Aberrated Multidimensional EEG Characteristics in Patients with Generalized Anxiety Disorder: A Machine-Learning Based Analysis Framework
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. EEG Data Acquisition and Preprocessing
2.3. EEG Features Extraction
2.3.1. PSD Calculation
2.3.2. FE Calculation
2.3.3. PLI Calculation
2.4. Feature Selection via Statistical Analysis
2.5. Machine Learning for Classification
3. Results
4. Discussion
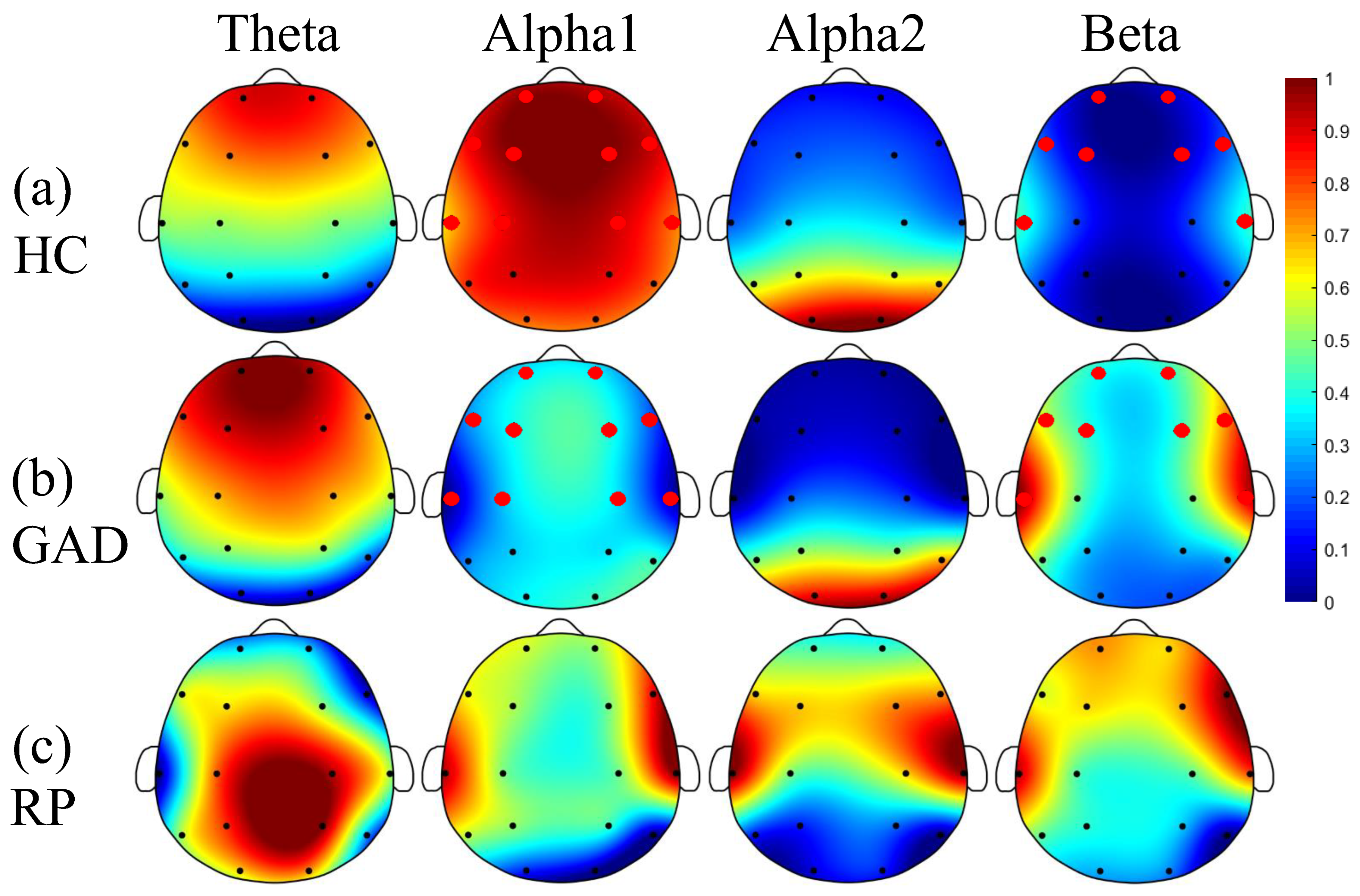
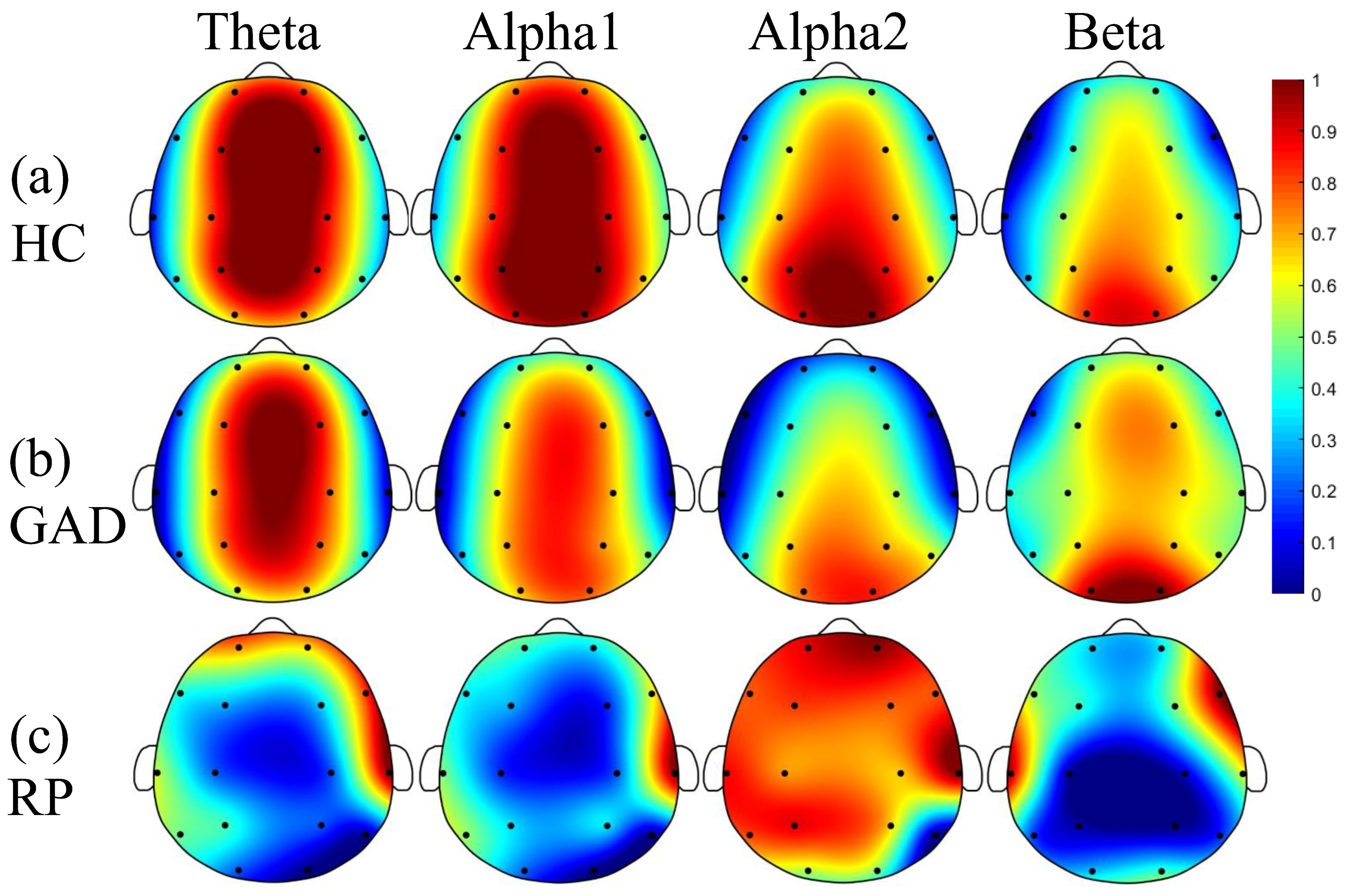
4.1. Striking EEG Rhythm in GAD
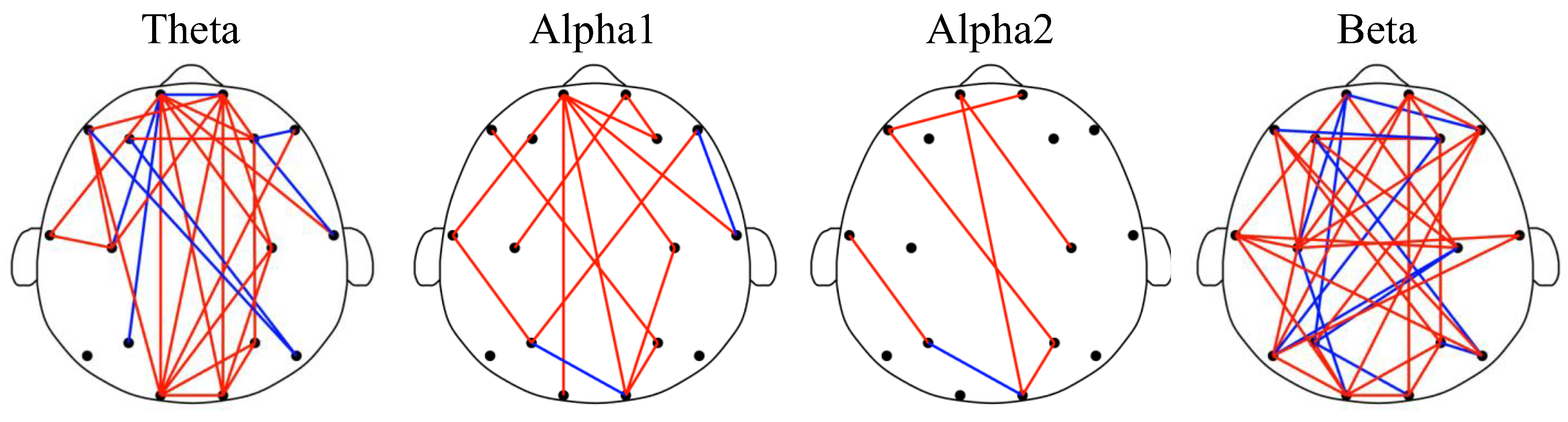
4.2. Reduced FCs and Dominant Brain Areas
4.3. Excellent Classification Performance for GAD Detection
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tyrer, P.; Baldwin, D. Generalised anxiety disorder. Lancet 2006, 368, 2156–2166. [Google Scholar] [CrossRef]
- Tempesta, D.; Mazza, M.; Serroni, N.; Moschetta, F.S.; Di Giannantonio, M.; Ferrara, M.; De Berardis, D. Neuropsychological functioning in young subjects with generalized anxiety disorder with and without pharmacotherapy. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 45, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.B.; Sareen, J. Generalized Anxiety Disorder. N. Engl. J. Med. 2015, 373, 2059–2068. [Google Scholar] [CrossRef]
- Yu, W.; Singh, S.S.; Calhoun, S.; Zhang, H.; Zhao, X.; Yang, F. Generalized anxiety disorder in urban China: Prevalence, awareness, and disease burden. J. Affect. Disord. 2018, 234, 89–96. [Google Scholar] [CrossRef]
- Kessler, R.C.; Petukhova, M.; Sampson, N.A.; Zaslavsky, A.M.; Wittchen, H.-U. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int. J. Methods Psychiatr. Res. 2012, 21, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, Y.; Wang, H.; Liu, Z.; Yu, X.; Yan, J.; Yu, Y.; Kou, C.; Xu, X.; Lu, J.; et al. Prevalence of mental disorders in China: A cross-sectional epidemiological study. Lancet Psychiatry 2019, 6, 211–224. [Google Scholar] [CrossRef]
- Maslowsky, J.; Mogg, K.; Bradley, B.P.; Mcclure-Tone, E.; Ernst, M.; Pine, D.S.; Monk, C.S. A Preliminary Investigation of Neural Correlates of Treatment in Adolescents with Generalized Anxiety Disorder. J. Child Adolesc. Psychopharmacol. 2010, 20, 105–111. [Google Scholar] [CrossRef]
