Recent Developments in the Field of Optical Immunosensors Focusing on a Label-Free, White Light Reflectance Spectroscopy-Based Immunosensing Platform
Abstract
:1. Introduction
Labeled vs. Label-Free Optical Immunosensors
2. Newest Developments in Optical Immunosensors: Bioanalytical Applications
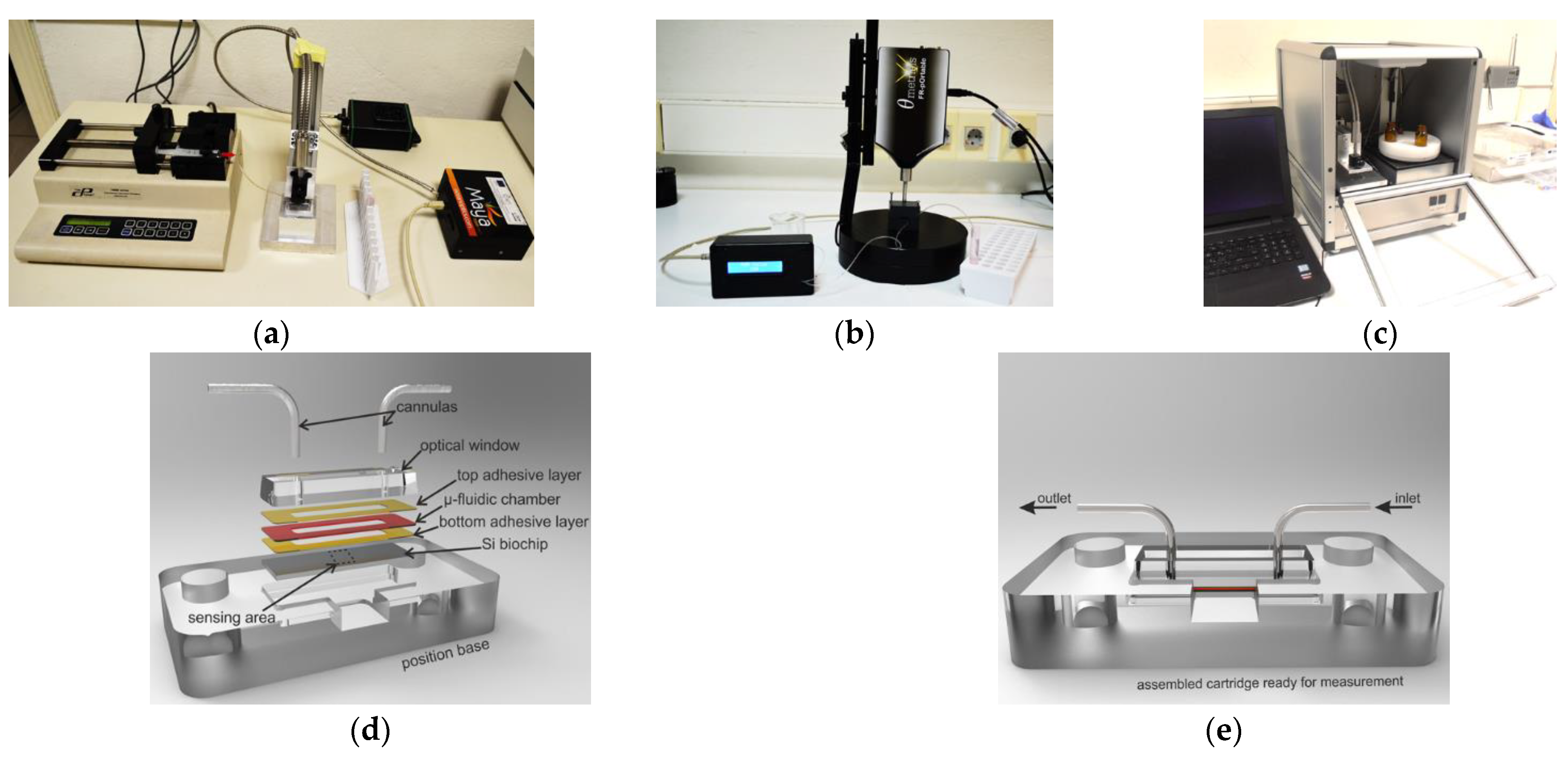
3. WLRS-Based Optical Immunosensors
4. Discussion and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aydin, M.; Aydin, E.B.; Sezgintürk, M.K. Advances in immunosensor technology. Adv. Clin. Chem. 2021, 102, 1–62. [Google Scholar] [CrossRef] [PubMed]
- Gil Rosa, B.; Akingbade, O.E.; Guo, X.; Gonzalez-Macia, L.; Crone, M.A.; Cameron, L.P.; Freemont, P.; Choy, K.L.; Güder, F.; Yeatman, E.; et al. Multiplexed immunosensors for point-of-care diagnostic applications. Biosens. Bioelectron. 2022, 203, 114050. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zong, C.; Li, G.; Wang, J.; Kong, T.; Li, F.; Chang, J. High throughput immunosensor chip coupled with a fluorescent DNA dendrimer for ultrasensitive detection of cardiac troponin T. RSC Adv. 2021, 11, 27523–27529. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Tannenberg, R.; Tscheuschner, G.; Ponader, M.; Weller, M.G. Cocaine detection by a laser-induced immunofluorometric biosensor. Biosensors 2021, 11, 313. [Google Scholar] [CrossRef]
- Singh, H.; Singh, S.; Bhardwaj, S.K.; Kaur, G.; Khatri, M.; Deep, A.; Bhardwaj, N. Development of carbon quantum dot-based lateral flow immunoassay for sensitive detection of aflatoxin M1 in milk. Food Chem. 2022, 393, 133374. [Google Scholar] [CrossRef]
- Peng, X.; Wang, Y.; Wen, W.; Chen, M.-M.; Zhang, X.; Wang, S. Simple MoS2-nanofiber paper-based fluorescence immunosensor for point-of-care detection of programmed cell death protein 1. Anal. Chem. 2021, 93, 8791–8798. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Shi, L.; Zou, X.; Wang, T.; Wang, D.; Gong, Z.; Fan, M. Fluorescence immunoassay rapid detection of 2019-nCoV antibody based on the fluorescence resonance energy transfer between graphene quantum dots and Ag@Au nanoparticle. Microchem. J. 2022, 173, 107046. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Ueda, H. Recent advances in quenchbody, a fluorescent immunosensor. Sensors 2021, 21, 1223. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Chen, L.; Li, B.; Dong, H.; Liu, H.; Yang, X.; Ueda, H.; Dong, J. Quench-release-based fluorescent immunosensor for the rapid detection of tumor necrosis factor α. ACS Omega 2021, 6, 31009–31016. [Google Scholar] [CrossRef]
- Ning, X.; Yasuda, T.; Kitaguchi, T.; Ueda, H. Construction of fluorescent immunosensor quenchbody to detect His-tagged recombinant proteins produced in bioprocess. Sensors 2021, 21, 4993. [Google Scholar] [CrossRef]
- Liang, J.; Dong, H.; Wang, H.; Yi, Z.; Jiang, G.; Inagaki, T.; Gomez-Sanchez, C.E.; Dong, J.; Ueda, H. Creation of a quick and sensitive fluorescent immunosensor for detecting the mineracorticoid steroid hormone aldosterone. J. Steroid Biochem. Mol. Biol. 2022, 221, 106118. [Google Scholar] [CrossRef] [PubMed]
- Makarov, N.S.; Drobizhev, M.; Rebane, A. Two-photon absorption standards in the 550–1600 nm excitation wavelength range. Opt. Express 2008, 16, 4029–4047. [Google Scholar] [CrossRef] [PubMed]
- Meiling, T.T.; Cywiński, P.J.; Löhmannsröben, H.-G. Two-photon excitation fluorescence spectroscopy of quantum dots: Photophysical properties and application in bioassays. J. Phys. Chem. C 2018, 122, 9641–9647. [Google Scholar] [CrossRef]
- Parravicini, j.; Tomaselli, A.; Hasani, E.; Tomassini, D.; Manfredi, N.; Tartara, L. Practical two-photon absorption cross sections and spectra of eosin and hematoxylin. J. Biophotonics 2020, 13, e202000141. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.; Wang, Y.; Feng, Q.; Wei, Y.; Ji, J.; Zhang, W. Progress of new label-free techniques for biosensors: A review. Crit. Rev. Biotechnol. 2016, 36, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Makarona, E.; Petrou, P.; Kakabakos, S.; Misiakos, K.; Raptis, I. Point-of-need bioanalytics based on planar optical interferometry. Biotechnol. Adv. 2016, 34, 209–233. [Google Scholar] [CrossRef]
- Zanchetta, G.; Lanfranco, R.; Giavazzi, F.; Bellini, T.; Buscaglia, M. Emerging applications of label-free optical biosensors. Nanophotonics 2017, 6, 627–645. [Google Scholar] [CrossRef]
- Gauglitz, G. Direct optical detection in bioanalysis: An update. Anal. Bioanal. Chem. 2010, 398, 2363–2372. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, J.; Yang, Z.; Wilkinson, J.S.; Zhou, X. Optical biosensors based on refractometric sensing schemes: A review. Biosens. Bioelectron. 2019, 144, 111693. [Google Scholar] [CrossRef]
- Choi, J.-H.; Lee, J.-H.; Son, J.; Choi, J.-W. Nobel-metal assisted surface plasmon resonance immunosensors. Sensors 2020, 20, 1003. [Google Scholar] [CrossRef] [Green Version]
- Souto, D.E.P.; Volpe, J.; Gonçalves, C.C.; Ramos, C.H.I.; Kubota, L.T. A brief review on the strategy of developing SPR-based biosensors for application to the diagnosis of neglected tropical diseases. Talanta 2019, 205, 120122. [Google Scholar] [CrossRef] [PubMed]
- Gauglitz, G.; Nahm, W. Observation of spectral interferences for the determination of volume and surface effects of thin films. Fresenius J. Anal. Chem. 1991, 341, 279–283. [Google Scholar] [CrossRef]
- Gauglitz, G.; Brecht, A.; Kraus, G.; Mahm, W. Chemical and biochemical sensors based on interferometry at thin (multi-) layers. Sens. Actuators B Chem. 1993, 11, 21–27. [Google Scholar] [CrossRef]
