A Room-Temperature Surface Acoustic Wave Ammonia Sensor Based on rGO/DPP2T-TT Composite Films
Abstract
:1. Introduction
2. Experimental Methods
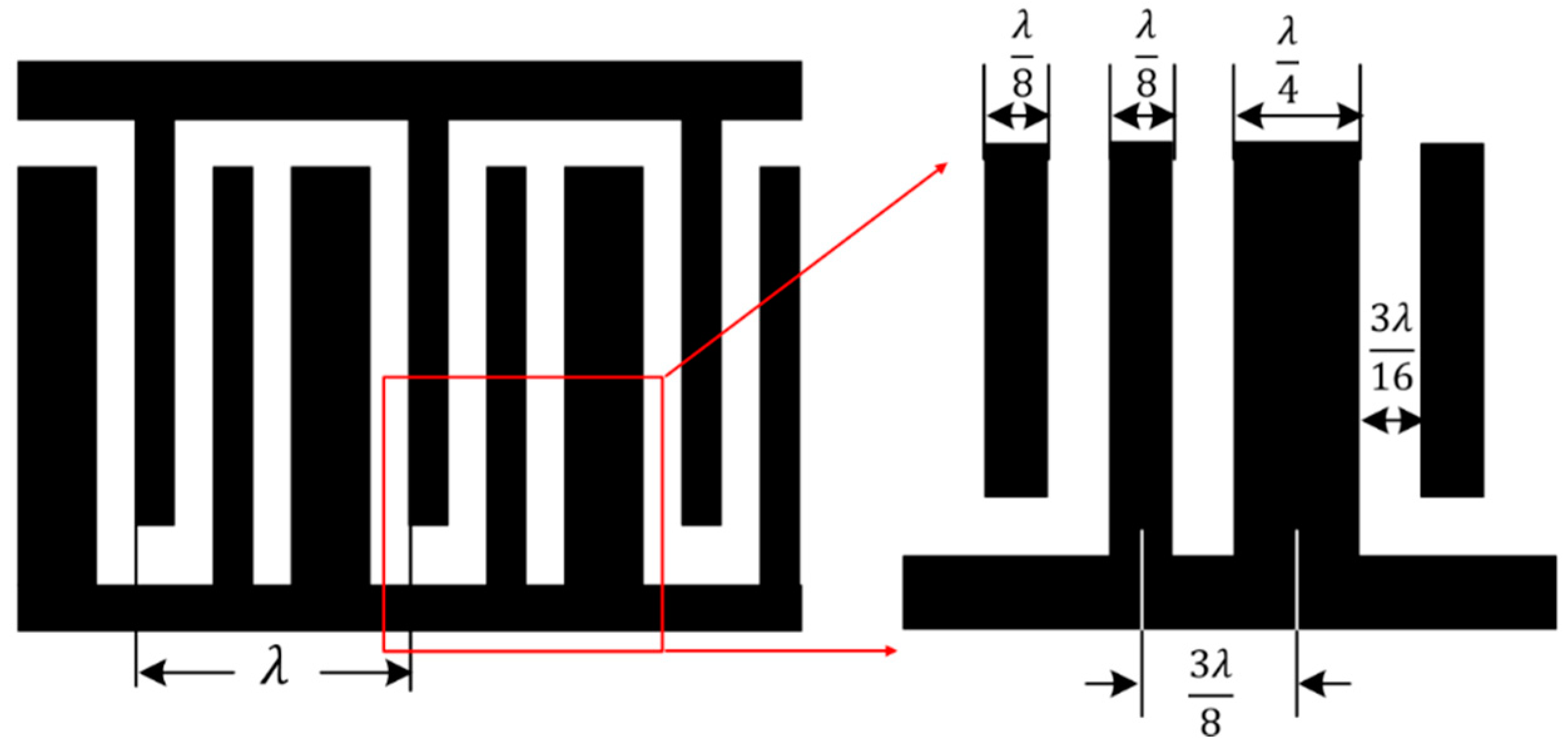
2.1. Device Fabrication
2.2. Preparation of rGO/DPP2T-TT
2.3. Experimental Setup
3. Results and Discussion
3.1. Analysis of the rGO/DPP2T-TT Composite Film
3.2. Mechanism of Gas Sensing
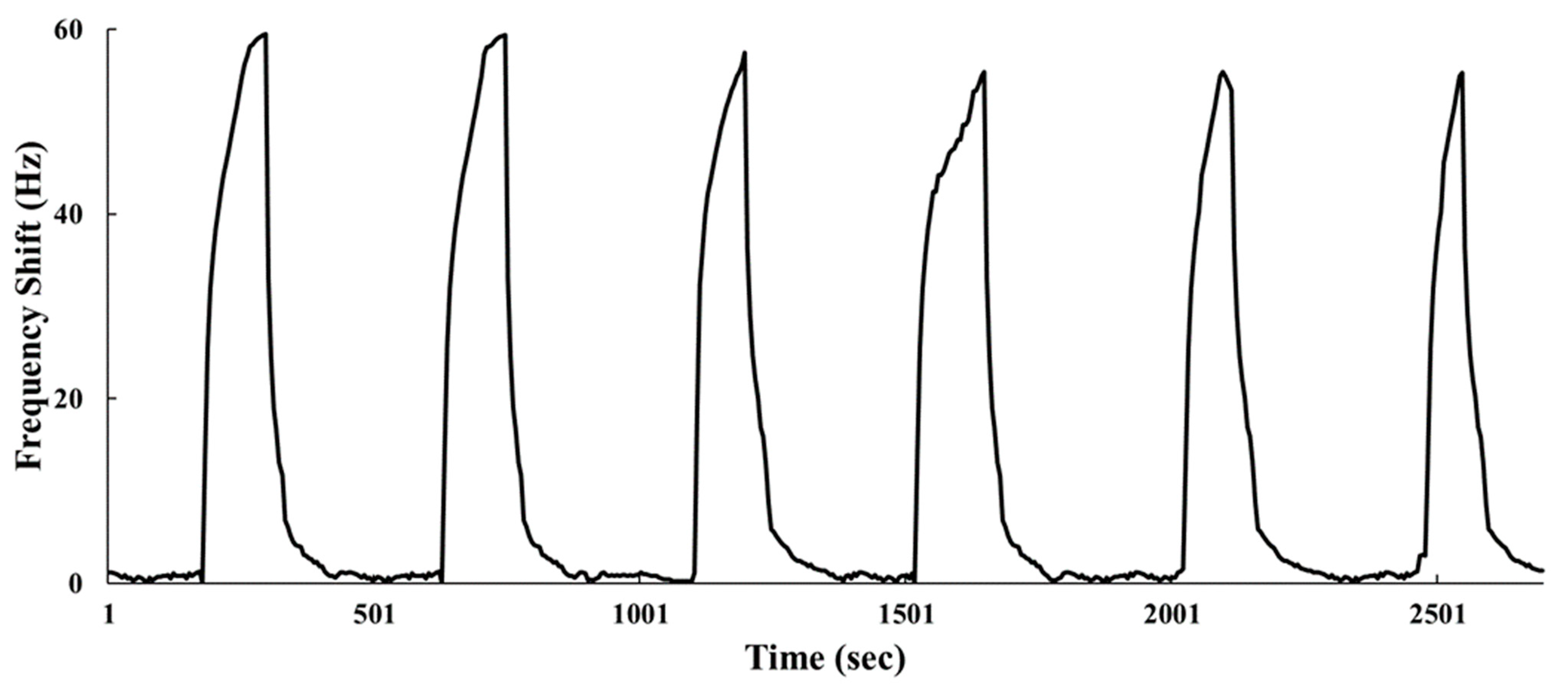
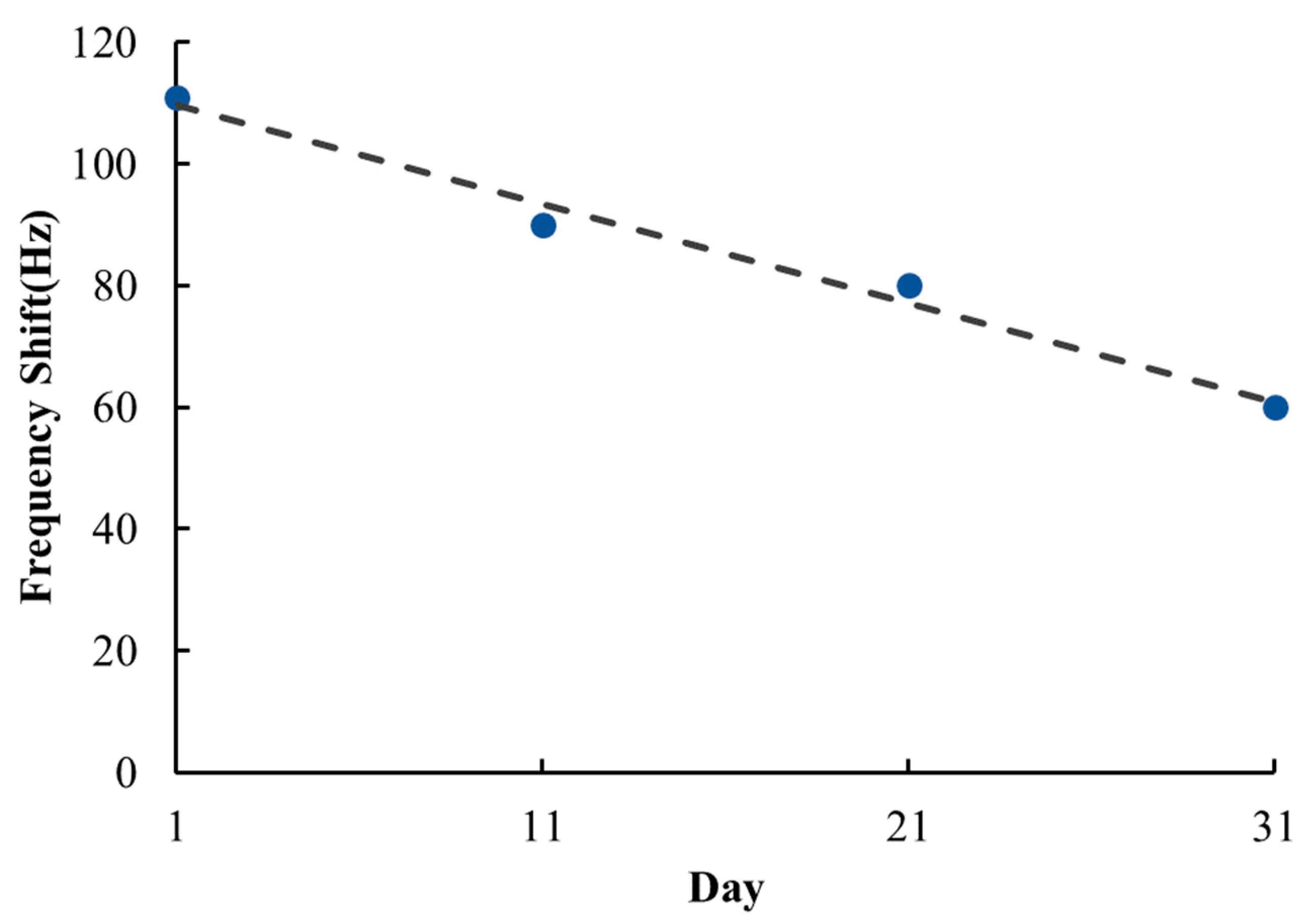
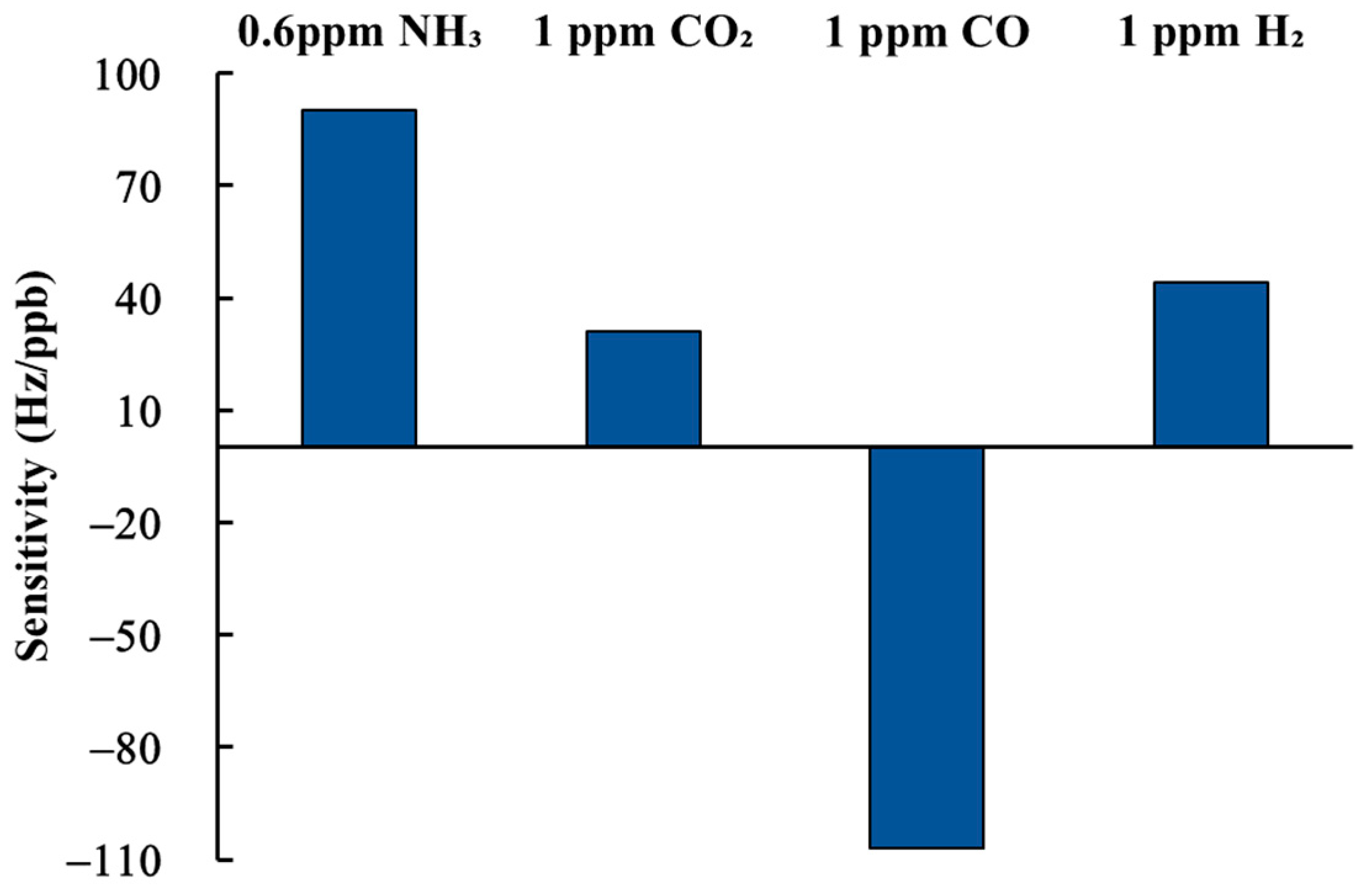
3.3. Gas Sensing Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Franklin, B.A.; Brook, R.; Pope, C.A., III. Air Pollution and Cardiovascular Disease. Curr. Probl. Cardiol. 2015, 40, 207–238. [Google Scholar] [CrossRef] [PubMed]
- Hoek, G.; Krishnan, R.M.; Beelen, R.; Peters, A.; Ostro, B.; Brunekreef, B.; Kaufman, J.D. Long-term air pollution exposure and cardio-respiratory mortality: A review. Environ. Health 2013, 12, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timmer, B.; Olthuis, W.; Van Den Berg, A. Ammonia sensors and their applications-a review. Sens. Actuators B 2005, 107, 666–677. [Google Scholar] [CrossRef]
- Krishnan, S.T.; Devadhasan, J.P.; Kim, S. Recent analytical approaches to detect exhaled breath ammonia with special reference to renal patients. Anal. Bioanal. Chem. 2017, 409, 21–31. [Google Scholar] [CrossRef]
