Laser-Induced Breakdown Spectroscopy Associated with the Design of Experiments and Machine Learning for Discrimination of Brachiaria brizantha Seed Vigor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Standard Classification of the Seeds
2.2. Laser-Induced Breakdown Spectroscopy System
2.3. Optimization of Instrumental Parameters for LIBS Analyses
2.4. Classification Training and Prediction Methodologies
3. Results and Discussion
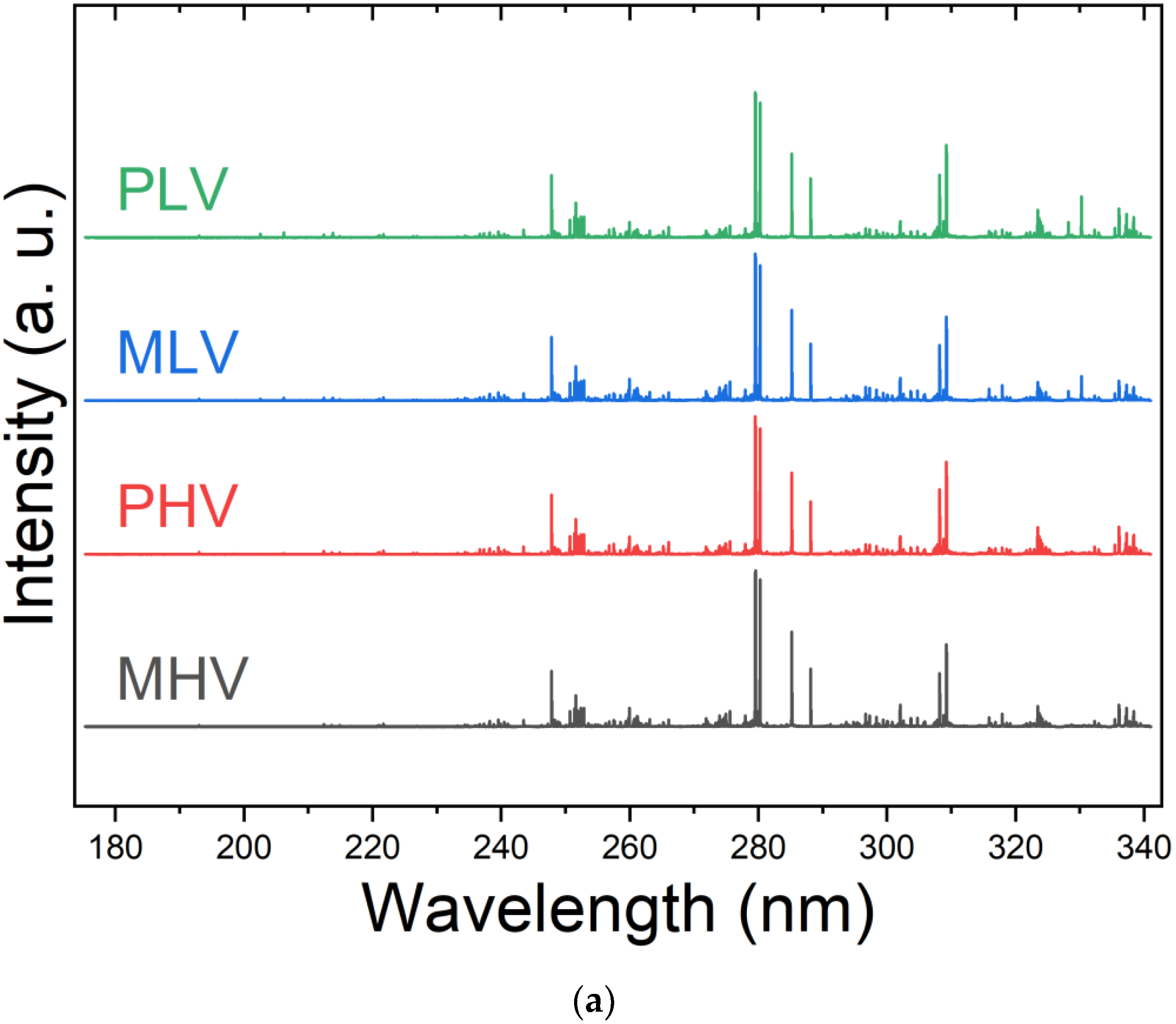
Optimization of LIBS Spectra
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Finch-Savage, W.E.; Bassel, G.W. Seed vigour and crop establishment: Extending performance beyond adaptation. J. Exp. Bot. 2016, 67, 567–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcos Filho, J. Seed vigor testing: An overview of the past, present and future perspective. Sci. Agric. 2015, 72, 363–374. [Google Scholar] [CrossRef] [Green Version]
- TeKrony, D.M.; Egli, D.B. Relationship of Seed Vigor to Crop Yield: A Review. Crop Sci. 1991, 31, 816–822. [Google Scholar] [CrossRef]
- Rajjou, L.; Duval, M.; Gallardo, K.; Catusse, J.; Bally, J.; Job, C.; Job, D. Seed Germination and Vigor. Annu. Rev. Plant Biol. 2012, 63, 507–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, I.C.; Franca, T.; Nicolodelli, G.; Morais, C.P.; Marangoni, B.; Bacchetta, G.; Milori, D.M.B.P.; Alves, C.Z.; Cena, C. Fast and Accurate Discrimination of Brachiaria brizantha (A.Rich.) Stapf Seeds by Molecular Spectroscopy and Machine Learning. ACS Agric. Sci. Technol. 2021, 1, 443–448. [Google Scholar] [CrossRef]
- Oliveira, A.M.S.; Nery, M.C.; Ribeiro, K.G.; Rocha, A.S.; Cunha, P.T. Accelerated aging for evaluation of vigor in Brachiaria brizantha ‘Xaraés’ seeds. J. Seed Sci. 2020, 42. [Google Scholar] [CrossRef]
- Senesi, G.S.; Romano, R.A.; Marangoni, B.S.; Nicolodelli, G.; Villas-Boas, P.R.; Benites, V.M.; Milori, D.M.B.P. Laser-Induced Breakdown Spectroscopy Associated with Multivariate Analysis Applied to Discriminate Fertilizers of Different Nature. J. Appl. Spectrosc. 2017, 84, 923–928. [Google Scholar] [CrossRef] [Green Version]
- Larios, G.; Nicolodelli, G.; Ribeiro, M.; Canassa, T.; Reis, A.R.; Oliveira, S.L.; Alves, C.Z.; Marangoni, B.S.; Cena, C. Soybean seed vigor discrimination by using infrared spectroscopy and machine learning algorithms. Anal. Methods 2020, 12, 4303–4309. [Google Scholar] [CrossRef]
- Belchior, V.; Botelho, B.G.; Casal, S.; Oliveira, L.S.; Franca, A.S. FTIR and Chemometrics as Effective Tools in Predicting the Quality of Specialty Coffees. Food Anal. Methods 2020, 13, 275–283. [Google Scholar] [CrossRef]
- Jiang, L.; Mehedi Hassan, M.; Jiao, T.; Li, H.; Chen, Q. Rapid detection of chlorpyrifos residue in rice using surface-enhanced Raman scattering coupled with chemometric algorithm. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 261, 119996. [Google Scholar] [CrossRef]
