Computational Modelling for Electrical Impedance Spectroscopy-Based Diagnosis of Oral Potential Malignant Disorders (OPMD)
Abstract
:1. Introduction
1.1. Objectives
1.2. Background: Electrical Impedance Spectroscopy as a Diagnostic Tool
1.3. Computational Modelling of EIS
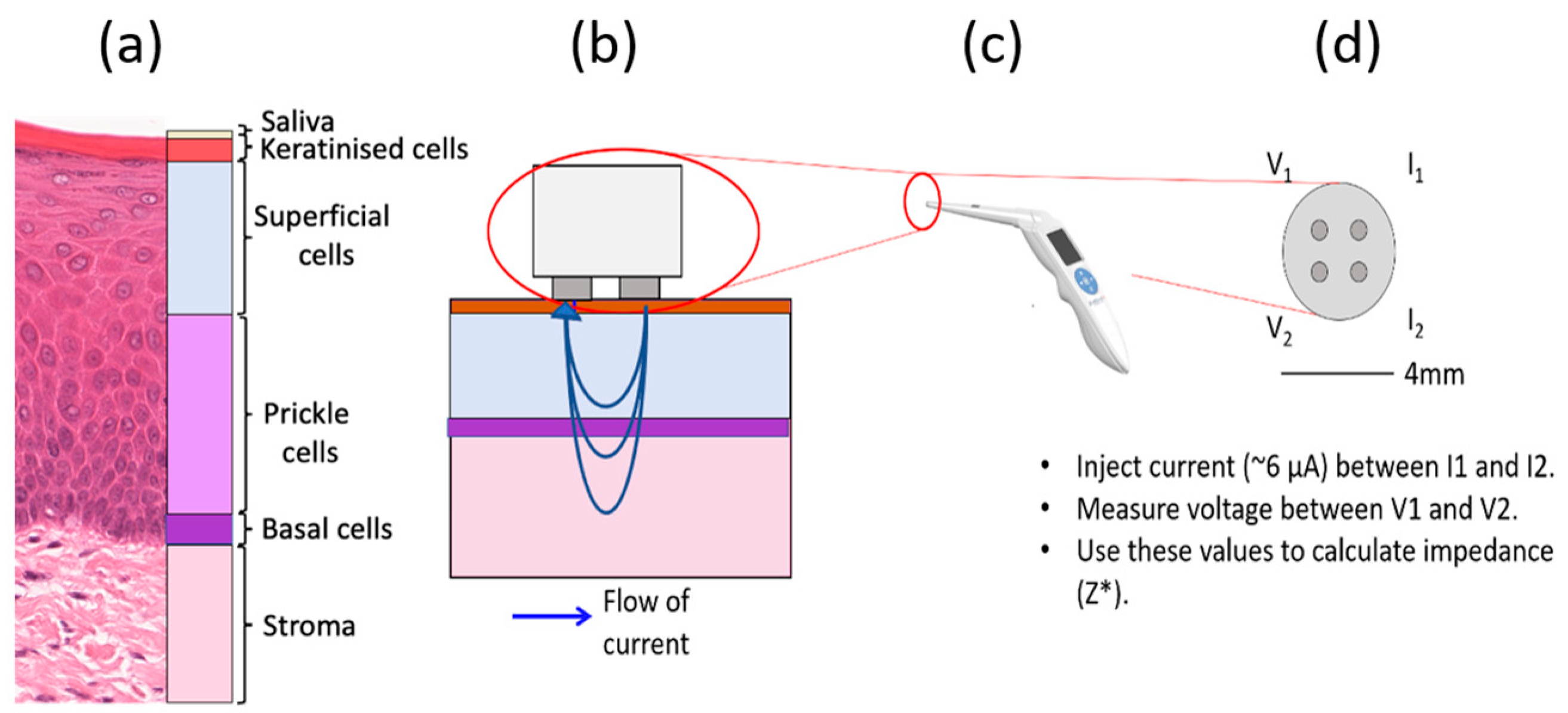
1.4. Oral Tissue Structure and EIS Measurements
2. Materials and Methods
2.1. Histology Analysis
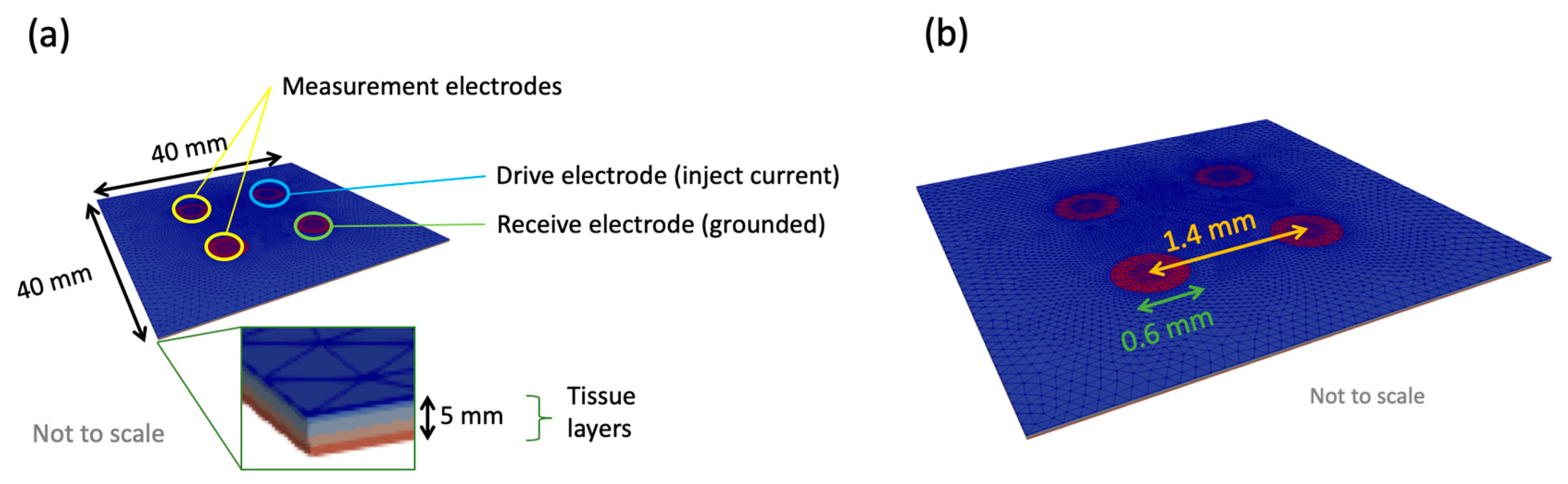
2.2. Finite Element Modelling
2.2.1. General Application of Finite Element Modelling (FEM) to EIS
2.2.2. Multiscale Approach
2.2.3. The Cellular Scale Model
2.2.4. The Tissue Scale Model
3. Results
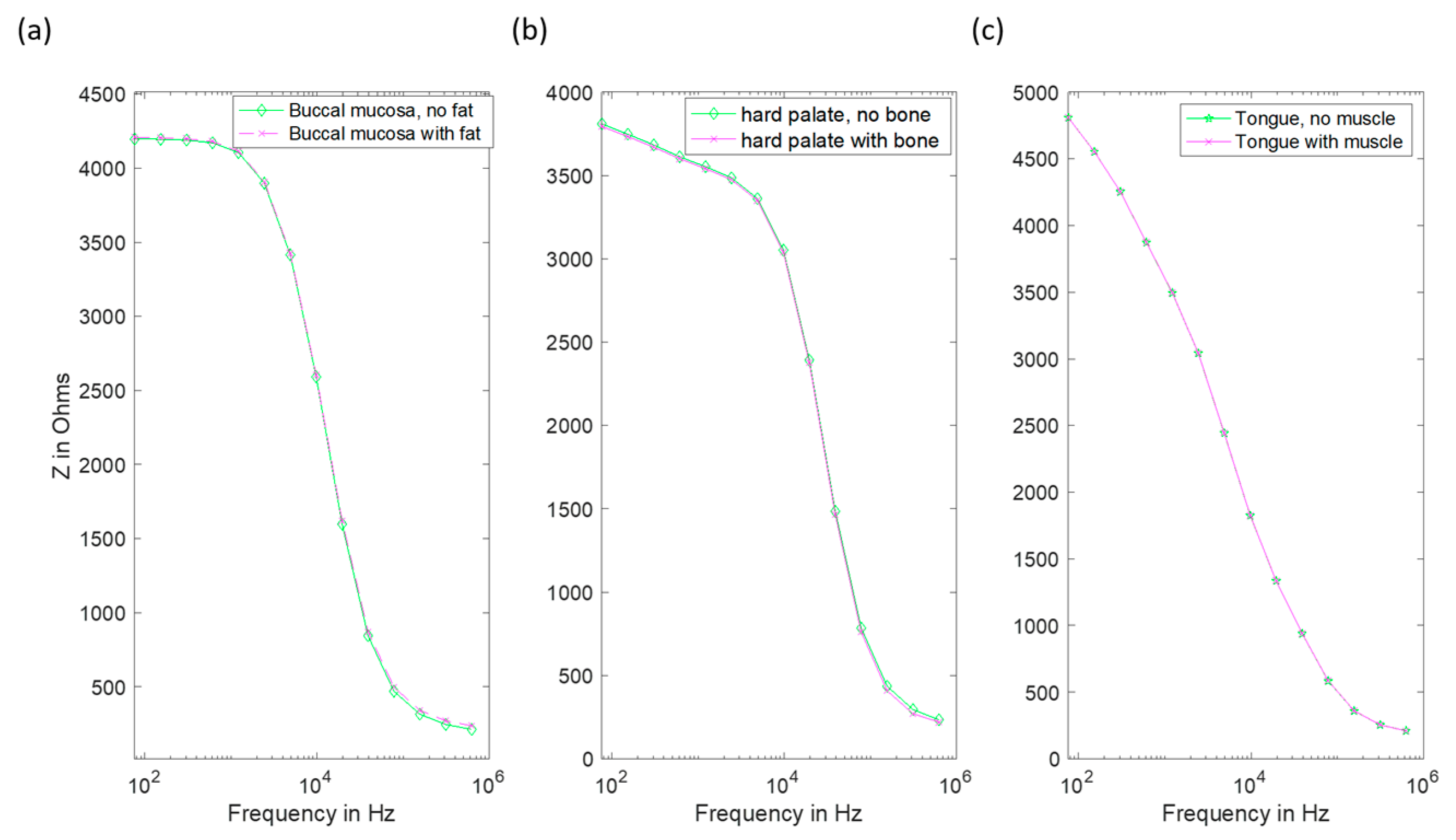
3.1. Effect of Keratinised Layer Properties
3.1.1. Frequency-Independent Keratin Properties
3.1.2. Frequency-Dependent Keratin Properties
3.2. Effect of Dysplasia
3.3. Effect of Underlying Tissue
4. Discussion
5. Conclusions
- Simulations suggest differences between spectra obtained from healthy and non-keratinous OPMD tissue, with good agreement with the low-frequency impedance and general form of real measurements.
- The exception is keratinised OPMD tissue, where higher than expected impedance values are predicted.
- Given the overlap of predicted spectra for keratinised and non-keratinised severe and moderate dysplasia (OPMD), it is unlikely that EIS will provide a reliable method of differentiating between these tissue types.
- Our results suggest that high impedance readings obtained from OPMD tissue can be caused by increased keratinisation. Measurements at frequencies in the range 5–50 kHz may provide the optimum range to differentiate between tissue types.
- The presence of different sub-stromal underlying tissue types according to oral site is unlikely to have a significant impact on the measured impedance spectra.
- Extracellular space thickness in oral tissue and how it varies with dysplasia.
- Keratinised layer electrical properties.
