A Comparison of the Photophysical, Electrochemical and Cytotoxic Properties of meso-(2-, 3- and 4-Pyridyl)-BODIPYs and Their Derivatives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization
2.1.1. General
2.1.2. 1,3,5,7-Tetramethyl-8-(N-methyl-2-pyridyl)-BODIPY Iodide (2catPy)
2.1.3. 1,3,5,7-Tetramethyl-8-(N-methyl-3-pyridyl)-BODIPY Iodide (3catPy)
2.1.4. 2,6-Dichloro-8-(2-pyridyl)-1,3,5,7-tetramethyl-BODIPY (2PyCl2)
2.1.5. 2,6-Dichloro-8-(3-pyridyl)-1,3,5,7-tetramethyl-BODIPY (3PyCl2)
2.1.6. 2,6-Dichloro-8-(N-methyl-2-pyridyl)-1,3,5,7-tetramethyl-BODIPY (2catPyCl2)
2.1.7. 2,6-Dichlo-8-(N-methyl-3-pyridyl)-1,3,5,7-tetramethyl-BODIPY (3catPyCl2)
2.1.8. Anion Exchange
2.2. Spectroscopy Methods
2.3. Electrochemistry
2.4. X-ray Crystallography
2.5. Computational Methods
2.6. Cell Toxicity
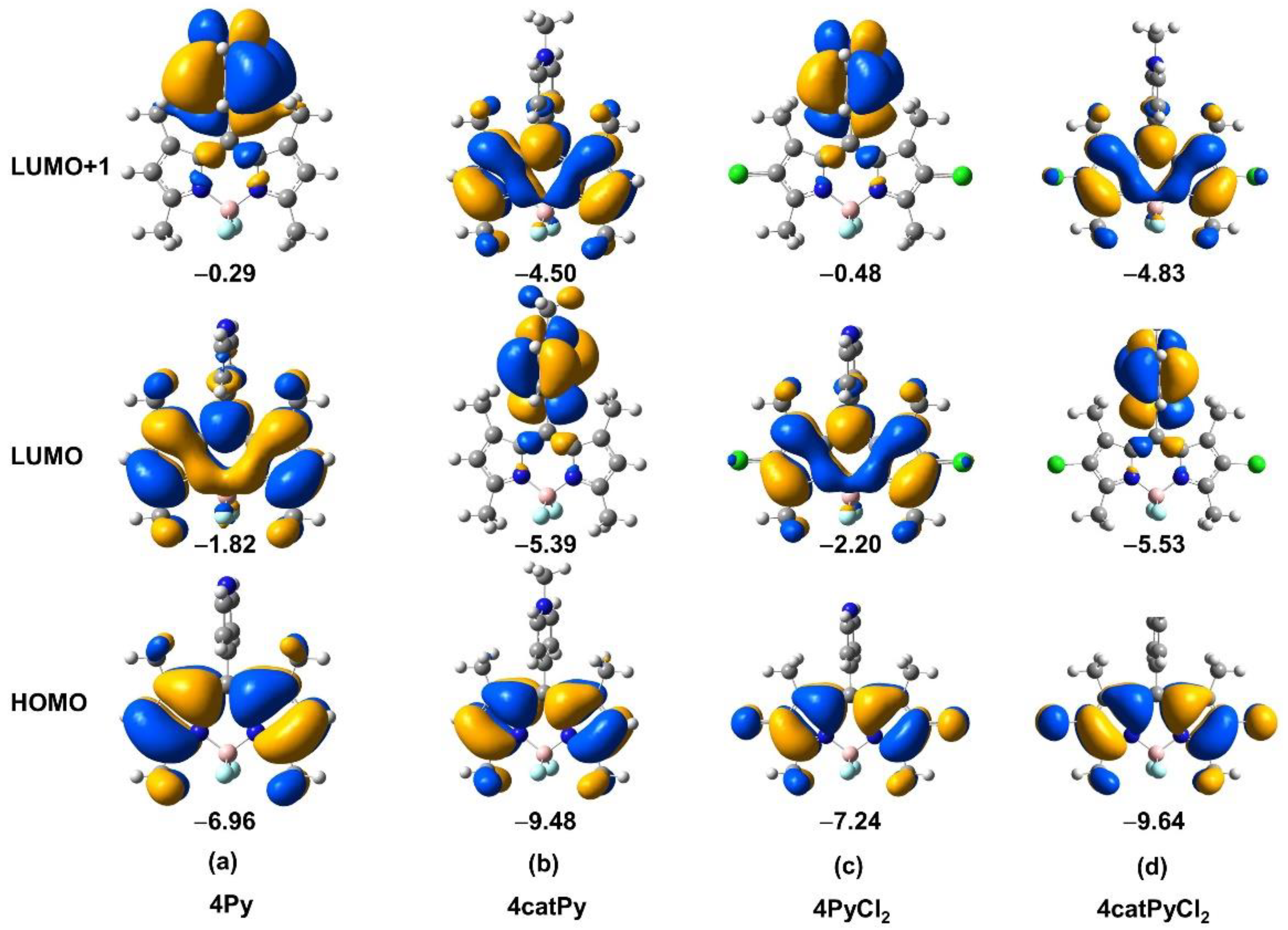
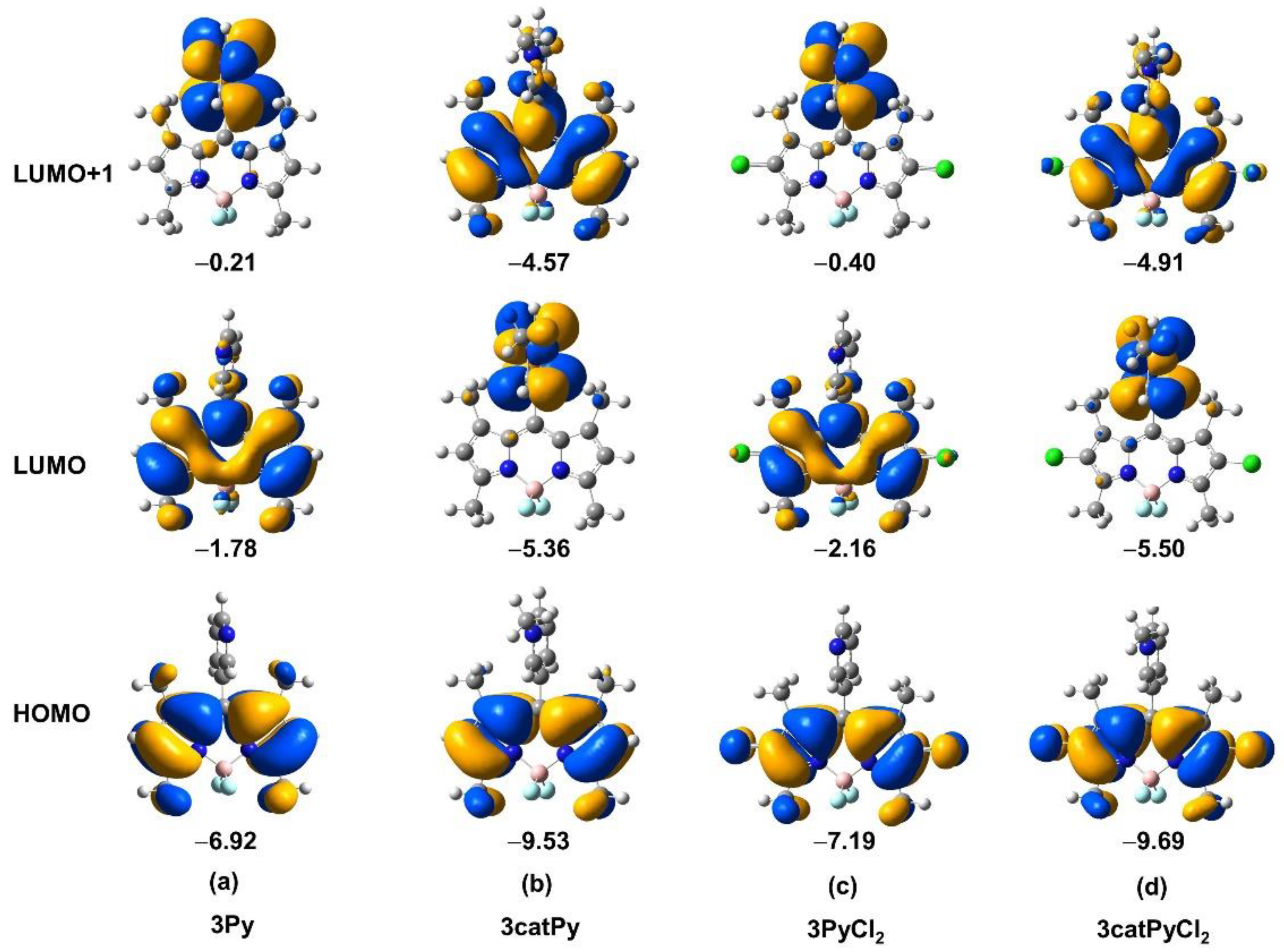
3. Results and Discussion
3.1. Synthesis
3.2. X-ray Analysis
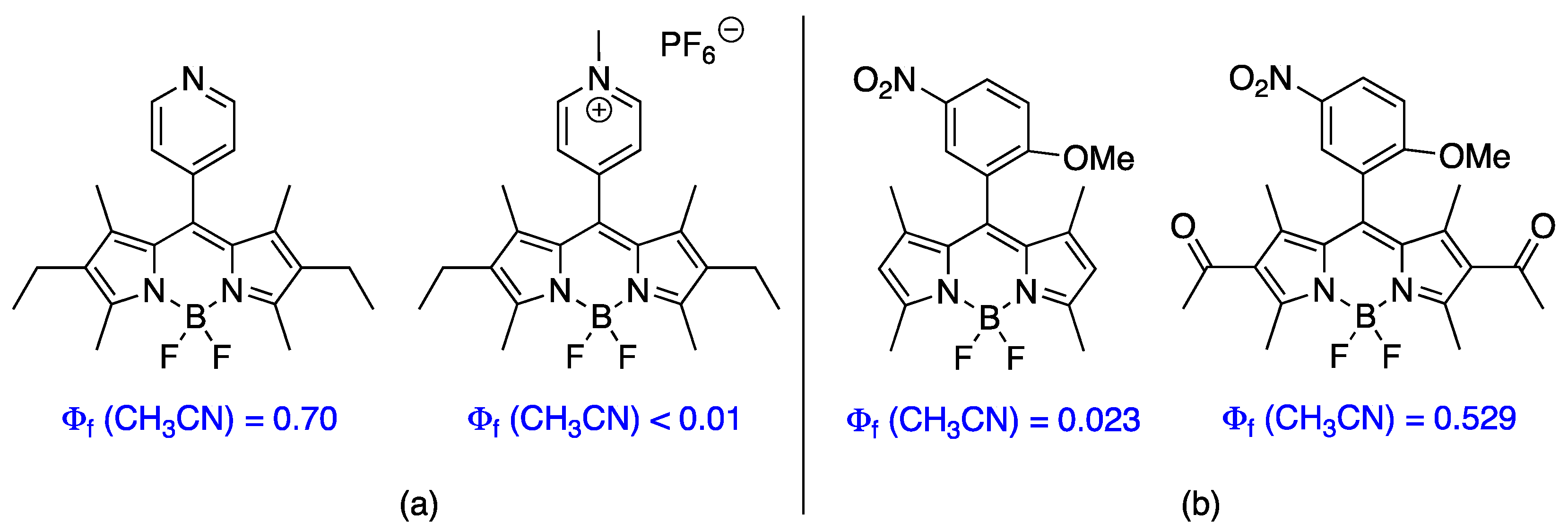
3.3. Spectroscopic Properties
3.4. Electrochemical Properties
3.5. Cytotoxicity Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Treibs, A.; Häberle, N. Über die Synthese und die Elektronenspektrenms-substituierter porphine. Just. Lieb. Ann. Chem. 1968, 718, 183–207. [Google Scholar] [CrossRef] [PubMed]
- Loudet, A.; Burgess, K. Bodipy dyes and their derivatives: Syntheses and spectroscopic properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef] [PubMed]
- Krumova, K.; Cosa, G. Bodipy dyes with tunable redox potentials and functional groups for further tethering: Preparation, electrochemical, and spectroscopic characterization. J. Am. Chem. Soc. 2010, 132, 17560–17569. [Google Scholar] [CrossRef] [PubMed]
- Mula, S.; Ray, A.K.; Banerjee, M.; Chaudhuri, T.; Dasgupta, K.; Chattopadhyay, S. Design and development of a new pyrromethene dye with improved photostability and lasing efficiency: theoretical rationalization of photophysical and photochemical properties. J. Org. Chem. 2008, 73, 2146–2154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wang, M.; Ndung’U, C.; Bobadova-Parvanova, P.; Fronczek, F.R.; Smith, K.M.; Vicente, M.G.H. Synthesis and investigation of BODIPYs with restricted meso-8-aryl rotation. J. Porphyr. Phthalocyanines 2020, 24, 869–877. [Google Scholar] [CrossRef]