- Magalhães, A.B.; Senesi, G.S.; Ranulfi, A.; Massaiti, T.; Marangoni, B.S.; Nery da Silva, M.; Villas Boas, P.R.; Ferreira, E.; Novelli, V.M.; Cristofani-Yaly, M.; et al. Discrimination of Genetically Very Close Accessions of Sweet Orange (Citrus sinensis L. Osbeck) by Laser-Induced Breakdown Spectroscopy (LIBS). Molecules 2021, 26, 3092. [Google Scholar] [CrossRef] [PubMed]
- Nicolodelli, G.; Senesi, G.S.; Romano, R.A.; Cabral, J.; Perazzoli, I.L.O.; Marangoni, B.S.; Villas-Boas, P.R.; Milori, D.M.B.P. Laser-induced breakdown spectroscopy of environmental and synthetic samples using non-intensified CCD: Optimization of the excitation wavelength. Appl. Phys. B Lasers Opt. 2017, 123, 127. [Google Scholar] [CrossRef]
- Larios, G.S.; Nicolodelli, G.; Senesi, G.S.; Ribeiro, M.C.S.; Xavier, A.A.P.; Milori, D.M.B.P.; Alves, C.Z.; Marangoni, B.S.; Cena, C. Laser-Induced Breakdown Spectroscopy as a Powerful Tool for Distinguishing High- and Low-Vigor Soybean Seed Lots. Food Anal. Methods 2020, 13, 1691–1698. [Google Scholar] [CrossRef]
- Senesi, G.S.; Cabral, J.; Menegatti, C.R.; Marangoni, B.; Nicolodelli, G. Recent advances and future trends in LIBS applications to agricultural materials and their food derivatives: An overview of developments in the last decade (2010–2019). Part II. Crop plants and their food derivatives. TrAC—Trends Anal. Chem. 2019, 118, 453–469. [Google Scholar] [CrossRef]
- Ribeiro, M.C.S.; Senesi, G.S.; Cabral, J.S.; Cena, C.; Marangoni, B.S.; Kiefer, C.; Nicolodelli, G. Evaluation of rice varieties using LIBS and FTIR techniques associated with PCA and machine learning algorithms. Appl. Opt. 2020, 59, 10043. [Google Scholar] [CrossRef]
- Liu, X.; Feng, X.; Liu, F.; Peng, J.; He, Y. Rapid Identification of Genetically Modified Maize Using Laser-Induced Breakdown Spectroscopy. Food Bioprocess Technol. 2019, 12, 347–357. [Google Scholar] [CrossRef]
- He, Y.; Zhao, Y.; Zhang, C.; Li, Y.; Bao, Y.; Liu, F. Discrimination of Grape Seeds Using Laser-Induced Breakdown Spectroscopy in Combination with Region Selection and Supervised Classification Methods. Foods 2020, 9, 199. [Google Scholar] [CrossRef] [Green Version]
- Singh, J.; Kumar, R.; Awasthi, S.; Singh, V.; Rai, A.K. Laser Induced breakdown spectroscopy: A rapid tool for the identification and quantification of minerals in cucurbit seeds. Food Chem. 2017, 221, 1778–1783. [Google Scholar] [CrossRef]
