Wearable Antennas for Sensor Networks and IoT Applications: Evaluation of SAR and Biological Effects
Abstract
:1. Introduction
2. Methods, Models, and Experimental Procedures
2.1. Methods and Models
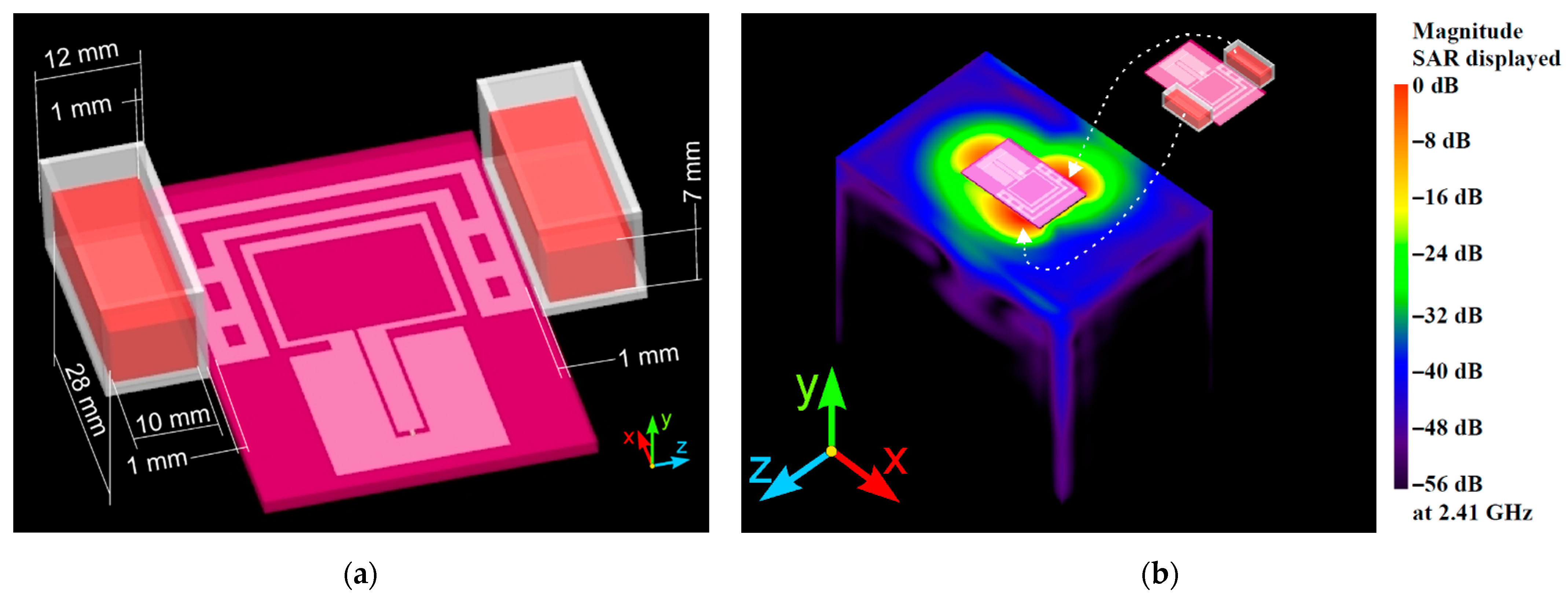
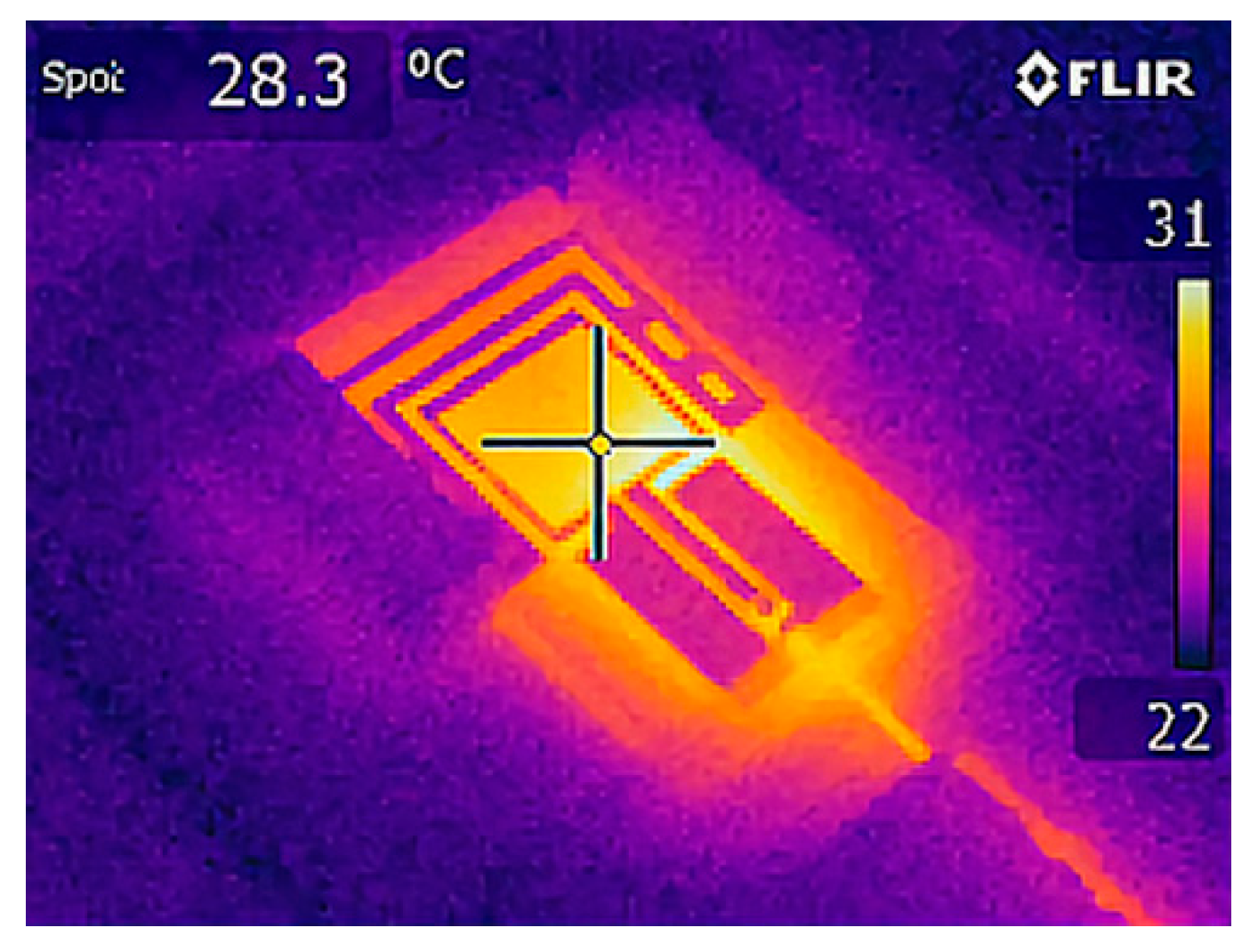
2.1.1. Numerical Method and Models
2.1.2. Experimental Method
2.2. Experimental Procedures