- Erbas-Cakmak, S.; Akkaya, E.U. Toward singlet oxygen delivery at a measured rate: A self-reporting photosensitizer. Org. Lett. 2014, 16, 2946–2949. [Google Scholar] [CrossRef] [Green Version]
- Gibbs, J.H.; Zhou, Z.; Kessel, D.; Fronczek, F.R.; Pakhomova, S.; Vicente, M.G.H. Synthesis, spectroscopic, and in vitro investigations of 2,6-diiodo-BODIPYs with PDT and bioimaging applications. J. Photochem. Photobiol. B Biol. 2015, 145, 35–47. [Google Scholar] [CrossRef] [Green Version]
- Hendricks, J.A.; Keliher, E.J.; Wan, D.; Hilderbrand, S.A.; Weissleder, R.; Mazitschek, R. Synthesis of [18F] BODIPY: Bifunctional reporter for hybrid optical/positron emission tomography imaging. Angew. Chem. Int. Ed. 2012, 51, 4603–4606. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.; Wu, Q.; Wang, J.; Wei, Y.; Hao, E.; Jiao, L. Red to near-Infrared isoindole BODIPY fluorophores: Synthesis, crystal structures, and spectroscopic and electrochemical properties. J. Org. Chem. 2016, 81, 3761–3770. [Google Scholar] [CrossRef]
- Zhao, N.; Williams, T.M.; Zhou, Z.; Fronczek, F.R.; Sibrian-Vazquez, M.; Jois, S.D.; Vicente, M.G.H. Synthesis of BODIPY-peptide conjugates for fluorescence labeling of EGFR overexpressing cells. Bioconjug. Chem. 2017, 28, 1566–1579. [Google Scholar] [CrossRef] [Green Version]
- Zhao, N.; Xuan, S.; Zhou, Z.; Fronczek, F.R.; Smith, K.M.; Vicente, M.G.H. Synthesis, spectroscopic and cellular properties of near-IR [a]phenanthrene-fused 4,4-difluoro-4-bora-3a,4a-diaza-s-indacenes. J. Org. Chem. 2017, 82, 9744–9750. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, N.E.; Meng, Q.; Griffin, K.E.; Singh, S.S.; Dahal, A.; Zhou, Z.; Fronczek, F.R.; Mathis, J.M.; Jois, S.D.; Vicente, M.G.H. Synthesis, characterization, and evaluation of near-IR boron dipyrromethene bioconjugates for labeling of adenocarcinomas by selectively targeting the epidermal growth factor receptor. J. Med. Chem. 2019, 62, 3323–3335. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Zhao, J.; Cui, X.; Ma, J. Photoswitching of triplet–triplet annihilation upconversion showing large emission shifts using a photochromic fluorescent dithienylethene-bodipy triad as a triplet acceptor/emitter. Chem. Commun. 