Exploration of Emotion Dynamics Sensing Using Trapezius EMG and Fingertip Temperature
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Apparatus
2.3. Stimuli
2.4. Procedure
2.5. Physiological Data Recording
2.6. Data Analysis
3. Results
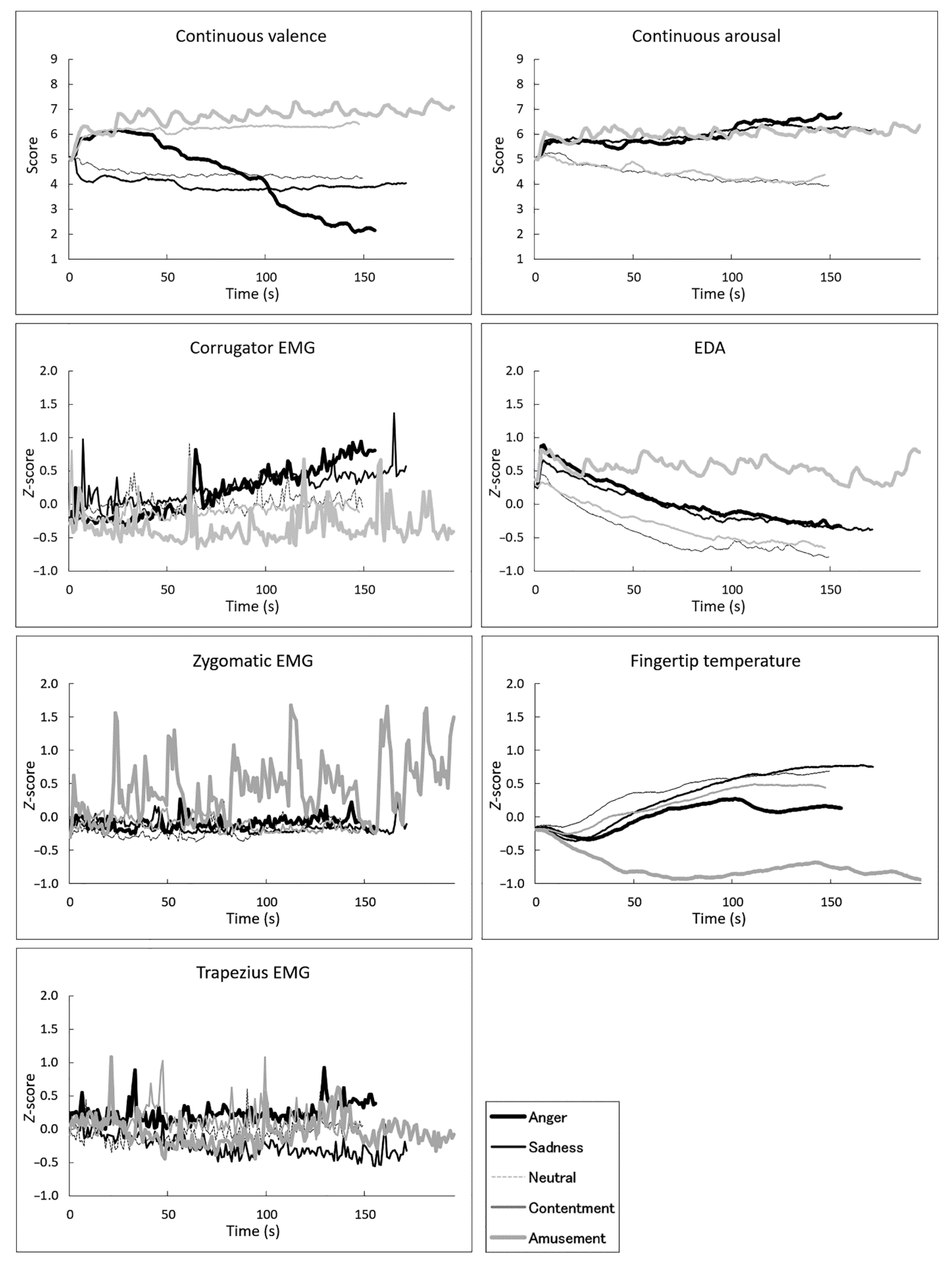
3.1. Overview
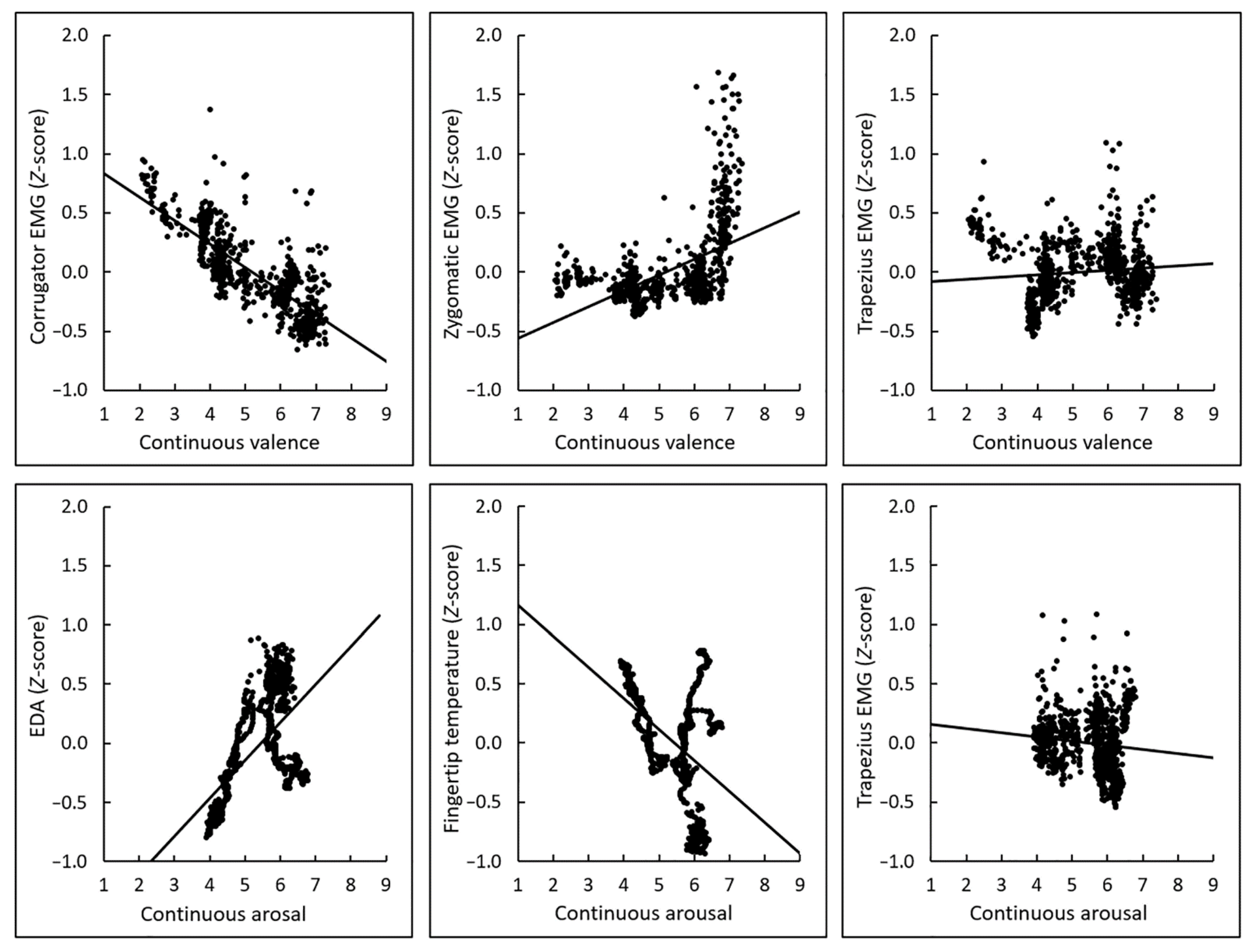
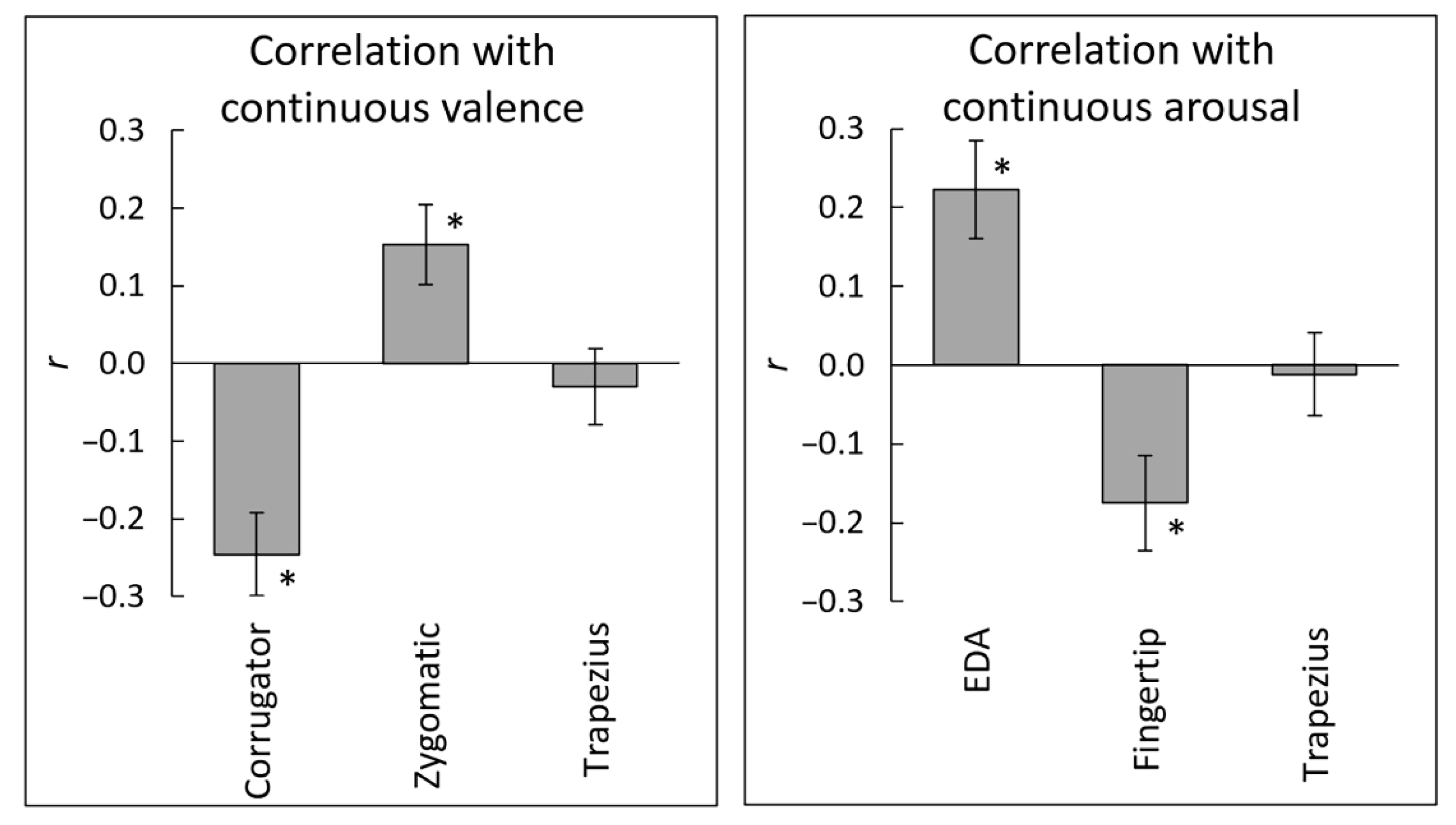
3.2. Correlations between Subjective Ratings and Physiological Activity
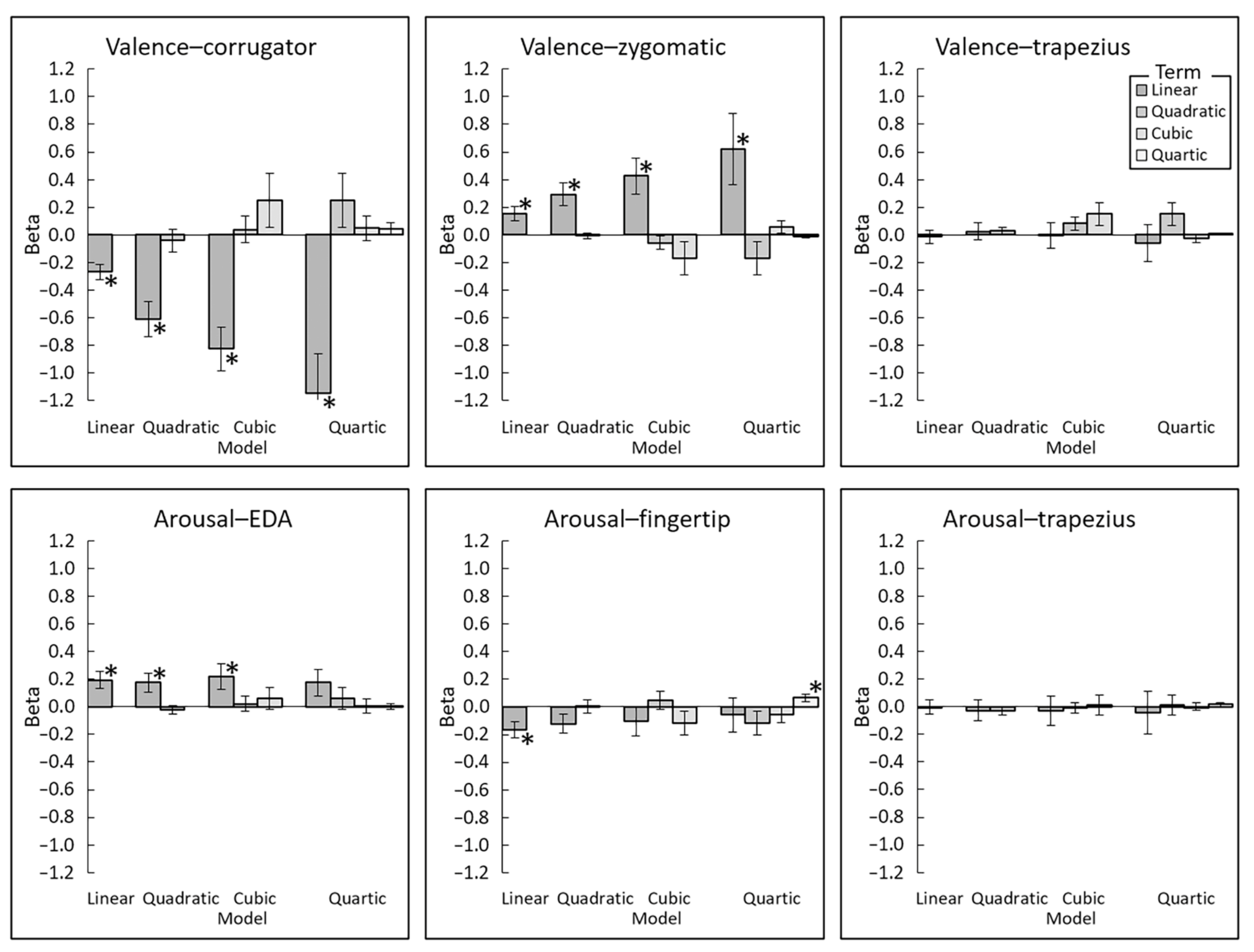
3.3. Polynomial Regression Modeling of Subjective–Physiological Concordance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lyubomirsky, S. Why are some people happier than others? The role of cognitive and motivational processes in well-being. Am. Psychol. 2001, 56, 239–249. [Google Scholar] [CrossRef]
- Burr, D.A.; Castrellon, J.J.; Zald, D.H.; Samanez-Larkin, G.R. Emotion dynamics across adulthood in everyday life: Older adults are more emotionally stable and better at regulating desires. Emotion 2021, 21, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Rozin, A.; Rozin, P.; Goldberg, E. The feeling of music past: How listeners remember musical affect. Music Percept. 2004, 22, 15–39. [Google Scholar] [CrossRef]
- Schäfer, T.; Zimmermann, D.; Sedlmeier, P. How we remember the emotional intensity of past musical experiences. Front. Psychol. 2014, 5, 911. [Google Scholar] [CrossRef]
- Strijbosch, W. When the parts of the sum are greater than the whole: Assessing the peak-and-end-theory for a heterogeneous, multi-episodic tourism experience. J. Dest. Mark. Manag. 2021, 20, 100607. [Google Scholar] [CrossRef]
