1. Introduction
Colorectal and stomach cancers have ranked 3–5 globally since 2012 [
1]. Owing to the development of preventive diagnostic medicine, endoscopic technology is rapidly developing, and the demands for diagnosis and laparoscopic surgery are increasing simultaneously [
2]. Early detection of gastrointestinal (colon/stomach) cancer is possible using endoscopic diagnosis, and a five-year survival rate of over 90% can be guaranteed through biopsy and surgical treatment. Safe surgery involves promptly extracting the tumor by accurately locating it. Furthermore, the purpose of laparoscopic surgery is to quickly identify the location of the tumor and to accurately and safely extract it, thereby shortening the overall operation time. This surgical technique involves a simple operation with minimal side effects and pain, and the patient recovers rapidly; however, determining the location of the mucosal resection to remove the tumor from the gastrointestinal cavity is difficult [
2,
3,
4,
5]. More specifically, in laparoscopic gastrointestinal cancer (or colon cancer) surgery, malignant tumors present in the visceral cavity due to mucous membranes cannot be seen in the parietal (or cecum) of the cavity, and to remove the tumor, the location of the tumor present in the visceral (or cyclic folds) must be determined. Therefore, three days before surgery, an endoscope is used to find tumors present in the visceral (or cyclic folds) and a marker (or a drug that can indicate the location of the tumor) is installed around the tumor. Additionally, during surgery, the marker position inside the organ (visceral or circular folds) can be found using a device that can detect the marker outside the organ (parallel or cecum). Since the marker’s location is the location of the tumor, the exact location can be identified by removing the tumor, and this method can be the exact determinant of the incision site for tumor removal. Hence, markers used during laparoscopic surgery play a very important role in surgery. It is important to locate the marker quickly and extracting the tumor quickly at the scheduled surgery (anesthesia) time can be a very important factor directly related to the patient’s life protection. Thus, it is very important to design a high-performance maker, and the reason for this is to propose an idea on how the operator can easily perceive markers when they are found in a chaotic surgical site.
Commonly used methods for locating tumors are fluorescent staining using indocyanine green, ink tattooing, and local autologous labeling [
6,
7,
8,
9,
10]. These methods have some side effects, although they can determine the location of the tumor [
8]. Fluorescent staining using indocyanine green is expensive and causes allergic reactions owing to the presence of iodine. The ink spot method involves a long procedure and causes granuloma formation and peritonitis [
7,
8]. Additionally, autologous local markers bleed profusely and cause cirrhosis of the liver. Furthermore, autologous blood localization is difficult and time-consuming, which makes this method economically disadvantageous. To overcome these disadvantages, techniques for locating a tumor using a sensor that can detect a clip are being studied [
11,
12,
13,
14,
15,
16]. More specifically, a clip is attached to the tumor site, and research is underway on a method for locating this clip using a sensor, thereby locating the tumor that needs resection. Analyzing the research case, the time to locate the tumor must be shortened. In addition, the sensor should be able to detect tumors in the stomach and large intestine simultaneously and be robust. At this time, the time required to locate the tumor through the sensor should be 5 s.
Radio frequency identification (RFID) [
11] and the open–close clip closure method [
14] have the advantage of being able to simultaneously detect tumors in the stomach and colon. In addition, the clip and the sensor are integrated to ensure robustness. However, the RFID method [
11] requires 40.5 and 38.4 s to detect a tumor in the stomach and colon, respectively, and the open–close clip closure method [
14] has a relatively long time as 24.9 and 18.7 s, respectively, for detecting tumors in the stomach and colon.
A sensor using RFID technology can only detect the location of a malignant tumor in the stomach [
16]. The time required for this detection is as long as 25 s [
12,
13,
15]. This technology uses a magnet, and a wire is connected between the clip and magnet sensor. However, the wire connection is not sturdy and wobbles. Therefore, the location of the tumor changes as much as the length of the wire, which makes it difficult to determine the exact location of the tumor and may cause the extraction to fail. In addition, only tumors in the colon can be detected, and the time required for tumor detection is 456, 342, and 15–90 s, respectively. The characteristic of [
11,
12,
13,
14,
15,
16] is that the time required to detect a tumor exceeds 5 s; therefore, these technologies are unsuitable for use in the operating room. To overcome these shortcomings, a sensor capable of locating a tumor in the stomach and colon simultaneously and accurately is required.
