Flexible Machine Learning Algorithms for Clinical Gait Assessment Tools
Abstract
:1. Introduction
2. Materials and Methods
2.1. Algorithm Development for Laboratory-Based 3D CGA Data
2.1.1. 3D CGA Patient Recordings
2.1.2. 3D CGA Data Processing and Spatio-Temporal Parameter Computation
2.1.3. Implementation, Training and Accuracy Testing of the AI Tool
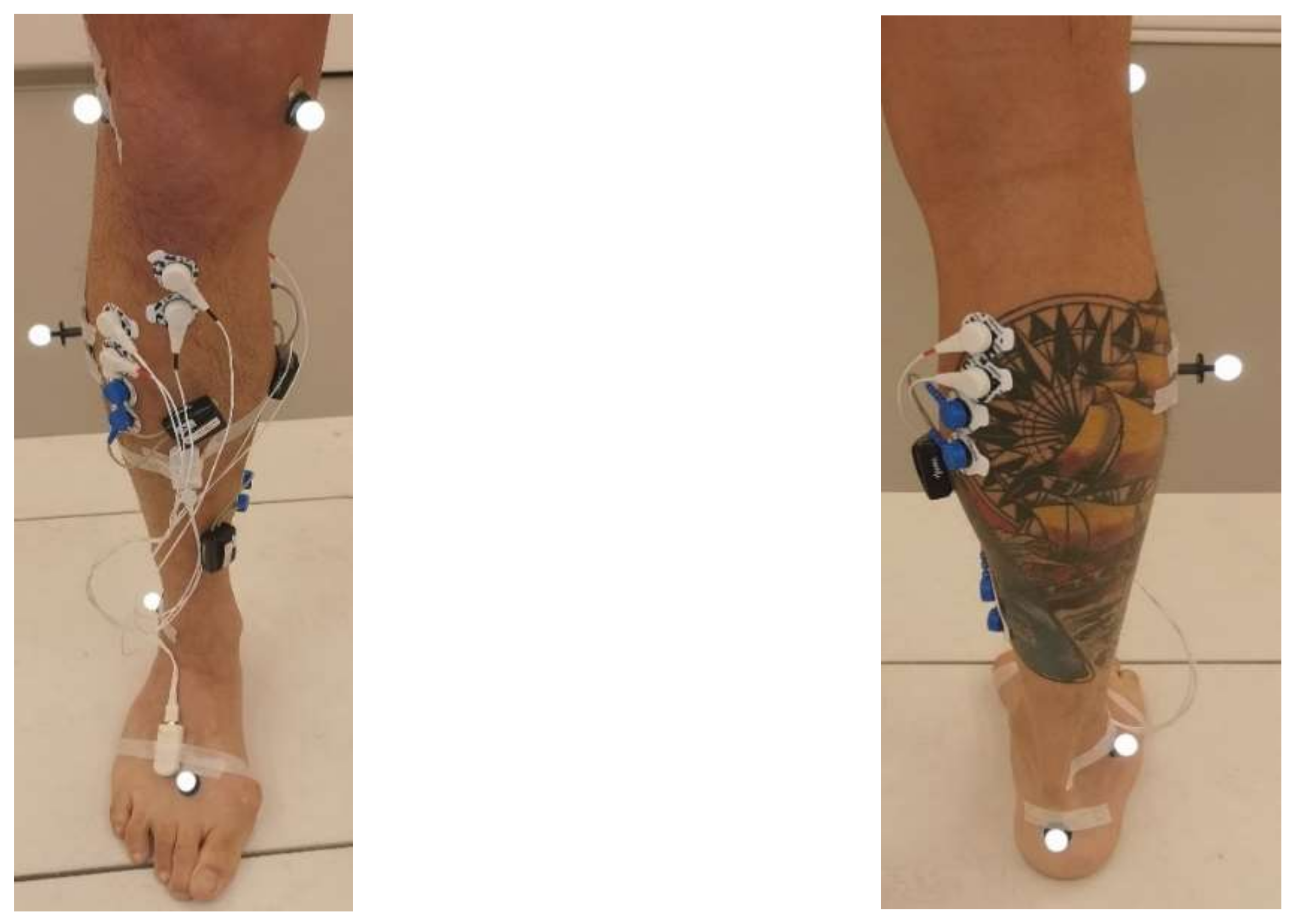
2.2. Experimental Validation with IMU System and Clinical Use Case
Experimental Procedure and Data Collection
3. Results
3.1. Laboratory-Based 3D CGA
3.1.1. Training and Test Datasets
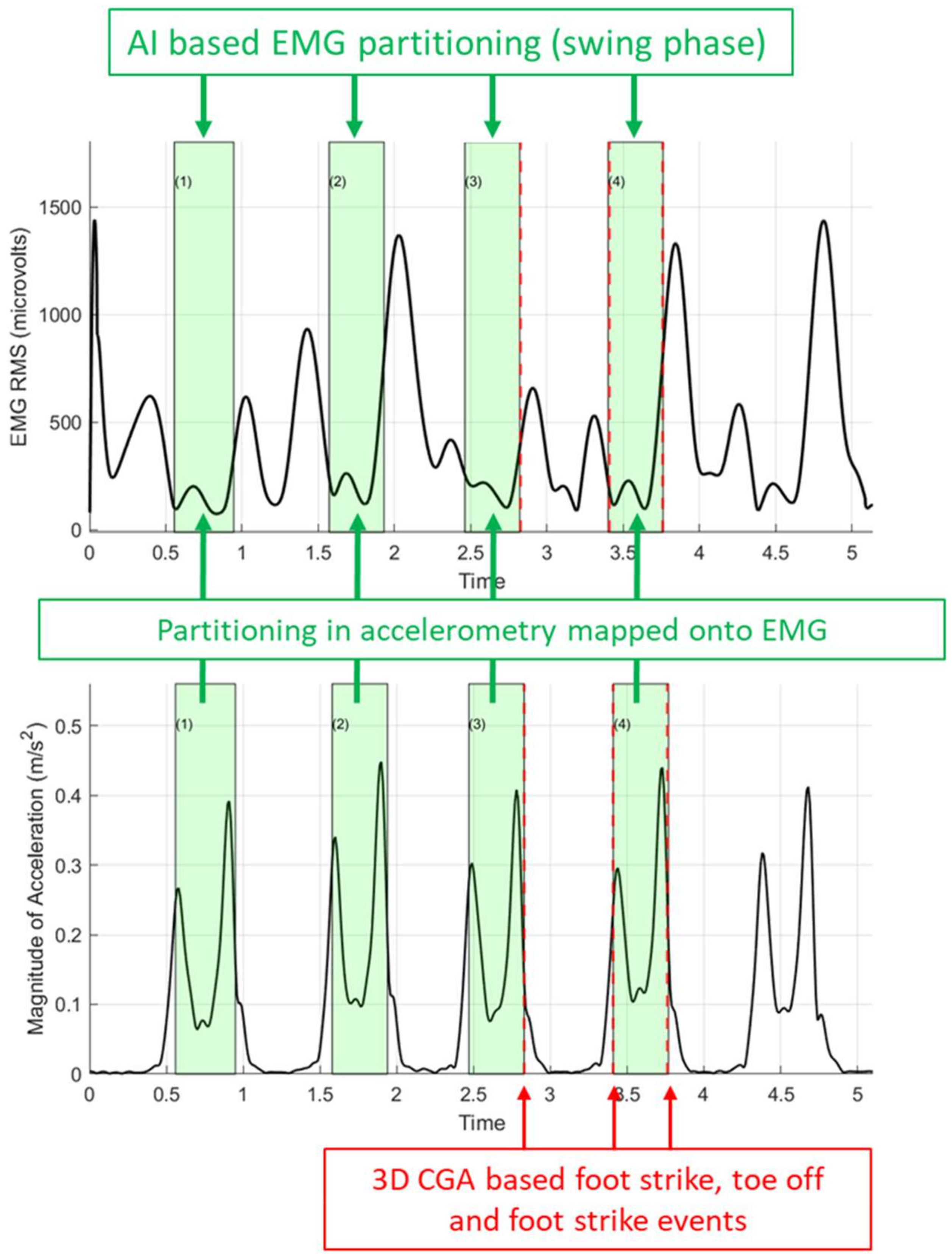
3.1.2. Partitioning Accuracy
3.2. Wearable Sensor-Based CGA
3.2.1. Participant Characteristics
3.2.2. Datasets and Algorithm Training
3.2.3. Partitioning Accuracy
3.2.4. Clinical Use Case
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jang, S.H. The recovery of walking in stroke patients: A review. Int. J. Rehabil. Res. 2010, 33, 285–289. [Google Scholar] [CrossRef]
- Langhorne, P.; Coupar, F.; Pollock, A. Motor recovery after stroke: A systematic review. Lancet Neurol. 2009, 8, 741–754. [Google Scholar] [CrossRef]
- van de Port, I.; Kwakkel, G.; Lindeman, E. Community ambulation in patients with chronic stroke: How is it related to gait speed? J. Rehabil. Med. 2008, 40, 23–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wren, T.A.L.; Tucker, C.A.; Rethlefsen, S.A.; Gorton, G.E.; Õunpuu, S. Clinical efficacy of instrumented gait analysis: Systematic review 2020 update. Gait Posture 2020, 80, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.; Esquenazi, A.; Benedetti, M.G.; Desloovere, K. Gait analysis: Clinical facts. Eur. J. Phys. Rehabil. Med. 2016, 52, 560–574. [Google Scholar] [PubMed]
- Ferrarin, M.; Rabuffetti, M.; Bacchini, M.; Casiraghi, A.; Castagna, A.; Pizzi, A.; Montesano, A.; Palsy, C. Does gait analysis change clinical decision-making in poststroke patients? Results from a pragmatic prospective observational study. Eur. J. Phys. Rehabil. Med. 2015, 51, 171–184. [Google Scholar] [PubMed]