- Strijbosch, W.; Mitas, O.; van Gisbergen, M.; Doicaru, M.; Gelissen, J.; Bastiaansen, M. From experience to memory: On the robustness of the peak-and-end-rule for complex, heterogeneous experiences. Front. Psychol. 2019, 10, 1705. [Google Scholar] [CrossRef]
- Houben, M. The relation between short-term emotion dynamics and psychological well-being: A meta-analysis. Psychol. Bull. 2015, 141, 901–930. [Google Scholar] [CrossRef]
- Li, S.; Scott, N.; Walters, G. Current and potential methods for measuring emotion in tourism experiences: A review. Curr. Issues Tour. 2015, 18, 805–827. [Google Scholar] [CrossRef]
- Sato, W.; Kochiyama, T.; Yoshikawa, S. Physiological correlates of subjective emotional valence and arousal dynamics while viewing films. Biol. Psychol. 2020, 157, 107974. [Google Scholar] [CrossRef]
- Dimberg, U. Facial electromyography and emotional reactions. Psychophysiology 1990, 27, 481–494. [Google Scholar] [CrossRef]
- Boucsein, W. Electrodermal Activity, 2nd ed.; Springer: New York, NY, USA, 2012. [Google Scholar]
- Ioannou, S.; Gallese, V.; Merla, A. Thermal infrared imaging in psychophysiology: Potentialities and limits. Psychophysiology 2014, 51, 951–963. [Google Scholar] [CrossRef]
- Sato, W.; Murata, K.; Uraoka, Y.; Shibata, K.; Yoshikawa, S.; Furuta, M. Emotional valence sensing using a wearable facial EMG device. Sci. Rep. 2021, 11, 5757. [Google Scholar] [CrossRef]
- Dan-Glauser, E.S.; Gross, J.J. Emotion regulation and emotion coherence: Evidence for strategy-specific effects. Emotion 2013, 13, 832–842. [Google Scholar] [CrossRef]
- Golland, Y.; Hakim, A.; Aloni, T.; Schaefer, S.; Levit-Binnun, N. Affect dynamics of facial EMG during continuous emotional experiences. Biol. Psychol. 2018, 139, 47–58. [Google Scholar] [CrossRef]