This study proposes a ferromagnetic clip and clip-detection sensor that can simultaneously locate malignant tumors in the stomach and colon quickly and accurately. The clip is constructed using a ferromagnetic material to ensure sturdiness and miniaturization, such that it can pass through the working channel (diameter: 4 mm) of a gastroscope and trocar. The introduction presents the motivation for the research,
Section 2 and
Section 3 describe the design methods and experimental results, and
Section 4 and
Section 5 present the discussion and conclusion.
2. Analysis of the Magnetic Coupling
In laparoscopic surgery, malignant tumors present in the colon (or stomach) interior (circular folds or visceral) are not visible outside (cecum or parietal) of the colon, so it is not easy to locate the tumor in the cavity [
4]. Therefore, to locate the tumor on the inner wall of the stomach (circular folds or visceral), the tumor is located through an endoscope three days before surgery (flow chart on the left), as shown in
Figure 1, and then the clip is pre-installed around the tumor. Then, during laparoscopic surgery, the trocar is docked to locate the detector (picture on the right) and the clip present inside the colon (or stomach) in the cavity.
If the detector finds the location of the clip, an alarm (picture on the right) is generated in the detector to guide the location of the clip. Therefore, the tumor can be safely removed, and the surgery is over. In the process of finding a clip using a detector, sine waves generated in the oscillation are transferred to the detector, as shown in
Figure 2. A magnet sensor is attached to the end of the detector.
At this time, if the current for the sine wave generated from the oscillator reaches the sensor, the current is generated in the sensor and the magnetic flux density (
B) is formed as in Equation (1). Assuming that the permeability is constant, if the magnetic flux density (
B1) of the sensor is generated, a magnetic field (
H1) is formed as in Equation (2) [
15,
16,
17,
18,
19].
where the
B and
µ0 are magnetic flux density and permeability of free space (4
π × 10
−7 H/m). Therefore, the energy (
H1 and
ω1) generated from the magnet sensor passes through the mucous membrane of the colon (or stomach). After that, energy is transmitted (TX) to the neodymium magnet (NeFe35) present inside the clip as shown in
Figure 3, where
ω is angular frequency which is
2πf and the
f is frequency.
A neodymium magnet is a magnet with the strongest magnetic force among permanent magnets. Neodymium magnets have excellent machinability of various shapes. Therefore, the neodymium magnet is made of iron and boron, and it has the maximum magnetic energy among permanent magnets. Due to the ferromagnetic properties of the neodymium magnet, a strong magnetic flux density (
B2) is generated as in Equation (1), and the magnetic field (
H2) increases. Therefore, as in Equation (3), the neodymium magnet operates as a magnet by generating a strong magnetic force (
F). At this time,
D means the size of the neodymium magnet, and as the size of the magnet increases, the magnetic flux density (
B2) increases. Thus, the magnetic field
H2 will increase, and the magnetic force
F will increase. Additionally, a magnetic field (
H2) is generated along with the induction frequency (
ω2) [
15].
As a result,
ω1 and
H1 generated from the sensor are coupled to
ω2 and
H2 generated from the neodymium magnet to form
H1 and
H2 as shown in Equation (4), and
ω1 and
ω2 become
ω0 as shown in Equation (5). Therefore, signals are exchanged (TX/RX) between the sensor and the clip (neodymium magnet) [
17].