- Zhuo, S.; Xun, M.; Li, M.; Kong, X.; Shao, R.; Zheng, T.; Pan, D.; Li, J.; Li, Q. Rapid and label-free optical assay of S-layer protein with high sensitivity using TiO2-coated porous silicon-based microfluidic biosensor. Sens. Actuators B Chem. 2020, 321, 128524. [Google Scholar] [CrossRef]
- Su, Q.; Wu, F.; Xu, P.; Dong, A.; Liu, C.; Wan, Y.; Qian, W. Interference Effect of silica colloidal crystal films and their applications to biosensing. Anal. Chem. 2019, 91, 6080–6087. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Wan, Y.; Wang, L.; Zhou, L.; Ma, N.; Qian, W. Construction of Optical Interference Fibrin and Thrombolysis Analysis with Silica Colloidal Crystal Films. Langmuir 2021, 37, 7264–7272. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Xu, P.; Zhou, L.; Wu, F.; Dong, A.; Wan, Y.; Qian, W. Real-time and label-free monitoring of biomolecular interactions within complex biological media using a silica colloidal crystal film. ACS Appl. Mater. Interfaces 2020, 12, 35950–35957. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, L.; Ma, N.; Su, Q.; Wan, Y.; Zhang, Y.; Wu, F.; Qian, W. Real-time monitoring of immunoglobulin G levels in milk using an ordered porous layer interferometric optical sensor. Talanta 2022, 237, 122958. [Google Scholar] [CrossRef]
- Avci, O.; Ünlü, N.L.; Özkumur, A.Y.; Ünlü, M.S. Interferometric reflectance imaging sensor (IRIS)—A platform technology for multiplexed diagnostics and digital detection. Sensors 2015, 15, 17649–17665. [Google Scholar] [CrossRef] [Green Version]
- Gagni, P.; Sola, L.; Cretich, M.; Chiari, M. Development of a high-sensitivity immunoassay for amyloid-beta 1-42 using a silicon microarray platform. Biosens. Bioelectron. 2013, 47, 490–495. [Google Scholar] [CrossRef]
- Monroe, M.R.; Daaboul, G.G.; Tuysuzoglu, A.; Lopez, C.A.; Little, F.F.; Unlü, M.S. Single nanoparticle detection for multiplexed protein diagnostics with attomolar sensitivity in serum and unprocessed whole blood. Anal. Chem. 2013, 85, 3698–3706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, S.; Freedman, D.S.; Massari, P.; Cabodi, M.; Ünlü, M.S. A mass-tagging approach for enhanced sensitivity of dynamic cytokine detection using a label-free biosensor. Langmuir 2013, 29, 5369–5376. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.A.; Daaboul, G.G.; Vedula, R.S.; Ozkumur, E.; Bergstein, D.A.; Geisbert, T.W.; Fawcett, H.E.; Goldberg, B.B.; Connor, J.H.; Unlü, M.S. Label-free multiplexed virus detection using spectral reflectance imaging. Biosens. Bioelectron. 2011, 26, 3432–3437. [Google Scholar] [CrossRef] [Green Version]
- Bakhshpour, M.; Chiodi, E.; Celebi, I.; Saylan, Y.; Ünlü, N.L.; Ünlü, M.S.; Denizli, A. Sensitive and real-time detection of IgG using interferometric reflecting imaging sensor system. Biosens. Bioelectron. 2022, 201, 113961. [Google Scholar] [CrossRef]
- Daaboul, G.G.; Vedula, R.S.; Ahn, S.; Lopez, C.A.; Reddington, A.; Ozkumur, E.; Ünlü, M.S. LED-based interferometric reflectance imaging sensor for quantitative dynamic monitoring of biomolecular interactions. Biosens. Bioelectron. 2011, 26, 2221–2227. [Google Scholar] [CrossRef] [PubMed]
- Celebi, I.; Geib, M.T.; Chiodi, E.; Lortlar Ünlü, N.; Ekiz Kanik, F.; Ünlü, S. Instrument-free protein microarray fabrication for accurate affinity measurements. Biosensors 2020, 10, 158. [Google Scholar] [CrossRef] [PubMed]
- Chiodi, E.; Marn, A.M.; Geib, M.T.; Ekiz Kanik, F.; Rejman, J.; AnKrapp, D.; Ünlü, M.S. Highly multiplexed label-free imaging sensor for accurate quantification of small-molecule binding kinetics. ACS Omega 2020, 5, 25358–25364. [Google Scholar] [CrossRef]
- Zavali, M.; Petrou, P.S.; Kakabakos, S.E.; Kitsara, M.; Raptis, I.; Beltsios, K.; Misiakos, K. Label-free kinetic study of biomolecular interactions by white light reflectance spectroscopy. Micro Nano Lett. 2006, 1, 94–98. [Google Scholar] [CrossRef]
- Goustouridis, D.; Manoli, K.; Chatzandroulis, S.; Sanopoulou, M.; Raptis, I. Characterization of polymer layers for silicon micromachined bilayer chemical sensors using white light interferometry. Sens. Actuators B Chem. 2005, 111–112, 549–554. [Google Scholar] [CrossRef]
- Kitsara, M.; Petrou, P.; Kontziampasis, D.; Misiakos, K.; Makarona, E.; Raptis, I.; Beltsios, K. Biomolecular layer thickness evaluation using white light reflectance spectroscopy. Microelectron. Eng. 2010, 87, 802–805. [Google Scholar] [CrossRef]
- Nandi, S.K.; Singh, D.; Upadhay, J.; Gupta, N.; Dhiman, N.; Mittal, S.K.; Mahindroo, N. Identification of tear-based protein and non-protein biomarkers: Its application in diagnosis of human diseases using biosensors. Int. J. Biol. Macromol. 2021, 193, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Krausz, A.D.; Korley, F.K.; Burns, M.A. The current state of traumatic brain injury biomarker measurement methods. Biosensors 2021, 11, 319. [Google Scholar] [CrossRef] [PubMed]
- Drobysh, M.; Ramanaviciene, A.; Viter, R.; Chen, C.F.; Samukaite-Bubniene, U.; Ratautaite, V.; Ramanavicius, A. Biosensors for the determination of SARS-CoV-2 virus and diagnosis of COVID-19 infection. Int. J. Mol. Sci. 2022, 23, 666. [Google Scholar] [CrossRef] [PubMed]
- El-Sherif, D.M.; Abouzid, M.; Gaballah, M.S.; Ahmed, A.A.; Adeel, M.; Sheta, S.M. New approach in SARS-CoV-2 surveillance using biosensor technology: A review. Environ. Sci. Pollut. Res. Int. 2022, 29, 1677–1695. [Google Scholar] [CrossRef]