- Van der Velde, J.; Laan, E.; Everaerd, W. Vaginismus, a component of a general defensive reaction. an investigation of pelvic floor muscle activity during exposure to emotion-inducing film excerpts in women with and without vaginismus. Int. Urogynecol. J. Pelvic. Floor Dysfunct. 2001, 12, 328–331. [Google Scholar] [CrossRef]
- Krantz, G.; Forsman, M.; Lundberg, U. Consistency in physiological stress responses and electromyographic activity during induced stress exposure in women and men. Integr. Psychol. Behav. Sci. 2004, 39, 105–118. [Google Scholar] [CrossRef]
- Luijcks, R.; Hermens, H.J.; Bodar, L.; Vossen, C.J.; Van Os, J.; Lousberg, R. Experimentally induced stress validated by EMG activity. PLoS ONE 2014, 9, e95215. [Google Scholar] [CrossRef]
- Lundberg, U.; Kadefors, R.; Melin, B.; Palmerud, G.; Hassmen, P.; Engstrom, M.; Dohns, I.E. Psychophysiological stress and EMG activity of the trapezius muscle. Int. J. Behav. Med. 1994, 1, 354–370. [Google Scholar] [CrossRef]
- Marsman, A.; Luijcks, R.; Vossen, C.; van Os, J.; Lousberg, R. The impact of adverse childhood experiences on EMG reactivity: A proof of concept study. PLoS ONE 2019, 14, e0216657. [Google Scholar] [CrossRef]
- Pourmohammadi, S.; Maleki, A. Stress detection using ECG and EMG signals: A comprehensive study. Comput. Methods Programs Biomed. 2020, 193, 105482. [Google Scholar] [CrossRef]
- Eijckelhof, B.H.; Huysmans, M.A.; Bruno Garza, J.L.; Blatter, B.M.; van Dieen, J.H.; Dennerlein, J.T.; van der Beek, A.J. The effects of workplace stressors on muscle activity in the neck-shoulder and forearm muscles during computer work: A systematic review and meta-analysis. Eur. J. Appl. Physiol. 