- Electrical properties and morphology of the saliva layer.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Essat, M.; Cooper, K.; Bessey, A.; Clowes, M.; Chilcott, J.B.; Hunter, K.D. Diagnostic accuracy of conventional oral examination for detecting oral cavity cancer and potentially malignant disorders in patients with clinically evident oral lesions: Systematic review and meta-analysis. Head Neck 2022, 44, 998–1013. [Google Scholar] [CrossRef] [PubMed]
- Speight, P.M.; Abram, T.J.; Floriano, P.N.; James, R.; Vick, J.; Thornhill, M.H.; Murdoch, C.; Freeman, C.; Hegarty, A.M.; D’Apice, K.; et al. Interobserver agreement in dysplasia grading: Toward an enhanced gold standard for clinical pathology trials. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015, 120, 474–482. [Google Scholar] [CrossRef] [Green Version]
- Heath, J.P.; Harding, J.H.; Sinclair, D.C.; Dean, J.S. The Analysis of Impedance Spectra for Core-Shell Microstructures: Why a Multiformalism Approach is Essential. Adv. Funct. Mater. 2019, 29, 1904036. [Google Scholar] [CrossRef]
- Schwan, H. Electrical Properties of Tissue and Cell Suspensions. Adv. Biol. Med. Phys. 1957, 5, 147–209. [Google Scholar] [CrossRef]
- Emran, S.; Lappalainen, R.; Kullaa, A.M.; Myllymaa, S. Concentric Ring Probe for Bioimpedance Spectroscopic Measurements: Design and Ex Vivo Feasibility Testing on Pork Oral Tissues. Sensors 2018, 18, 3378. [Google Scholar] [CrossRef] [Green Version]
- Nicander, I. Electrical Impedance Related to Experimental Induced Changes of Human Skin and Oral Mucosa; Karolinska Institue: Stockholm, Sweden, 1998. [Google Scholar]
- González-Correa, C.A.; Brown, B.H.; Smallwood, R.H.; Kalia, N.; Stoddard, C.J.; Stephenson, T.J.; Haggie, S.J.; Slater, D.N.; Bardhan, K.D. Virtual biopsies in Barrett’s esophagus using an impedance probe. Ann. N. Y. Acad. Sci. 1999, 873, 313–321. [Google Scholar] [CrossRef]
- Brown, B.H.; Tidy, J.A.; Boston, K.; Blackett, A.D.; Smallwood, R.H.; Sharp, F. Relation between tissue structure and imposed electrical current flow in cervical neoplasia. Lancet 2000, 355, 892–895. [Google Scholar] [CrossRef]