- Mollarasouli, F.; Zare-Shehneh, N.; Ghaedi, M. A review on corona virus disease 2019 (COVID-19): Current progress, clinical features and bioanalytical diagnostic methods. Microchim. Acta 2022, 189, 103. [Google Scholar] [CrossRef]
- Drobysh, M.; Ramanaviciene, A.; Viter, R.; Ramanavicius, A. Affinity sensors for the diagnosis of COVID-19. Micromachines 2021, 12, 390. [Google Scholar] [CrossRef]
- Pisco, M.; Cusano, A. Lab-on-fiber technology: A roadmap toward multifunctional plug and play platforms. Sensors 2020, 20, 4705. [Google Scholar] [CrossRef]
- Soares, M.S.; Vidal, M.; Santos, N.F.; Costa, F.M.; Marques, C.; Pereira, S.O.; Leitão, C. Immunosensing based on optical fiber technology: Recent advances. Biosensors 2021, 11, 305. [Google Scholar] [CrossRef]
- Plikusiene, I.; Maciulis, V.; Ramanavicius, A.; Ramanaviciene, A. Spectroscopic ellipsometry and quartz crystal microbalance with dissipation for the assessment of polymer layers and for the application in biosensing. Polymers 2022, 14, 1056. [Google Scholar] [CrossRef]
- Kaur, J.; Srivastava, R.; Borse, V. Recent advances in point-of-care diagnostics for oral cancer. Biosens. Bioelectron. 2021, 178, 112995. [Google Scholar] [CrossRef]
- Serebrennikova, K.V.; Berlina, A.N.; Sotnikov, D.V.; Zherdev, A.V.; Dzantiev, B.B. Raman scattering-based biosensing: New prospects and opportunities. Biosensors 2021, 11, 512. [Google Scholar] [CrossRef] [PubMed]
- Thakare, S.; Shaikh, A.; Bodas, D.; Gajbhiye, V. Application of dendrimer-based nanosensors in immunodiagnosis. Colloids Surf. B 2022, 209, 112174. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Huang, Q.; Xiao, Z.; Liu, M.; Zhu, Y.; Chen, Q.; Li, Y.; Ai, K. Nanomaterial-based biosensor developing as a route toward in vitro diagnosis of early ovarian cancer. Mater. Today Bio 2022, 13, 100218. [Google Scholar] [CrossRef] [PubMed]
- Raji, M.A.; Chinnappan, R.; Shibl, A.; Suaifan, G.; Weber, K.; Cialla-May, D.; Popp, J.; El Shorbagy, E.; Al-Kattan, K.; Zourob, M. Low-cost colorimetric diagnostic screening assay for methicillin resistant Staphylococcus aureus. Talanta 2021, 225, 121946. [Google Scholar] [CrossRef]
- Bu, T.; Bai, F.; Zhao, S.; Sun, X.; Jia, P.; He, K.; Wang, Y.; Li, Q.; Wang, L. Dual-modal immunochromatographic test for sensitive detection of zearalenone in food samples based on biosynthetic Staphylococcus aureus-mediated polymer dot-nanocomposites. Anal. Chem. 2022, 94, 5546–5554. [Google Scholar] [CrossRef]
- Kalkal, A.; Kadian, S.; Kumar, S.; Manik, G.; Sen, P.; Kumar, S.; Packirisamy, G. Ti3C2-MXene decorated with nanostructured silver as a dual-energy acceptor for the fluorometric neuron specific enolase detection. Biosens. Bioelectron. 2022, 195, 113620. [Google Scholar] [CrossRef]
- Han, C.; Chen, R.; Wu, X.; Shi, N.; Duan, T.; Xu, K.; Huang, T. Fluorescence turn-on immunosensing of HE4 biomarker and ovarian cancer cells based on target-triggered metal-enhanced fluorescence of carbon dots. Anal. Chim. Acta 2021, 1187, 339160. [Google Scholar] [CrossRef]
- Jia, B.; Liao, X.; Sun, C.; Fang, L.; Zhou, L.; Kong, W. Development of a quantum dot nanobead-based fluorescent strip immunosensor for on-site detection of aflatoxin B1 in lotus seeds. Food Chem. 2021, 356, 129614. [Google Scholar] [CrossRef]
- Kamal, Z.; Zarei Ghobadi, M.; Mohseni, S.M.; Ghourchian, H. High-performance porphyrin-like graphene quantum dots for immuno-sensing of Salmonella typhi. Biosens. Bioelectron. 2021, 188, 113334. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Zhang, P.; Yan, Z.; Zhou, Y.; Du, Y.; Qu, C.; Song, Y.; Zhou, D.; Qu, S.; et al. Cell-based fluorescent microsphere incorporated with carbon dots as a sensitive immunosensor for the rapid detection of Escherichia coli O157 in milk. Biosens. Bioelectron. 2021, 179, 113057. [Google Scholar] [CrossRef]
- Liu, J.; Xing, Y.; Zhou, X.; Chen, G.Y.; Shi, H. Light-sheet skew rays enhanced U-shaped fiber-optic fluorescent immunosensor for Microcystin-LR. Biosens. Bioelectron. 2021, 176, 112902. [Google Scholar] [CrossRef]
- Deng, F.; Li, Y.; Qiao, L.; Goldys, E. A CRISPR/Cas12a-assisted on-fibre immunosensor for ultrasensitive small protein detection in complex biological samples. Anal. Chim. Acta 2022, 1192, 339351. [Google Scholar] [CrossRef] [PubMed]
- Monash, A.; Marciano, D.; Colvin, A.S.; Fass, R.; Dvash, Y.; Rosen, O. Phosphorescent palladium-tetrabenzoporphyrin indicators for immunosensing of small molecules with a novel optical device. Talanta 2021, 224, 121927. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Chen, X.; Huang, J.; Zhang, X.; Sun, J.; Yang, L. Point-of-care testing of methylamphetamine with a portable optical fiber immunosensor. Anal. Chim. Acta 2022, 1192, 339345. [Google Scholar] [CrossRef] [PubMed]
- Roda, A.; Cavalera, S.; Di Nardo, F.; Calabria, D.; Rosati, S.; Simoni, P.; Colitti, B.; Baggiani, C.; Roda, M.; Anfossi, L. Dual lateral flow optical/chemiluminescence immunosensors for the rapid detection of salivary and serum IgA in patients with COVID-19 disease. Biosens. Bioelectron. 2021, 172, 112765. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.B.D.; Silva, J.R.D.; Rodrigues, M.C.; Vieira, J.A.; Andrade, I.A.; Nagata, T.; Santos, A.S.; Silva, S.W.D.; Rocha, M.; Báo, S.N.; et al. Detection of SARS-CoV-2 virus via dynamic light scattering using antibody-gold nanoparticle bioconjugates against viral spike protein. Talanta 2022, 243, 123355. [Google Scholar] [CrossRef]