2013, 113, 2897–2912. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.H.; Byun, S.; Park, M.S.; Sohn, J.H. Predicting Individuals’ Experienced Fear From Multimodal Physiological Responses to a Fear-Inducing Stimulus. Adv. Cogn. Psychol. 2020, 16, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Kistler, A.; Mariauzouls, C.; von Berlepsch, K. Fingertip temperature as an indicator for sympathetic responses. Int. J. Psychophysiol. 1998, 29, 35–41. [Google Scholar] [CrossRef]
- Yoshihara, K.; Tanabe, H.C.; Kawamichi, H.; Koike, T.; Yamazaki, M.; Sudo, N.; Sadato, N. Neural correlates of fear-induced sympathetic response associated with the peripheral temperature change rate. Neuroimage 2016, 134, 522–531. [Google Scholar] [CrossRef]
- Levenson, R.W.; Carstensen, L.L.; Friesen, W.V.; Ekman, P. Emotion, physiology, and expression in old age. Psychol. Aging 1991, 6, 28–35. [Google Scholar] [CrossRef]
- Sinha, R.; Parsons, O.A. Multivariate response patterning of fear and anger. Cogn. Emot. 1996, 10, 173–198. [Google Scholar] [CrossRef]
- Ohata, M.; Zhou, L.; Yada, Y.; Yokoyama, I.; Arihara, K. 2,3-Dimethylpyrazine (3DP) and 2,5-dimethyl-4-hydroxy-3(2H)-furanone (DMHF) generated by the Maillard reaction in foods affect autonomic nervous activity and central nervous activity in human. Biosci. Biotechnol. Biochem. 2020, 84, 1894–1902. [Google Scholar] [CrossRef]
- Cacioppo, J.T.; Berntson, G.G.; Larsen, J.T.; Poehlmann, K.M.; Ito, T.A. The psychophysiology of emotion. In The Handbook of Emotion; Lewis, J.M., Haviland-Jones, J.M., Eds.; Guildford Press: New York, NY, USA, 2000; pp. 173–191. [Google Scholar]
