Relation between Cortical Activation and Effort during Robot-Mediated Walking in Healthy People: A Functional Near-Infrared Spectroscopy Neuroimaging Study (fNIRS)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Protocol
- -
- full assistance (A100%): the participant is passive;
- -
- 50 % assistance (A50%): movement is partially assisted;
- -
- no assistance (A0%): the participant is fully active;
- -
- 25% resistance (A−25%): movement is resisted.
2.3. The Robotic Device
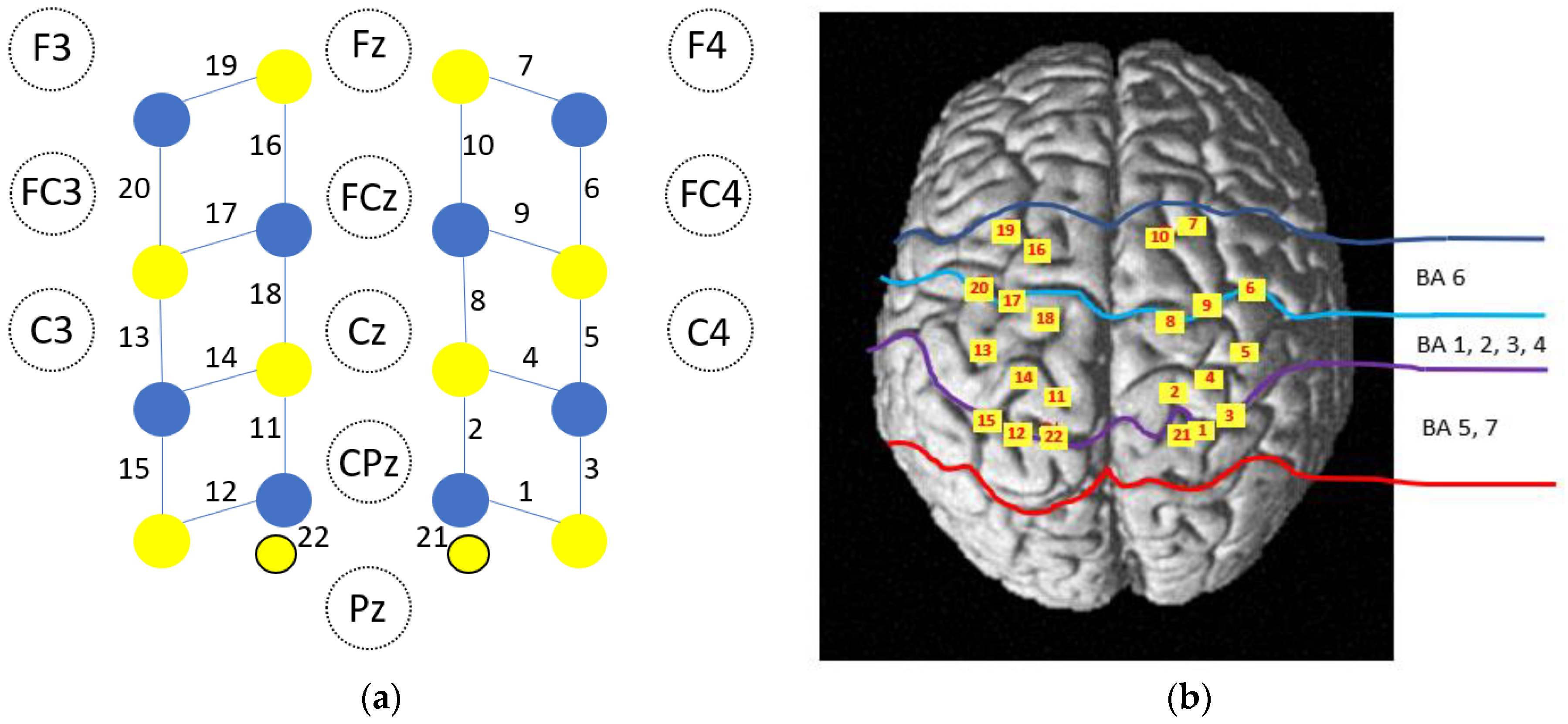
2.4. fNIRS Data Acquisition
2.5. Preprocessing of fNIRS Data
- Trials in which the participant stopped at least twice or for more than 5 s were discarded.
- Identification and exclusion of bad channels: channels were considered as bad and excluded from the analysis if the coefficient of variation ([standard deviation/mean] × 100) of the raw data was >33%. The function hmrPruneChannels was used (SNRthresh = 3).
- Optical density conversion: raw data were converted into optical density with the hmrIntensity2OD function.
- Identification of motion artifacts: time sections were considered as containing motion artifacts if the signal for any given active channel changed by more than 30 times the standard deviation or by more than 5 during a 0.5 s period. The hmrMotionArtifactByChannel function was used (tMotion = 0.5, tMask = 1, STDEVthresh = 30, AMPthresh = 5).