- Rickwood, D.; Bradford, S. The role of self-help in the treatment of mild anxiety disorders in young people: An evidence-based review. Psychol. Res. Behav. Manag. 2012, 5, 25–36. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Chai, F.; Zhang, H.; Liu, X.; Xie, P.; Zheng, L.; Yang, L.; Li, L.; Fang, D. Cortical functional activity in patients with generalized anxiety disorder. BMC Psychiatry 2016, 16, 217. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Li, B.; Jiang, Y.H.; Jiao, W.D.; Lan, H.; Zhu, C.G. A new method for automatically modelling brain functional networks. Biomed. Signal Processing Control 2018, 45, 70–79. [Google Scholar] [CrossRef]
- Schoenberg, P.L.A. Linear and Nonlinear EEG-Based Functional Networks in Anxiety Disorders. Anxiety Disord. Rethink. Underst. Recent Discov. 2020, 1191, 35–59. [Google Scholar]
- Byeon, H. Exploring Factors for Predicting Anxiety Disorders of the Elderly Living Alone in South Korea Using Interpretable Machine Learning: A Population-Based Study. Int. J. Environ. Res. Public Health 2021, 18, 7625. [Google Scholar] [CrossRef]
- Khalid, A.; Kim, B.S.; Chung, M.K.; Ye, J.C.; Jeon, D. Tracing the evolution of multi-scale functional networks in a mouse model of depression using persistent brain network homology. Neuroimage 2014, 101, 351–363. [Google Scholar] [CrossRef]
- Ponten, S.C.; Bartolomei, F.; Stam, C.J. Small-world networks and epilepsy: Graph theoretical analysis of intracerebrally recorded mesial temporal lobe seizures. Clin. Neurophysiol. 2007, 118, 918–927. [Google Scholar] [CrossRef]
- Micheloyannis, S.; Pachou, E.; Stam, C.J.; Breakspear, M.; Bitsios, P.; Vourkas, M.; Erimaki, S.; Zervakis, M. Small-world networks and disturbed functional connectivity in schizophrenia. Schizophr. Res. 2006, 87, 60–66. [Google Scholar] [CrossRef]
- Cejnek, M.; Vysata, O.; Valis, M.; Bukovsky, I. Novelty detection-based approach for Alzheimer’s disease and mild cognitive impairment diagnosis from EEG. Med. Biol. Eng. Comput. 2021, 59, 2287–2296. [Google Scholar] [CrossRef]
- Zhang, J.; Idaji, M.J.; Villringer, A.; Nikulin, V.V. Neuronal biomarkers of Parkinson’s disease are present in healthy aging. Neuroimage 2021, 243, 118512. [Google Scholar] [CrossRef] [PubMed]
