during Simulated Pivot Landings. Sensors 2022, 22, 4433. https://doi.org/10.3390/s22124433
Baek SY, Ajdaroski M, Shahshahani PM, Beaulieu ML, Esquivel AO, Ashton-Miller JA. A Comparison of Inertial Measurement Unit and Motion Capture Measurements of Tibiofemoral Kinematics during Simulated Pivot Landings. Sensors. 2022; 22(12):4433. https://doi.org/10.3390/s22124433
Chicago/Turabian StyleBaek, So Young, Mirel Ajdaroski, Payam Mirshams Shahshahani, Mélanie L. Beaulieu, Amanda O. Esquivel, and James A. Ashton-Miller. 2022. "A Comparison of Inertial Measurement Unit and Motion Capture Measurements of Tibiofemoral Kinematics during Simulated Pivot Landings" Sensors 22, no. 12: 4433. https://doi.org/10.3390/s22124433