- Gandhi, S.V.; Walker, D.C.; Brown, B.H.; Anumba, D.O. Comparison of human uterine cervical electrical impedance measurements derived using two tetrapolar probes of different sizes. Biomed. Eng. Online 2006, 5, 62. [Google Scholar] [CrossRef] [Green Version]
- Mohr, P.; Birgersson, U.; Berking, C.; Henderson, C.; Trefzer, U.; Kemeny, L.; Sunderkotter, C.; Dirschka, T.; Motley, R.; Frohm-Nilsson, M.; et al. Electrical impedance spectroscopy as a potential adjunct diagnostic tool for cutaneous melanoma. Ski. Res. Technol. 2013, 19, 75–83. [Google Scholar] [CrossRef]
- Braun, R.P.; Mangana, J.; Goldinger, S.; French, L.; Dummer, R.; Marghoob, A.A. Electrical Impedance Spectroscopy in Skin Cancer Diagnosis. Dermatol. Clin. 2017, 35, 489–493. [Google Scholar] [CrossRef]
- Halter, R.J.; Schned, A.; Heaney, J.; Hartov, A.; Schutz, S.; Paulsen, K.D. Electrical impedance spectroscopy of benign and malignant prostatic tissues. J. Urol. 2008, 179, 1580–1586. [Google Scholar] [CrossRef]
- Wilkinson, B.A.; Smallwood, R.H.; Keshtar, A.; Lee, J.A.; Hamdy, F.C. Electrical impedance spectroscopy and the diagnosis of bladder pathology: A pilot study. J. Urol. 2002, 168, 1563–1567. [Google Scholar] [CrossRef]
- Anumba, D.O.C.; Stern, V.; Healey, J.T.; Dixon, S.; Brown, B.H. Value of cervical electrical impedance spectroscopy to predict spontaneous preterm delivery in asymptomatic women: The ECCLIPPx prospective cohort study. Ultrasound Obstet. Gynecol. 2021, 58, 293–302. [Google Scholar] [CrossRef]
- Murdoch, C.; Brown, B.H.; Hearnden, V.; Speight, P.M.; D’Apice, K.; Hegarty, A.M.; Tidy, J.A.; Healey, T.J.; Highfield, P.E.; Thornhill, M.H. Use of electrical impedance spectroscopy to detect malignant and potentially malignant oral lesions. Int. J. Nanomed. 2014, 9, 4521–4532. [Google Scholar] [CrossRef] [Green Version]