2.2.1. Erythrocytes, Isolation, and Preparation of the Cell Suspensions
2.2.2. Experimental Groups
2.2.3. Exposure Setups and Conditions
2.2.4. Duration of Exposure
2.3. Statistical Analysis
3. Results and Discussion
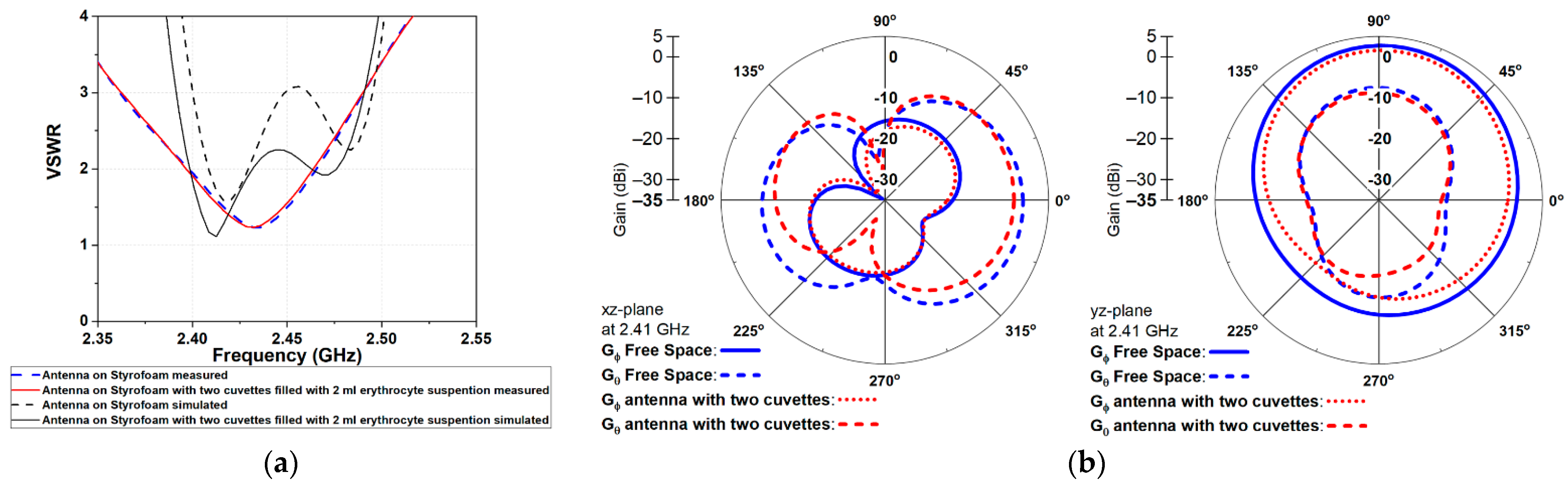
3.1. Wearable Antenna Performance
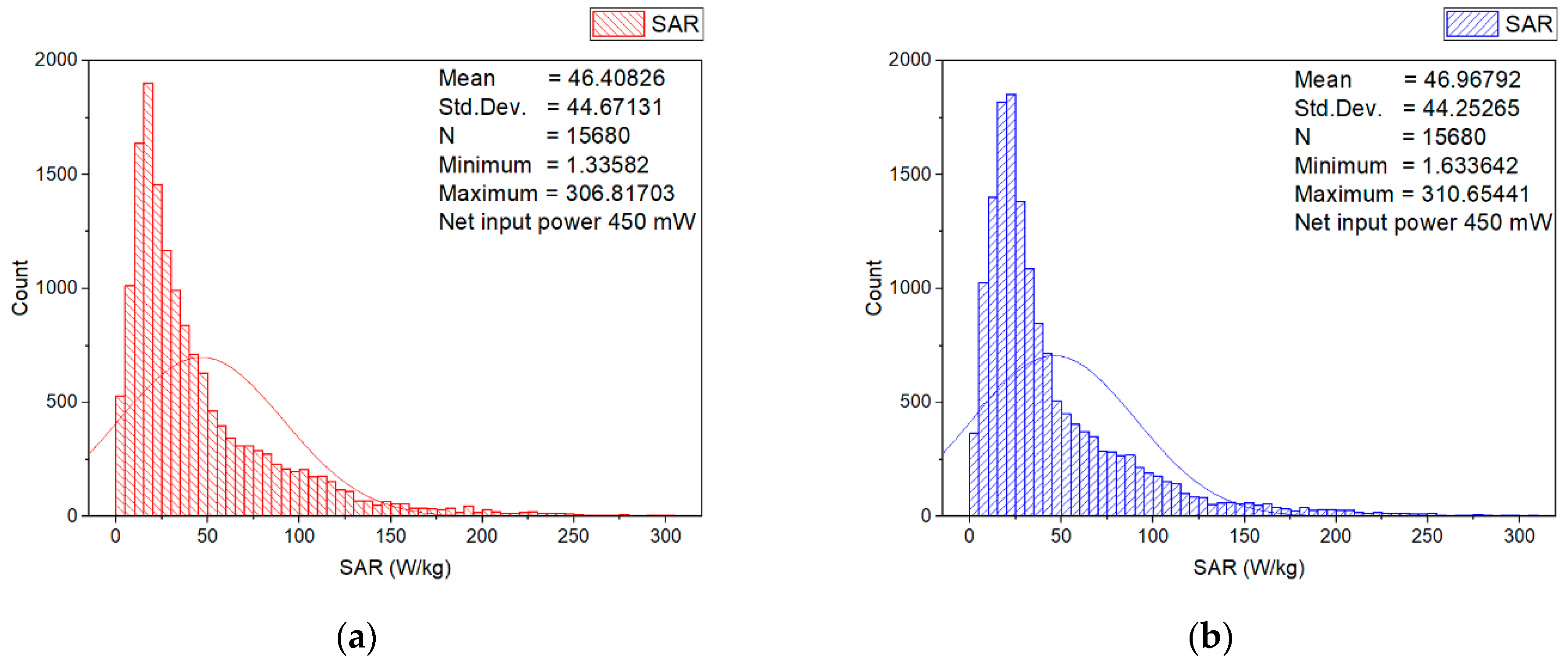
3.2. Numerical Estimation of the Induced Specific Absorption Rate