- Myndrul, V.; Coy, E.; Bechelany, M.; Iatsunskyi, I. Photoluminescence label-free immunosensor for the detection of Aflatoxin B1 using polyacrylonitrile/zinc oxide nanofibers. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111401. [Google Scholar] [CrossRef]
- Gan, W.; Xu, Z.; Li, Y.; Bi, W.; Chu, L.; Qi, Q.; Yang, Y.; Zhang, P.; Gan, N.; Dai, S.; et al. Rapid and sensitive detection of Staphylococcus aureus by using a long-period fiber grating immunosensor coated with egg yolk antibody. Biosens. Bioelectron. 2022, 199, 113860. [Google Scholar] [CrossRef]
- Zhong, J.; Liu, S.; Zou, T.; Yan, W.; Zhou, M.; Liu, B.; Rao, X.; Wang, Y.; Sun, Z.; Wang, Y. All fiber-optic immunosensors based on elliptical core helical intermediate-period fiber grating with low-sensitivity to environmental disturbances. Biosensors 2022, 12, 99. [Google Scholar] [CrossRef]
- Costa, R.; Costa, J.; Sagastizábal, I.; Brandão, A.; Moreira, P.; Mafra, I.; Silva, A.F.; Pereira, C.M. Electrochemical and optical biosensing platforms for the immunorecognition of hazelnut Cor a 14 allergen. Food Chem. 2021, 361, 130122. [Google Scholar] [CrossRef]
- Funabashi, R.; Miyakawa, K.; Yamaoka, Y.; Yoshimura, S.; Yamane, S.; Jeremiah, S.S.; Shimizu, K.; Ozawa, H.; Kawakami, C.; Usuku, S.; et al. Development of highly sensitive and rapid antigen detection assay for diagnosis of COVID-19 utilizing optical waveguide immunosensor. J. Mol. Cell Biol. 2021, 13, 763–766. [Google Scholar] [CrossRef] [PubMed]
- Gwiazda, M.; Bhardwaj, S.K.; Kijeńska-Gawrońska, E.; Swieszkowski, W.; Sivasankaran, U.; Kaushik, A. Impedimetric and plasmonic sensing of collagen I using a half-antibody-supported, Au-modified, self-assembled monolayer system. Biosensors 2021, 11, 227. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhang, Z.H.; Chen, J.; Mao, Z.; Zhu, H.; Liu, Y.; Zhu, Z.; Chen, H. One dimensional magneto-optical nanocomplex from silver nanoclusters and magnetite nanorods containing ordered mesocages for sensitive detection of PD-L1. Biosens. Bioelectron. 2021, 189, 113385. [Google Scholar] [CrossRef]
- Leitão, C.; Leal-Junior, A.; Almeida, A.R.; Pereira, S.O.; Costa, F.M.; Pinto, J.L.; Marques, C. Cortisol AuPd plasmonic unclad POF biosensor. Biotechnol. Rep. 2021, 29, e00587. [Google Scholar] [CrossRef]
- Luo, L.; Yang, J.; Li, Z.; Xu, H.; Guo, L.; Wang, L.; Wang, Y.; Luo, L.; Wang, J.; Zhang, P.; et al. Label-free differentiation and quantification of ricin, abrin from their agglutinin biotoxins by surface plasmon resonance. Talanta 2022, 238, 122860. [Google Scholar] [CrossRef]
- Kausaite-Minkstimiene, A.; Popov, A.; Ramanaviciene, A. Surface plasmon resonance immunosensor with antibody-functionalized magnetoplasmonic nanoparticles for ultrasensitive quantification of the CD5 biomarker. ACS Appl. Mater. Interfaces 2022, 14, 20720–20728. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, N.; Han, C.; Chen, Z.; Zhai, X.; Li, Z.; Zheng, K.; Zhu, J.; Wang, X.; Zou, X.; et al. Competitive immunosensor for sensitive and optical anti-interference detection of imidacloprid by surface-enhanced Raman scattering. Food Chem. 2021, 358, 129898. [Google Scholar] [CrossRef] [PubMed]
- Ran, Y.; Long, J.; Xu, Z.; Yin, Y.; Hu, D.; Long, X.; Zhang, Y.; Liang, L.; Liang, H.; Guan, B.O. Harmonic optical microfiber Bragg grating immunosensor for the accelerative test of cardiac biomarker (cTn-I). Biosens. Bioelectron. 2021, 179, 113081. [Google Scholar] [CrossRef]
- Székács, I.; Adányi, N.; Szendrő, I.; Székács, A. Direct and competitive optical grating immunosensors for determination of Fusarium mycotoxin zearalenone. Toxins 2021, 13, 43. [Google Scholar] [CrossRef]
- Kim, K.R.; Lee, K.W.; Chun, H.J.; Lee, D.; Kim, J.H.; Yoon, H.C. Wash-free operation of smartphone-integrated optical immunosensor using retroreflective microparticles. Biosens. Bioelectron. 2022, 196, 113722. [Google Scholar] [CrossRef]
- Maeda, T.; Kanamori, R.; Choi, Y.J.; Taki, M.; Noda, T.; Sawada, K.; Takahashi, K. Bio-interface on freestanding nanosheet of microelectromechanical system optical interferometric immunosensor for label-free attomolar prostate cancer marker detection. Sensors 2022, 22, 1356. [Google Scholar] [CrossRef]
- Nabok, A.; Al-Jawdah, A.M.; Gémes, B.; Takács, E.; Székács, A. An optical planar waveguide-based immunosensors for determination of Fusarium mycotoxin zearalenone. Toxins 2021, 13, 89. [Google Scholar] [CrossRef]
- Kawasaki, D.; Yamada, H.; Sueyoshi, K.; Hisamoto, H.; Endo, T. Imprinted photonic crystal-film-based smartphone-compatible label-free optical sensor for SARS-CoV-2 testing. Biosensors 2022, 12, 200. [Google Scholar] [CrossRef] [PubMed]
- Plikusiene, I.; Maciulis, V.; Juciute, S.; Maciuleviciene, R.; Balevicius, S.; Ramanavicius, A.; Ramanaviciene, A. Investigation and comparison of specific antibodies’ affinity interaction with SARS-CoV-2 wild-type, B.1.1.7, and B.1.351 spike protein by total internal reflection ellipsometry. Biosensors 2022, 12, 351. [Google Scholar] [CrossRef] [PubMed]
- Karachaliou, C.-E.; Koukouvinos, G.; Pissaridi, K.; Ladikos, D.; Goustouridis, D.; Raptis, I.; Livaniou, E.; Kakabakos, S.; Petrou, P. Fast and accurate determination of minute ochratoxin A levels in cereal flours: Towards application at the field. Eng. Proc. 2022, 16, 14. [Google Scholar]
- Koukouvinos, G.; Karachaliou, C.E.; Raptis, I.; Petrou, P.; Livaniou, E.; Kakabakos, S. Fast and sensitive determination of the fungicide carbendazim in fruit juices with an immunosensor based on white light reflectance spectroscopy. Biosensors 2021, 11, 153. [Google Scholar] [CrossRef]