- Motion artifact correction: sections marked as motion artifacts were corrected with principal component analysis as movement is the principal source of variance. We used the hmrMotionCorrectPCA function (nSv = 0.8).
- Filtering periodic noise: respiration, cardiac activity and high frequency noise were attenuated with hmrBandpassFilt (hpf = 0, lpf = 0.1).
- Concentration conversion: corrected optical density data were converted into relative concentration changes with the modified Beer–Lambert law. The age-dependent differential path length factor (DPF) value was calculated for each participant according to the formula proposed by Scholkman and Wolf [35]. Values of DPF for each wavelength were averaged for the group according to the mean age. They were respectively 6.25 and 5.43 for the 760 and 840 nm wavelengths.
- Short channel contribution and hemodynamic response function (HRF) estimation: the contribution of short separation channels and estimation of the HRF were removed with a Kalman filter dynamic estimator with the hmrDeconvTB_SS3rd function (t range = [−10, 35], gstd = 1, gms = 1, rhoSD_ssThresh = 1).
2.6. Data Analysis
3. Results
3.1. Number of Valid Trials and Duration of Trials
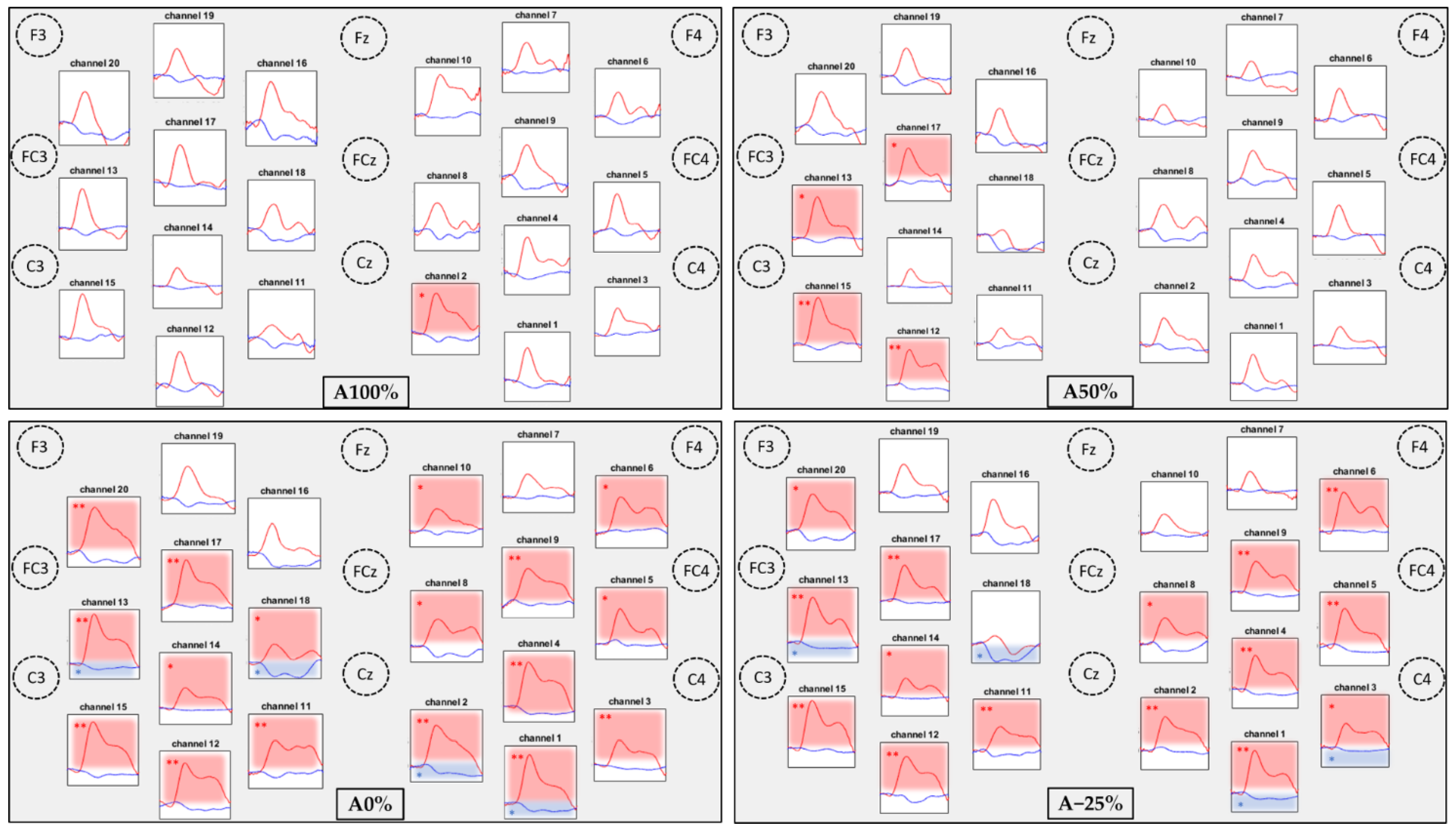
3.2. Comparisons of the Hemodynamic Response between the Conditions
3.3. Comparisons of Task Related Hemodynamic Responses between the 4 Conditions
4. Discussion
4.1. Neuronal Activation Is Increased with Higher Levels of Effort
4.2. Feasibility of fNIRS to Explore Brain Activity during Gait in a Rehabilitation Setting
4.3. Implications for Robot Mediated Gait Training in the Rehabilitation Setting
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| fNIRS | Functional near-infrared spectroscopy |
| HbO2 | Oxyhemoglobin |
| HbR | Deoxyhemoglobin |
| RAGT | Robot-assisted gait training |
| PET | Positron emission tomography |
| fMRI | Functional magnetic resonance imaging |
| EEG | Electroencephalography |
| SMA | Supplementary motor area |
| M1 | Primary motor cortex |
| PMC | Premotor cortex |
| PFC | Prefrontal cortex |
| IMU | Inertial measurement units |
| COM | Center of mass |
| DPF | Differential path length factor |
| HRF | hemodynamic response function |
| FDR | False discovery rate |
| NIRS-SPM | Statistical parametric mapping for near-infrared spectroscopy |
References
- Park, J.; Kim, T.-H. The Effects of Balance and Gait Function on Quality of Life of Stroke Patients. NeuroRehabilitation 2019, 44, 37–41. [Google Scholar] [CrossRef]
- Mehrholz, J.; Pohl, M.; Platz, T.; Kugler, J.; Elsner, B. Electromechanical and Robot-Assisted Arm Training for Improving Activities of Daily Living, Arm Function, and Arm Muscle Strength after Stroke. Cochrane Database Syst. Rev. 2018, 9, CD006876. [Google Scholar] [CrossRef]
- Rodgers, H.; Bosomworth, H.; Krebs, H.I.; van Wijck, F.; Howel, D.; Wilson, N.; Finch, T.; Alvarado, N.; Ternent, L.; Fernandez-Garcia, C.; et al. Robot-Assisted Training Compared with an Enhanced Upper Limb Therapy Programme and with Usual Care for Upper Limb Functional Limitation after Stroke: The RATULS Three-Group RCT. Health Technol. Assess. 2020, 24, 1–232. [Google Scholar] [CrossRef]