3.3. Experimental Results on the Stability of Human Erythrocyte Membranes
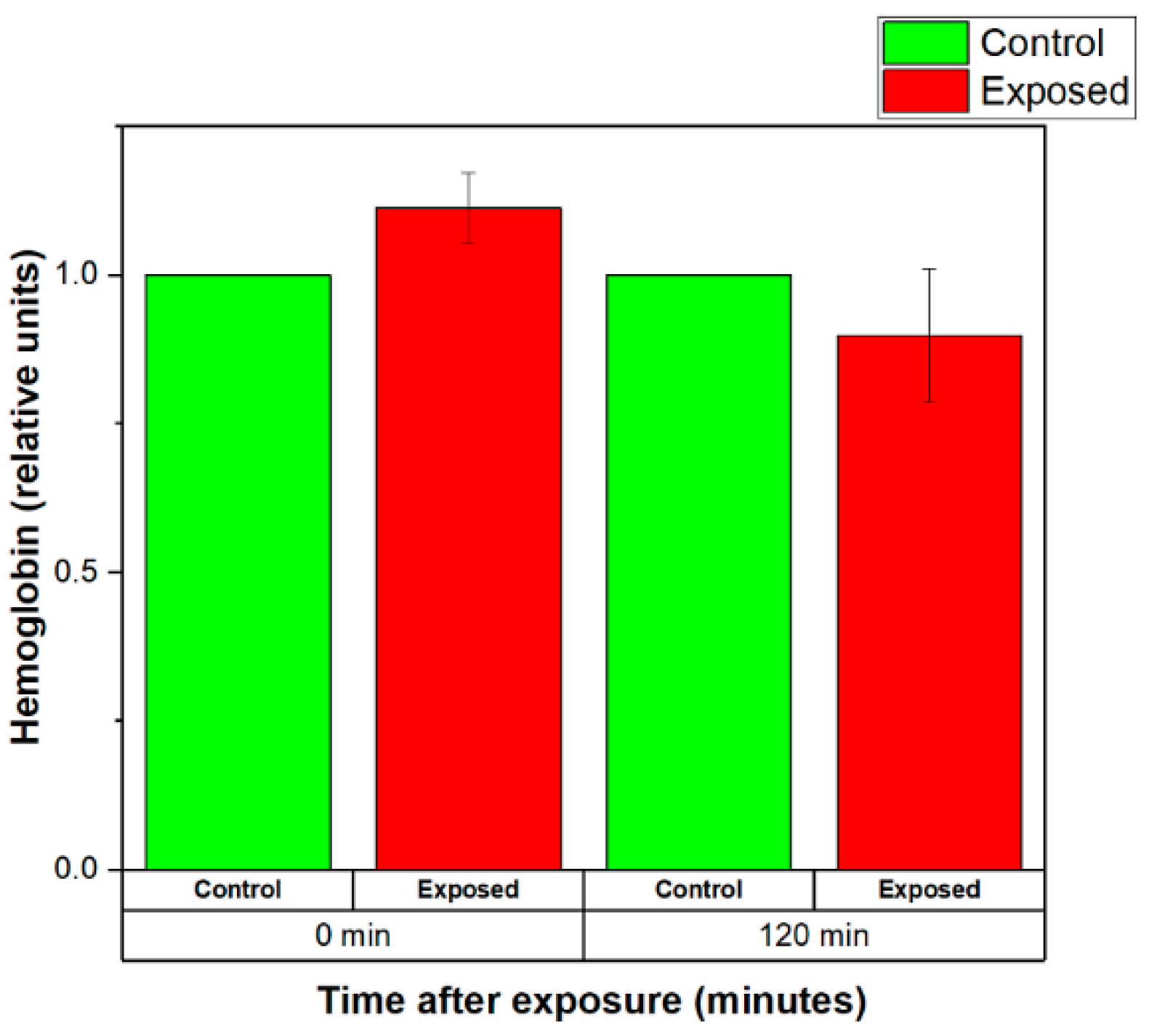
3.3.1. Effects of Weak EMFs Emitted by a Wearable Antenna on the Stability of the RBCs Membranes
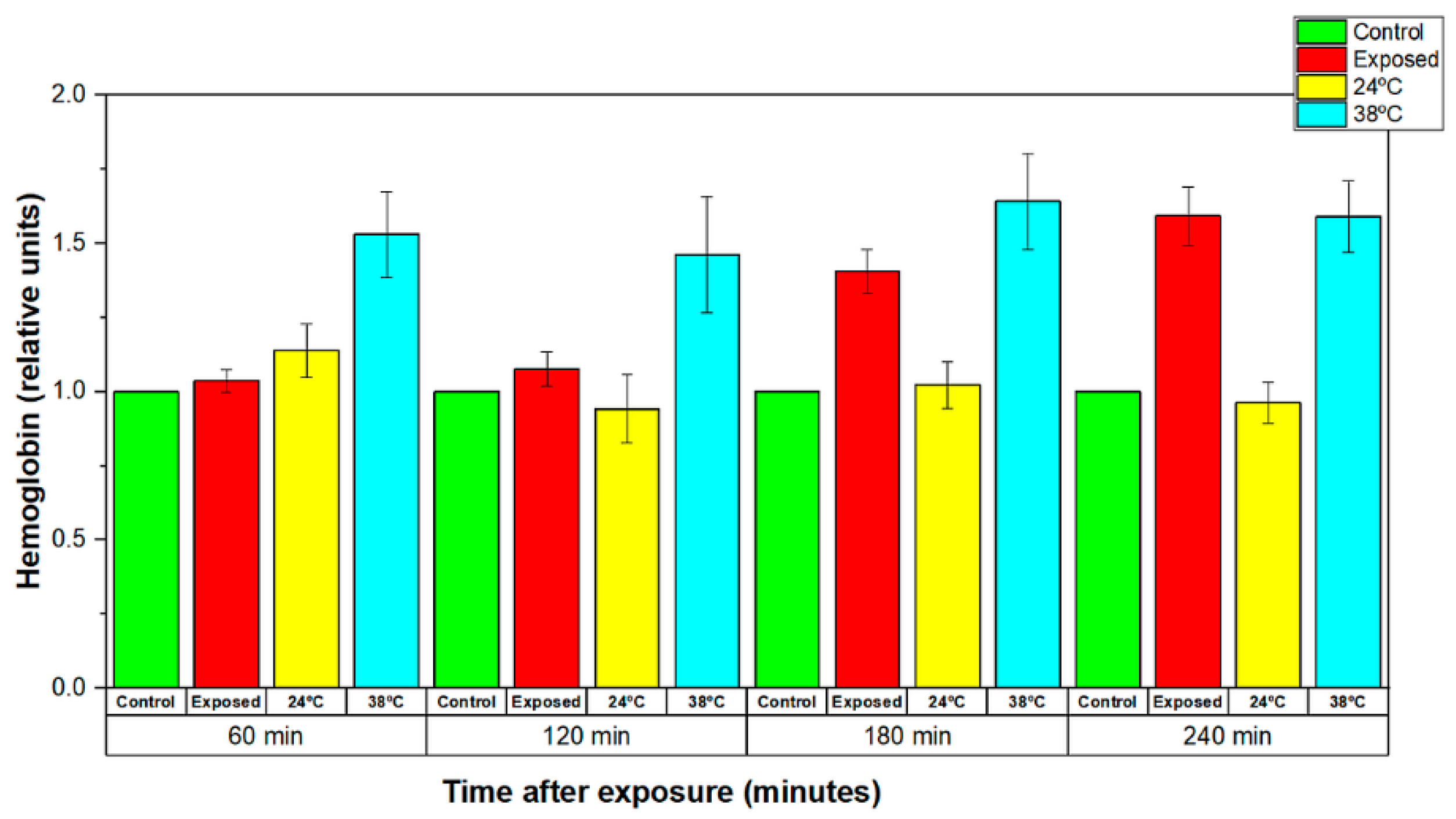
3.3.2. Effects of Strong EMFs Emitted by a Wearable Antenna on the Stability of the RBCs Membranes
3.3.3. Comparison of the Effects Induced by EMF and Conventional Heat Exposure on the Stability of Human Erythrocyte Membranes
3.3.4. Discussion
3.3.5. Comparison
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Statista. Available online: https://www.statista.com/statistics/487291/global-connected-wearable-devices/ (accessed on 14 February 2022).