- Tsounidi, D.; Koukouvinos, G.; Christianidis, V.; Legaki, E.; Giogli, V.; Panagiotopoulou, K.; Taka, S.; Ekaterinidi, Z.; Kakabakos, S.; Raptis, I.; et al. Development of a point-of-care system based on white light reflectance spectroscopy: Application in CRP determination. Biosensors 2021, 11, 268. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulou, M.; Tzialla, K.; Voulgari, A.; Dikeoulia, M.; Raptis, I.; Kakabakos, S.E.; Petrou, P. Rapid detection of Salmonella typhimurium in drinking water by a white light reflectance spectroscopy immunosensor. Sensors 2021, 21, 2683. [Google Scholar] [CrossRef] [PubMed]
- Koukouvinos, G.; Petrou, P.; Goustouridis, D.; Misiakos, K.; Kakabakos, S.; Raptis, I. Development and bioanalytical applications of a white light reflectance spectroscopy label-free sensing platform. Biosensors 2017, 7, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zavali, M.; Petrou, P.S.; Goustouridis, D.; Raptis, I.; Misiakos, K.; Kakabakos, S.E. A regenerable flow-through affinity sensor for label-free detection of proteins and DNA. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2010, 878, 237–242. [Google Scholar] [CrossRef]
- Petrou, P.S.; Ricklin, D.; Zavali, M.; Raptis, I.; Kakabakos, S.E.; Misiakos, K.; Lambris, J.D. Real-time label-free detection of complement activation products in human serum by white light reflectance spectroscopy. Biosens. Bioelectron. 2009, 24, 3359–3364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koukouvinos, G.; Petrou, P.S.; Misiakos, K.; Drygiannakis, D.; Raptis, I.; Goustouridis, D.; Kakabakos, S.E. A label-free flow-through immunosensor for determination of total- and free-PSA in human serum samples based on white-light reflectance spectroscopy. Sens. Actuators B Chem. 2015, 209, 1041–1048. [Google Scholar] [CrossRef]
- Koukouvinos, G.; Petrou, P.; Misiakos, K.; Drygiannakis, D.; Raptis, I.; Stefanitsis, G.; Martini, S.; Nikita, D.; Goustouridis, D.; Moser, I.; et al. Simultaneous determination of CRP and D-dimer in human blood plasma samples with white light reflectance spectroscopy. Biosens. Bioelectron. 2016, 84, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Koukouvinos, G.; Τsialla, Z.; Petrou, P.S.; Misiakos, K.; Goustouridis, D.; Ucles Moreno, A.; Fernandez-Alba, A.R.; Raptis, I.; Kakabakos, S.E. Fast simultaneous detection of three pesticides by a White Light Reflectance Spectroscopy sensing platform. Sens. Actuators B Chem. 2017, 238, 1214–1223. [Google Scholar] [CrossRef]
- Koukouvinos, G.; Metheniti, A.; Karachaliou, C.E.; Goustouridis, D.; Livaniou, E.; Misiakos, K.; Raptis, I.; Kondili, A.; Miniati, P.; Petrou, P.; et al. White light reflectance spectroscopy biosensing system for fast quantitative prostate specific antigen determination in forensic samples. Talanta 2017, 175, 443–450. [Google Scholar] [CrossRef]
- Koukouvinos, G.; Goustouridis, D.; Misiakos, K.; Kakabakos, S.; Raptis, I.; Petrou, P. Rapid C-reactive protein determination in whole blood with a white light reflectance spectroscopy label-free immunosensor for point-of-care applications. Sens. Actuators B Chem. 2018, 260, 282–288. [Google Scholar] [CrossRef]
- Stavra, E.; Petrou, P.S.; Koukouvinos, G.; Kiritsis, C.; Pirmettis, I.; Papadopoulos, M.; Goustouridis, D.; Economou, A.; Misiakos, K.; Raptis, I.; et al. Simultaneous determination of paraquat and atrazine in water samples with a white light reflectance spectroscopy biosensor. J. Hazard. Mater. 2018, 359, 67–75. [Google Scholar] [CrossRef]
- Anastasiadis, V.; Koukouvinos, G.; Petrou, P.S.; Economou, A.; Dekker, J.; Harjanne, M.; Heimala, P.; Goustouridis, D.; Raptis, I.; Kakabakos, S.E. Multiplexed mycotoxins determination employing white light reflectance spectroscopy and silicon chips with silicon oxide areas of different thickness. Biosens. Bioelectron. 2020, 153, 112035. [Google Scholar] [CrossRef]
- Blouchos, P.; Moschopoulou, G.; Kintzios, S.; Glezakos, T.J.; Ferentinos, C.; Yialouris, C.; Livaniou, E.; Zikos, C.; Kakabakos, S.E.; Petrou, P.S.; et al. FOODSCAN: A Novel and automated biosensor platform for pesticide residue detection. In Proceedings of the 13th International Conference on Environmental Science and Technology (CEST 2013), Athens, Greece, 5–7 September 2013. [Google Scholar]
- Zikos, C.; Evangelou, A.; Karachaliou, C.E.; Gourma, G.; Blouchos, P.; Moschopoulou, G.; Yialouris, C.; Griffiths, J.; Johnson, G.; Petrou, P.; et al. Commercially available chemicals as immunizing haptens for the development of a polyclonal antibody recognizing carbendazim and other benzimidazole-type fungicides. Chemosphere 2015, 119, S16–S20. [Google Scholar] [CrossRef]
- Diamandis, E.P.; Christopoulos, T.K. The biotin-(strept)avidin system: Principles and applications in biotechnology. Clin. Chem. 1991, 37, 625–636. [Google Scholar] [CrossRef]
- Anastasiadis, V.; Raptis, I.; Economou, A.; Kakabakos, S.E.; Petrou, P.S. Fast deoxynivalenol determination in cereals using a white light reflectance spectroscopy immunosensor. Biosensors 2020, 10, 154. [Google Scholar] [CrossRef] [PubMed]
- Stavra, E.; Petrou, P.S.; Koukouvinos, G.; Economou, A.; Goustouridis, D.; Misiakos, K.; Raptis, I.; Kakabakos, S.E. Fast, sensitive and selective determination of herbicide glyphosate in water samples with a white light reflectance spectroscopy immunosensor. Talanta 2020, 214, 120854. [Google Scholar] [CrossRef] [PubMed]