- Khouri, N.; Desailly, E. Contribution of clinical gait analysis to single-event multi-level surgery in children with cerebral palsy. Orthop. Traumatol. Surg. Res. 2017, 103, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Prasanth, H.; Caban, M.; Keller, U.; Courtine, G.; Ijspeert, A.; Vallery, H.; von Zitzewitz, J. Wearable Sensor-Based Real-Time Gait Detection: A Systematic Review. Sensors 2021, 21, 2727. [Google Scholar] [CrossRef] [PubMed]
- Morbidoni, C.; Cucchiarelli, A.; Agostini, V.; Knaflitz, M.; Fioretti, S.; Di Nardo, F. Machine-Learning-Based Prediction of Gait Events from EMG in Cerebral Palsy Children. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Taborri, J.; Palermo, E.; Rossi, S.; Cappa, P. Gait partitioning methods: A systematic review. Sensors 2016, 16, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caldas, R.; Mundt, M.; Potthast, W.; de Lima Neto, F.B.; Markert, B. A systematic review of gait analysis methods based on inertial sensors and adaptive algorithms. Gait Posture 2017, 57, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Jourdan, T.; Debs, N.; Frindel, C. The Contribution of Machine Learning in the Validation of Commercial Wearable Sensors for Gait Monitoring in Patients: A Systematic Review. Sensors 2021, 21, 4808. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Chen, J.; Wang, F.; Chen, Y.; Yang, W.; Yang, C. Machine-learning-based children’s pathological gait classification with low-cost gait-recognition system. BioMed. Eng. OnLine 2021, 20, 60. [Google Scholar] [CrossRef] [PubMed]
- Caderby, T.; Yiou, E.; Peyrot, N.; Bonazzi, B.; Dalleau, G. Detection of swing heel-off event in gait initiation using force-plate data. Gait Posture 2013, 37, 463–466. [Google Scholar] [CrossRef] [Green Version]
- Kidziński, Ł.; Delp, S.; Schwartz, M. Automatic real-time gait event detection in children using deep neural networks. PLoS ONE 2019, 14, e0211466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamani, N.; Russel, P.; Lantz, H.; Hoeppner, M.P.; Meadows, J.R.S.; Vijay, N.; Mauceli, E.; di Palma, F.; Lindblad-Toh, K.; Jern, P.; et al. Unsupervised genome-wide recognition of local relationship patterns. BMC Genom. 2013, 14, 347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zurbenko, I.; Porter, P.S.; Gui, R.; Rao, S.; Ku, J.; Eskridge, J. Detecting Discontinuities in Time Series of Upper-Air Data: Development and Demonstration of an Adaptive Filter Technique. J. Clim. 1996, 9, 3548–3560. [Google Scholar] [CrossRef] [Green Version]