- Silva, T.V.; Hubinger, S.Z.; Gomes Neto, J.A.; Milori, D.M.B.P.; Ferreira, E.J.; Ferreira, E.C. Potential of Laser Induced Breakdown Spectroscopy for analyzing the quality of unroasted and ground coffee. Spectrochim. Acta Part B At. Spectrosc. 2017, 135, 29–33. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; He, Z.; Liu, F.; Chen, R. Fast Identification of Soybean Seed Varieties Using Laser-Induced Breakdown Spectroscopy Combined With Convolutional Neural Network. Front. Plant Sci. 2021, 12, 2180. [Google Scholar] [CrossRef]
- de Morais, C.P.; Nicolodelli, G.; Mitsuyuki, M.C.; Mounier, S.; Milori, D.M.B.P. Optimization of laser-induced breakdown spectroscopy parameters from the design of experiments for multi-element qualitative analysis in river sediment. Spectrochim. Acta Part B At. Spectrosc. 2021, 177, 106066. [Google Scholar] [CrossRef]
- Vadillo, J.M.; Fernández Romero, J.M.; Rodríguez, C.; Laserna, J.J. Effect of plasma shielding on laser ablation rate of pure metals at reduced pressure. Surf. Interface Anal. 1999, 27, 1009–1015. [Google Scholar] [CrossRef]
- Aziz, A.; Basheer, F.; Sengar, A.; Irfanullah; Khan, S.U.; Farooqi, I.H. Biological wastewater treatment (anaerobic-aerobic) technologies for safe discharge of treated slaughterhouse and meat processing wastewater. Sci. Total Environ. 2019, 686, 681–708. [Google Scholar] [CrossRef] [PubMed]
- GONDAL, M.; HUSSAIN, T.; YAMANI, Z.; BAIG, M. The role of various binding materials for trace elemental analysis of powder samples using laser-induced breakdown spectroscopy. Talanta 2007, 72, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Castle, B.C.; Talabardon, K.; Smith, B.W.; Winefordner, J.D. Variables Influencing the Precision of Laser-Induced Breakdown Spectroscopy Measurements. Appl. Spectrosc. 1998, 52, 649–657. [Google Scholar] [CrossRef]
- Senesi, G.S.; Nicolodelli, G.; Milori, D.M.B.P.; De Pascale, O. Depth profile investigations of surface modifications of limestone artifacts by laser-induced breakdown spectroscopy. Environ. Earth Sci. 2017, 76, 565. [Google Scholar] [CrossRef]
- Krüger, A.L.; Nicolodelli, G.; Villas-Boas, P.R.; Watanabe, A.; Milori, D.M.B.P. Quantitative Multi-Element Analysis in Soil Using 532 nm and 1064 nm Lasers in LIBS Technique. Plasma Chem. Plasma Process. 2020, 40, 1417–1427. [Google Scholar] [CrossRef]
- Durakovic, B. Design of experiments application, concepts, examples: State of the art. Period. Eng. Nat. Sci. 2017, 5. [Google Scholar] [CrossRef]
- Castro, J.P.; Pereira-Filho, E.R. Twelve different types of data normalization for the proposition of classification, univariate and multivariate regression models for the direct analyses of alloys by laser-induced breakdown spectroscopy (LIBS). J. Anal. At. Spectrom. 2016, 31, 2005–2014. [Google Scholar] [CrossRef]
- Vera Candioti, L.; De Zan, M.M.; Cámara, M.S.; Goicoechea, H.C. Experimental design and multiple response optimization. Using the desirability function in analytical methods development. Talanta 2014, 124, 123–138. [Google Scholar] [CrossRef]