2015, 51, 1803–1806. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhang, X.; Li, K.; Zhu, S.; Guo, Z.; Zhang, L.; Wang, F.; Fei, Q.; Luo, S.; Shi, P. Forster resonance energy transfer switchable self-assembled micellar nanoprobe: Ratiometric fluorescent trapping of endogenous H2S generation via fluvastatin-stimulated upregulation. J. Am. Chem. Soc. 2015, 137, 8490–8498. [Google Scholar] [CrossRef] [PubMed]
- Bañuelos, J. BODIPY dye, the most versatile fluorophore ever? Chem. Rec. 2016, 16, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, M.J.; Garcia-Moreno, I.; Agarrabeitia, A.R.; Duran-Sampedro, G.; Costela, A.; Sastre, R.; López Arbeloa, F.; Bañuelos Prieto, J.; López Arbeloa, I. Red-edge-wavelength finely-tunable laser action from new BODIPY Dyes. Phys. Chem. Chem. Phys. 2010, 12, 7804–7811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Durán, C.F.; Esnal, I.; Valois-Escamilla, I.; Urías-Benavides, A.; Bañuelos, J.; López Arbeloa, I.; García-Moreno, I.; Peña-Cabrera, E. Near-IR BODIPY dyes à la carte-programmed orthogonal functionalization of rationally designed building blocks. Chem. Eur. J. 2015, 22, 1048–1061. [Google Scholar] [CrossRef] [Green Version]
- Niu, S.L.; Massif, C.; Ulrich, G.; Ziessel, R.; Renard, P.-Y.; Romieu, A. Water-solubilisation and bio-conjugation of a red-emitting Bodipy marker. Org. Biomol. Chem. 2011, 9, 66–69. [Google Scholar] [CrossRef]
- Xuan, S.; Zhao, N.; Zhou, Z.; Fronczek, F.R.; Vicente, M.G.H. Synthesis and in vitro studies of a series of carborane-containing boron dipyrromethenes (BODIPYs). J. Med. Chem. 2016, 59, 2109–2117. [Google Scholar] [CrossRef] [Green Version]
- Fan, G.; Yang, L.; Chen, Z. Water-soluble BODIPY and Aza-BODIPY Dyes: Synthetic progress and applications. Front. Chem. Sci. Eng. 2014, 8, 405–417. [Google Scholar] [CrossRef]
- Bura, T.; Ziessel, R. Water-soluble phosphonate-substituted BODIPY derivatives with tunable emission channels. Org. Lett. 2011, 13, 3072–3075. [Google Scholar] [CrossRef] [PubMed]
- Wories, H.J.; Koek, J.H.; Lodder, G.; Lugtenburg, J.; Fokkens, R.; Driessen, O.; Mohn, G.R. A novel water-soluble fluorescent probe: Synthesis, luminescence and biological properties of the sodium salt of the 4-sulfonato-3,3′,5,5′-tetramethyl-2,2′-pyrromethen-1,1′-BF2 complex. Rec. Trav. Chim. Pays-Bas 1985, 104, 288–291. [Google Scholar] [CrossRef]
