Mechanisms of NO2 Detection in Hybrid Structures Containing Reduced Graphene Oxide: A Review
Abstract
:1. Introduction—Problems of NO2 Detection
2. Graphene and Graphene Oxide as a Gas Sensing Structure
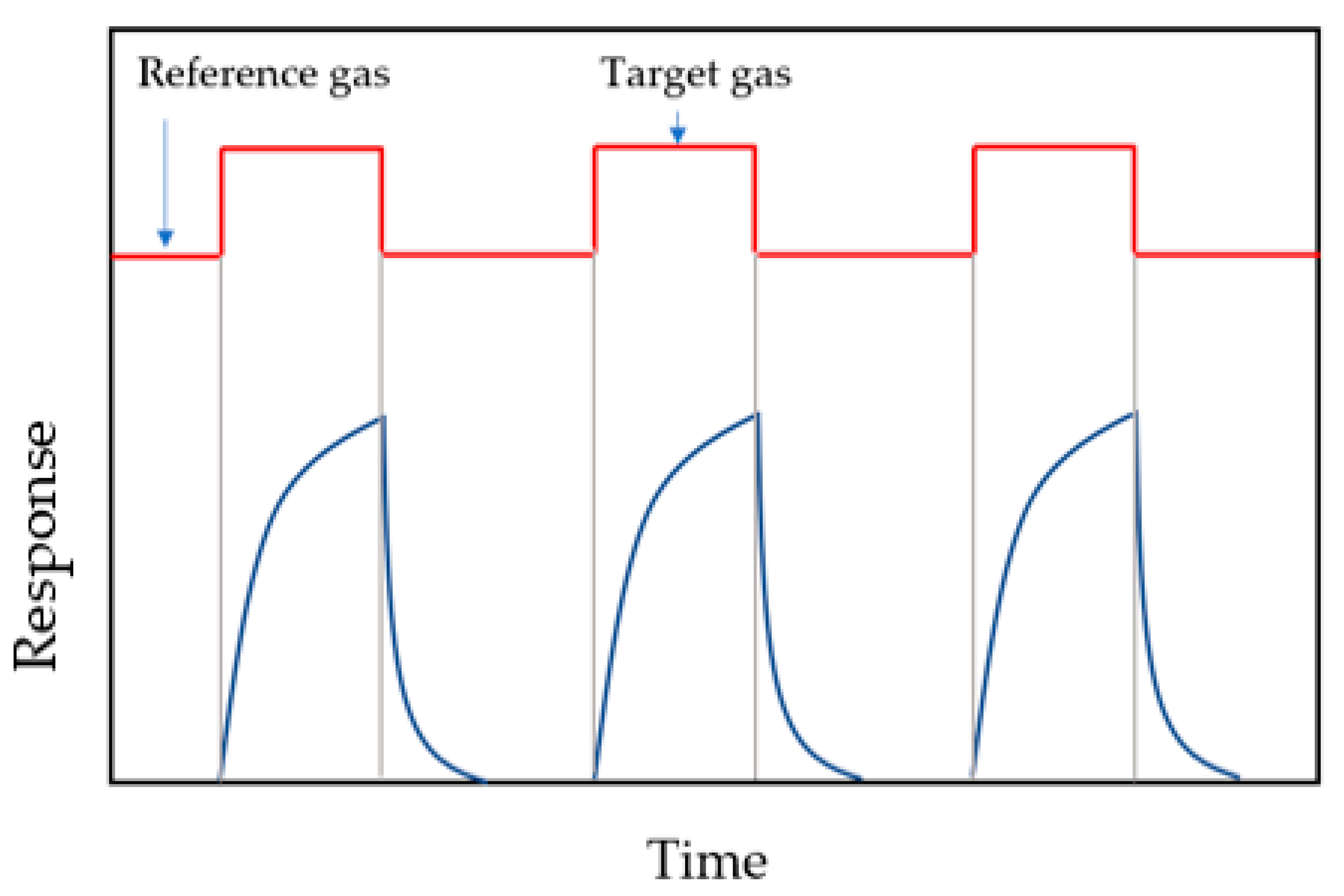
3. Principle of Operation of a Semiconductor Resistance Sensor
4. Mechanism of NO2 Detection in rGO Hybrid Materials
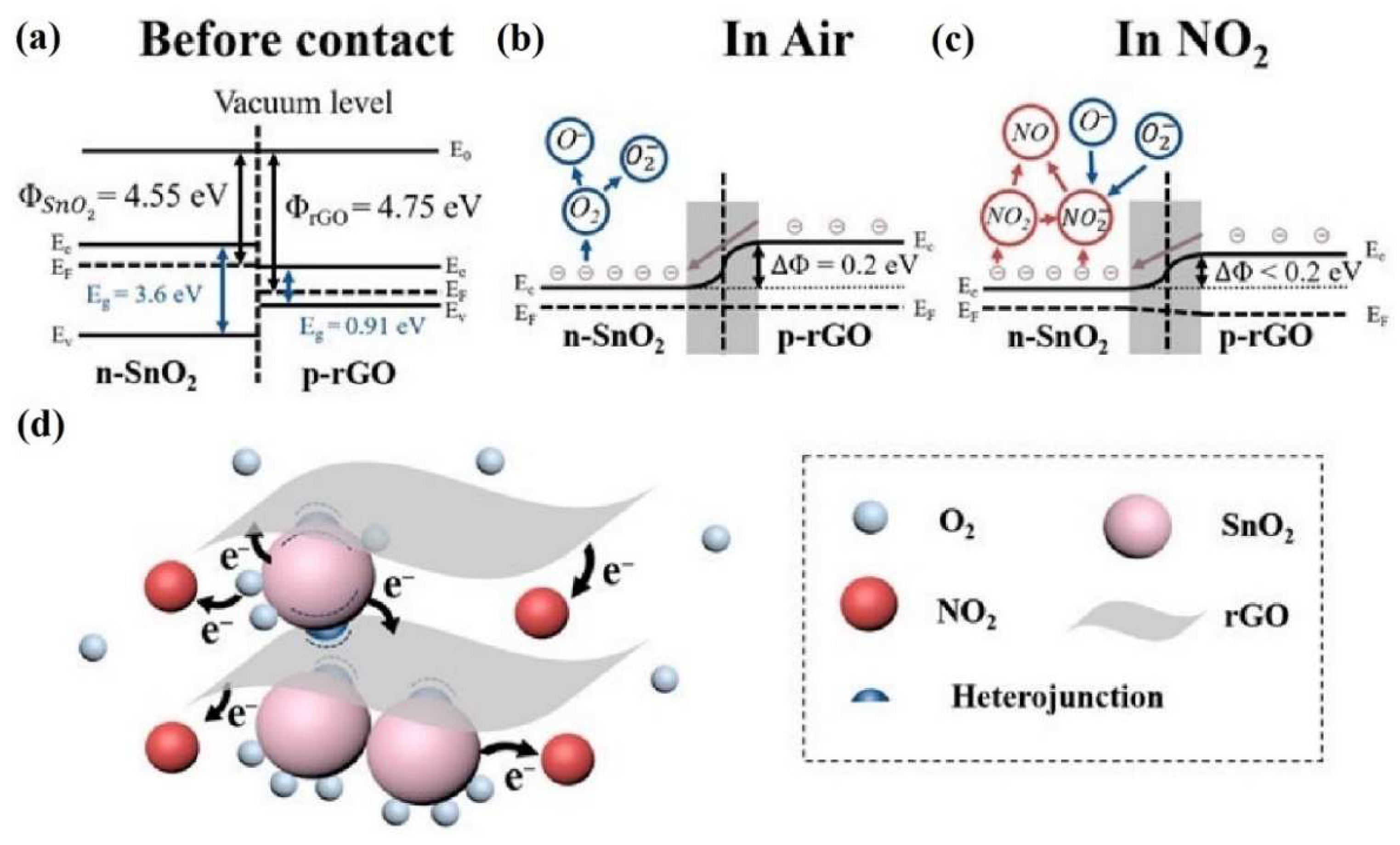
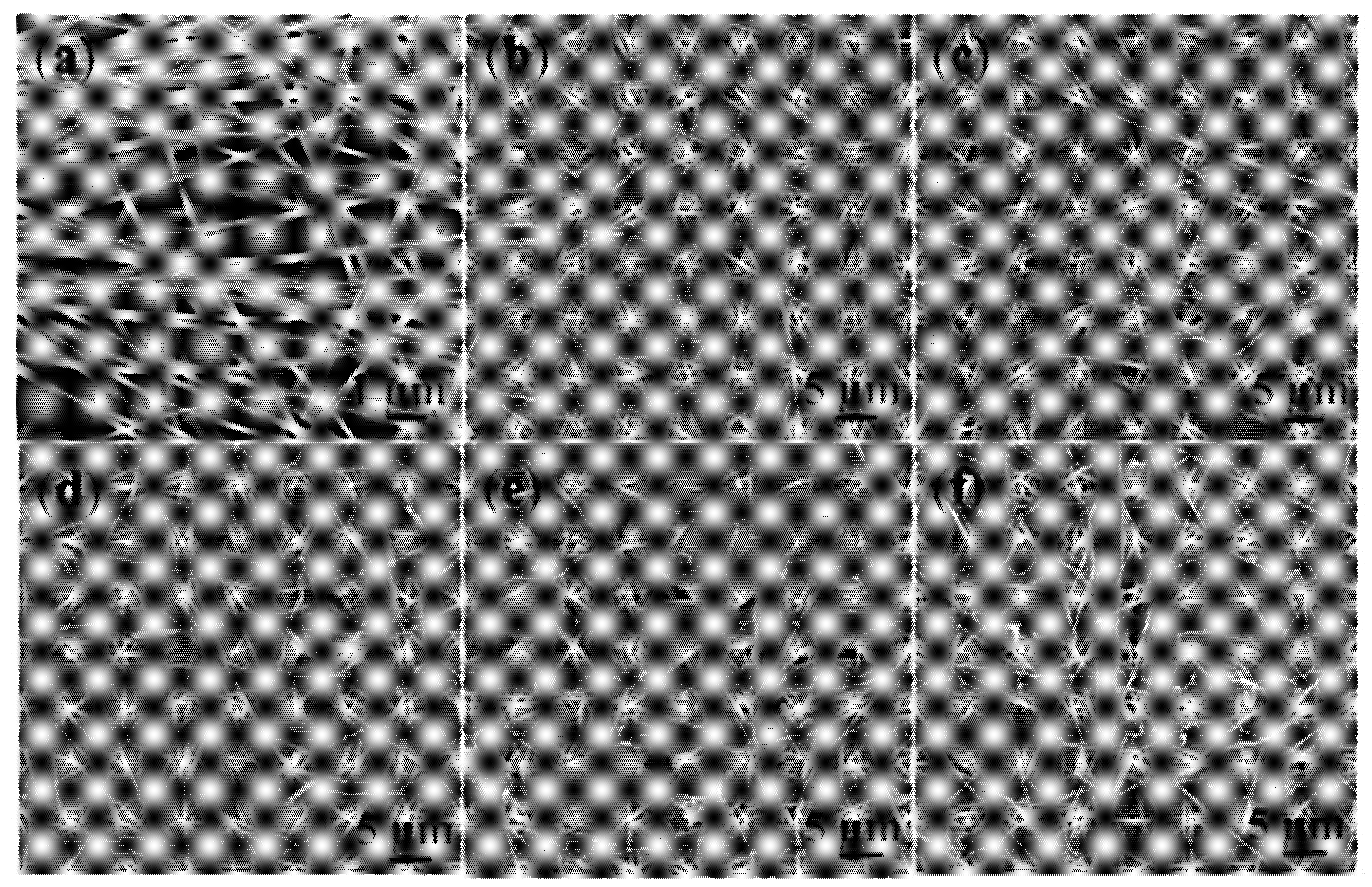
4.1. Reduced Graphene Oxide and Tin (IV) Oxide (rGO/SnO2)
- reduced graphene oxide has a favored porosity and broad specific surface area that provides more active sites for NO2 adsorption;
- high carrier mobility of rGO allows it to increase an electron transport between NO2 molecules and conduction band of rGO/SnO2 hybrid;
- p–n heterojunctions may enhance the NO2 adsorption due to more highly active sites, and it can cause a broader electron depletion layer.
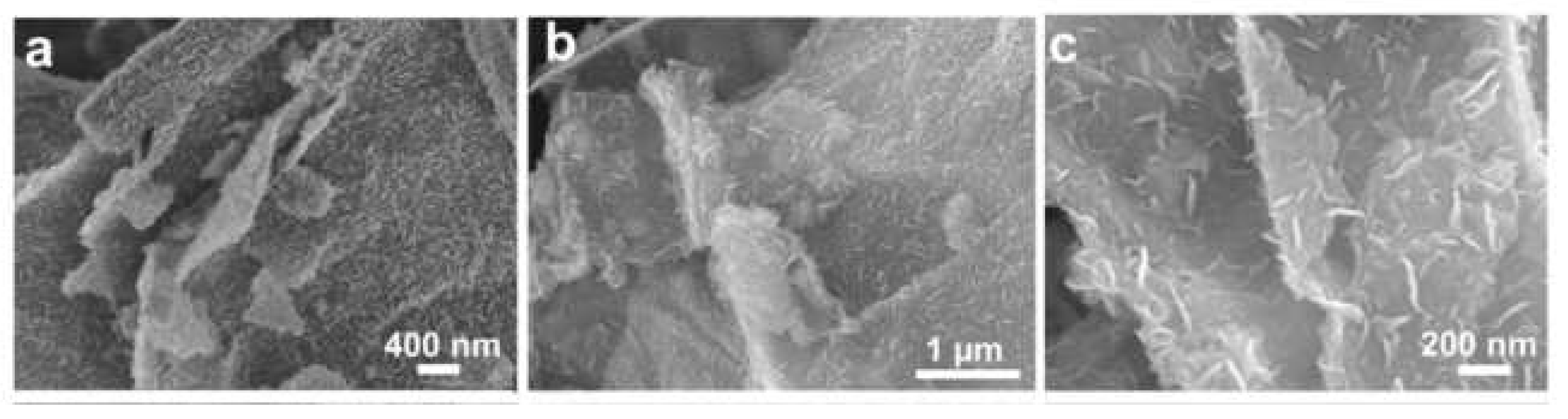
4.2. Reduced Graphene Oxide and Tin (IV) Sulfide (rGO/SnS2)
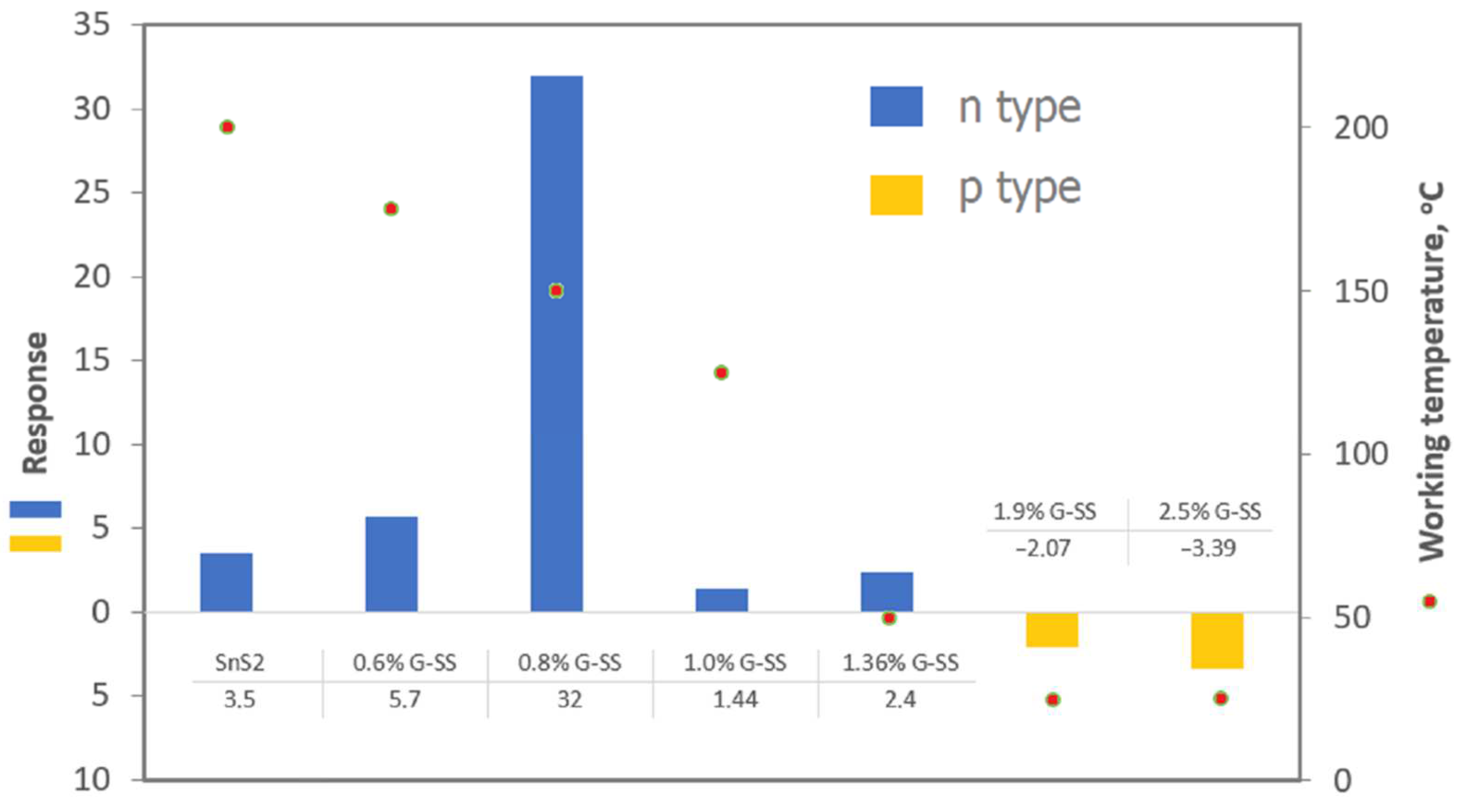
4.3. Reduced Graphene Oxide and Zinc Oxide (rGO/ZnO)
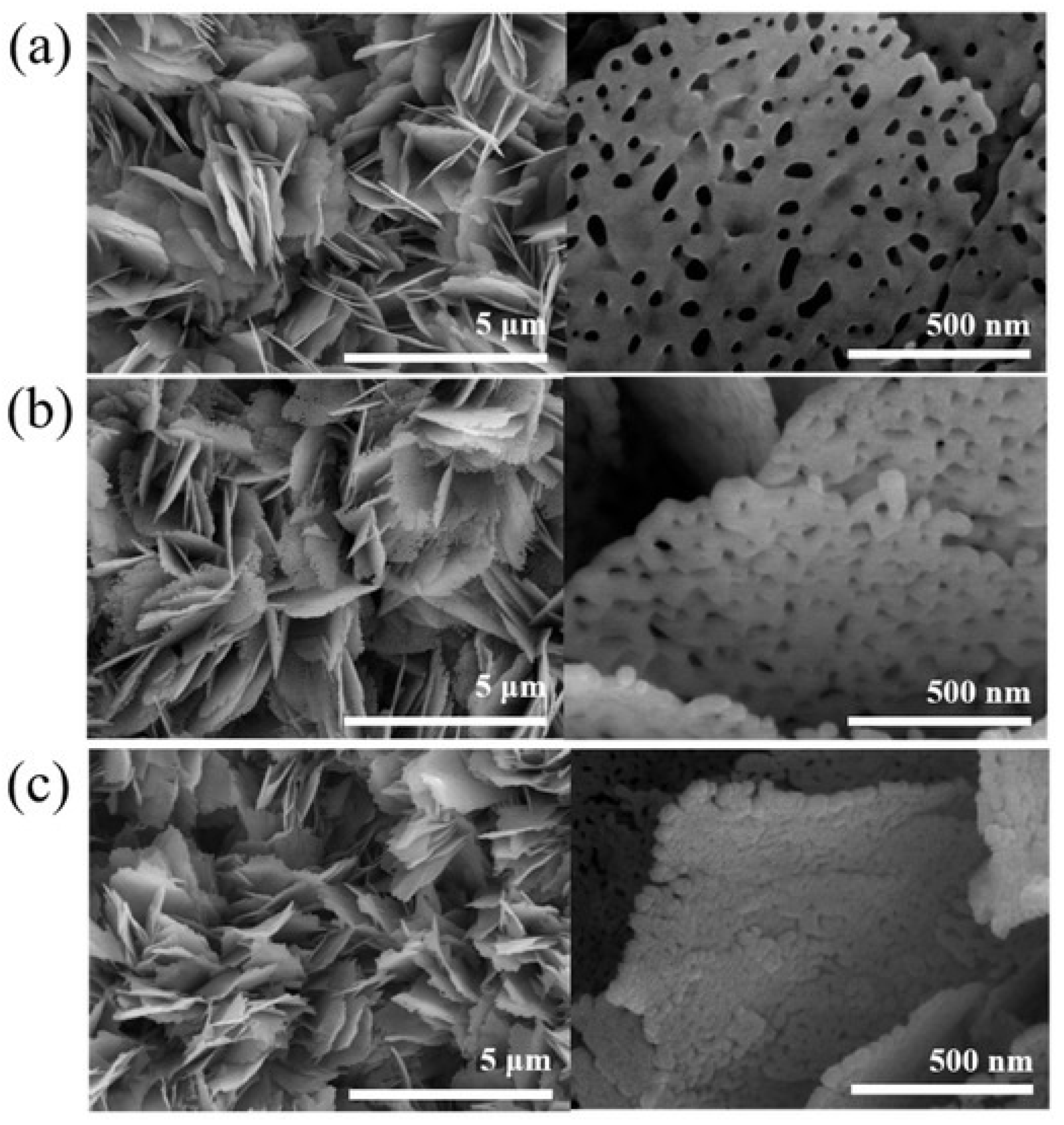
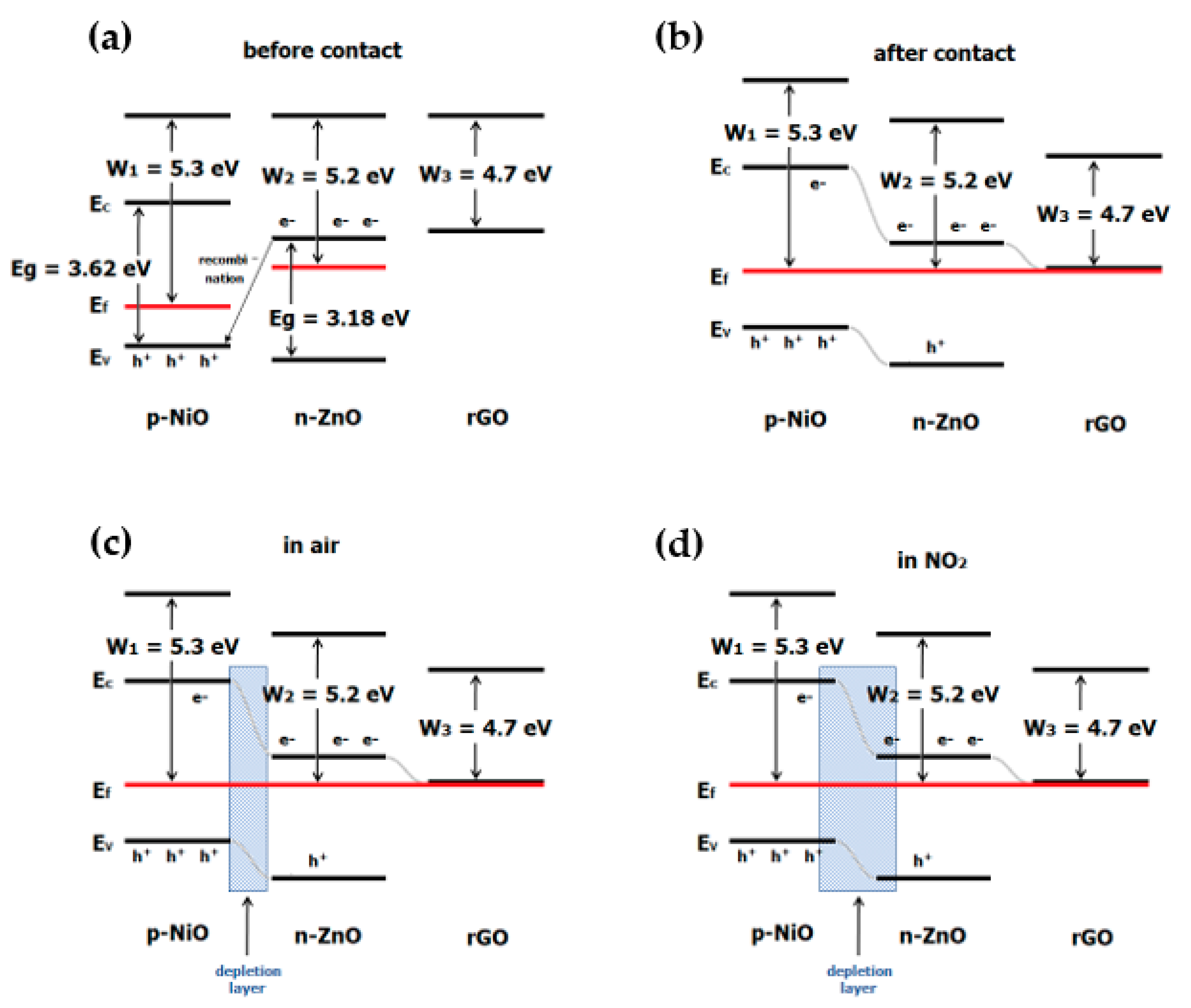
4.4. Reduced Graphene Oxide, Nickel Oxide and Zinc Oxide (rGO/NiO/ZnO)
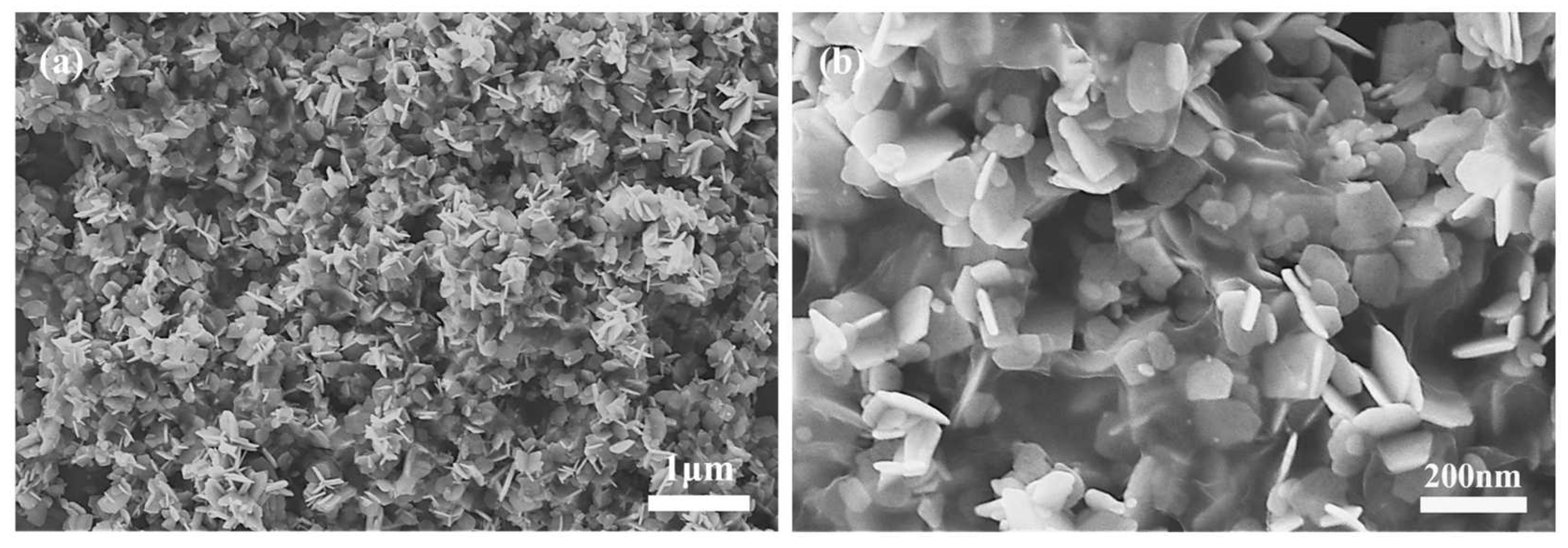
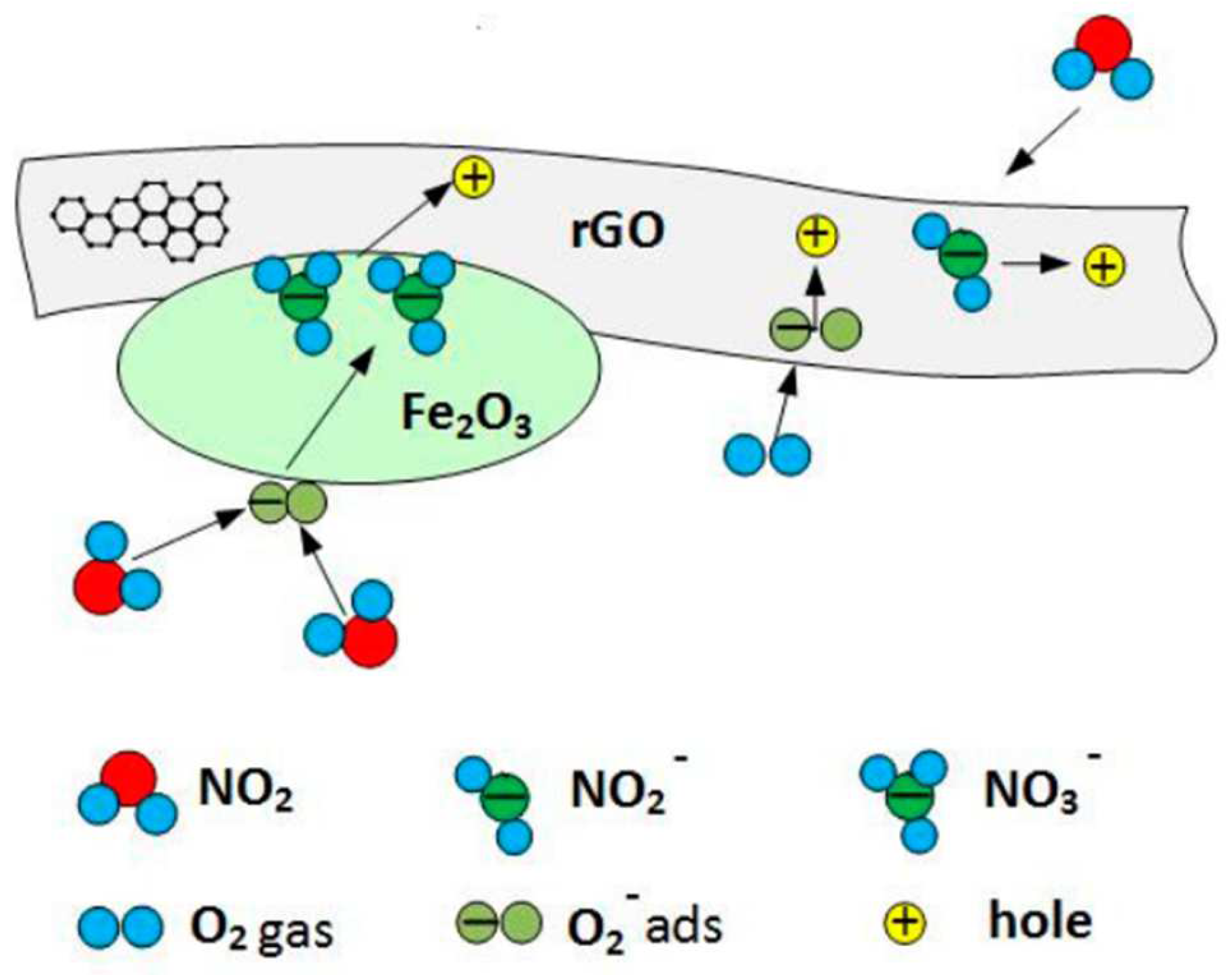
4.5. Reduced Graphene Oxide and Iron (III) Oxide (rGO/Fe2O3)
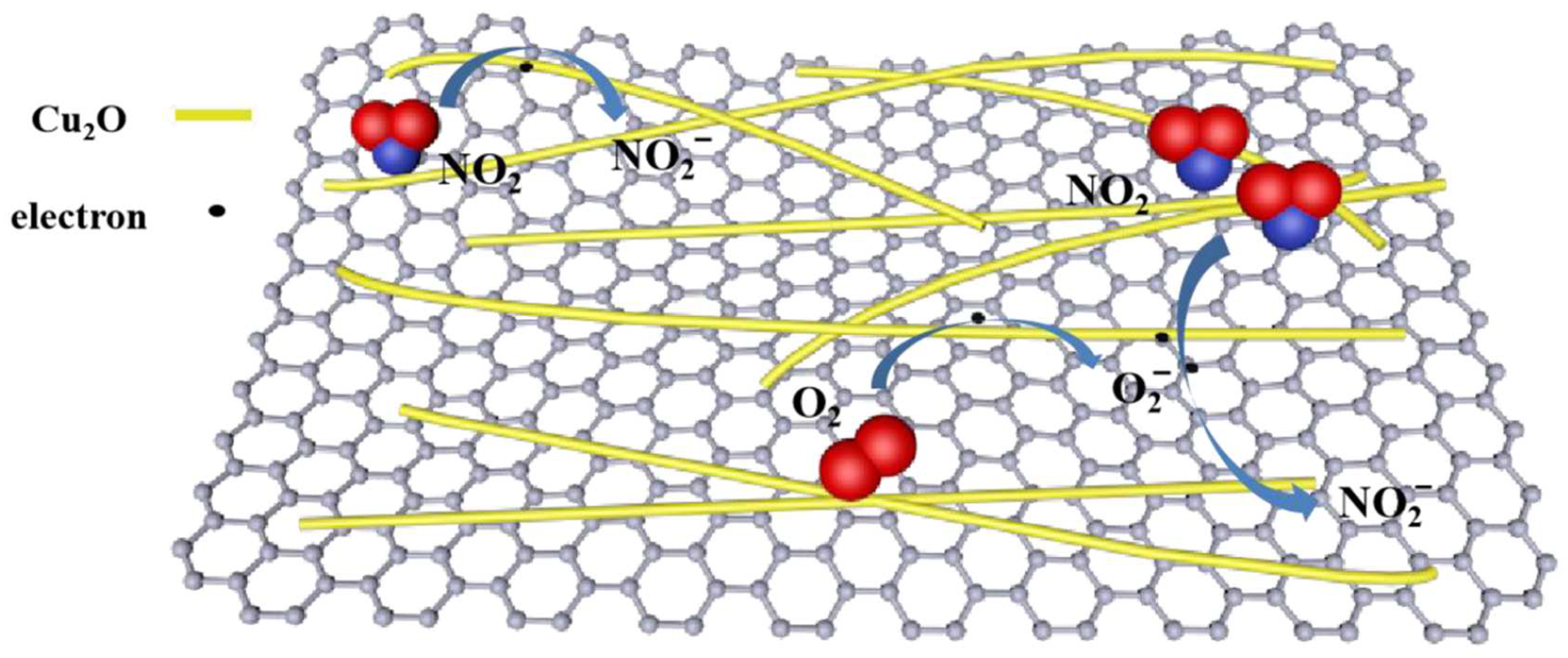
4.6. Reduced Graphene Oxide, Polypyrrole and Copper (I) Oxide (rGO/PPy/Cu2O)
5. Remarks on WBS–rGO Hybrid Sensor Structures
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kumunda, C.; Adekunle, A.S.; Mamba, B.B.; Hlongwa, N.W.; Nkambule, T.T.I. Electrochemical Detection of Environmental Pollutants Based on Graphene Derivatives: A Review. Front. Mater. 2021, 7. [Google Scholar] [CrossRef]
- B, G.R.; M, S.B.R.; Kailasa, S.; Maseed, H.; Bikshalu, K.; K, V.R. Comparative gas sensing analysis of green and chemically reduced graphene oxide. Mater. Res. Express 2019, 6, 115624. [Google Scholar] [CrossRef]
- Park, J.; Kim, Y.; Park, S.Y.; Sung, S.J.; Jang, H.W.; Park, C.R. Band gap engineering of graphene oxide for ultrasensitive NO2 gas sensing. Carbon 2019, 159, 175–184. [Google Scholar] [CrossRef]
- Marcos-Viquez, A.L.; Miranda, A.; Cruz-Irisson, M.; Pérez, L.A. Tin carbide monolayers as potential gas sensors. Mater. Lett. 2021, 294, 129751. [Google Scholar] [CrossRef]
- Gomaa, M.; Sayed, M.; Patil, V.; Boshta, M.; Patil, P. Gas sensing performance of sprayed NiO thin films toward NO2 gas. J. Alloy. Compd. 2021, 885, 160908. [Google Scholar] [CrossRef]