- di Natale, C.; Paolesse, R.; Martinelli, E.; Capuano, R. Solid-state gas sensors for breath analysis: A review. Anal. Chim. Acta 2014, 824, 1–17. [Google Scholar] [CrossRef]
- Li, H.Y.; Lee, C.S.; Kim, D.H.; Lee, J.H. Flexible room-temperature NH3 sensor for ultrasensitive selective and humidity independent gas detection. ACS Appl. Mater. Interfaces 2018, 10, 27858–27867. [Google Scholar] [CrossRef]
- Wang, K.H.; Hsieh, J.C.; Chen, C.C.; Zan, H.W.; Meng, H.F.; Kuo, S.Y.; Nguyễn, M.T.N. A low-cost, portable and easy-operated salivary urea sensor for point-of-care application. Biosens. Bioelectron. 2019, 132, 352–359. [Google Scholar] [CrossRef]
- Romero, A.C.; Bergamaschi, C.T.; de Souza, D.N.; Nogueira, F.N. Salivary alterations in rats with experimental chronic kidney disease. PLoS ONE 2016, 11, e0148742. [Google Scholar] [CrossRef] [Green Version]
- Nylander, C.; Armgarth, M.; Lundström, I. An ammonia detector based on a conducting polymer. Anal. Chem. Symp. Ser 1983, 17, 203–207. [Google Scholar]
- Chen, G.; Liu, R.X.; Shon, H.K.; Wang, Y.Q.; Song, J.F.; Li, X.M.; He, T. Open porous hydrophilic supported thin-film composite forward osmosis membrane via co-casting for treatment of high-salinity wastewater. Desalination 2017, 405, 76–84. [Google Scholar] [CrossRef]
- Stassen, I.; Styles, M.; Grenci, G.; Gorp, H.V.; Vanderlinden, W.; Feyter, S.D.; Falcaro, P.; Vos, D.D.; Vereecken, P.; Ameloot, R. Chemical vapour deposition of zeolitic imidazolate framework thin films. Nat. Mater. 2016, 15, 304. [Google Scholar] [CrossRef] [Green Version]