- Niu, S.-L.; Ulrich, G.; Ziessel, R.; Kiss, A.; Renard, P.-Y.; Romieu, A. Water-soluble BODIPY derivatives. Org. Lett. 2009, 11, 2049–2052. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, Y. A water-soluble sulfonate-BODIPY based fluorescent probe for selective detection of HOCl/OCl- in aqueous media. Analyst 2014, 139, 2986–2989. [Google Scholar] [CrossRef] [PubMed]
- Ao, H.-W.; Zhu, X.-Y.; Guo, X.-F.; Wang, H. An amphiphilic fluorescent probe designed for extracellular visualization of nitric oxide released from living cells. Anal. Chem. 2016, 88, 9014–9021. [Google Scholar]
- Li, X.; Gao, X.; Shi, W.; Ma, H. Design strategies for water-soluble small molecular chromogenic and fluorogenic probes. Chem. Rev. 2014, 114, 590–659. [Google Scholar] [CrossRef]
- Komatsu, T.; Urano, Y.; Fujikawa, Y.; Kobayashi, T.; Kojima, H.; Terai, T.; Hanaoka, K.; Nagano, T. Development of 2,6-carboxy-substituted boron dipyrromethene (BODIPY) as a novel scaffold of ratiometric fluorescent probes for live cell imaging. Chem. Commun. 2009, 45, 7015–7017. [Google Scholar] [CrossRef]
- Dilek, Ö.; Bane, S.L. Synthesis, spectroscopic properties and protein labeling of water-soluble 3,5-disubstituted boron dipyrromethenes. Bioorg. Med. Chem. Lett. 2009, 19, 6911–6913. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, A.L.; Griffin, K.E.; Zhou, Z.; Fronczek, F.R.; Smith, K.M.; Vicente, M.G.H. Syntheses of 1,2,3-triazole-BODIPYs bearing up to three carbohydrate units. New J. Chem. 2018, 42, 8241–8246. [Google Scholar] [CrossRef]
- Lu, Z.; Mei, L.; Zhang, X.; Wang, Y.; Zhao, Y.; Li, C. Water-soluble BODIPY-conjugated glycopolymers as fluorescent probes for live cell imaging. Polym. Chem. 2013, 4, 5743–5750. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, J.; Vegesna, G.; Luo, F.-T.; Green, S.A.; Liu, H. Highly water-soluble neutral BODIPY dyes with controllable fluorescence quantum yields. Org. Lett. 2010, 13, 438–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harriman, A.; Mallon, L.J.; Ulrich, G.; Ziessel, R. Rapid intersystem crossing in closely-spaced but orthogonal molecular dyads. Chem. Phys. Chem. 2007, 8, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- LaMaster, D.J.; Kaufman, N.E.M.; Bruner, A.S.; Vicente, M.G.H. Structure based modulation of electron dynamics in meso-(4-pyridyl)-BODIPYs: A computational and synthetic approach. J. Phys. Chem. A 2018, 122, 6372–6380. [Google Scholar] [CrossRef] [PubMed]