- World Health Organization, Ambient (outdoor) air pollution. Available online: https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health (accessed on 28 May 2022).
- Yi, N.; Cheng, Z.; Li, H.; Yang, L.; Zhu, J.; Zheng, X.; Chen, Y.; Liu, Z.; Zhu, H.; Cheng, H. Stretchable, ultrasensitive, and low-temperature NO2 sensors based on MoS2@rGO nanocomposites. Mater. Today Phys. 2020, 15, 100265. [Google Scholar] [CrossRef]
- Wu, J.; Wu, Z.; Ding, H.; Wei, Y.; Huang, W.; Yang, X.; Li, Z.; Qiu, L.; Wang, X. Flexible, 3D SnS2/Reduced graphene oxide heterostructured NO2 sensor. Sensors Actuators B Chem. 2019, 305, 127445. [Google Scholar] [CrossRef]
- Ganapathi, K.; Kaur, M.; Shaheera, M.; Pathak, A.; Gadkari, S.; Debnath, A. Highly sensitive NO2 sensor based on ZnO nanostructured thin film prepared by SILAR technique. Sensors Actuators B Chem. 2021, 335, 129678. [Google Scholar] [CrossRef]
- Procek, M.; Pustelny, T.; Stolarczyk, A. Influence of External Gaseous Environments on the Electrical Properties of ZnO Nanostructures Obtained by a Hydrothermal Method. Nanomaterials 2016, 6, 227. [Google Scholar] [CrossRef] [Green Version]
- Khudadad, A.I.; Yousif, A.A.; Abed, H.R. Effect of heat treatment on WO3 nanostructures based NO2 gas sensor low-cost device. Mater. Chem. Phys. 2021, 269, 124731. [Google Scholar] [CrossRef]
- Zhou, L.; Hu, Z.; Wang, P.; Gao, N.; Zhai, B.; Ouyang, M.; Zhang, G.; Chen, B.; Luo, J.; Jiang, S.; et al. Enhanced NO2 sensitivity of SnO2 SAW gas sensors by facet engineering. Sensors Actuators B: Chem. 2022, 361, 131735. [Google Scholar] [CrossRef]
- Sangale, S.S.; Jadhav, V.V.; Shaikh, S.F.; Shinde, P.V.; Ghule, B.G.; Raut, S.D.; Tamboli, M.S.; Al-Enizi, A.M.; Mane, R.S. Facile one-step hydrothermal synthesis and room-temperature NO2 sensing application of α-Fe2O3 sensor. Mater. Chem. Phys. 2020, 246, 122799. [Google Scholar] [CrossRef]
- Pustelny, T.; Ignac-Nowicka, J.; Opilski, Z. Optical investigations on layered methalphthalicyanine nanostructures affected by NO2 applying the surface plasmon resonance method. Opt. Appl. 2004, 34, 563–572. [Google Scholar]
- Pustelny, T.; Setkiewicz, M.; Drewniak, S.; Maciak, E.; Stolarczyk, A.; Urbańczyk, M.; Procek, M.; Gut, K.; Opilski, Z.; Pasternak, I.; et al. The sensibility of resistance sensor structures with graphene to the action of selected gaseous media. Bull. Pol. Acad. Sci. Tech. Sci. 2013, 61, 293–300. [Google Scholar] [CrossRef]
- Han, T.; Gao, S.; Wang, Z.; Fei, T.; Liu, S.; Zhang, T. Investigation of the effect of oxygen-containing groups on reduced graphene oxide-based room-temperature NO2 sensor. J. Alloy. Compd. 2019, 801, 142–150. [Google Scholar] [CrossRef]