- Tsounidi, D.; Koukouvinos, G.; Petrou, P.; Misiakos, K.; Zisis, G.; Goustouridis, D.; Raptis, I.; Kakabakos, S.E. Rapid and sensitive label-free determination of aflatoxin M1 levels in milk through a white light reflectance spectroscopy immunosensor. Sens. Actuators B Chem. 2019, 282, 104–111. [Google Scholar] [CrossRef]
- Karachaliou, C.E.; Kostopoulos, I.V.; Vassilakopoulou, V.; Klimentzou, P.; Paravatou-Petsotas, M.; Voelter, W.; Kalbacher, H.; Zikos, C.; Tsitsilonis, O.; Livaniou, E. Development of a specific IgY-based ELISA for prothymosin alpha, a bioactive polypeptide with diagnostic and therapeutic potential. Heliyon 2019, 5, e02616. [Google Scholar] [CrossRef] [Green Version]
- Karachaliou, C.E.; Vassilakopoulou, V.; Livaniou, E. IgY technology: Methods for developing and evaluating avian immunoglobulins for the in vitro detection of biomolecules. World J. Methodol. 2021, 11, 243–262. [Google Scholar] [CrossRef]
- Viegas Barroso, J.F.; Halder, M.E.; Whelan, M. EURL ECVAM Recommendation on Non-Animal-Derived Antibodies. Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC120199 (accessed on 4 February 2022).
- Liu, S.; Xu, Y.; Jiang, X.; Tan, H.; Ying, B. Translation of aptamers toward clinical diagnosis and commercialization. Biosens. Bioelectron. 2022, 208, 114168. [Google Scholar] [CrossRef]
- Ali, G.K.; Omer, K.M. Molecular imprinted polymer combined with aptamer (MIP-aptamer) as a hybrid dual recognition element for bio(chemical) sensing applications. Talanta 2022, 236, 122878. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Wu, L.; Ding, L.; Effah, C.Y.; Wu, Y.; Xiong, Y.; He, L. Construction and bioapplications of aptamer-based dual recognition strategy. Biosens. Bioelectron. 2022, 195, 113661. [Google Scholar] [CrossRef]
- Arshavsky-Graham, S.; Heuer, C.; Jiang, X.; Segal, E. Aptasensors versus immunosensors-Which will prevail? Eng. Life Sci. 2022, 22, 319–333. [Google Scholar] [CrossRef]

| Labeled/ Label-Free | Optical Signal/Method | Antibodies (Abs) and Signal-Sensitive Material(s) Employed | Immunoassay Format | Analyte/Application Area | Ref. |
|---|---|---|---|---|---|
| Labeled | Fluorescence | Commercially available monoclonal Abs immobilized on graphene quantum dots (energy donor) along with Ti3C2-MXene decorated with silver nanoparticles (energy acceptor) | non-competitive (direct-type) a | Neuron Specific Enolase/Health | [56] |
| Labeled | Fluorescence | Commercially available Abs immobilized on carbon dots and silver nanoparticles | non-competitive (sandwich-type) b | Human Epididymis Protein 4 and/or ovarian cancer cells/Health | [57] |
| Labeled | Fluorescence (FRET-based) | Commercially available Abs immobilized on iron porphyrin bio-mimicking graphene quantum dots | non-competitive (direct-type) | Salmonella typhi VI antigen in human serum/Health | [59] |
| Labeled | Fluorescence | In-house developed mouse monoclonal Abs immobilized through protein A onto cell-based fluorescent microspheres containing carbon dots | non-competitive (direct-type) | Escherichia coli O157:H7 in milk/Food Analysis | [60] |
| Labeled | Fluorescence | Commercially available mouse monoclonal Absimmobilized on quantum dot nanobeads | competitive | Aflatoxin B1 in lotus seeds/Food Analysis | [58] |
| Labeled | Fluorescence (Evanescent-wave-based) | Commercially available Abs labeled with Cy5.5 along with a light-sheet skew rays enhanced U-shaped optical fiber | competitive | Microcystin-LR in drinking and lake water/Food Analysis & Environmental Pollution Monitoring | [61] |
| Labeled | Fluorescence | Antibodies indirectly immobilized on a glass fiber surface and used as an antibody-analyte-aptamer structure along with a CRISPR/Cas12a fluorescence detection system | non-competitive (sandwich-type) | Small proteins of biomedical interest, e.g., INF-γ in human serum, whole blood, perspiration and saliva samples/Health | [62] |
| Labeled | Fluorescence/Colorimetry | Monoclonal Abs coupled to Staphylococcus aureus-biosynthesized polymer dots, which can generate colorimetric-fluorescent dual signals | non-competitive (direct-type) | Zearalenone in millet and corn samples/Food analysis | [55] |
| Labeled | Phosphorescence (quenching-based) | Commercially available rabbit polyclonal Abs along with a phosphorescent tracer, i.e., analyte labeled with palladium-tetrabenzoporphyrin | competitive | Estrone and Estradiol/Health & Environmental Pollution Monitoring | [63] |
| Labeled | Chemiluminescence | Commercially available biotinylated Abs combined with streptavidin-biotinylated HRP nanocomposites and a HRP/chemiluminescent substrate; an optical fiber was used for signal guidance, on which an analyte-protein bioconjugate was immobilized | competitive | Methamphetamine in human blood, urine and oral fluid/Forensic Analysis | [64] |
| Labeled | Chemiluminescence | Commercially available mouse monoclonal Abs labeled with HRP along with a proper chemiluminescent substrate embedded on glass fiber pad of a sensor-strip | non-competitive (direct-type) | IgAs against proteins of SARS-CoV-2 in human serum and saliva/Health | [65] |
| Colorimetry | Commercially available mouse monoclonal Abs immobilized onto gold nanoparticles | non-competitive (direct-type) | |||
| Labeled | Colorimetry | Commercially available Abs Immobilized onto cotton swabs (capture Abs) and blue-colored polymeric nanobeads (detection Abs) | non-competitive (sandwich-type) | Methicillin resistant Staphylococcus aureus (MRSA)/Health | [54] |