- Bütefisch, C.; Hummelsheim, H.; Denzler, P.; Mauritz, K.H. Repetitive Training of Isolated Movements Improves the Outcome of Motor Rehabilitation of the Centrally Paretic Hand. J. Neurol. Sci. 1995, 130, 59–68. [Google Scholar] [CrossRef]
- Kwakkel, G.; Wagenaar, R.C.; Twisk, J.W.; Lankhorst, G.J.; Koetsier, J.C. Intensity of Leg and Arm Training after Primary Middle-Cerebral-Artery Stroke: A Randomised Trial. Lancet 1999, 354, 191–196. [Google Scholar] [CrossRef]
- Moucheboeuf, G.; Griffier, R.; Gasq, D.; Glize, B.; Bouyer, L.; Dehail, P.; Cassoudesalle, H. Effects of Robotic Gait Training after Stroke: A Meta-Analysis. Ann. Phys. Rehabil. Med. 2020, 63, 518–534. [Google Scholar] [CrossRef]
- Mehrholz, J.; Thomas, S.; Kugler, J.; Pohl, M.; Elsner, B. Electromechanical-Assisted Training for Walking after Stroke. Cochrane Database Syst. Rev. 2020, 10, CD006185. [Google Scholar] [CrossRef]
- Chollet, F.; DiPiero, V.; Wise, R.J.; Brooks, D.J.; Dolan, R.J.; Frackowiak, R.S. The Functional Anatomy of Motor Recovery after Stroke in Humans: A Study with Positron Emission Tomography. Ann. Neurol. 1991, 29, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Cramer, S.C.; Nelles, G.; Benson, R.R.; Kaplan, J.D.; Parker, R.A.; Kwong, K.K.; Kennedy, D.N.; Finklestein, S.P.; Rosen, B.R. A Functional MRI Study of Subjects Recovered from Hemiparetic Stroke. Stroke 1997, 28, 2518–2527. [Google Scholar] [CrossRef]
- García-Cossio, E.; Severens, M.; Nienhuis, B.; Duysens, J.; Desain, P.; Keijsers, N.; Farquhar, J. Decoding Sensorimotor Rhythms during Robotic-Assisted Treadmill Walking for Brain Computer Interface (BCI) Applications. PLoS ONE 2015, 10, e0137910. [Google Scholar] [CrossRef] [Green Version]
- Bishnoi, A.; Holtzer, R.; Hernandez, M.E. Brain Activation Changes While Walking in Adults with and without Neurological Disease: Systematic Review and Meta-Analysis of Functional Near-Infrared Spectroscopy Studies. Brain Sci. 2021, 11, 291. [Google Scholar] [CrossRef]
- Pelicioni, P.H.S.; Tijsma, M.; Lord, S.R.; Menant, J. Prefrontal Cortical Activation Measured by FNIRS during Walking: Effects of Age, Disease and Secondary Task. PeerJ 2019, 7, e6833. [Google Scholar] [CrossRef] [Green Version]
- Miyai, I.; Tanabe, H.C.; Sase, I.; Eda, H.; Oda, I.; Konishi, I.; Tsunazawa, Y.; Suzuki, T.; Yanagida, T.; Kubota, K. Cortical Mapping of Gait in Humans: A near-Infrared Spectroscopic Topography Study. Neuroimage 2001, 14, 1186–1192. [Google Scholar] [CrossRef]
- Kurz, M.J.; Wilson, T.W.; Arpin, D.J. Stride-Time Variability and Sensorimotor Cortical Activation during Walking. Neuroimage 2012, 59, 1602–1607. [Google Scholar] [CrossRef]
- Kim, H.Y.; Yang, S.P.; Park, G.L.; Kim, E.J.; You, J.S.H. Best Facilitated Cortical Activation during Different Stepping, Treadmill, and Robot-Assisted Walking Training Paradigms and Speeds: A Functional near-Infrared Spectroscopy Neuroimaging Study. NeuroRehabilitation 2016, 38, 171–178. [Google Scholar] [CrossRef]
- Hepp-Reymond, M.; Kirkpatrick-Tanner, M.; Gabernet, L.; Qi, H.X.; Weber, B. Context-Dependent Force Coding in Motor and Premotor Cortical Areas. Exp. Brain Res. 1999, 128, 123–133. [Google Scholar] [CrossRef]
- Dettmers, C.; Ridding, M.C.; Stephan, K.M.; Lemon, R.N.; Rothwell, J.C.; Frackowiak, R.S. Comparison of Regional Cerebral Blood Flow with Transcranial Magnetic Stimulation at Different Forces. J. Appl. Physiol. 1996, 81, 596–603. [Google Scholar] [CrossRef]