- Guo, L.; Hao, Y.-W.; Li, P.-L.; Song, J.-F.; Yang, R.-Z.; Fu, X.-Y.; Xie, S.-Y.; Zhao, J.; Zhang, Y.-L. Improved NO2 Gas Sensing Properties of Graphene Oxide Reduced by Two-beam-laser Interference. Sci. Rep. 2018, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Black, N.; Liu, C.; Pearce, R.; Li, B.; Maier, S.; Cohen, L.; Gallop, J.; Hao, L. Graphene gas sensing using a non-contact microwave method. Nanotechnology 2017, 28, 395501. [Google Scholar] [CrossRef]
- Hu, H.; Yang, X.; Guo, X.; Khaliji, K.; Biswas, S.R.; De Abajo, F.J.G.; Low, T.; Sun, Z.; Dai, Q. Gas identification with graphene plasmons. Nat. Commun. 2019, 10, 1–7. [Google Scholar] [CrossRef]
- Majhi, S.; Mirzaei, A.; Kim, H.; Kim, S. Reduced Graphene Oxide (rGO)-Loaded Metal-Oxide Nanofiber Gas Sensors: An Overview. Sensors 2021, 21, 1352. [Google Scholar] [CrossRef]
- Armano, A.; Agnello, S. Two-Dimensional Carbon: A Review of Synthesis Methods, and Electronic, Optical, and Vibrational Properties of Single-Layer Graphene. C 2019, 5, 67. [Google Scholar] [CrossRef] [Green Version]
- Sur, U.K. Graphene: A Rising Star on the Horizon of Materials Science. Int. J. Electrochem. 2012, 2012, 1–12. [Google Scholar] [CrossRef]
- Pisarkiewicz, T.; Maziarz, W.; Małolepszy, A.; Stobiński, L.; Michoń, D.; Rydosz, A. Multilayer Structure of Reduced Graphene Oxide and Copper Oxide as a Gas Sensor. Coatings 2020, 10, 1015. [Google Scholar] [CrossRef]
- Chatterjee, S.G.; Chatterjee, S.; Ray, A.K.; Chakraborty, A.K. Graphene–metal oxide nanohybrids for toxic gas sensor: A review. Sensors Actuators B: Chem. 2015, 221, 1170–1181. [Google Scholar] [CrossRef]
- Rowley-Neale, S.J.; Randviir, E.P.; Dena, A.S.A.; Banks, C.E. An overview of recent applications of reduced graphene oxide as a basis of electroanalytical sensing platforms. Appl. Mater. Today 2018, 10, 218–226. [Google Scholar] [CrossRef]
- Łukowiec, D.; Kubacki, J.; Kałużyński, P.; Procek, M.; Wacławek, S.; Radoń, A. Formation and role in gas sensing properties of spherical and hollow silver nanoparticles deposited on the surface of electrochemically exfoliated graphite. Appl. Surf. Sci. 2021, 580, 152316. [Google Scholar] [CrossRef]