- Karatsidis, A.; Jung, M.; Schepers, M.H.; Bellusci, G.; de Zee, M.; Veltink, P.H.; Andersen, M.S. Musculoskeletal model-based inverse dynamic analysis under ambulatory conditions using inertial motion capture. Med. Eng. Phys. 2019, 65, 68–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Condition | Step Length Left (Mean (m)) | Step Length Right (Mean (m)) | Step Length Left std (m) | Step Length Right std (m) | Gait Speed (m/s) | Number of Included Steps |
|---|---|---|---|---|---|---|
| Barefoot | 0.37 | 0.389 | 0.052 | 0.038 | 0.876 | 1 |
| Barefoot | 0.465 | 0.473 | 0.013 | 0.03 | 1.019 | 1 |
| Barefoot | 0.282 | 0.444 | 0.037 | 0.013 | 0.389 | 2 |
| Barefoot | 0.457 | 0.417 | 0.012 | 0.02 | 0.797 | 1 |
| Mean | 0.394 | 0.431 | 0.029 | 0.025 | 0.770 | |
| std | 0.086 | 0.036 | 0.019 | 0.011 | 0.270 |
| Condition | Step Length Left (Mean (m)) | Step Length Right (Mean (m)) | Step Length Left (std (m)) | Step Length Right (std (m)) | Gait Speed (m/s) | Number of Steps Left | Number of Steps Right |
|---|---|---|---|---|---|---|---|
| Barefoot | 0.56 | 0.609 | 0.047 | 0.043 | 1.292 | 16 | 16 |
| Barefoot | 0.318 | 0.345 | 0.027 | 0.021 | 0.837 | 24 | 23 |
| Shoes/Orthotics | 0.341 | 0.36 | 0.052 | 0.041 | 0.87 | 7 | 7 |
| Barefoot | 0.471 | 0.496 | 0.032 | 0.044 | 1.053 | 23 | 21 |
| Barefoot | 0.451 | 0.452 | 0.036 | 0.037 | 1.028 | 20 | 20 |
| Barefoot | 0.298 | 0.315 | 0.037 | 0.045 | 0.823 | 23 | 22 |
| Barefoot | 0.306 | 0.457 | 0.022 | 0.016 | 0.399 | 5 | 4 |
| Barefoot | 0.507 | 0.501 | 0.049 | 0.013 | 1.256 | 19 | 19 |
| Barefoot | 0.446 | 0.423 | 0.022 | 0.016 | 0.814 | 25 | 22 |
| Barefoot | 0.538 | 0.502 | 0.026 | 0.063 | 1.153 | 24 | 23 |
| Barefoot | 0.389 | 0.439 | 0.043 | 0.025 | 0.771 | 21 | 20 |
| Barefoot | 0.573 | 0.604 | 0.023 | 0.017 | 0.998 | 20 | 18 |
| Barefoot | 0.511 | 0.521 | 0.05 | 0.03 | 1.092 | 23 | 21 |