- Kaviyarasua, K.; Magdalanec, C.M.; Kanimozhie, K.; Kennedya, J.; Siddhardhag, B.; Reddyh, E.S.; Rottei, N.K.; Sharmai, C.S.; Themaa, F.T.; Letsholathebej, D.; et al. Elucidation of photocatalysis, photoluminescence and antibacterial studies of ZnO thin films by spin coating method. J. Photochem. Photobiol. B Biol. 2017, 173, 466–475. [Google Scholar] [CrossRef]
- Luo, X.N.; Ma, K.; Jiao, T.F.; Xing, R.R.; Zhang, L.X.; Zhou, J.X.; Li, B.B. Graphene oxide-polymer composite Langmuir films constructed by interfacial thiol-ene photopolymerization. Nanoscale Res. Lett. 2017, 12, 99. [Google Scholar] [CrossRef] [Green Version]
- Hung, T.T.; Chung, M.H.; Chiu, J.J.; Yang, M.W.; Tien, T.N.; Shen, C.Y. Poly(4-styrenesulfonic acid) doped polypyrrole/tungsten oxide/reduced graphene oxide nanocomposite films based surface acoustic wave sensors for NO sensing behavior. Org. Electron. 2021, 88, 106006. [Google Scholar] [CrossRef]
- Bai, H.; Shi, G. Gas sensors based on conducting polymers. Sensors 2007, 7, 267–307. [Google Scholar] [CrossRef] [Green Version]
- Tiggemanna, L.; Ballena, S.; Bocalona, C.; Graboskia, A.M.; Manzoli, A.; Herrmann, P.S.P.; Steffens, J.; Valduga, E.; Steffens, C. Low-cost gas sensors with polyaniline film for aroma detection. J. Food Eng. 2016, 180, 16–21. [Google Scholar] [CrossRef]