- Drewniak, S.; Pustelny, T.; Muzyka, R.; Stolarczyk, A.; Konieczny, G. Investigations of selected physical properties of graphite oxide and thermally exfoliated/reduced graphene oxide in the aspect of their applications in photonic gas sensors. Photon- Lett. Pol. 2015, 7, 2. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.-C.; Dai, J.; Hu, G.; Yu, W.-B.; Ogbeide, O.; De Luca, A.; Huang, X.; Su, B.-L.; Li, Y.; Udrea, F.; et al. Machine-intelligent inkjet-printed α-Fe2O3/rGO towards NO2 quantification in ambient humidity. Sensors Actuators B: Chem. 2020, 321, 128446. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H.-M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Stobinski, L.; Lesiak, B.; Malolepszy, A.; Mazurkiewicz, M.; Mierzwa, B.; Zemek, J.; Jiricek, P.; Bieloshapka, I. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J. Electron Spectrosc. Relat. Phenom. 2014, 195, 145–154. [Google Scholar] [CrossRef]
- Drewniak, S.; Muzyka, R.; Stolarczyk, A.; Pustelny, T.; Kotyczka-Morańska, M.; Setkiewicz, M. Studies of Reduced Graphene Oxide and Graphite Oxide in the Aspect of Their Possible Application in Gas Sensors. Sensors 2016, 16, 103. [Google Scholar] [CrossRef]
- Botas, C.; Álvarez, P.; Blanco, C.; Santamaria, R.; Granda, M.; Gutiérrez, M.D.; Rodriguez-Reinoso, F.; Menéndez, R. Critical temperatures in the synthesis of graphene-like materials by thermal exfoliation–reduction of graphite oxide. Carbon 2013, 52, 476–485. [Google Scholar] [CrossRef] [Green Version]
- Muzyka, R.; Drewniak, S.; Pustelny, T.; Chrubasik, M.; Gryglewicz, G. Characterization of Graphite Oxide and Reduced Graphene Oxide Obtained from Different Graphite Precursors and Oxidized by Different Methods Using Raman Spectroscopy. Materials 2018, 11, 1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drewniak, S.; Procek, M.; Muzyka, R.; Pustelny, T. Comparison of Gas Sensing Properties of Reduced Graphene Oxide Obtained by Two Different Methods. Sensors 2020, 20, 3175. [Google Scholar] [CrossRef]
- Imae, I. Reduction of Graphene Oxide Using an Environmentally Friendly Method and Its Application to Energy-Related Materials. Coatings 2021, 11, 297. [Google Scholar] [CrossRef]
- Sali, S.; Mackey, H.R.; Abdala, A.A. Effect of Graphene Oxide Synthesis Method on Properties and Performance of Polysulfone-Graphene Oxide Mixed Matrix Membranes. Nanomaterials 2019, 9, 769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarcan, R.; Todor-Boer, O.; Petrovai, I.; Leordean, C.; Astilean, S.; Botiz, I. Reduced graphene oxide today. J. Mater. Chem. C 2019, 8, 1198–1224. [Google Scholar] [CrossRef]
- Lawal, A.T. Graphene-based nano composites and their applications. A review. Biosens. Bioelectron. 2019, 141, 111384. [Google Scholar] [CrossRef]