Therefore, when the detector detects a clip,
H0 and
ω0 are converted into pulse waves through the Schmitt trigger circuit, and the pulse waves are converted into direct current (DC) form that can get sound from the speaker through the regulator. Therefore, DC signal is transmitted to the speaker and in order to provide clean sound quality without noise, we can convert it into pulse by applying the Schmitt trigger circuit. Therefore, it is possible to increase the accurate search efficiency for the position of the clip. However, an important fact is the analysis of the distance between the magnet and the sensor. In the difference between the magnet and the sensor, the coupling frequency (
fo) is the same, but the magnetic flux density (
B) and the magnetic field (
H0) are changed. From the simulation results, the magnet and sensor are in inverse proportion to each other. As shown in
Figure 4, if the distance between the magnet and sensor is increased,
H and
B are decreased, and, therefore,
F is weakened as given by Equation (6) [
18,
19]. Therefore, as the distance (
d) between the sensor and the clip increases, the magnetic field (
H) and magnetic flux density (
B) decrease, so that the magnetic force (
F) decreases as the square of the distance [
15,
16,
17].
In contrast, as the distance between the magnet and sensor is decreased, H and B are shortened, and therefore F becomes strong. Additionally, to increase H, the size of the magnet must be increased. Therefore, the size of the magnet must also be considered when determining H that is sufficient to pass through tissues. The inner diameter of the magnet in this study is determined to be 1.5 mm (dn), height (hn) is 3.4 mm, thickness (tin) is 0.5 mm, and total diameter (D) is 2.5 mm. Additionally, the diameter (tc) and height (hc) of the clip are 2.7 mm, respectively, and the working channels of the detector are 2.8 mm (stomach) and 3.2 mm (colon), respectively.
Considering the thickness of the mucosa between the magnet present in the clip and sensor (t
m) (top: 2 cm) and the cavity distance (d) between the mucosa and sensor,
F is calculated as 838.4, as shown in
Figure 5, measured in N. Additionally,
H and
B are calculated as 1.035 A/m and 1.33 T, respectively, and the polarization direction is changed by 0–10°.
3. Design and Fabrication of the Clip–Detector
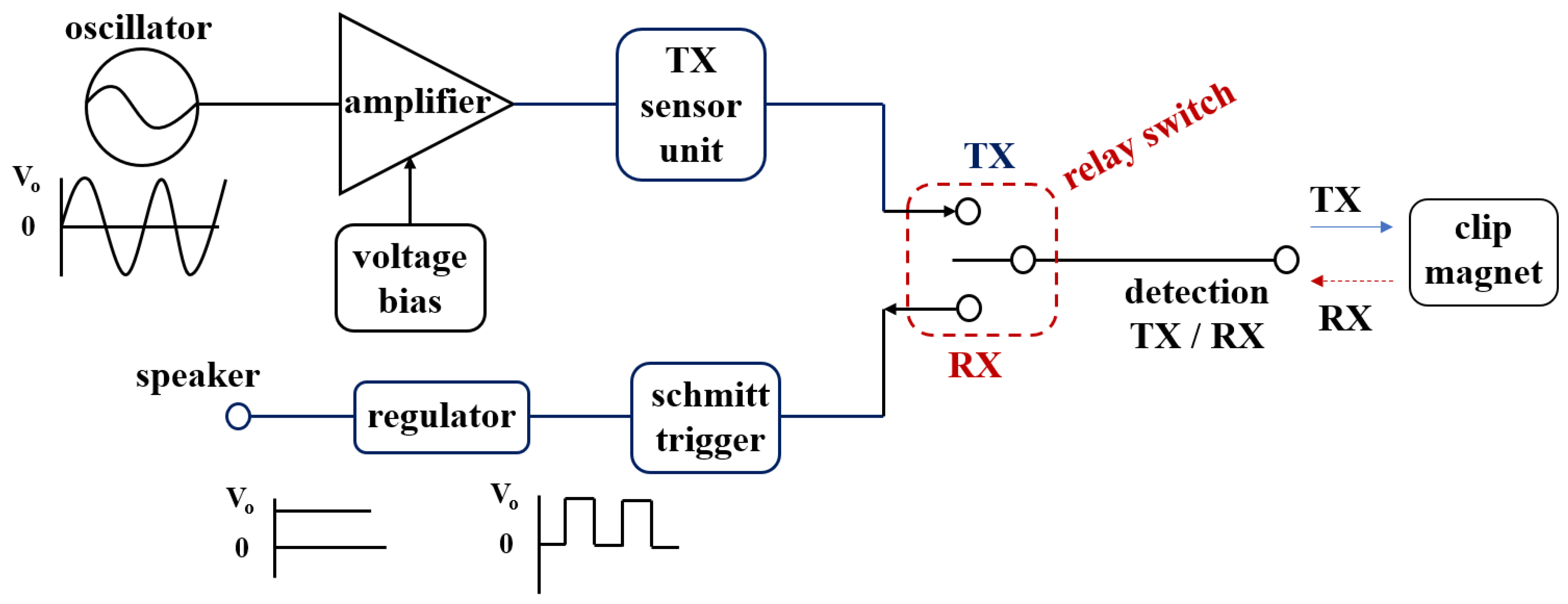
Figure 6 shows the schematic circuit and fabricated printed circuit board containing the clip–detector. As shown in the figure, the circuit consists of a Colpitts oscillator, amplifier, Schmitt trigger, regulator, relay switch, and speaker.