- Russell, J.A.; Weiss, A.; Mendelsohn, G.A. Affect grid: A single-item scale of pleasure and arousal. J. Pers. Soc. Psychol. 1989, 57, 493–502. [Google Scholar] [CrossRef]
- Ruef, A.M.; Levenson, R.W. Continuous measurement of emotion: The affect rating dial. In Handbook of Emotion Elicitation and Assessment; Coan, J.A., Allen, J.J.B., Eds.; Oxford University Press: New York, NY, USA, 2007; pp. 286–297. [Google Scholar]
- Epstein, S. The stability of behavior: II. Implications for psychological research. Am. Psychol. 1980, 35, 790–806. [Google Scholar] [CrossRef]
- Zhang, Q.J.; Wang, L.P. Aggregating and testing intra-individual correlations: Methods and comparisons. Multivar. Behav. Res. 2014, 49, 130–148. [Google Scholar] [CrossRef]
- Holmes, A.; Friston, K.J. Generalisability, random effects and population inference. Neuroimage 1998, 7, S754. [Google Scholar] [CrossRef]
- Plant, R.R.; Turner, G. Millisecond precision psychological research in a world of commodity computers: New hardware, new problems? Behav. Res. Methods 2009, 41, 598–614. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Ostertagova, E. Modelling using polynomial regression. Procedia Eng. 2012, 48, 500–506. [Google Scholar] [CrossRef]
- Gross, J.J.; Levenson, R.W. Emotion elicitation using films. Cogn. Emo. 1995, 9, 87–108. [Google Scholar] [CrossRef]
- Sato, W.; Noguchi, M.; Yoshikawa, S. Emotion elicitation effect of films in a Japanese sample. Soc. Behav. Pers. 2007, 35, 863–874. [Google Scholar] [CrossRef]
- Mauss, I.B.; Levenson, R.W.; McCarter, L.; Wilhelm, F.H.; Gross, J.J. The tie that binds? Coherence among emotion experience, behavior, and physiology. Emotion 2005, 5, 175–190. [Google Scholar] [CrossRef]