- Walker, D.C.; Brown, B.H.; Smallwood, R.H.; Hose, D.R.; Jones, D.M. Modelled current distribution in cervical squamous tissue. Physiol. Meas. 2002, 23, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Walker, D.C.; Smallwood, R.H.; Keshtar, A.; Wilkinson, B.A.; Hamdy, F.C.; Lee, J.A. Modelling the electrical properties of bladder tissue-quantifying impedance changes due to inflammation and oedema. Physiol. Meas. 2005, 26, 251–268. [Google Scholar] [CrossRef] [Green Version]
- Huclova, S.; Erni, D.; Froehlich, J. Modelling effective dielectric properties of materials containing diverse types of biological cells. J. Phys. D-Appl. Phys. 2010, 43, 365405. [Google Scholar] [CrossRef]
- Huclova, S.; Erni, D.; Frohlich, J. Modelling and validation of dielectric properties of human skin in the MHz region focusing on skin layer morphology and material composition. J. Phys. D-Appl. Phys. 2012, 45, 025301. [Google Scholar] [CrossRef]
- Walker, D. Modelling the Electrical Properties of Cervical Epithelium; University of Sheffield: Sheffield, UK, 2001. [Google Scholar]
- Miller, C.E.; Henriquez, C.S. Finite-Element Analysis of Bioelectric Phenomena. Crit. Rev. Biomed. Eng. 1990, 18, 207–233. [Google Scholar]
- ANSYS. ANSYS MAPDL 18.2; ANSYS: Houston, TX, USA, 2021. [Google Scholar]
- Gabriel, S.; Lau, R.W.; Gabriel, C. The dielectric properties of biological tissues. 2. Measurements in the frequency range 10 Hz to 20 GHz. Phys. Med. Biol. 1996, 41, 2251–2269. [Google Scholar] [CrossRef] [Green Version]
- Marzec, E. Electric properties of non-irradiated and gamma-irradiated keratin. Radiat. Phys. Chem. 2000, 59, 477–481. [Google Scholar] [CrossRef]
- Marzec, E.; Olszewski, J. Influence of water and temperature on the electrical conductivity of the human nail. J. Therm. Anal. Calorim. 2019, 138, 2185–2191. [Google Scholar] [CrossRef] [Green Version]
- Johnsen, G. Skin Electrical Properties and Physical Aspects of Hydration of Keratinized Tissues; University of Oslo: Oslo, Norway, 2010. [Google Scholar]
- Gabriel, C. Compilation of the Dielectric Properties of Body Tissues at RF and Microwave Frequencies; King’s Coll London: London, UK, 1996. [Google Scholar]