- Meager, I.; Ashraf, R.S.; Mollinger, S.; Schroeder, B.C.; Bronstein, H.; Beatrup, D.; Vezie, M.S.; Kirchartz, T.; Salleo, A.; Nelson, J.; et al. Photocurrent enhancement from diketopyrrolopyrrole polymer solar cells through alkyl-chain branching point manipulation. J. Am. Chem. Soc. 2013, 135, 11537–11540. [Google Scholar] [CrossRef]
- Chang, J.; Lin, Z.; Li, J.; Lim, S.L.; Wang, F.; Li, G.; Zhang, J.; Wu, J. Enhanced polymer thin film transistor performance by carefully controlling the solution self-assembly and film alignment with slot die coating. Adv. Electron. Mater. 2015, 1, 1500036. [Google Scholar] [CrossRef]
- Hung, T.T.; Chung, M.H.; Lin, G.S.; Shen, C.Y. Piezoelectric microsensor for selective detection of low concentrations of ammonia. Solid State Electron. 2021, 186, 108191. [Google Scholar] [CrossRef]
- Novikov, S.; Lebedeva, N.; Satrapinski, A.; Walden, J.; Davydov, V.; Lebedev, A. Graphene based sensor for environmental monitoring of NO2. Sens. Actuators B Chem. 2016, 236, 1054–1060. [Google Scholar] [CrossRef]
- Karaduman, I.; Er, E.; Çelikkan, H.; Erk, N.; Acar, S. Room-temperature ammonia gas sensor based on reduced graphene oxide nanocomposites decorated by Ag, Au and Pt nanoparticles. J. Alloys Compd. 2017, 722, 569–578. [Google Scholar] [CrossRef]
- Hanma, K.; Hunsinger, B.J. A Triple Transit Suppression Technique. In Proceedings of the 1976 Ultrasonics Symposium, Annapolis, MD, USA, 29 September 1976. [Google Scholar]