| Label-free | Dynamic light scattering (DLS) | Commercially available rabbit polyclonal Abs immobilized onto gold nanoparticles | non-competitive (direct-type) | SARS-CoV-2 virus/Health | [66] |
| Label-free | Scattering of evanescence light | Commercially available monoclonal Abs conjugated to light scattering particles (capture Abs)/immobilized on the surface of optical waveguide film (detection Abs) | non-competitive (sandwich-type) | Nucleocapsid protein of SARS-CoV-2 virus/Health | [71] |
| Label-free | Retroreflectometry | Commercially available mouse monoclonal Abs immobilized on PMMA-biosensing chips (capture Abs)/conjugated to retroreflective microparticles (detection Abs) | non-competitive (sandwich-type) | Creatine kinase-myocardial band in buffer and spiked human serum/Health | [80] |
| Label-free | Fabry–Pérot interferometry (FPI) | Commercially available Abs immobilized on parylene-C nanosheet/Si | non-competitive (direct-type) | Prostate Specific Antigen/Health | [81] |
| Label-free | Polarization interferometry | Commercially available polyclonal Abs immobilized on a waveguide composed of Si3N4/SiO2 on Si | non-competitive (direct-type) | Fusarium mycotoxin Zearalenone in buffer/Food Analysis | [82] |
| Label-free | Refractometry | In-house developed avian (hen) polyclonal Abs (IgYs) immobilized on the grating region of a long-period fiber grating (LPFG) sensor | non-competitive (direct-type) | Staphylococcus aureus in water samples/Food Analysis | [68] |
| Label-free | Refractometry | Commercially available goat polyclonal Abs immobilized on the surface of an elliptical core helical intermediate-period fiber grating (E-HIPFG) sensor | non-competitive (direct-type) | Human immunoglobulin G/Health | [69] |
| Label-free | Refractometry | Commercially available monoclonal Abs immobilized on the surface of a Microfiber Bragg grating sensor | non-competitive (direct-type) | Cardiac Troponin I (cTn-I)/Health | [78] |
| Label-free | Photoluminescence | Commercially available monoclonal Abs immobilized on polyacrylonitrile/zinc oxide (PAN/ZnO) nanofibers | non-competitive (direct-type) | Aflatoxin B1 in buffer/Food Analysis | [67] |
| Label-free | SPR | Commercially available Abs immobilized on the surface of a gold chip -along with an aptamer conjugated to magnetite nanorods containing ordered mesocages and silver nanoclusters (MNOM@AgNCs) | non-competitive (sandwich-type) | Programmed death ligand 1 (PD-L1) in human plasma/Health | [73] |
| Label-free | SPR | Commercially available polyclonal Absimmobilized onto a gold/palladium coated unclad plastic optical fiber | non-competitive (direct-type) | Cortisol in buffer/Health and/or Food Analysis | [74] |
| Label-free | SPR | Tailor-made rabbit (IgGs) and avian (IgYs) polyclonal Abs immobilized on gold-coated glass surface | non-competitive (direct-type) | Hazelnut allergen Cor a14/Food Analysis | [70] |
| Label-free | SPR | Enzymatically half-reduced mouse monoclonal Abs immobilized on self-assembled gold nanoparticles | non-competitive (direct-type) | Collagen I/Health | [72] |
| Label-free | SPR | Mouse (and humanized) monoclonal Abs immobilized on the chip surface through protein G | non-competitive (sandwich-type) | Ricin, abrin biotoxins in human plasma as well as crude extracts of food samples/Health and/or Food Analysis | [75] |
| Label-free | SPR | Pair of mouse monoclonal Abs, immobilized on the gold sensing surface (capture antibodies) or coupled onto gold-coated magnetic nanoparticles (detection antibodies) leading to signal amplification | non-competitive (sandwich-type) | CD5 in spiked human sera/Health | [76] |
| Label-free | Surface Enhanced Raman Scattering (SERS) | Commercially available Abs immobilized on Fe3O4 magnetic nanoparticles (for signal enhancement) along with analyte labeled with a Raman-tag and gold (core)/silver(shell) bimetallic nanocuboid (Raman probe/tracer) | competitive | Imidacloprid in water /Environmental Pollution Monitoring | [77] |
| Label-free | Optical Waveguide Lightmode Spectroscopy (OWLS) | In-house developed rabbit polyclonal Abs either directly (a) or indirectly, i.e., through an analyte-bioconjugate (b) immobilized on SiO2-TiO2 chips | (a) non-competitive (direct-type); (b) competitive | Fusarium mycotoxin zearalenone in maize/Food analysis | [79] |
| Label-free | Light Diffraction | Abs against the SARS-CoV-2 spike protein immobilized on a polymer-type imprinted photonic crystal film | non-competitive (direct-type) | SARS-CoV-2 spike protein/Health | [83] |
| Label-free | Total internal reflection ellipsometry | Recombinant spike proteins of three SARS-CoV-2 variants (wild type, B.1.1.7. and B.1.351) immobilized on gold-coated SPR sensor disc | non-competitive (direct-type) | Abs circulating in human sera after vaccination with the Vaxzevria vaccine which could recognize the spike proteins of three SARS-CoV-2 variants/Health | [84] |
| Label-free | White light reflectance spectroscopy (WLRS) | In-house developed rabbit polyclonal Abs immobilized through an analyte-bioconjugate onto a Si/SiO2 chip | competitive | Ochratoxin A in cereal flours/Food Analysis | [85] |
| Label-free | WLRS | In-house developed rabbit polyclonal Abs immobilized through an analyte-bioconjugate onto a Si/SiO2 chip | competitive | Carbendazim in fruit juices/Food Analysis | [86] |