- Bonavolontà, C.; Vettoliere, A.; Falco, G.; Aramo, C.; Rendina, I.; Ruggiero, B.; Silvestrini, P.; Valentino, M. Reduced graphene oxide on silicon-based structure as novel broadband photodetector. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Sieradzka, M.; Ślusarczyk, C.; Biniaś, W.; Fryczkowski, R. The Role of the Oxidation and Reduction Parameters on the Properties of the Reduced Graphene Oxide. Coatings 2021, 11, 166. [Google Scholar] [CrossRef]
- Osmieri, L.; Videla, A.H.A.M.; Specchia, S. The use of different types of reduced graphene oxide in the preparation of Fe-N-C electrocatalysts: Capacitive behavior and oxygen reduction reaction activity in alkaline medium. J. Solid State Electrochem. 2016, 20, 3507–3523. [Google Scholar] [CrossRef]
- Loryuenyong, V.; Totepvimarn, K.; Eimburanapravat, P.; Boonchompoo, W.; Buasri, A. Preparation and Characterization of Reduced Graphene Oxide Sheets via Water-Based Exfoliation and Reduction Methods. Adv. Mater. Sci. Eng. 2013, 2013, 923403. [Google Scholar] [CrossRef] [Green Version]
- Bing, C.; Jiahao, Y.; Xiaoying, L.; Qi, J.; Guoping, W.; Linghua, J. Effects of reduction method on reduced graphene oxide and its electrochemical energy storage performance. Diam. Relat. Mater. 2021, 114, 108305. [Google Scholar] [CrossRef]
- Spilarewicz-Stanek, K.; Kisielewska, A.; Ginter, J.; Bałuszyńska, K.; Piwoński, I. Elucidation of the function of oxygen moieties on graphene oxide and reduced graphene oxide in the nucleation and growth of silver nanoparticles. RSC Adv. 2016, 6, 60056–60067. [Google Scholar] [CrossRef] [Green Version]
- Lesiak, B.; Trykowski, G.; Tóth, J.; Biniak, S.; Kövér, L.; Rangam, N.; Stobinski, L.; Malolepszy, A. Chemical and structural properties of reduced graphene oxide—dependence on the reducing agent. J. Mater. Sci. 2020, 56, 3738–3754. [Google Scholar] [CrossRef]
- Gao, H.; Ma, Y.; Song, P.; Leng, J.; Wang, Q. Gas sensor based on rGO/ZnO aerogel for efficient detection of NO2 at room temperature. J. Mater. Sci. Mater. Electron. 2021, 32, 10058–10069. [Google Scholar] [CrossRef]
- Zhu, X.; Zhou, Y.; Guo, Y.; Ren, H.; Gao, C. Nitrogen dioxide sensing based on multiple-morphology cuprous oxide mixed structures anchored on reduced graphene oxide nanosheets at room temperature. Nanotechnology 2019, 30, 455502. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, L.; Li, Q.; Du, Y.; Ruan, S. Reduced graphene oxide/α-Fe2O3 hybrid nanocomposites for room temperature NO2 sensing. Sensors Actuators B: Chem. 2017, 241, 109–115. [Google Scholar] [CrossRef]
- Cheng, M.; Wu, Z.; Liu, G.; Zhao, L.; Gao, Y.; Zhang, B.; Liu, F.; Yan, X.; Liang, X.; Sun, P.; et al. Highly sensitive sensors based on quasi-2D rGO/SnS2 hybrid for rapid detection of NO2 gas. Sensors Actuators B: Chem. 2019, 291, 216–225. [Google Scholar] [CrossRef]