- Ueno, T.; Urano, Y.; Kojima, H.; Nagano, T. Mechanism-based molecular design of highly selective fluorescence probes for nitrative stress. J. Am. Chem. Soc. 2006, 128, 10640–10641. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wu, T.; Fan, J.; Li, Z.; Jiang, N.; Wang, J.; Dou, B.; Sun, S.; Song, F.; Peng, X. A BODIPY-based fluorescent dye for mitochondria in living cells, with low cytotoxicity and high photostability. Org. Biomol. Chem. 2013, 11, 555–558. [Google Scholar] [CrossRef]
- Luo, G.-G.; Fang, K.; Wu, J.-H.; Dai, J.-C.; Zhao, Q.-H. Noble-metal-free BODIPY-cobaloxime photocatalysts for visible-light-driven hydrogen production. Phys. Chem. Chem. Phys. 2014, 16, 23884–23894. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision D.01 Computational Chemistry Software; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Wang, Y.-W.; Li, M.; Shen, Z.; You, X.-Z. Meso-pyridine substituted boron-dipyrromethene (BDP) dye as a pH probe: Synthesis, crystal structure and spectroscopic properties. Chin. J. Inorg. Chem. 2008, 24, 1247–1252. [Google Scholar]
- Raza, M.K.; Gautam, S.; Howlader, P.; Bhattacharyya, A.; Kondaiah, P.; Chakravarty, A.R. Pyriplatin-boron-dipyrromethene conjugates for imaging and mitochondria-targeted photodynamic therapy. Inorg. Chem. 2018, 57, 14374–14385. [Google Scholar] [CrossRef] [Green Version]
- Olmsted, J. Calorimetric determinations of absolute fluorescence quantum yields. J. Phys. Chem. 1979, 83, 2581–2584. [Google Scholar] [CrossRef]
- Bartelmess, J.; Weare, W.W.; Latortue, N.; Duong, C.; Jones, D.S. Meso-pyridyl BODIPYs with tunable chemical, optical and electrochemical properties. New J. Chem. 2013, 37, 2663–2668. [Google Scholar] [CrossRef]
- Zimbron, J.M.; Passador, K.; Gatin-Fraudet, B.; Bachelet, C.-M.; Plazuk, D.; Chamoreau, L.-M.; Botuha, C.; Thorimbert, S.; Salmain, M. Synthesis, photophysical properties and living cell imaging of theranostic harf-sandwich iridium-4,4-difluoro-4-bora-3a,4a-diaza-s-indacene (BODIPY) dyads. Organometallics 2017, 36, 3435–3442. [Google Scholar] [CrossRef]