- Carlier, S.; Van Der Paelt, S.; Ongenae, F.; De Backere, F.; De Turck, F. Empowering Children with ASD and Their Parents: Design of a Serious Game for Anxiety and Stress Reduction. Sensors 2020, 20, 966. [Google Scholar] [CrossRef] [Green Version]
- Newson, J.J.; Thiagarajan, T.C. EEG Frequency Bands in Psychiatric Disorders: A Review of Resting State Studies. Front. Hum. Neurosci. 2019, 12, 521. [Google Scholar] [CrossRef]
- Hickey, B.A.; Chalmers, T.; Newton, P.; Lin, C.-T.; Sibbritt, D.; Mclachlan, C.S.; Clifton-Bligh, R.; Morley, J.; Lal, S. Smart Devices and Wearable Technologies to Detect and Monitor Mental Health Conditions and Stress: A Systematic Review. Sensors 2021, 21, 3461. [Google Scholar] [CrossRef] [PubMed]
- Oathes, D.J.; Ray, W.J.; Yamasaki, A.S.; Borkovec, T.D.; Castonguay, L.G.; Newman, M.G.; Nitschke, J. Worry, generalized anxiety disorder, and emotion: Evidence from the EEG gamma band. Biol. Psychol. 2008, 79, 165–170. [Google Scholar] [CrossRef] [Green Version]
- Al-Ezzi, A.; Kamel, N.; Faye, I.; Gunaseli, E. Review of EEG, ERP, and Brain Connectivity Estimators as Predictive Biomarkers of Social Anxiety Disorder. Front. Psychol. 2020, 11, 730. [Google Scholar] [CrossRef]
- Beaty, R.E.; Seli, P.; Schacter, D.L. Network neuroscience of creative cognition: Mapping cognitive mechanisms and individual differences in the creative brain. Curr. Opin. Behav. Sci. 2019, 27, 22–30. [Google Scholar] [CrossRef]
- Friston, K.J. Functional and effective connectivity in neuroimaging: A synthesis. Hum. Brain Mapp. 1994, 2, 56–78. [Google Scholar] [CrossRef]
- Xing, M.; Tadayonnejad, R.; Macnamara, A.; Ajilore, O.; Leow, A. EEG based functional connectivity reflects cognitive load during emotion regulation. In Proceedings of the 2016 IEEE 13th International Symposium on Biomedical Imaging (ISBI), Prague, Czech Republic, 13–16 April 2016. [Google Scholar]
- Mokatren, L.S.; Ansari, R.; Cetin, A.E.; Leow, A.D.; Ajilore, O.A.; Klumpp, H.; Yarman Vural, F.T. EEG Classification by Factoring in Sensor Spatial Configuration. IEEE Access 2021, 9, 19053–19065. [Google Scholar] [CrossRef]