- Fridlund, A.J.; Cacioppo, J.T. Guidelines for human electromyographic research. Psychophysiology 1986, 23, 567–589. [Google Scholar] [CrossRef]
- Schumann, N.P.; Bongers, K.; Guntinas-Lichius, O.; Scholle, H.C. Facial muscle activation patterns in healthy male humans: A multi-channel surface EMG study. J. Neurosci. Methods 2010, 187, 120–128. [Google Scholar] [CrossRef]
- Kasman, D.; Wolf, S. Surface EMG Made Easy: A Beginner’s Guide for Rehabilitation Clinicians; Noraxon USA, Inc.: Scottsdale, AZ, USA, 2002. [Google Scholar]
- De Luca, C.J.; Gilmore, L.D.; Kuznetsov, M.; Roy, S.H. Filtering the surface EMG signal: Movement artifact and baseline noise contamination. J. Biomech. 2010, 43, 1573–1579. [Google Scholar] [CrossRef]
- Van Boxtel, A. Optimal signal bandwidth for the recording of surface EMG activity of facial, jaw, oral, and neck muscles. Psychophysiology 2001, 38, 22–34. [Google Scholar] [CrossRef] [PubMed]
- JASP Team. JASP; (Version 0.14.1); [Computer Software]; 2020. [Google Scholar]
- Sato, W.; Yoshikawa, S.; Fushiki, T. Facial EMG activity is associated with hedonic experiences but not nutritional values while viewing food images. Nutrients 2020, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Brydges, C.R.; Gaeta, L. An introduction to calculating Bayes factors in JASP for speech, language, and hearing research. J. Speech Lang. Hear. Res. 2019, 62, 4523–4533. [Google Scholar] [CrossRef] [PubMed]
- Jeffreys, H. The Theory of Probability; Oxford University Press: Oxford, UK, 1961. [Google Scholar]
- Kahneman, D. Thinking, Fast and Slow; Farrar Straus & Giroux: New York, NY, USA, 2011. [Google Scholar]