In particular, the Colpitts oscillator is composed of parallel capacities (
Cx:
C1//
C2, see
Figure 6) as shown in Equation (7), and thus the quality factor (Q) is increased due to high
Cx. This high Q is a decisive factor in accurately locating the clip. Therefore, the oscillation frequency
ωr is determined by the adjustment of the
Cx value as shown in Equation (8). However, the inductance
Lx may act as a resistor
Rx as shown in
Figure 6.
The Colpitts oscillator is connected to a crystal and the frequency of the crystal is set to operate from 1.56 to 1.57 kHz. Therefore, the oscillator generates a sine wave with a frequency of 1.56–1.57 kHz, and this sine wave is transmitted to the sensor of the detector through TX of the relay switch (S/W).
The sine wave (H1 and ω1) output from the sensor of the detector is transmitted to the clip, and the signals (H2 and ω2) generated from the clip are combined with the signal generated from the sensor to generate the coupling signal (H0 and ω0). Therefore, the sensor and clip exchange signal with each other. The signal detected in the clip is transferred to the RX of the relay switch and converted into a pulse wave through the Schmitt trigger circuit, improving the sound quality of the detected signal and suppressing the noise. The pulse wave provides a function so that sound can be heard from the speaker by converting it into direct current (DC) form through the regulator. Therefore, the speaker notifies the fact that the clip has been detected by generating an alarm.
During the PCB fabrication process, the fabrication of the substrate used a Fr4 substrate with a dielectric constant of 4.6 and a thickness of 3.2 T. The magnet is used for combination of the nickel and gold, and the material of the coating with the clip is used by parylene C. In addition, the detector is used for 3D printing technique which is shown in
Figure 7.
5. Conclusions
This study presents a method for locating a malignant tumor in the stomach and colon using a sensor in the cavity during laparoscopic cancer surgery. Determining the extent of excision for removing gastric and colonic tumors is difficult. Therefore, after installing the clip around the tumor, locating the clip using a detector and extracting the tumor is a feasible solution.
Since the proposed clip–detector employs magnetic field coupling based on a neodymium magnet (clip) detection technology, tissue penetration is easy. Additionally, the amplitude of magnetic coupling has a relatively high gain at 3.33 V and 1.59 kHz. Therefore, if the detector locates the clip installed around the tumor, an alarm is audible from the speaker at an amplitude of 3.20 V (frequency: 1.59 kHz). Therefore, the tumor can be located quickly using the alarm in the operating room.
The designed clip–detector does not have any side effects, unlike the conventional ink tattoo, indocyanine green fluorescence staining, and autologous blood maker methods, and it can quickly locate the tumor, thereby reducing the burden between doctors and patients. Because the clip–detector uses ferrite and a coil, it is inexpensive and easy to manufacture. Additionally, the clip–detector can be mass produced owing to low unit price. Since the clip–detector requires an endoscope, the demand for endoscopes and laparoscopic surgery is expected to increase in the future.