- Al-Ezzi, A.; Yahya, N.; Kamel, N.; Faye, I.; Alsaih, K.; Gunaseli, E. Severity Assessment of Social Anxiety Disorder Using Deep Learning Models on Brain Effective Connectivity. IEEE Access 2021, 9, 86899–86913. [Google Scholar] [CrossRef]
- Park, S.M.; Jeong, B.; Oh, D.Y.; Choi, C.-H.; Jung, H.Y.; Lee, J.-Y.; Lee, D.; Choi, J.-S. Identification of Major Psychiatric Disorders From Resting-State Electroencephalography Using a Machine Learning Approach. Front. Psychiatry 2021, 12, 1398. [Google Scholar] [CrossRef]
- Liu, X.; Li, G.; Wang, S.; Wan, F.; Sun, Y.; Wang, H.; Bezerianos, A.; Li, C.; Sun, Y. Toward practical driving fatigue detection using three frontal EEG channels: A proof-of-concept study. Physiol. Meas. 2021, 42, 044003. [Google Scholar] [CrossRef]
- Chen, W.; Wang, Z.; Xie, H.; Yu, W. Characterization of surface EMG signal based on fuzzy entropy. IEEE Trans. Neural Syst. Rehabil. Eng. 2007, 15, 266–272. [Google Scholar] [CrossRef]
- Aydore, S.; Pantazis, D.; Leahy, R.M. A note on the phase locking value and its properties. Neuroimage 2013, 74, 231–244. [Google Scholar] [CrossRef] [Green Version]
- Stam, C.J.; Nolte, G.; Daffertshofer, A. Phase lag index: Assessment of functional connectivity from multi channel EEG and MEG with diminished bias from common sources. Hum. Brain Mapp. 2007, 28, 1178–1193. [Google Scholar]
- Massullo, C.; Carbone, G.A.; Farina, B.; Panno, A.; Capriotti, C.; Giacchini, M.; Machado, S.; Budde, H.; Murillo-Rodriguez, E.; Imperatori, C. Dysregulated brain salience within a triple network model in high trait anxiety individuals: A pilot EEG functional connectivity study. Int. J. Psychophysiol. 2020, 157, 61–69. [Google Scholar] [CrossRef]
- Sviderskaia, N.E.; Prudnikov, V.N.; Antonov, A.G. Characteristics of EEG signs of anxiety in human. Zhurnal Vyss. Nervn. Deiatelnosti Im. I P Pavlov. 2001, 51, 158–165. [Google Scholar]
- Buchsbaum, M.S.; Hazlett, E.; Sicotte, N.; Stein, M.; Wu, J.; Zetin, M. Topographic EEG changes with benzodiazepine administration in generalized anxiety disorder. Biol. Psychiatry 1985, 20, 832–842. [Google Scholar] [CrossRef]
- Li, G.; Huang, S.; Xu, W.; Jiao, W.; Jiang, Y.; Gao, Z.; Zhang, J. The impact of mental fatigue on brain activity: A comparative study both in resting state and task state using EEG. BMC Neurosci. 2020, 21, 20. [Google Scholar] [CrossRef] [PubMed]