- Hu, W.; Liu, M.; Zhang, X.-F.; Wang, Y.; Wang, Y.; Lan, H.; Zhao, H. Can BODIPY-electron acceptor conjugates act as heavy atom-free excited triplet state and singlet oxygen photosensitizers via photoinduced charge separation-charge recombination mechanism? J. Phys. Chem. C 2019, 123, 15944–15955. [Google Scholar] [CrossRef]
- Ulrich, G.; Ziessel, R. Convenient and efficient synthesis of functionalized oligopyridine ligands bearing accessory pyrromethene-BF2 fluorophores. J. Org. Chem. 2004, 69, 2070–2083. [Google Scholar] [CrossRef] [PubMed]
- Ragab, S.S.; Swaminathan, S.; Baker, J.D.; Raymo, F.M. Activation of BODIPY fluorescence by the photoinduced dealkylation of a pyridinium quencher. Phys. Chem. Chem. Phys. (PCCP) 2013, 15, 14851–14855. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Bao, X.; Zhou, J.; Peng, F.; Ren, H.; Dong, X.; Zhao, W. A mitochondria-targeted turn-on fluorescent probe for the detection of glutathione in living cells. Bios. Bioelect. 2016, 85, 164–170. [Google Scholar] [CrossRef]
- Zhang, J.; Ji, X.; Zhou, J.; Chen, Z.; Dong, X.; Zhao, W. Pyridinium-substituted BODIPY as NIR fluorescent probe for simultaneous sensing of hydrogen sulphide/glutathione and cysteine/homocysteine. Sens. Actuators B 2018, 257, 1076–1082. [Google Scholar] [CrossRef]
- Koyanagi, Y.; Kawaguchi, S.; Fujii, K.; Kimura, Y.; Sasamori, T.; Tokitoh, N.; Matano, Y. Effects of counter anions, p-substituents, and solvents on optical and photophysical properties of 2-phenylbenzo[b]phospholium salts. Dalton Trans. 2017, 46, 9517–9527. [Google Scholar] [CrossRef]
- Duran-Sampedro, G.; Agarrabeitia, A.R.; Garcia-Moreno, I.; Costela, A.; Bañuelos, J.; Arbeloa, T.; Arbeloa, I.L.; Chiara, J.L.; Ortiz, M.J. Chlorinated BODIPYs: Surprizingly efficient and highly photostable laser dyes. Eur. J. Org. Chem. 2012, 2012, 6335–6350. [Google Scholar] [CrossRef]
- Jin, Y.; Lv, M.; Tao, Y.; Xu, S.; He, J.; Zhang, J.; Zhao, W. A water-soluble BODIPY-based fluorescent probe for rapid and selective detection of hypochlorous acid in living cells. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 219, 569–575. [Google Scholar] [CrossRef]