- Yoon, T.; Vanden Noven, M.L.; Nielson, K.A.; Hunter, S.K. Brain Areas Associated with Force Steadiness and Intensity during Isometric Ankle Dorsiflexion in Men and Women. Exp. Brain Res. 2014, 232, 3133–3145. [Google Scholar] [CrossRef] [Green Version]
- Derosière, G.; Alexandre, F.; Bourdillon, N.; Mandrick, K.; Ward, T.E.; Perrey, S. Similar Scaling of Contralateral and Ipsilateral Cortical Responses during Graded Unimanual Force Generation. Neuroimage 2014, 85 Pt 1, 471–477. [Google Scholar] [CrossRef]
- Shibuya, K.; Kuboyama, N.; Yamada, S. Complementary Activation of the Ipsilateral Primary Motor Cortex during a Sustained Handgrip Task. Eur. J. Appl. Physiol. 2016, 116, 171–178. [Google Scholar] [CrossRef]
- Tyagi, O.; Zhu, Y.; Johnson, C.; Mehta, R.K.; Sasangohar, F.; Erraguntla, M.; Qaraqe, K. Neural Signatures of Handgrip Fatigue in Type 1 Diabetic Men and Women. Front. Hum. Neurosci. 2020, 14, 564969. [Google Scholar] [CrossRef]
- Kim, H. Cerebral Hemodynamics Predicts the Cortical Area and Coding Scheme in the Human Brain for Force Generation by Wrist Muscles. Behav. Brain Res. 2020, 396, 112865. [Google Scholar] [CrossRef]
- Shi, P.; Li, A.; Yu, H. Response of the Cerebral Cortex to Resistance and Non-Resistance Exercise under Different Trajectories: A Functional Near-Infrared Spectroscopy Study. Front. Neurosci. 2021, 15, 685920. [Google Scholar] [CrossRef]
- Wagner, J.; Solis-Escalante, T.; Grieshofer, P.; Neuper, C.; Müller-Putz, G.; Scherer, R. Level of Participation in Robotic-Assisted Treadmill Walking Modulates Midline Sensorimotor EEG Rhythms in Able-Bodied Subjects. Neuroimage 2012, 63, 1203–1211. [Google Scholar] [CrossRef]
- Thickbroom, G.W.; Phillips, B.A.; Morris, I.; Byrnes, M.L.; Mastaglia, F.L. Isometric Force-Related Activity in Sensorimotor Cortex Measured with Functional MRI. Exp. Brain Res. 1998, 121, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Dettmers, C.; Fink, G.R.; Lemon, R.N.; Stephan, K.M.; Passingham, R.E.; Silbersweig, D.; Holmes, A.; Ridding, M.C.; Brooks, D.J.; Frackowiak, R.S. Relation between Cerebral Activity and Force in the Motor Areas of the Human Brain. J. Neurophysiol. 1995, 74, 802–815. [Google Scholar] [CrossRef]
- Keisker, B.; Hepp-Reymond, M.-C.; Blickenstorfer, A.; Kollias, S.S. Differential Representation of Dynamic and Static Power Grip Force in the Sensorimotor Network. Eur. J. Neurosci. 2010, 31, 1483–1491. [Google Scholar] [CrossRef]
- Alexandre, F.; Heraud, N.; Oliver, N.; Varray, A. Cortical Implication in Lower Voluntary Muscle Force Production in Non-Hypoxemic COPD Patients. PLoS ONE 2014, 9, e100961. [Google Scholar] [CrossRef] [Green Version]
- Harada, T.; Miyai, I.; Suzuki, M.; Kubota, K. Gait Capacity Affects Cortical Activation Patterns Related to Speed Control in the Elderly. Exp. Brain Res. 2009, 193, 445–454. [Google Scholar] [CrossRef]
- Oh, S.; Song, M.; Kim, J. Validating Attentive Locomotion Training Using Interactive Treadmill: An FNIRS Study. J. Neuroeng. Rehabil. 2018, 15, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleh, S.; Sandroff, B.M.; Vitiello, T.; Owoeye, O.; Hoxha, A.; Hake, P.; Goverover, Y.; Wylie, G.; Yue, G.; DeLuca, J. The Role of Premotor Areas in Dual Tasking in Healthy Controls and Persons with Multiple Sclerosis: An FNIRS Imaging Study. Front. Behav. Neurosci. 2018, 12, 296. [Google Scholar] [CrossRef] [Green Version]