- Sivakumar, R.; Krishnamoorthi, K.; Vadivel, S.; Govindasamy, S. Progress towards a novel NO2 gas sensor based on SnO2/RGO hybrid sensors by a facial hydrothermal approach. Diam. Relat. Mater. 2021, 116, 108418. [Google Scholar] [CrossRef]
- Rigoletto, M.; Calza, P.; Gaggero, E.; Laurenti, E. Hybrid materials for the removal of emerging pollutants in water: Classification, synthesis, and properties. Chem. Eng. J. Adv. 2022, 10. [Google Scholar] [CrossRef]
- Choudhari, A.; Bhanvase, B.A.; Saharan, V.K.; Salame, P.; Hunge, Y. Sonochemical preparation and characterization of rGO/SnO2 nanocomposite: Electrochemical and gas sensing performance. Ceram. Int. 2020, 46, 11290–11296. [Google Scholar] [CrossRef]
- Li, L.; He, S.; Liu, M.; Zhang, C.; Chen, W. Three-Dimensional Mesoporous Graphene Aerogel-Supported SnO2 Nanocrystals for High-Performance NO2 Gas Sensing at Low Temperature. Anal. Chem. 2015, 87, 1638–1645. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Xie, G.; Zhang, Q. Enhanced Room Temperature NO2 Sensing Performance of RGO Nanosheets by Building RGO/SnO2 Nanocomposite System. Sensors 2019, 19, 4650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Marikutsa, A.V.; Rumyantseva, M.N.; Gaskov, A.; Knotko, A.; Li, X. p-n Transition-Enhanced Sensing Properties at Room Temperature. IEEE Sens. J. 2020, 20, 4562–4570. [Google Scholar] [CrossRef]
- Li, G.; Shen, Y.; Zhou, P.; Hao, F.; Fang, P.; Wei, D.; Meng, D.; San, X. Design and application of highly responsive and selective rGO-SnO2 nanocomposites for NO2 monitoring. Mater. Charact. 2020, 163, 110284. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Sun, F.; Li, T.; Zhang, T.; Qin, S. Humidity-Insensitive NO2 Sensors Based on SnO2/rGO Composites. Front. Chem. 2021, 9, 681313. [Google Scholar] [CrossRef]
- Wu, L.; Shao, H.; Yang, C.; Feng, X.; Han, L.; Zhou, Y.; Du, W.; Sun, X.; Xu, Z.; Zhang, X.; et al. SnS2 Nanosheets with RGO Modification as High-Performance Anode Materials for Na-Ion and K-Ion Batteries. Nanomaterials 2021, 11, 1932. [Google Scholar] [CrossRef]
- Shafiei, M.; Bradford, J.; Khan, H.; Piloto, C.; Wlodarski, W.; Li, Y.; Motta, N. Low-operating temperature NO2 gas sensors based on hybrid two-dimensional SnS2-reduced graphene oxide. Appl. Surf. Sci. 2018, 462, 330–336. [Google Scholar] [CrossRef]
- Liu, S.; Yu, B.; Zhang, H.; Fei, T.; Zhang, T. Enhancing NO2 gas sensing performances at room temperature based on reduced graphene oxide-ZnO nanoparticles hybrids. Sensors Actuators B: Chem. 2014, 202, 272–278. [Google Scholar] [CrossRef]