- Marangoni, B.S.; Silva, K.S.G.; Nicolodelli, G.; Senesi, G.S.; Cabral, J.S.; Villas-Boas, P.R.; Silva, C.S.; Teixeira, P.C.; Nogueira, A.R.A.; Benites, V.M.; et al. Phosphorus quantification in fertilizers using laser induced breakdown spectroscopy (LIBS): A methodology of analysis to correct physical matrix effects. Anal. Methods 2016, 8, 78–82. [Google Scholar] [CrossRef] [Green Version]







| Cultivar | Vigor | Quantity |
|---|---|---|
| Marandu | High Vigor | 20 |
| Paiaguás | High Vigor | 40 |
| Marandu | Low Vigor | 40 |
| Paiaguás | Low Vigor | 20 |
| Total | 120 | |
| Experiment | Laser Pulse Energy | Delay Time | Signal Acquisition Time | OD | |||
|---|---|---|---|---|---|---|---|
| Coded | Real (mJ) | Coded | Real (µs) | Coded | Real (µs) | ||
| 1 | 1 | 54.86 | 1 | 1.50 | 1 | 20.00 | 0.64 |
| 2 | 1 | 54.86 | 1 | 1.50 | −1 | 1.00 | 0.63 |
| 3 | 1 | 54.86 | −1 | 0.50 | 1 | 20.00 | 0.95 |
| 4 | 1 | 54.86 | −1 | 0.50 | −1 | 1.00 | 0.68 |
| 5 | −1 | 29.73 | 1 | 1.50 | 1 | 20.00 | 0.64 |
| 6 | −1 | 29.73 | 1 | 1.50 | −1 | 1.00 | 0.49 |
| 7 | −1 | 29.73 | −1 | 0.50 | 1 | 20.00 | 0.33 |
| 8 | −1 | 29.73 | −1 | 0.50 | −1 | 1.00 | 0.30 |
| 9 * | 0 | 42.29 | 0 | 1.00 | 0 | 11.00 | 0.42 |
| 10 * | 0 | 42.29 | 0 | 1.00 | 0 | 11.00 | 0.83 |
| 11 * | 0 | 42.29 | 0 | 1.00 | 0 | 11.00 | 0.67 |
| Algorithm | Hyperparameter | Values | PCs |
|---|---|---|---|
| KNN | K-Neighbours | 1 to 45 | 1 to 20 |
| LDA | Solver | “svd”, “lsqr”, “eigen” | |
| QDA | Regularization | 0.1, 0.2, 0.3, 0.4, 0.5 | |
| SVM | Regularization (C) | 0.1, 10, 100, 1000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cioccia, G.; Pereira de Morais, C.; Babos, D.V.; Milori, D.M.B.P.; Alves, C.Z.; Cena, C.; Nicolodelli, G.; Marangoni, B.S. Laser-Induced Breakdown Spectroscopy Associated with the Design of Experiments and Machine Learning for Discrimination of Brachiaria brizantha Seed Vigor. Sensors 2022, 22, 5067. https://doi.org/10.3390/s22145067
Cioccia G, Pereira de Morais C, Babos DV, Milori DMBP, Alves CZ, Cena C, Nicolodelli G, Marangoni BS. Laser-Induced Breakdown Spectroscopy Associated with the Design of Experiments and Machine Learning for Discrimination of Brachiaria brizantha Seed Vigor. Sensors. 2022; 22(14):5067. https://doi.org/10.3390/s22145067
Chicago/Turabian StyleCioccia, Guilherme, Carla Pereira de Morais, Diego Victor Babos, Débora Marcondes Bastos Pereira Milori, Charline Z. Alves, Cícero Cena, Gustavo Nicolodelli, and Bruno S. Marangoni. 2022. "Laser-Induced Breakdown Spectroscopy Associated with the Design of Experiments and Machine Learning for Discrimination of Brachiaria brizantha Seed Vigor" Sensors 22, no. 14: 5067. https://doi.org/10.3390/s22145067