- Kerdraon, J.; Previnaire, J.G.; Tucker, M.; Coignard, P.; Allegre, W.; Knappen, E.; Ames, A. Evaluation of Safety and Performance of the Self Balancing Walking System Atalante in Patients with Complete Motor Spinal Cord Injury. Spinal Cord Ser. Cases 2021, 7, 71. [Google Scholar] [CrossRef]
- Lancaster, J.L.; Woldorff, M.G.; Parsons, L.M.; Liotti, M.; Freitas, C.S.; Rainey, L.; Kochunov, P.V.; Nickerson, D.; Mikiten, S.A.; Fox, P.T. Automated Talairach Atlas Labels for Functional Brain Mapping. Hum. Brain Mapp. 2000, 10, 120–131. [Google Scholar] [CrossRef]
- Ye, J.C.; Tak, S.; Jang, K.E.; Jung, J.; Jang, J. NIRS-SPM: Statistical Parametric Mapping for near-Infrared Spectroscopy. Neuroimage 2009, 44, 428–447. [Google Scholar] [CrossRef] [PubMed]
- Scholkmann, F.; Wolf, M. General Equation for the Differential Pathlength Factor of the Frontal Human Head Depending on Wavelength and Age. J. Biomed. Opt. 2013, 18, 105004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huppert, T.J.; Hoge, R.D.; Diamond, S.G.; Franceschini, M.A.; Boas, D.A. A Temporal Comparison of BOLD, ASL, and NIRS Hemodynamic Responses to Motor Stimuli in Adult Humans. Neuroimage 2006, 29, 368–382. [Google Scholar] [CrossRef] [Green Version]
- Zama, T.; Shimada, S. Simultaneous Measurement of Electroencephalography and Near-Infrared Spectroscopy during Voluntary Motor Preparation. Sci. Rep. 2015, 5, 16438. [Google Scholar] [CrossRef] [Green Version]
- Hoshi, Y. Hemodynamic Signals in FNIRS. Prog. Brain Res. 2016, 225, 153–179. [Google Scholar] [CrossRef]
- Nishiyori, R.; Bisconti, S.; Ulrich, B. Motor Cortex Activity during Functional Motor Skills: An FNIRS Study. Brain Topogr. 2016, 29, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Gentile, E.; Ricci, K.; Delussi, M.; Brighina, F.; de Tommaso, M. Motor Cortex Function in Fibromyalgia: A Study by Functional Near-Infrared Spectroscopy. Pain Res. Treat. 2019, 2019, 2623161. [Google Scholar] [CrossRef] [Green Version]
- Hirth, C.; Obrig, H.; Valdueza, J.; Dirnagl, U.; Villringer, A. Simultaneous Assessment of Cerebral Oxygenation and Hemodynamics during a Motor Task. A Combined near Infrared and Transcranial Doppler Sonography Study. Adv. Exp. Med. Biol. 1997, 411, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Ito, M.; Suto, T.; Kameyama, M.; Suda, M.; Yamagishi, Y.; Ohshima, A.; Uehara, T.; Fukuda, M.; Mikuni, M. Time Courses of Brain Activation and Their Implications for Function: A Multichannel near-Infrared Spectroscopy Study during Finger Tapping. Neurosci. Res. 2007, 58, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Dravida, S.; Noah, J.A.; Zhang, X.; Hirsch, J. Comparison of Oxyhemoglobin and Deoxyhemoglobin Signal Reliability with and without Global Mean Removal for Digit Manipulation Motor Tasks. Neurophotonics 2018, 5, 011006. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-L.; Wagner, J.; Heugel, N.; Sugar, J.; Lee, Y.-W.; Conant, L.; Malloy, M.; Heffernan, J.; Quirk, B.; Zinos, A.; et al. Functional Near-Infrared Spectroscopy and Its Clinical Application in the Field of Neuroscience: Advances and Future Directions. Front. Neurosci. 2020, 14, 724. [Google Scholar] [CrossRef]