- Bai, S.; Han, J.; Meng, J.C.; Sun, L.; Sun, J.; Zhao, Y.; Tang, P.; Luo, R.; Li, D.; Chen, A. NiO/ZnO composite decorated on rGO for detection of NO2. Sensors Actuators B: Chem. 2021, 339, 129720. [Google Scholar] [CrossRef]
- Gao, R.; Gao, L.; Zhang, X.; Gao, S.; Xu, Y.; Cheng, X.; Guo, G.; Ye, Q.; Zhou, X.; Major, Z.; et al. The controllable assembly of the heterojunction interface of the ZnO@rGO for enhancing the sensing performance of NO2 at room temperature and sensing mechanism. Sensors Actuators B: Chem. 2021, 342, 130073. [Google Scholar] [CrossRef]
- Galstyan, V.; Comini, E.; Kholmanov, I.; Ponzoni, A.; Sberveglieri, V.; Poli, N.; Faglia, G.; Sberveglieri, G. Graphene-zinc Oxide Based Nanomaterials for Gas Sensing Devices. Procedia Eng. 2016, 168, 1172–1175. [Google Scholar] [CrossRef] [Green Version]
- Geng, X.; Zhang, C.; Olivier, M.-G.; Debliquy, M. Room Temperature NO2 Responses of Visible-Light Activated Nanosheet rGO@ZnO1−x Sensors. Proceedings 2017, 1, 411. [Google Scholar] [CrossRef] [Green Version]
- Ngo, Y.-L.T.; Hur, S.H. Low-temperature NO2 gas sensor fabricated with NiO and reduced graphene oxide hybrid structure. Mater. Res. Bull. 2016, 84, 168–176. [Google Scholar] [CrossRef]
- Liang, Y.-C.; Chang, Y.-C.; Zhao, W.-C. Design and Synthesis of Novel 2D Porous Zinc Oxide-Nickel Oxide Composite Nanosheets for Detecting Ethanol Vapor. Nanomaterials 2020, 10, 1989. [Google Scholar] [CrossRef]
- Pisarkiewicz, T.; Maziarz, W.; Małolepszy, A.; Stobiński, L.; Michoń, D.A.; Szkudlarek, A.; Pisarek, M.; Kanak, J.; Rydosz, A. Nitrogen Dioxide Sensing using Multilayer Structure of Reduced Graphene Oxide and α-Fe2O3. Sensors 2021, 21, 1011. [Google Scholar] [CrossRef]
- Tang, X.; Tian, C.; Zou, C. Highly sensitive and selective room-temperature NO2 gas sensor based on novel Fe2O3 nanorings/reduced graphene oxide heterojunction nanocomposites. Optik 2021, 241, 166951. [Google Scholar] [CrossRef]
- Huang, M.; Wang, Y.; Ying, S.; Wu, Z.; Liu, W.; Chen, D.; Peng, C. Synthesis of Cu2O-Modified Reduced Graphene Oxide for NO2 Sensors. Sensors 2021, 21, 1958. [Google Scholar] [CrossRef]
- Nia, P.M.; Meng, W.P.; Lorestani, F.; Mahmoudian, M.; Alias, Y. Electrodeposition of copper oxide/polypyrrole/reduced graphene oxide as a nonenzymatic glucose biosensor. Sensors Actuators B: Chem. 2015, 209, 100–108. [Google Scholar] [CrossRef]