- Pfurtscheller, G.; Da Silva, F.H.L. Event-related EEG/MEG synchronization and desynchronization: Basic principles. Clin. Neurophysiol. 1999, 110, 1842–1857. [Google Scholar] [CrossRef]
- Miskovic, V.; Ashbaugh, A.R.; Santesso, D.L.; Mccabe, R.E.; Antony, M.M.; Schmidt, L.A. Frontal brain oscillations and social anxiety: A cross-frequency spectral analysis during baseline and speech anticipation. Biol. Psychol. 2010, 83, 125–132. [Google Scholar] [CrossRef]
- Engel, A.K.; Fries, P.; Singer, W. Dynamic predictions: Oscillations and synchrony in top-down processing. Nat. Rev. Neurosci. 2001, 2, 704–716. [Google Scholar] [CrossRef]
- Knyazev, G.G.; Bocharov, A.V.; Levin, E.A.; Savostyanov, A.N.; Slobodskoj-Plusnin, J.Y. Anxiety and oscillatory responses to emotional facial expressions. Brain Res. 2008, 1227, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Gordeev, S.A. Clinical-psychophysiological studies of patients with panic attacks with and without agoraphobic disorders. Neurosci. Behav. Physiol. 2008, 38, 633–637. [Google Scholar] [CrossRef]
- Cao, Z.; Lin, C.-T. Inherent Fuzzy Entropy for the Improvement of EEG Complexity Evaluation. IEEE Trans. Fuzzy Syst. 2018, 26, 1032–1035. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.T.; Zhuang, J.; Yu, W.X.; Wang, Z.Z. Measuring complexity using FuzzyEn, ApEn, and SampEn. Med. Eng. Phys. 2009, 31, 61–68. [Google Scholar] [CrossRef]
- Klimesch, W.; Doppelmayr, M.; Pachinger, T.; Ripper, B. Brain oscillations and human memory: EEG correlates in the upper alpha and theta band. Neurosci. Lett. 1997, 238, 9–12. [Google Scholar] [CrossRef]
- Al-Ezzi, A.; Kamel, N.; Faye, I.; Gunaseli, E. Analysis of Default Mode Network in Social Anxiety Disorder: EEG Resting-State Effective Connectivity Study. Sensors 2021, 21, 4098. [Google Scholar] [CrossRef]
- Hanaoka, A.; Kikuchi, M.; Komuro, R.; Oka, H.; Kidani, T.; Ichikawa, S. EEG coherence analysis in never-medicated patients with panic disorder. Clin. EEG Neurosci. 2005, 36, 42–48. [Google Scholar] [CrossRef] [Green Version]