- Atanasov, N.T.; Atanasova, G.L.; Atanasov, B.N. Wearable Textile Antennas with High Body-Area Isolation: Design, Fabrication, and Characterization Aspects. In Modern Printed Circuit Antennas, 1st ed.; Al-Rizzo, H., Ed.; IntechOpen: London, UK, 2020; Volume 1, pp. 143–157. [Google Scholar] [CrossRef] [Green Version]
- Alomainy, A.; Hao, Y. Antennas for Wearable Devices. In Antenna for Portable Devices, 1st ed.; Chen, Z.N., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2007; pp. 197–229. [Google Scholar]
- Jiang, Z.H.; Yue, T.; Werner, D.H. Metamaterial-Enabled and Microwave Circuit Integrated Wearable Antennas for Off-Body Communications. In Electromagnetics of Body Area Networks: Antennas, Propagation and RF Systems, 1st ed.; Werner, D.H., Jiang, Z.H., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 27–59. [Google Scholar]
- Sayem, A.S.M.; Simorangkir, R.B.V.B.; Esselle, K.P.; Lalbakhsh, A.; Gawade, D.R.; O’Flynn, B.; Buckley, J.L. Flexible and Transparent Circularly Polarized Patch Antenna for Reliable Unobtrusive Wearable Wireless Communications. Sensors 2022, 22, 1276. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Vital, D.; Werner, D.H.; Wu, Y.; Bhardwaj, S. Dual-Polarized Embroidered Textile Armband Antenna Array With Omnidirectional Radiation for On-/Off-Body Wearable Applications. IEEE Trans. Antennas Propag. 2020, 68, 2575–2584. [Google Scholar] [CrossRef]
- Jiang, Z.H.; Cui, Z.; Yue, T.; Zhu, Y.; Werner, D.H. Compact, Highly Efficient, and Fully Flexible Circularly Polarized Antenna Enabled by Silver Nanowires for Wireless Body-Area Networks. IEEE Trans. Biomed. Circuits Syst. 2017, 11, 920–932. [Google Scholar] [CrossRef]
- Monti, G.; Corchia, L.; Paiano, E.; De Pascali, G.; Tarricone, L.; Tomassoni, C.; Sorrentino, R. Textile Wearable Antenna for Firefighters Positioning. In Proceedings of the 2019 URSI Asia-Pacific Radio Science Conference (AP-RASC), New Delhi, India, 9–15 March 2019; pp. 1–4. [Google Scholar] [CrossRef]
- Corchia, L.; De Benedetto, E.; Monti, G.; Cataldo, A.; Tarricone, L. Wearable antennas for applications in remote assistance to elderly people. In Proceedings of the 2017 IEEE International Workshop on Measurement and Networking (M&N), Naples, Italy, 27–29 September 2017; pp. 1–6. [Google Scholar] [CrossRef]
- Monti, G.; Corchia, L.; Tarricone, L. Textile Logo Antennas. In Proceedings of the 2014 Mediterranean Microwave Symposium (MMS2014), Marrakech, Marocco, 12–14 December 2014; pp. 1–5. [Google Scholar] [CrossRef]
- Mikulić, D.; Šopp, E.; Bonefačić, D.; Šipuš, Z. Textile Slotted Waveguide Antennas for Body-Centric Applications. Sensors 2022, 22, 1046. [Google Scholar] [CrossRef]
- Ibanez Labiano, I.; Arslan, D.; Ozden Yenigun, E.; Asadi, A.; Cebeci, H.; Alomainy, A. Screen Printing Carbon Nanotubes Textiles Antennas for Smart Wearables. Sensors 2021, 21, 4934. [Google Scholar] [CrossRef]
- Atanasova, G.L.; Atanasov, N.T. Impact of Electromagnetic Properties of Textile Materials on Performance of a Low-Profile Wearable Antenna Backed by a Reflector. In Proceedings of the 2020 International Workshop on Antenna Technology (iWAT), Bucharest, Romania, 25–28 February 2020; pp. 1–4. [Google Scholar] [CrossRef]
- Atanasova, G.; Atanasov, N. Small Antennas for Wearable Sensor Networks: Impact of the Electromagnetic Properties of the Textiles on Antenna Performance. Sensors 2020, 20, 5157. [Google Scholar] [CrossRef]
- Bait-Suwailam, M.M.; Labiano, I.; Alomainy, A. Impedance Enhancement of Textile Grounded Loop Antenna Using High-Impedance Surface (HIS) for Healthcare Applications. Sensors 2020, 20, 3809. [Google Scholar] [CrossRef]
- Zradziński, P.; Karpowicz, J.; Gryz, K.; Morzyński, L.; Młyński, R.; Swidziński, A.; Godziszewski, K.; Ramos, V. Modelling the Influence of Electromagnetic Field on the User of a Wearable IoT Device Used in a WSN for Monitoring and Reducing Hazards in the Work Environment. Sensors 2020, 20, 7131. [Google Scholar] [CrossRef]