- Chung, M.H.; Hwang, R.C.; Chiu, J.J.; Yang, M.W.; Hung, T.T.; Shen, C.Y. Enhanced Sensitive Surface Acoustic Wave Device Designed for Nitric Oxide Gas Detection. Sens. Mater. 2019, 31, 2195–2212. [Google Scholar] [CrossRef]
- Bai, S.; Shen, X.; Zhu, G.; Yuan, A.; Zhang, J.; Ji, Z.; Qiu, D. The Influence of Wrinkling in Reduced Graphene Oxide on Their Adsorption and Catalytic Properties. Carbon 2013, 60, 157–168. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Z.; He, D.; Yang, H.; Jin, D.; Qu, J.; Zhang, Y. Comparative Study on the Adsorption Capacities of the Three Black Phosphorus-Based Materials for Methylene Blue in Water. Sustainability 2020, 12, 8335. [Google Scholar] [CrossRef]
- Stobinski, L.; Lesiak, B.; Malolepszy, A.; Mazurkiewicz, M.; Mierzwa, B.; Zemek, J.; Jiricek, P.; Bieloshapka, I. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J. Electron Spectrosc. Relat. Phenom. 2014, 195, 145–154. [Google Scholar] [CrossRef]
- Devkota, J.; Ohodnicki, P.R.; Greve, D.W. SAW sensors for chemical vapors and gases. Sensors 2017, 17, 801. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Chen, X.; Zhu, S.; Zhou, Z.; Yao, Y.; Quan, W.; Liu, B. Enhanced sensitivity of ammonia sensor using graphene/polyaniline nanocomposite. Sens. Actuators B 2013, 178, 485–493. [Google Scholar] [CrossRef]
- Comini, E.; Faglia, G.; Sberveglier, G. Solid State Gas Sensing; Springer: Berlin/Heidelberg, Germany, 2009; p. 289. [Google Scholar]