| Label-free | WLRS | Commercially available rabbit polyclonal Abs immobilized through Salmonella LPS onto a Si/SiO2 chip | competitive | Salmonella typhimurium in drinking water/Food Analysis | [88] |
| Label-free | WLRS | Commercially available goat polyclonal Abs immobilized onto a Si/SiO2 chip (capture Abs); the same Abs were used for detection | non-competitive (sandwich-type) | C-Reactive Protein in human blood/Health | [87] |
| Analyte | Functionalizing Biomolecule | Type of Antibodies (Abs) | Immunoassay Format | Signal Enhancement | Matrix/ Sample | Analysis Time | Regeneration Cycles | Refs |
|---|---|---|---|---|---|---|---|---|
| Ochratoxin A (OTA) | OTA-Ovalbumin (OTA-OVA) conjugate | In-house developed rabbit polyclonal Abs | Competitive | Yes (Biotinylated secondary a Ab/streptavidin) | Cereal flours | 25 min | N/A | [85] |
| Carbendazim | Benzimidazole derivative-Oligolysine conjugate | In-house developed rabbit polyclonal Abs | Competitive | Yes (Biotinylated secondary Ab/streptavidin) | Fruit juices | 28 min | 12 | [86] |
| Salmonella typhimurium (bacteria cells) | Salmonella LPS | Commercially available rabbit polyclonal Abs | Competitive | Yes (Biotinylated secondary Ab/streptavidin) | Water | 15 min | 15 | [88] |
| C-reactive protein (CRP) | anti-CRP Abs | Commercially available goat polyclonal Abs for capture and detection | Non-competitive (sandwich-type) | No | Human plasma | 12 min | N/A | [87] |
| Deoxynivalenol (DON) | DON-OVA conjugate | Commercially availabl emouse monoclonal Abs | Competitive | Yes (Secondary Ab) | Cereals | 12 min | 20 | [102] |
| Aflatoxin B1 (AFB1) and Fumonisin B1 (FB1) | AFB1-Bovine Serum Albumin (AFB1-BSA) & FB1-OVA conjugates | Commercially available mouse monoclonal Abs | Competitive | Yes (Secondary Ab) | Wheat and maize | 12 min | N/A | [98] |
| Glyphosate | Glyphosate-BSA conjugate | Commercially available avian polyclonal Abs (IgYs) | Competitive | Yes (Secondary Ab) | Water | 25 min | 14 | [103] |
| Atrazine and Paraquat | Atrazine-BSA & Paraquat-BSA conjugates | Commercially available rabbit polyclonal anti-paraquat Abs and mouse monoclonal anti-atrazine Abs | Competitive | Yes (Secondary Ab) | Water | 12 min | 20 | [97] |
| Aflatoxin M1 (AFM1) | AFM1-BSA conjugate | Commercially available rabbit polyclonal Abs | Competitive | Yes (Biotinylated secondary Ab/streptavidin) | Milk | 25 min | 25 | [104] |
| C-Reactive Protein (CRP) | anti-CRP Abs | Commercially available goat polyclonal Abs for capture and detection | Non-competitive (sandwich-type) | Yes (Biotinylated detection Ab/str) | Whole blood | 12 min | N/A | [96] |
| Prostate Specific Antigen (PSA) | anti-PSA Abs | Commercially available goat polyclonal Abs for capture and detection | Non-competitive (sandwich-type) | Yes (Biotinylated detection Ab/streptavidin) | Forensic samples/semen samples | 10 min | 24 | [95] |
| Chlorpyrifos, Imazalil and Thiabendazole | Chlorpyrifos-BSA, Imazalil-BSA & Thiabendazole-BSA conjugates | Commercially available monoclonal Abs | Competitive | Yes (Secondary Ab) | Water, wine | 10 min | 30 | [94] |
| CRP and D-dimer | anti-CRP Abs | Commercially available goat polyclonal Abs for capture and detection. | Non-competitive (sandwich-type) | No | Human plasma | 45 min | 25 | [93] |
| anti-D dimer Abs | Commercially available goat polyclonal Abs for capture and mouse monoclonal Abs for detection | Non-competitive (sandwich-type) | Yes (Biotinylated detection Ab/streptavidin) | |||||
| PSA (total and free) | anti-totalPSA & anti-freePSA Abs | Commercially available goat polyclonal anti-total PSA Abs for capture and detection and a pair of mouse monoclonal anti-freePSA Abs | Non-competitive (sandwich-type) | Yes (Biotinylated detection Ab/streptavidin) | Human serum | 65 min | 20 | [92] |
| Mouse IgG | anti-mouse IgG Abs | Commercially available goat polyclonal Abs | Non-competitive (direct-type) | No | Buffer | 1 min | 7 | [90] |
| Complement Activation Products (C3b) | anti-C3b Abs | Tailor-made mouse monoclonal Abs | Non-competitive (direct-type) | No | Human serum | 1 min | N/A | [91] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karachaliou, C.-E.; Koukouvinos, G.; Goustouridis, D.; Raptis, I.; Kakabakos, S.; Livaniou, E.; Petrou, P. Recent Developments in the Field of Optical Immunosensors Focusing on a Label-Free, White Light Reflectance Spectroscopy-Based Immunosensing Platform. Sensors 2022, 22, 5114. https://doi.org/10.3390/s22145114
Karachaliou C-E, Koukouvinos G, Goustouridis D, Raptis I, Kakabakos S, Livaniou E, Petrou P. Recent Developments in the Field of Optical Immunosensors Focusing on a Label-Free, White Light Reflectance Spectroscopy-Based Immunosensing Platform. Sensors. 2022; 22(14):5114. https://doi.org/10.3390/s22145114
Chicago/Turabian StyleKarachaliou, Chrysoula-Evangelia, Georgios Koukouvinos, Dimitrios Goustouridis, Ioannis Raptis, Sotirios Kakabakos, Evangelia Livaniou, and Panagiota Petrou. 2022. "Recent Developments in the Field of Optical Immunosensors Focusing on a Label-Free, White Light Reflectance Spectroscopy-Based Immunosensing Platform" Sensors 22, no. 14: 5114. https://doi.org/10.3390/s22145114
APA StyleKarachaliou, C.-E., Koukouvinos, G., Goustouridis, D., Raptis, I., Kakabakos, S., Livaniou, E., & Petrou, P. (2022). Recent Developments in the Field of Optical Immunosensors Focusing on a Label-Free, White Light Reflectance Spectroscopy-Based Immunosensing Platform. Sensors, 22(14), 5114. https://doi.org/10.3390/s22145114