- IEEE Standard for Safety Levels with Respect to Human Exposure to Electric, Magnetic, and Electromagnetic Fields, 0 Hz to 300 GHz. In IEEE Std C95.1-2019 (Revision of IEEE Std C95.1-2005/ Incorporates IEEE Std C95.1-2019/Cor 1-2019); IEEE: Manhattan, NY, USA, 2019. [CrossRef]
- Rahmat-Samii, Y.; Guterman, J.; Moreira, A.A.; Peixeiro, C. Integrated antennas for wireless personal communications. In Modern Antenna Handbook, 1st ed.; Balanis, C., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 1079–1142. [Google Scholar]
- International Commission on Non-Ionizing Radiation Protection (ICNIRP). Guidelines for Limiting Exposure to Electro-magnetic Fields (100 kHz to 300 GHz). Health Phys. 2020, 118, 483–524. [Google Scholar] [CrossRef]
- Lin, J.C.; Bernardi, P.; Pisa, S.; Cavagnaro, M.; Piuzzi, E. Antennas for Biological Experiments. In Modern Antenna Handbook, 1st ed.; Balanis, C., Ed.; John Wiley & Sons: Hoboken: NJ, USA, 2008; pp. 1429–1460. [Google Scholar]
- Kostoff, R.N.; Heroux, P.; Aschner, M.; Tsatsakis, A. Adverse health effects of 5G mobile networking technology under real-life conditions. Toxicol. Lett. 2020, 323, 35–40. [Google Scholar] [CrossRef]
- Abdel Aziz, I.; El-Khozondar, H.J.; Shabat, M.M.; Elwasife, K.Y.; Mohamed-Osman, A. Effect of electromagnetic field on body weight and blood indices in albino rats and the therapeutic action of vitamin C or E. Rom. J. Biophys. 2010, 20, 235–244. [Google Scholar]
- Hassan, N.S.; Rafaat, B.M.; Aziz, S.W. Modulatory role of grape seed extract on erythrocyte hemolysis and oxidative stress induced by microwave radiation in rats. Int. J. Integr. Biol. 2010, 10, 106–111. [Google Scholar]
- Kiel, J.L.; Erwin, D.N. Microwave and thermal interactions with oxidative hemolysis. Physiol. Chem. Phys. Med. NMR 1984, 16, 317–323. [Google Scholar] [PubMed]
- Markov, M. Thermal vs. nonthermal mechanisms of interactions between electromagnetic fields and biological systems. In Bioelectromagnetics Current Concepts, 1st ed.; Ayrapetyan, S.N., Markov, M.S., Eds.; Springer: Dordrecht, The Netherland, 2006; pp. 1–15. [Google Scholar] [CrossRef]
- Pilla, A.A. Nonthermal electromagnetic fields: From first messenger to therapeutic applications. Electromagn. Biol. Med. 2013, 32, 123–136. [Google Scholar] [CrossRef]
- Lin, J.C. 5G Communication Technology and Coronavirus Disease [Health Matters]. IEEE Microw. Mag. 2020, 21, 16–19. [Google Scholar] [CrossRef]
- Kim, Y.A.; Elemesov, R.E.; Akoev, V.R.; Abdrasilov, B.S. Study of Erythrocyte Hemolysis on Exposure to Strong 2.45-GHz Electromagnetic Radiation. Biophysics 2005, 50, S44–S50. [Google Scholar]
- Sajin, G.; Kovacs, E.; Moraru, R.P.; Savopol, T.; Sajin, M. Cell membrane permeabilization of human erythrocytes by athermal 2450-MHz microwave radiation. IEEE Trans. Microw. Theory Tech. 2000, 48, 2072–2075. [Google Scholar] [CrossRef]
- Popovic, M.; Hagness, S.C.; Taflove, A. Finite-difference time-domain analysis of a complete transverse electromagnetic cell loaded with liquid biological media in culture dishes. IEEE Trans. Biomed. Eng. 1998, 45, 1067–1076. [Google Scholar] [CrossRef] [Green Version]
- Kouzmanova, M.; Atanasova, G.; Atanasov, N.; Tasheva, S. Alterations in hemolysis after in vitro exposure of human erythrocytes to GSM900 electromagnetic field. In Proceedings of the 4th International Workshop “Biological Effects of EMF”, Crete, Greece, 16–20 October 2006; pp. 96–101. [Google Scholar]
- IEEE Standard for Safety Levels with Respect to Human Exposure to Radio Frequency Electromagnetic Fields, 3 kHz to 300 GHz. In IEEE Std C95.1-2005 (Revision of IEEE Std C95.1-1991); IEEE: Manhattan, NY, USA, 2006. [CrossRef]