- Thompson, B.L.; Heiden, Z. Redox chemistry of BODIPY dyes. In BODIPY Dyes—A Privilege Molecular Scaffold with Tunable Properties; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Carpenter, B.L.; Situ, X.; Scholle, F.; Bartelmess, J.; Weare, W.W.; Ghiladi, R.A. Antiviral, antifungal and antibacterial activities of a BODIPY-based photosensitizer. Molecules 2015, 20, 10604–10621. [Google Scholar] [CrossRef] [Green Version]
- Caruso, E.; Banfi, S.; Barbieri, P.; Leva, B.; Orlandi, V.T. Synthesis and antibacterial activity of novel cationic BODIPY photosensitizers. J. Photochem. Photobiol. B Biol. 2012, 114, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Hu, M.; Zhang, R.; Zhu, Y.; Gu, K.; Bai, J.; Li, J.; Dong, X.; Zhao, W. Discovery of meso-(meta-pyridinium) BODIPY photosensitizers: In vitro and in vivo evaluations for antimicrobial photodynamic therapy. J. Med. Chem. 2021, 64, 18143–18157. [Google Scholar] [CrossRef] [PubMed]




| Solvent | BODIPY | λabs (nm) | λem (nm) | Stokes Shift (nm) | Φf | ᵋ (M−1 cm −1) |
|---|---|---|---|---|---|---|
| CH3CN | 2Py | 502 | 514 | 12 | 0.04 a | 87,500 |
| 3Py | 502 | 514 | 12 | 0.43 a | 92,900 | |
| 4Py [18] | 501 | 515 | 14 | 0.31 | 72,100 | |
| 2PyCl2 | 530 | 545 | 15 | 0.17 a | 64,280 | |
| 3PyCl2 | 527 | 542 | 15 | 0.58 a | 57,540 | |
| 4PyCl2 [18] | 528 | 546 | 18 | 0.58 | 50,800 | |
| 2catPy (I−) | 520 | 544 | 24 | 0.13 b | 65,800 | |
| 2catPy (PF6−) | 520 | 546 | 26 | 0.23 b | 36,700 | |
| 3catPy (I−) | 510 | 532 | 22 | 0.006 b | 65,460 | |
| 3catPy (PF6−) | 510 | 529 | 19 | 0.006 b | 88,580 | |
| 4catPy (I−) [18] | 509 | 596 | 87 | 0.019 | 26,000 | |
| 4catPy (PF6−) | 509 | 603 | 94 | 0.010 b | 71,520 | |
| 2catPyCl2 (I−) | 530 | 547 | 17 | 0.17 b | 19,420 | |
| 3catPyCl2 (I−) | 540 | 560 | 20 | 0.23 b | 43,160 | |
| 4catPyCl2 (I−) [18] | 538 | 600 | 62 | 0.048 | 23,400 | |
| H2O | 2catPy (I−) | 520 | 542 | 22 | 0.001 b | 52,380 |
| 3catPy (I−) | 510 | 530 | 20 | 0.005 b | 37,320 | |
| 4catPy (I−) [18] | 509 | 600 | 91 | 0.004 | 31,600 | |
| 2catPyCl2 (I−) | 555 | 577 | 22 | 0.24 b | 21,000 | |
| 3catPyCl2 (I−) | 540 | 564 | 24 | 0.47 b | 35,180 | |
| 4catPyCl2 (I−) | 540 | 605 | 65 | 0.038 | 11,640 |