- Imperatori, C.; Farina, B.; Adenzato, M.; Valenti, E.M.; Murgia, C.; Della Marca, G.; Brunetti, R.; Fontana, E.; Ardito, R.B. Default mode network alterations in individuals with high-trait-anxiety: An EEG functional connectivity study. J. Affect. Disord. 2019, 246, 611–618. [Google Scholar] [CrossRef]
- Lackner, C.L.; Marshall, W.J.; Santesso, D.L.; Dywan, J.; Wade, T.; Segalowitz, S.J. Adolescent anxiety and aggression can be differentially predicted by electrocortical phase reset variables. Brain Cogn. 2014, 89, 90–98. [Google Scholar] [CrossRef]
- Xing, M.; Tadayonnejad, R.; Macnamara, A.; Ajilore, O.; Digangi, J.; Phan, K.L.; Leow, A.; Klumpp, H. Resting-state theta band connectivity and graph analysis in generalized social anxiety disorder. Neuroimage Clin. 2017, 13, 24–32. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Atif, O.; Tian, J.; Lee, J.; Park, D.; Chung, Y. Multi-level Hierarchical Complex Behavior Monitoring System for Dog Psychological Separation Anxiety Symptoms. Sensors 2022, 22, 1556. [Google Scholar] [CrossRef]
- Gavrilescu, M.; Vizireanu, N. Predicting Depression, Anxiety, and Stress Levels from Videos Using the Facial Action Coding System. Sensors 2019, 19, 3693. [Google Scholar] [CrossRef] [Green Version]
- Petrescu, L.; Petrescu, C.; Mitrut, O.; Moise, G.; Moldoveanu, A.; Moldoveanu, F.; Leordeanu, M. Integrating Biosignals Measurement in Virtual Reality Environments for Anxiety Detection. Sensors 2020, 20, 7088. [Google Scholar] [CrossRef] [PubMed]
- Demiris, G.; Magan, K.L.C.; Oliver, D.P.; Washington, K.T.; Chadwick, C.; Voigt, J.D.; Brotherton, S.; Naylor, M.D. Spoken words as biomarkers: Using machine learning to gain insight into communication as a predictor of anxiety. J. Am. Med. Inform. Assoc. 2020, 27, 929–933. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | GAD (n = 45) | HC (n = 36) | t | F | p-Value |
|---|---|---|---|---|---|
| Age (year) | 22–55 (41.8 ± 9.4) | 21–57 (36.9 ± 11.3) | 1.99 | 3.96 | 0.06 |
| Gender: male/female | 13/32 | 11/25 | - | - | - |
| Duration of illness (month) | 1–48 (7.9 ± 7.6) | - | - | - | - |
| HAMA | 27.1 ± 9.0 | 2.3 ± 0.9 | 16.63 | 222.18 | 1.14 × 10−24 |
| HAMD-17 | 10.6 ± 6.0 | 2.4 ± 0.8 | 17.70 | 253.59 | 2.24 ×10−26 |
| Models | Index (%) | All | Theta | Alpha1 | Alpha2 | Beta |
|---|---|---|---|---|---|---|