- Andrews, E.F.; Lim, H.B.; Xiao, D.; Khamas, S.; Starke, P.L.; Ang, S.P.; Barker, A.T.; Cook, G.G.; Coulton, L.A.; Scutt, A. Investigation of SAR uniformity in TEM cell exposed culture media. In Proceedings of the 2004 IEE Antenna Measurements and SAR, AMS 2004, Loughborough, UK, 25–26 May 2004; pp. 71–74. [Google Scholar] [CrossRef]
- Chen, B.; Guo, F.; Xiang, H. Visualization study of motion and deformation of red blood cells in a microchannel with straight, divergent and convergent sections. J. Biol. Phys. 2011, 37, 429–440. [Google Scholar] [CrossRef] [Green Version]
- Goodhead, L.K.; MacMillan, F.M. Measuring osmosis and hemolysis of red blood cells. Adv. Physiol. Educ. 2017, 41, 298–305. [Google Scholar] [CrossRef]
- Prastalo, R.; Tesanovic, G.; Sukalo, S. Mechanism of mobile phone radiation acting on biological systems. In Proceedings of the 6th International Conference on Telecommunications in Modern Satellite, Cable and Broadcasting Service, 2003. TELSIKS 2003, Nis, Yugoslavia, 1–3 October 2003; pp. 675–678. [Google Scholar] [CrossRef]
- Kouzmanova, M.; Atanasova, G.; Atanasov, N.; Tasheva, S. Effects of in vitro exposure to GSM900 electromagnetic field on human erythrocytes. Environmentalist 2007, 27, 423–428. [Google Scholar] [CrossRef]
- Sajin, G.; Al, D.; Savopol, T.; Roxana, M.; Eugenia, K. 2.45 GHz microwave radiation effects in nonthermal damaging of the human erythrocyte membrane. In Proceedings of the 1995 25th European Microwave Conference, Bologna, Italy, 4 September 1995; pp. 845–848. [Google Scholar] [CrossRef]
- George, S.; Ion, R.; Eugenia, K.; Tudor, S.; Alexandru, D.; Maria, S. Low Power Microwave Effects on Erythrocyte Membranes. In Proceedings of the 1997 27th European Microwave Conference, Jerusalem, Israel, 8–12 September 1997. [Google Scholar] [CrossRef]
- Nguyen, T.H.P.; Pham, V.T.H.; Baulin, V.; Croft, R.J.; Crawford, R.J.; Ivanova, E.P. The effect of a high frequency electromagnetic field in the microwave range on red blood cells. Sci. Rep. 2017, 7, 10798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Koyama, S.; Komatsubara, Y.; Suzuki, Y.; Taki, M.; Miyakoshi, J. Effects of a 2450 MHz high-frequency electromagnetic field with a wide range of SARs on the induction of heat-shock proteins in A172 cells. Bioelectromagnetics 2006, 27, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Sannino, A.; Zeni, O.; Romeo, S.; Massa, R.; Gialanella, G.; Grossi, G.; Manti, L.; Vijayalaxmi; Scarfì, M.R. Adaptive response in human blood lymphocytes exposed to non-ionizing radiofrequency fields: Resistance to ionizing radiation-induced damage. J. Radiat. Res. 2014, 55, 210–217. [Google Scholar] [CrossRef] [Green Version]
- Paolini, G.; Masotti, D.; Costanzo, A. Simulated Effects of Specific Absorption Rate and Thermal Variations on Keratinocytes and Epidermis Exposed to Radio-Frequency. In Proceedings of the 2018 EMF-Med 1st World Conference on Biomedical Applications of Electromagnetic Fields (EMF-Med), Split, Croatia, 10–13 September 2018; pp. 1–2. [Google Scholar] [CrossRef]



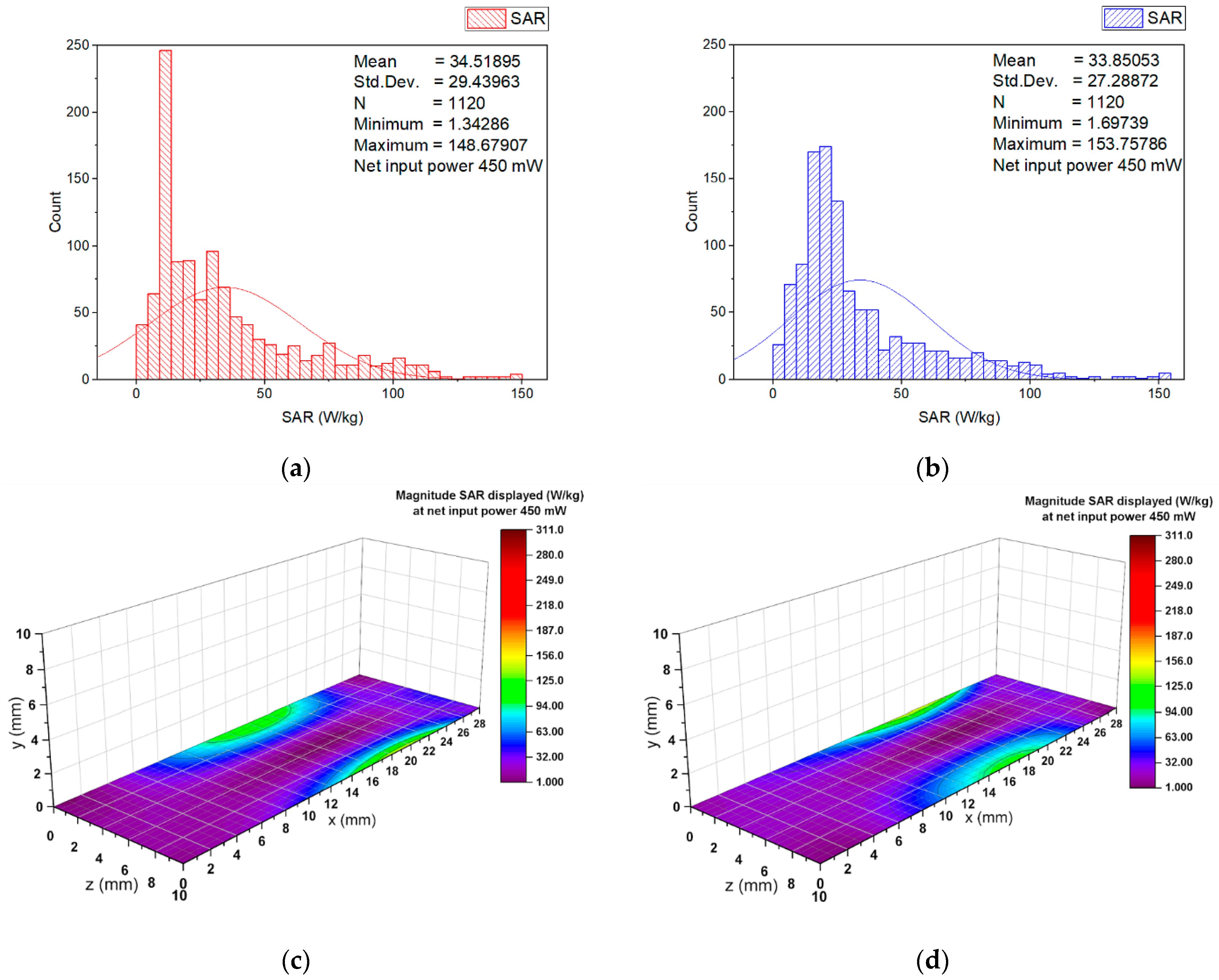





| Materials | εr′ 1 | σ 2 | Density 3 |
|---|---|---|---|
| Wearable antenna substrate (polyester) | 1.62 | 0.00118 | 1380 |
| Erythrocyte suspension | 58.20 | 2.59 | 1000 |
| Plastic cuvette | 2.2 | 0 | 880 |
| Homogeneous flat phantom | 40.805 | 2.33 | 1166 |
| Parameters | Antenna in Free Space | Antenna with Two Cuvettes |
|---|---|---|
| Impedance (Ohms) | 89.52 + j16.47 | 49.71 + j5.29 |
| VSWR | 1.87 | 1.10 |
| Dissipated power (% of net input power) | 47.57 | 65.04 |
| Radiation efficiency (%) | 52.43 | 36.40 |
| System efficiency (%) | 47.59 | 36.31 |
| SAR 1 | Input Power 6.3 mW | Input Power 450 mW |
|---|---|---|
| psSAR1g | 0.5758 | 41.13 |
| WSA SAR | 0.4149 | 29.6325 |
| SAR 1 | Input Power 6.3 mW | Input Power 450 mW |