- Peters, S.; Lim, S.B.; Louie, D.R.; Yang, C.-L.; Eng, J.J. Passive, yet Not Inactive: Robotic Exoskeleton Walking Increases Cortical Activation Dependent on Task. J. Neuroeng. Rehabil. 2020, 17, 107. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Miyai, I.; Ono, T.; Oda, I.; Konishi, I.; Kochiyama, T.; Kubota, K. Prefrontal and Premotor Cortices Are Involved in Adapting Walking and Running Speed on the Treadmill: An Optical Imaging Study. Neuroimage 2004, 23, 1020–1026. [Google Scholar] [CrossRef] [PubMed]
- de Belli, V.; Orcioli-Silva, D.; Beretta, V.S.; Vitório, R.; Zampier, V.C.; Nóbrega-Sousa, P.; da Conceição, N.R.; Gobbi, L.T.B. Prefrontal Cortical Activity During Preferred and Fast Walking in Young and Older Adults: An FNIRS Study. Neuroscience 2021, 473, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, K.A.; Fox, E.J.; Daly, J.J.; Rose, D.K.; Christou, E.A.; McGuirk, T.E.; Otzel, D.M.; Butera, K.A.; Chatterjee, S.A.; Clark, D.J. Prefrontal Over-Activation during Walking in People with Mobility Deficits: Interpretation and Functional Implications. Hum. Mov. Sci. 2018, 59, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Knaepen, K.; Mierau, A.; Swinnen, E.; Fernandez Tellez, H.; Michielsen, M.; Kerckhofs, E.; Lefeber, D.; Meeusen, R. Human-Robot Interaction: Does Robotic Guidance Force Affect Gait-Related Brain Dynamics during Robot-Assisted Treadmill Walking? PLoS ONE 2015, 10, e0140626. [Google Scholar] [CrossRef] [Green Version]
- Evarts, E.V.; Fromm, C.; Kröller, J.; Jennings, V.A. Motor Cortex Control of Finely Graded Forces. J. Neurophysiol. 1983, 49, 1199–1215. [Google Scholar] [CrossRef] [PubMed]
- Georgopoulos, A.P.; Ashe, J.; Smyrnis, N.; Taira, M. The Motor Cortex and the Coding of Force. Science 1992, 256, 1692–1695. [Google Scholar] [CrossRef] [Green Version]
- Maier, M.J.; Rosenbaum, D.; Haeussinger, F.B.; Brüne, M.; Enzi, B.; Plewnia, C.; Fallgatter, A.J.; Ehlis, A.-C. Forgiveness and Cognitive Control-Provoking Revenge via Theta-Burst-Stimulation of the DLPFC. Neuroimage 2018, 183, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Ashe, J. Force and the Motor Cortex. Behav. Brain Res. 1997, 86, 255–269. [Google Scholar] [CrossRef]
- Keisker, B.; Hepp-Reymond, M.-C.; Blickenstorfer, A.; Meyer, M.; Kollias, S.S. Differential Force Scaling of Fine-Graded Power Grip Force in the Sensorimotor Network. Hum. Brain Mapp. 2009, 30, 2453–2465. [Google Scholar] [CrossRef] [PubMed]
- Villiger, M.; Estévez, N.; Hepp-Reymond, M.-C.; Kiper, D.; Kollias, S.S.; Eng, K.; Hotz-Boendermaker, S. Enhanced Activation of Motor Execution Networks Using Action Observation Combined with Imagination of Lower Limb Movements. PLoS ONE 2013, 8, e72403. [Google Scholar] [CrossRef]
- Pinti, P.; Tachtsidis, I.; Hamilton, A.; Hirsch, J.; Aichelburg, C.; Gilbert, S.; Burgess, P.W. The Present and Future Use of Functional Near-Infrared Spectroscopy (FNIRS) for Cognitive Neuroscience. Ann. N. Y. Acad. Sci. 2020, 1464, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Takakusaki, K. Functional Neuroanatomy for Posture and Gait Control. J. Mov. Disord. 2017, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Vitorio, R.; Stuart, S.; Mancini, M. Executive Control of Walking in People with Parkinson’s Disease With Freezing of Gait. Neurorehabil. Neural Repair 2020, 34, 1138–1149. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.L.; Ramos-Murguialday, A.; Birbaumer, N.; Hoffmann, U.; Luft, A. Neurophysiology of Robot-Mediated Training and Therapy: A Perspective for Future Use in Clinical Populations. Front. Neurol. 2013, 4, 184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calabrò, R.S.; Cacciola, A.; Bertè, F.; Manuli, A.; Leo, A.; Bramanti, A.; Naro, A.; Milardi, D.; Bramanti, P. Robotic Gait Rehabilitation and Substitution Devices in Neurological Disorders: Where Are We Now? Neurol. Sci. 2016, 37, 503–514. [Google Scholar] [CrossRef]
- Uçar, D.E.; Paker, N.; Buğdaycı, D. Lokomat: A Therapeutic Chance for Patients with Chronic Hemiplegia. NeuroRehabilitation 2014, 34, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Husemann, B.; Müller, F.; Krewer, C.; Heller, S.; Koenig, E. Effects of Locomotion Training with Assistance of a Robot-Driven Gait Orthosis in Hemiparetic Patients after Stroke: A Randomized Controlled Pilot Study. Stroke 2007, 38, 349–354. [Google Scholar] [CrossRef] [Green Version]
- Miyai, I.; Suzuki, M.; Hatakenaka, M.; Kubota, K. Effect of Body Weight Support on Cortical Activation during Gait in Patients with Stroke. Exp. Brain Res. 2006, 169, 85–91. [Google Scholar] [CrossRef]
- Dettmers, C.; Lemon, R.N.; Stephan, K.M.; Fink, G.R.; Frackowiak, R.S. Cerebral Activation during the Exertion of Sustained Static Force in Man. Neuroreport 1996, 7, 2103–2110. [Google Scholar] [CrossRef]
- Ludman, C.N.; Cooper, T.G.; Ploutz-Synder, L.L.; Potchen, E.J.; Meyer, R.A. Force of Voluntary Exercise Does Not Affect Sensorimotor Cortex Activation as Detected by Functional MRI at 1.5 T. NMR Biomed. 1996, 9, 228–232. [Google Scholar] [CrossRef]
- Głowiński, S.; Ptak, M. A kinematic model of a humanoid lower limb exoskeleton with pneumatic actuators. Acta Bioeng. Biomech. 2022, 24, 145–157. [Google Scholar] [CrossRef]
- Zhao, M.; Bonassi, G.; Samogin, J.; Taberna, G.A.; Pelosin, E.; Nieuwboer, A.; Avanzino, L.; Mantini, D. Frequency-dependent modulation of neural oscillations across the gait cycle. Hum. Brain Mapp. 2022, 43, 3404–3415. [Google Scholar] [CrossRef] [PubMed]



| Brodmann Areas | p-Values—HbO2 | p-Values—HbR | |||||||
|---|---|---|---|---|---|---|---|---|---|
| A100% | A50% | A0% | A−25% | A100% | A50% | A0% | A−25% | ||
| CH1 | 5—Somatosensory Association Cortex | 0.0855 | 0.0367 | 0.0005 ** | 0.0005 ** | 0.0457 | 0.0123 | 0.0027 * | 0.0031 * |
| 7—Somatosensory Association Cortex | |||||||||
| CH2 | 3—Primary Somatosensory Cortex | 0.0010 * | 0.0533 | <0.0001 ** | 0.0001 ** | 0.3184 | 0.0828 | 0.0013 * | 0.4294 |
| 4—Primary Motor Cortex | |||||||||
| CH3 | 5—Somatosensory Association Cortex | 0.0224 | 0.0213 | 0.0049 ** | 0.0060 * | 0.7518 | 0.0239 | 0.0295 | 0.0069 * |
| CH4 | 3—Primary Somatosensory Cortex | 0.0294 | 0.1452 | 0.0002 ** | 0.0006 ** | 0.2347 | 0.0195 | 0.0161 | 0.0122 |
| 4—Primary Motor Cortex | |||||||||
| CH5 | 4—Primary Motor Cortex | 0.1508 | 0.1908 | 0.0240 * | 0.0010 ** | 0.1172 | 0.4139 | 0.3658 | 0.0425 |
| CH6 | 6—Pre-Motor and Supplementary Motor Cortex | 0.1633 | 0.0879 | 0.0090 * | 0.0008 ** | 0.1425 | 0.7326 | 0.7273 | 0.5253 |
| CH7 | 6—Pre-Motor and Supplementary Motor Cortex | 0.0979 | 0.5044 | 0.0450 | 0.2760 | 0.4147 | 0.5180 | 0.4229 | 0.3234 |