| BODIPY | λabs (nm) | Oscillator Strength | HOMO-LUMOBODIPY Gap (eV) | Rotation Barrier (kcal/mol) | S1-T Energy Difference (eV) |
|---|---|---|---|---|---|
| 2Py | 416 | 0.542 | 5.14 | 17.8 | |
| 3Py | 416 | 0.542 | 5.14 | 20.7 | |
| 4Py | 415 | 0.544 | 5.15 | 19.7 | |
| 2catPy | 435 | 0.537 | 4.88 | >35 | 0.057 |
| 3catPy | 429 | 0.490 | 4.95 | 25.3 | 0.025 |
| 4catPy | 427 | 0.550 | 4.98 | 25.1 | 0.012 |
| 2PyCl2 | 432 | 0.565 | 5.05 | 16.6 | |
| 3PyCl2 | 433 | 0.562 | 5.03 | 18.6 | |
| 4PyCl2 | 432 | 0.561 | 5.04 | 19.2 | |
| 2catPyCl2 | 460 | 0.558 | 4.72 | - | |
| 3catPyCl2 | 455 | 0.533 | 4.78 | - | |
| 4catPyCl2 | 451 | 0.567 | 4.82 | - |
| BODIPY | ||
|---|---|---|
| 2catPy | +0.99 a | −1.09; −1.29 |
| 3catPy | +0.89 | −1.26; −1.54 |
| 4catPy | +0.89 | −1.06 e |
| 2catPyCl2 | +1.10 b | −0.87; −1.16 |
| 3catPyCl2 | +1.10 c | −1.02; −1.42 |
| 4catPyCl2 | +1.03 d | −0.89 e |
| BODIPY | Dark Toxicity (IC50, mM) | Phototoxicity (IC50, mM) |
|---|---|---|
| 2Py | >200 | >100 |
| 3Py | >200 | >100 |
| 4Py | >200 | >100 |
| 2PyCl2 | >200 | >100 |
| 3PyCl2 | 200 | >100 |
| 2catPy | >200 | >100 |
| 3catPy | >200 | >100 |
| 4catPy | >200 | >100 |
| 2catPyCl2 | 80 | 80 |
| 3catPyCl2 | 120 | >100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ndung’u, C.; LaMaster, D.J.; Dhingra, S.; Mitchell, N.H.; Bobadova-Parvanova, P.; Fronczek, F.R.; Elgrishi, N.; Vicente, M.d.G.H. A Comparison of the Photophysical, Electrochemical and Cytotoxic Properties of meso-(2-, 3- and 4-Pyridyl)-BODIPYs and Their Derivatives. Sensors 2022, 22, 5121. https://doi.org/10.3390/s22145121
Ndung’u C, LaMaster DJ, Dhingra S, Mitchell NH, Bobadova-Parvanova P, Fronczek FR, Elgrishi N, Vicente MdGH. A Comparison of the Photophysical, Electrochemical and Cytotoxic Properties of meso-(2-, 3- and 4-Pyridyl)-BODIPYs and Their Derivatives. Sensors. 2022; 22(14):5121. https://doi.org/10.3390/s22145121
Chicago/Turabian StyleNdung’u, Caroline, Daniel J. LaMaster, Simran Dhingra, Nathan H. Mitchell, Petia Bobadova-Parvanova, Frank R. Fronczek, Noémie Elgrishi, and Maria da Graça H. Vicente. 2022. "A Comparison of the Photophysical, Electrochemical and Cytotoxic Properties of meso-(2-, 3- and 4-Pyridyl)-BODIPYs and Their Derivatives" Sensors 22, no. 14: 5121. https://doi.org/10.3390/s22145121