|---|---|---|
| Minimum SAR | 0.0187 | 1.3358 |
| Maximum SAR | 4.3492 | 310.6544 |
| Reference | Cell Type | Exposure Conditions | Duration | SAR 1 | Results |
|---|---|---|---|---|---|
| This work | Red blood cells—RBCs (Human erythrocytes) | 2.41 GHz Zigbee signal Wearable antenna 6.3 mW input power | 20 min | 0.4149 | RBCs membrane stabilization(decreased quantity of released hemoglobin (Hb)) |
| This work | RBCs (Human erythrocytes) | 2.41 GHz Zigbee signal Wearable antenna 6.3 mW input power | 120 min | 0.4149 | RBCs membrane destabilization (increased level of released Hb 90 min after the exposure) |
| This work | RBCs (Human erythrocytes) | 2.41 GHz Zigbee signal Wearable antenna 450 mW input power | 20 min | 29.6325 | Increased level of released Hb immediately after the end of the exposure, relaxation to control levels 120 min later |
| This work | RBCs (Human erythrocytes) | 2.41 GHz Zigbee signal Wearable antenna 450 mW input power | 120 min | 29.6325 | Increased level of released Hb immediately after the end of the exposure |
| [29] | RBCs (Human erythrocytes) | 2.45 GHz Continuous-wave (CW) irradiation chamber incident power density 0.84 mW/cm2 | 3600 min | NA | Hemolysis degree increases quasi-linearly with the exposure time |
| [29] | RBCs (Human erythrocytes) | 2.45 GHz CW irradiation chamber incident power density 5 mW/cm2 | 3600 min | NA | Osmotic resistance of exposed RBCs increases in time, while the osmotic resistance of the controls is constant |
| [31] | RBCs (Human erythrocytes) | 0.902 GHz GSM signal Nokia 6150 is programmed to emit at 902 MHz with 2 W erythrocyte suspensions in the main lob of the antenna radiation pattern | 20 min | NA | Statistically significant decrease in the hemoglobin level in irradiated suspensions |
| [37] | RBCs (Human erythrocytes) | 0.902 GHz GSM signal Nokia 6150 is programmed to emit 2 W erythrocyte suspensions at 2 cm distance from phone helical antenna | 20 min | NA | Statistically significant decrease in the hemoglobin level in irradiated suspensions |
| [40] | RBCs (New Zealand white rabbit erythrocytes) | 18 GHz CW Vari-Wave Model LT 1500 | 1 min | 3000 | Disturbance of the RBCs membranes |
| [41] | A172 cells (Human malignant glioblastoma) | 2.45 GHz CW rectangular waveguide (TE10 mode) | 120 min | 5–20 | No statistically significant changes in HSP 70, HSP27, and P-HSP27 in A172 cells |
| [41] | A172 cells (Human malignant glioblastoma) | 2.45 GHz CW rectangular waveguide (TE10 mode) | 120 min | 50 | A statistically significant increase in HSP 70 and p-HSP27 in A172 cells |
| [42] | Human peripheral blood lymphocytes | 1.95 GHz UMTS signal exposure chamber | 1200 min | 0.3 | No significant increase in the incidence of micronuclei compared with those of unexposed controls |
| [43] | Human epidermal cells—simulation model | 1.8 GHz CW Wearable antenna—simulation model Gain 4.69 dBi Input power 290 mW | NA | 1.125 | Temperature increaseof 0.17 °C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atanasov, N.T.; Atanasova, G.L.; Angelova, B.; Paunov, M.; Gurmanova, M.; Kouzmanova, M. Wearable Antennas for Sensor Networks and IoT Applications: Evaluation of SAR and Biological Effects. Sensors 2022, 22, 5139. https://doi.org/10.3390/s22145139
Atanasov NT, Atanasova GL, Angelova B, Paunov M, Gurmanova M, Kouzmanova M. Wearable Antennas for Sensor Networks and IoT Applications: Evaluation of SAR and Biological Effects. Sensors. 2022; 22(14):5139. https://doi.org/10.3390/s22145139
Chicago/Turabian StyleAtanasov, Nikolay Todorov, Gabriela Lachezarova Atanasova, Boyana Angelova, Momchil Paunov, Maria Gurmanova, and Margarita Kouzmanova. 2022. "Wearable Antennas for Sensor Networks and IoT Applications: Evaluation of SAR and Biological Effects" Sensors 22, no. 14: 5139. https://doi.org/10.3390/s22145139