| CH8 | 6—Pre-Motor and Supplementary Motor Cortex | 0.2277 | 0.0476 | 0.0086 * | 0.0298 * | 0.1868 | 0.0490 | 0.0811 | 0.0929 |
| CH9 | 6—Pre-Motor and Supplementary Motor Cortex | 0.1042 | 0.0158 | 0.0021 ** | 0.0012** | 0.0339 | 0.3884 | 0.3270 | 0.1213 |
| CH10 | 6—Pre-Motor and Supplementary Motor Cortex | 0.0161 | 0.2398 | 0.0276 * | 0.1054 | 0.3731 | 0.3617 | 0.3376 | 0.1917 |
| CH11 | 3—Primary Somatosensory Cortex | 0.2638 | 0.0517 | 0.0005 ** | 0.0028 ** | 0.0264 | 0.3252 | 0.3252 | 0.0316 |
| 5—Somatosensory Association Cortex | |||||||||
| CH12 | 5—Somatosensory Association Cortex | 0.1746 | 0.0002 ** | 0.0002 ** | 0.0003 ** | 0.1537 | 0.0637 | 0.0155 | 0.0507 |
| 7—Somatosensory Association Cortex | |||||||||
| CH13 | 3—Primary Somatosensory Cortex | 0.1763 | 0.0018 * | <0.0001 ** | <0.0001 ** | 0.1620 | 0.1957 | 0.0043 * | 0.0041 * |
| 4—Primary Motor Cortex | |||||||||
| CH14 | 3—Primary Somatosensory and Motor Cortex | 0.2355 | 0.0558 | 0.0155 * | 0.0187 * | 0.1696 | 0.2274 | 0.0633 | 0.0751 |
| 4—Primary Motor Cortex | |||||||||
| CH15 | 5—Somatosensory Association Cortex | 0.0508 | 0.0007 ** | 0.0003 ** | <0.0001 ** | 0.4901 | 0.1842 | 0.0237 | 0.2276 |
| CH16 | 6—Pre-Motor and Supplementary Motor Cortex | 0.1495 | 0.3151 | 0.1059 | 0.1241 | 0.0603 | 0.1517 | 0.0654 | 0.1247 |
| CH17 | 6—Pre-Motor and Supplementary Motor Cortex | 0.1271 | 0.0042 * | 0.0003 ** | 0.0005 ** | 0.3615 | 0.6941 | 0.4628 | 0.2602 |
| CH18 | 6—Pre-Motor and Supplementary Motor Cortex | 0.3579 | 0.8857 | 0.0226 * | 0.6183 | 0.0181 | 0.0026 | 0.0049 * | 0.0061 * |
| CH19 | 6—Pre-Motor and Supplementary Motor Cortex | 0.4030 | 0.1868 | 0.0625 | 0.0425 | 0.2159 | 0.1225 | 0.0389 | 0.1271 |
| CH20 | 6—Pre-Motor and Supplementary Motor Cortex | 0.5002 | 0.0396 | 0.0004 ** | 0.0057 * | 0.0604 | 0.0386 | 0.0190 | 0.0166 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonnal, J.; Monnet, F.; Le, B.-T.; Pila, O.; Grosmaire, A.-G.; Ozsancak, C.; Duret, C.; Auzou, P. Relation between Cortical Activation and Effort during Robot-Mediated Walking in Healthy People: A Functional Near-Infrared Spectroscopy Neuroimaging Study (fNIRS). Sensors 2022, 22, 5542. https://doi.org/10.3390/s22155542
Bonnal J, Monnet F, Le B-T, Pila O, Grosmaire A-G, Ozsancak C, Duret C, Auzou P. Relation between Cortical Activation and Effort during Robot-Mediated Walking in Healthy People: A Functional Near-Infrared Spectroscopy Neuroimaging Study (fNIRS). Sensors. 2022; 22(15):5542. https://doi.org/10.3390/s22155542
Chicago/Turabian StyleBonnal, Julien, Fanny Monnet, Ba-Thien Le, Ophélie Pila, Anne-Gaëlle Grosmaire, Canan Ozsancak, Christophe Duret, and Pascal Auzou. 2022. "Relation between Cortical Activation and Effort during Robot-Mediated Walking in Healthy People: A Functional Near-Infrared Spectroscopy Neuroimaging Study (fNIRS)" Sensors 22, no. 15: 5542. https://doi.org/10.3390/s22155542
APA StyleBonnal, J., Monnet, F., Le, B.-T., Pila, O., Grosmaire, A.-G., Ozsancak, C., Duret, C., & Auzou, P. (2022). Relation between Cortical Activation and Effort during Robot-Mediated Walking in Healthy People: A Functional Near-Infrared Spectroscopy Neuroimaging Study (fNIRS). Sensors, 22(15), 5542. https://doi.org/10.3390/s22155542