- Meng, H.; Yang, W.; Ding, K.; Feng, L.; Guan, Y. Cu2O nanorods modified by reduced graphene oxide for NH3 sensing at room temperature. J. Mater. Chem. A 2014, 3, 1174–1181. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, R.; Zhou, T.; Lou, Z.; Deng, J.; Zhang, T. Concave Cu2O octahedral nanoparticles as an advanced sensing material for benzene (C6H6) and nitrogen dioxide (NO2) detection. Sensors Actuators B: Chem. 2015, 223, 311–317. [Google Scholar] [CrossRef]
- Shen, Y.; Tian, F.H.; Chen, S.; Ma, Z.; Zhao, L.; Jia, X. Density functional theory study on the mechanism of CO sensing on Cu2O (111) surface: Influence of the pre-adsorbed oxygen atom. Appl. Surf. Sci. 2014, 288, 452–457. [Google Scholar] [CrossRef]
- Pan, J.; Liu, W.; Quan, L.; Han, N.; Bai, S.; Luo, R.; Feng, Y.; Li, D.; Chen, A. Cu2O and rGO Hybridizing for Enhancement of Low-Concentration NO2 Sensing at Room Temperature. Ind. Eng. Chem. Res. 2018, 57, 10086–10094. [Google Scholar] [CrossRef]
- Wan, X.; Wang, J.; Zhu, L.; Tang, J. Gas sensing properties of Cu2O and its particle size and morphology-dependent gas-detection sensitivity. J. Mater. Chem. A 2014, 2, 13641–13647. [Google Scholar] [CrossRef]
- Steinhauer, S. Gas Sensors Based on Copper Oxide Nanomaterials: A Review. Chemosensors 2021, 9, 51. [Google Scholar] [CrossRef]

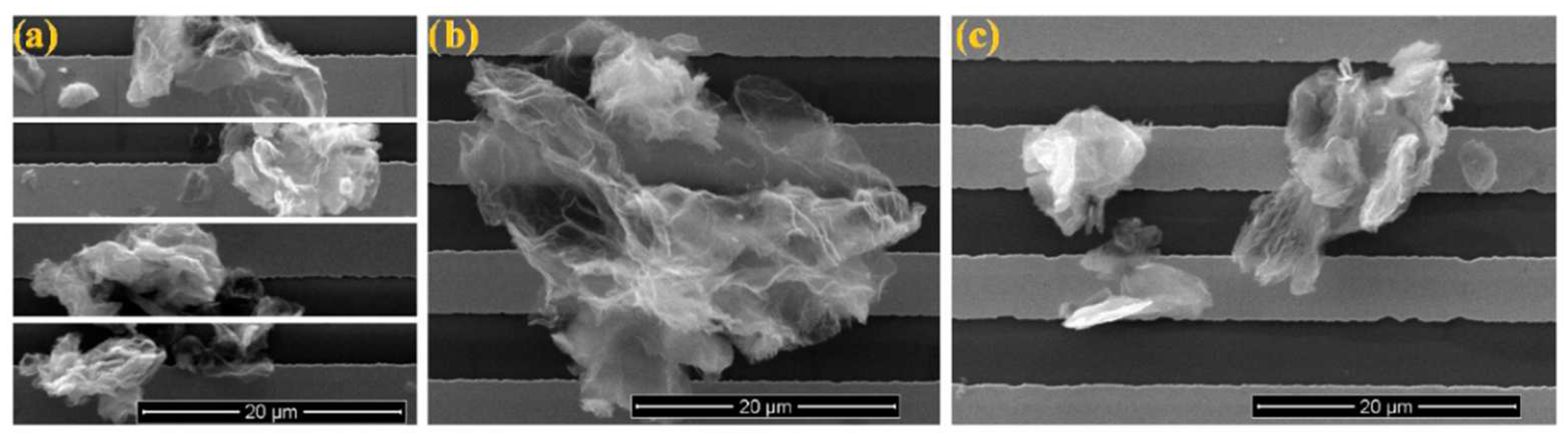

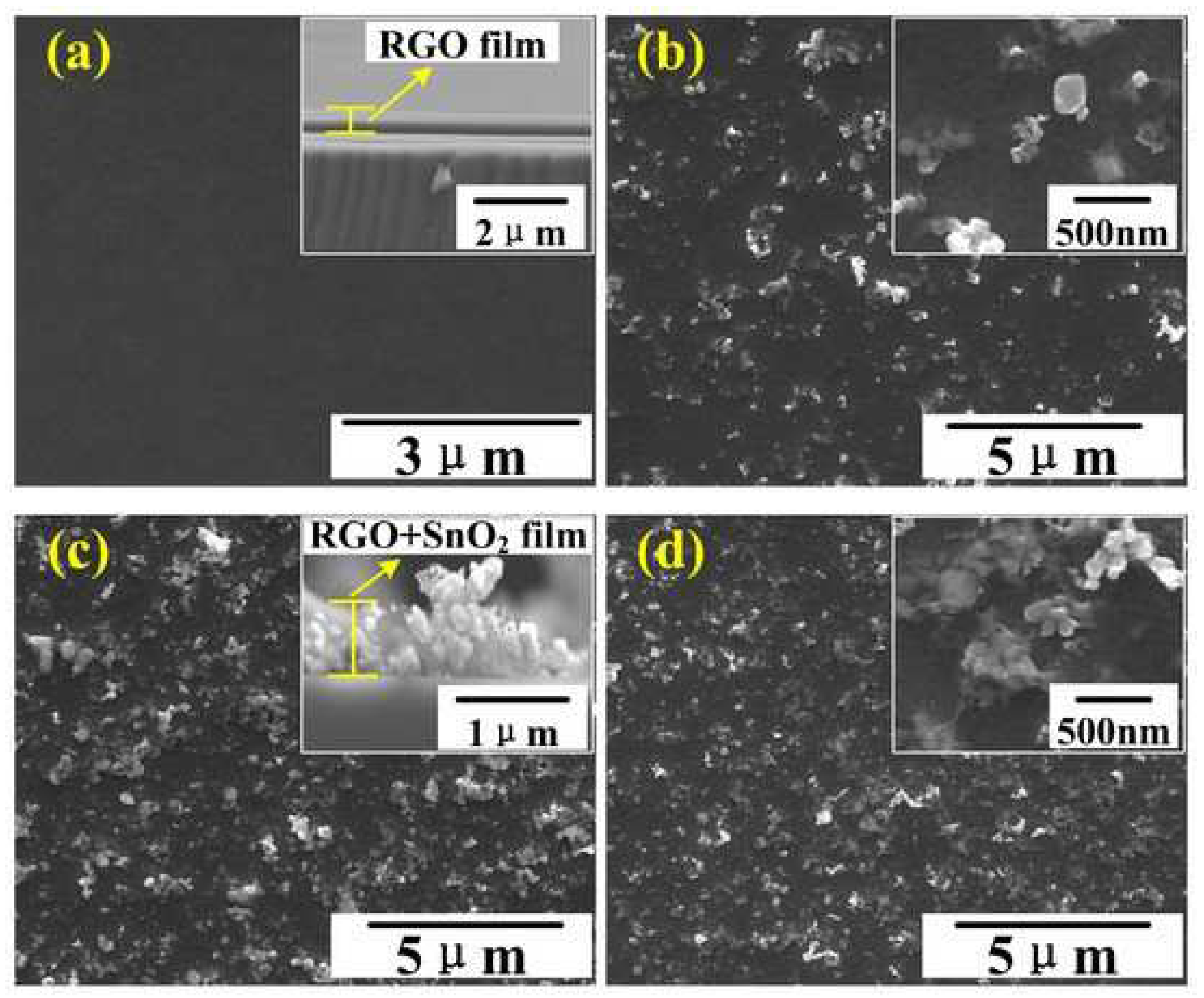



| Hybrid | Temperature (°C) | Concentration of NO2 (ppm) | Sensitivity | Equation of Sensitivity | Reaction/ Regeneration Time (s) | Ref. |
|---|---|---|---|---|---|---|
| rGO/SnO2 | 55 | 100 | 1.079 | -/373 | [53] | |
| rGO/SnS2 | 80 | 11.9 | 56.8% | 360/3180 | [59] | |
| rGO/ZnO | RT | 1 | 2.07 | 150/55 | [62] | |
| rGO/NiO/ZnO | 140 | 2 | 80.1 | 39/16 | [66] | |
| rGO/Fe2O3 | RT | 5 | 3.86 | 76/946 | [48] | |
| rGO/PPy/Cu2O | 25 | 50 | 42.5% | -/~200 1 | [69] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drewniak, S.; Drewniak, Ł.; Pustelny, T. Mechanisms of NO2 Detection in Hybrid Structures Containing Reduced Graphene Oxide: A Review. Sensors 2022, 22, 5316. https://doi.org/10.3390/s22145316
Drewniak S, Drewniak Ł, Pustelny T. Mechanisms of NO2 Detection in Hybrid Structures Containing Reduced Graphene Oxide: A Review. Sensors. 2022; 22(14):5316. https://doi.org/10.3390/s22145316
Chicago/Turabian StyleDrewniak, Sabina, Łukasz Drewniak, and Tadeusz Pustelny. 2022. "Mechanisms of NO2 Detection in Hybrid Structures Containing Reduced Graphene Oxide: A Review" Sensors 22, no. 14: 5316. https://doi.org/10.3390/s22145316
APA StyleDrewniak, S., Drewniak, Ł., & Pustelny, T. (2022). Mechanisms of NO2 Detection in Hybrid Structures Containing Reduced Graphene Oxide: A Review. Sensors, 22(14), 5316. https://doi.org/10.3390/s22145316