| SVM | Accuracy | 97.83 ± 0.40 | 70.92 ± 0.80 | 73.39 ± 0.56 | 63.13 ± 0.40 | 96.49 ± 0.33 |
| Sensitivity | 97.55 ± 0.31 | 74.00 ± 0.80 | 75.91 ± 0.65 | 66.64 ± 1.03 | 96.83 ± 0.34 | |
| Specificity | 97.78 ± 0.36 | 66.12 ± 1.16 | 69.76 ± 0.15 | 56.89 ± 2.23 | 95.82 ± 0.44 | |
| F1 | 97.95 ± 0.17 | 74.33 ± 0.55 | 76.91 ± 0.58 | 67.71 ± 0.49 | 96.83 ± 0.23 | |
| RF | Accuracy | 90.16 ± 0.92 | 69.59 ± 0.69 | 73.67 ± 0.91 | 69.46 ± 0.44 | 88.76 ± 0.52 |
| Sensitivity | 88.82 ± 1.08 | 70.16 ± 0.97 | 72.01 ± 0.70 | 68.49 ± 0.80 | 88.30 ± 0.98 | |
| Specificity | 91.69 ± 0.51 | 68.32 ± 0.46 | 77.90 ± 1.55 | 70.70 ± 0.98 | 89.71 ± 1.17 | |
| F1 | 91.44 ± 0.65 | 75.13 ± 0.72 | 79.31 ± 0.77 | 75.78 ± 0.66 | 90.45 ± 0.18 | |
| BP_Bagging | Accuracy | 95.51 ± 0.20 | 68.42 ± 0.80 | 71.37 ± 0.39 | 68.99 ± 0.33 | 93.41 ± 0.85 |
| Sensitivity | 88.74 ± 0.88 | 73.45 ± 0.66 | 71.94 ± 0.53 | 66.56 ± 0.48 | 88.54 ± 0.52 | |
| Specificity | 91.98 ± 0.97 | 66.23 ± 1.34 | 78.26 ± 1.56 | 68.78 ± 1.47 | 89.74 ± 0.84 | |
| F1 | 91.50 ± 0.38 | 72.22 ± 3.75 | 79.30 ± 0.42 | 73.87 ± 0.14 | 90.58 ± 0.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Z.; Li, G.; Fang, J.; Zhong, H.; Wang, J.; Sun, Y.; Shen, X. Aberrated Multidimensional EEG Characteristics in Patients with Generalized Anxiety Disorder: A Machine-Learning Based Analysis Framework. Sensors 2022, 22, 5420. https://doi.org/10.3390/s22145420
Shen Z, Li G, Fang J, Zhong H, Wang J, Sun Y, Shen X. Aberrated Multidimensional EEG Characteristics in Patients with Generalized Anxiety Disorder: A Machine-Learning Based Analysis Framework. Sensors. 2022; 22(14):5420. https://doi.org/10.3390/s22145420
Chicago/Turabian StyleShen, Zhongxia, Gang Li, Jiaqi Fang, Hongyang Zhong, Jie Wang, Yu Sun, and Xinhua Shen. 2022. "Aberrated Multidimensional EEG Characteristics in Patients with Generalized Anxiety Disorder: A Machine-Learning Based Analysis Framework" Sensors 22, no. 14: 5420. https://doi.org/10.3390/s22145420
APA StyleShen, Z., Li, G., Fang, J., Zhong, H., Wang, J., Sun, Y., & Shen, X. (2022). Aberrated Multidimensional EEG Characteristics in Patients with Generalized Anxiety Disorder: A Machine-Learning Based Analysis Framework. Sensors, 22(14), 5420. https://doi.org/10.3390/s22145420