- Li, W.; Li, X.; Cai, L.; Sun, Y.; Sun, M.; Xie, D. Reduced graphene oxide for room temperature ammonia (NH3) gas sensor. J. Nanosci. Nanotechnol. 2018, 18, 7927–7932. [Google Scholar] [CrossRef]






| NH3 Concentration (ppb) | 500 | 600 | 750 | 1100 | 1400 |
|---|---|---|---|---|---|
| Frequency shift (Hz) | 35 | 54 | 58 | 75 | 85 |
| Response time (s) | 30 | 57 | 35 | 130 | 112 |
| Recovery time (s) | 60 | 68 | 60 | 248 | 136 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, T.-T.; Chung, M.-H.; Wu, J.-Y.; Shen, C.-Y. A Room-Temperature Surface Acoustic Wave Ammonia Sensor Based on rGO/DPP2T-TT Composite Films. Sensors 2022, 22, 5280. https://doi.org/10.3390/s22145280
Hung T-T, Chung M-H, Wu J-Y, Shen C-Y. A Room-Temperature Surface Acoustic Wave Ammonia Sensor Based on rGO/DPP2T-TT Composite Films. Sensors. 2022; 22(14):5280. https://doi.org/10.3390/s22145280
Chicago/Turabian StyleHung, Tien-Tsan, Mei-Hui Chung, Jiun-Yi Wu, and Chi-Yen Shen. 2022. "A Room-Temperature Surface Acoustic Wave Ammonia Sensor Based on rGO/DPP2T-TT Composite Films" Sensors 22, no. 14: 5280. https://doi.org/10.3390/s22145280
APA StyleHung, T.-T., Chung, M.-H., Wu, J.-Y., & Shen, C.-Y. (2022). A Room-Temperature Surface Acoustic Wave Ammonia Sensor Based on rGO/DPP2T-TT Composite Films. Sensors, 22(14), 5280. https://doi.org/10.3390/s22145280