| Barefoot | 0.336 | 0.341 | 0.036 | 0.024 | 0.845 | 32 | 30 |
| Barefoot | 0.446 | 0.015 | 0.699 | 9 | 10 | ||
| Mean | 0.432 | 0.454 | 0.036 | 0.030 | 0.929 | 19.4 | 18.4 |
| std | 0.099 | 0.089 | 0.011 | 0.015 | 0.231 | 7.3 | 6.7 |
| Condition | Step Length Left (Mean (m)) | Step Length Right (Mean (m)) | Step Length Left std (Mean (m)) | Step Length Right std (m) | Gait Speed (Mean (m/s)) | Number of Steps Left | Number of Steps Right |
|---|---|---|---|---|---|---|---|
| Barefoot | 0.149 | 0.24 | 0.06 | 0.056 | 0.134 | 7 | 7 |
| Barefoot | 0.467 | 0.501 | 0.046 | 0.045 | 1.133 | 29 | 30 |
| Shoes/Orthotics | 0.439 | 0.485 | 0.053 | 0.053 | 0.979 | 43 | 41 |
| Barefoot | 0.254 | 0.032 | 0.014 | 0.01 | 0.184 | 27 | 21 |
| Shoes/Orthotics | 0.278 | 0.058 | 0.017 | 0.025 | 0.228 | 11 | 12 |
| Barefoot | 0.222 | 0.25 | 0.023 | 0.011 | 0.287 | 10 | 11 |
| Shoes/Orthotics | 0.284 | 0.362 | 0.024 | 0.022 | 0.449 | 8 | 8 |
| Barefoot | 0.475 | 0.14 | 0.024 | 0.022 | 0.237 | 24 | 22 |
| Shoes/Orthotics | 0.478 | 0.186 | 0.02 | 0.042 | 0.273 | 29 | 28 |
| Mean | 0.338 | 0.250 | 0.031 | 0.031 | 0.433 | 20.9 | 20 |
| std | 0.119 | 0.160 | 0.016 | 0.016 | 0.344 | 11.8 | 10.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greve, C.; Tam, H.; Grabherr, M.; Ramesh, A.; Scheerder, B.; Hijmans, J.M. Flexible Machine Learning Algorithms for Clinical Gait Assessment Tools. Sensors 2022, 22, 4957. https://doi.org/10.3390/s22134957
Greve C, Tam H, Grabherr M, Ramesh A, Scheerder B, Hijmans JM. Flexible Machine Learning Algorithms for Clinical Gait Assessment Tools. Sensors. 2022; 22(13):4957. https://doi.org/10.3390/s22134957
Chicago/Turabian StyleGreve, Christian, Hobey Tam, Manfred Grabherr, Aditya Ramesh, Bart Scheerder, and Juha M. Hijmans. 2022. "Flexible Machine Learning Algorithms for Clinical Gait Assessment Tools" Sensors 22, no. 13: 4957. https://doi.org/10.3390/s22134957
APA StyleGreve, C., Tam, H., Grabherr, M., Ramesh, A., Scheerder, B., & Hijmans, J. M. (2022). Flexible Machine Learning Algorithms for Clinical Gait Assessment Tools. Sensors, 22(13), 4957. https://doi.org/10.3390/s22134957