| Tissue Type (Normal for Site) | Mean Tissue Layer Thickness/μm | ||||||
|---|---|---|---|---|---|---|---|
| Case | Diagnosis | Oral Site | Basal | Prickle | Superficial | Keratinised | |
| 1 | Normal | Non-keratinised | Buccal mucosa | 12.4 ± 4.5 | 150.4 ± 28.8 | 274.9 ± 46.3 | n/a |
| 2 | Normal | Orthokeratinised | Hard palate | 15.7 ± 2.9 | 100.5 ± 8.5 | 182.0 ± 45.8 | 14.0 ± 1.8 |
| 3 | Severe dysplasia | Parakeratinised | ventral tongue | 22.1 ± 4.6 | 73.9 ± 13.5 | 261.7 ± 58.2 | 86.6 ± 34.0 |
| 4 | Severe dysplasia | Parakeratinised | lateral tongue | 25.4 ± 4.6 | 225.5 ± 36.6 | 289.5 ± 102.9 | 22.8 ± 7.0 |
| 5 | Moderate dysplasia | Parakeratinised | lateral tongue | 15.6 ± 4.1 | 168.0 ± 83.8 | 287.3 ± 102.9 | 42.9 ± 17.9 |
| 6 | Hyper-keratosis No dysplasia | Orthokeratinised | Hard palate | 15.1 ± 3.4 | 222.1 ± 16.2 | 363.3 ± 85.8 | 88.9 ± 21.5 |
| 7 | Severe dysplasia | Parakeratinised | lateral tongue | 28.0 ± 6.8 | 387.7 ± 53.8 | 356.9 ± 68.3 | 17.3 ± 8.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heath, J.P.; Hunter, K.D.; Murdoch, C.; Walker, D.C. Computational Modelling for Electrical Impedance Spectroscopy-Based Diagnosis of Oral Potential Malignant Disorders (OPMD). Sensors 2022, 22, 5913. https://doi.org/10.3390/s22155913
Heath JP, Hunter KD, Murdoch C, Walker DC. Computational Modelling for Electrical Impedance Spectroscopy-Based Diagnosis of Oral Potential Malignant Disorders (OPMD). Sensors. 2022; 22(15):5913. https://doi.org/10.3390/s22155913
Chicago/Turabian StyleHeath, James P., Keith D. Hunter, Craig Murdoch, and Dawn C. Walker. 2022. "Computational Modelling for Electrical Impedance Spectroscopy-Based Diagnosis of Oral Potential Malignant Disorders (OPMD)" Sensors 22, no. 15: 5913. https://doi.org/10.3390/s22155913
APA StyleHeath, J. P., Hunter, K. D., Murdoch, C., & Walker, D. C. (2022). Computational Modelling for Electrical Impedance Spectroscopy-Based Diagnosis of Oral Potential Malignant Disorders (OPMD). Sensors, 22(15), 5913. https://doi.org/10.3390/s22155913