- Lladó, A.; León, L.; Valls-Solé, J.; Mena, P.; Callejas, M.A.; Peri, J.M. Changes in the sympathetic skin response after thoracoscopic sympathectomy in patients with primary palmar hyperhidrosis. Clin. Neurophysiol. 2005, 116, 1348–1354. [Google Scholar] [CrossRef]
- Schestatsky, P.; Callejas, M.A.; Valls-Sole, J. Abnormal modulation of electrodermal activity by thermoalgesic stimuli in patients with primary palmar hyperhidrosis. J. Neurol. Neurosurg. Psychiatry 2011, 82, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.P. Research with the continuous response digital interface: A review with implications for future research. Philos. Music Educ. Rev. 1996, 4, 20–32. [Google Scholar]


| Association | t-Test | Bayesian | ||
|---|---|---|---|---|
| t | p | d | BF10 | |
| Valence–corrugator | 4.54 | <0.001 | 0.83 | 281.28 |
| Valence–zygomatic | 3.00 | 0.006 | 0.55 | 7.45 |
| Valence–trapezius | 0.61 | 0.549 | 0.11 | 0.23 |
| Arousal–EDA | 3.61 | 0.001 | 0.66 | 29.21 |
| Arousal–fingertip | 2.88 | 0.007 | 0.53 | 5.85 |
| Arousal–trapezius | 0.23 | 0.822 | 0.04 | 0.20 |
| Comparison | t-Test | Bayesian | ||
|---|---|---|---|---|
| t | p | d | BF10 | |
| Valence–corrugator vs. valence–trapezius | 3.09 | 0.004 | 0.83 | 9.13 |
| Valence–zygomatic vs. valence–trapezius | 1.65 | 0.109 | 0.55 | 0.66 |
| Arousal–EDA vs. arousal–fingertip | 0.67 | 0.508 | 0.12 | 0.24 |
| Arousal–EDA vs. arousal–trapezius | 3.31 | 0.002 | 0.61 | 14.91 |
| Association | Model | Term | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Linear | Quadratic | Cubic | Quartic | ||||||||||
| t | p | d | t | p | d | t | p | d | t | p | d | ||
| Valence–corrugator | Linear | 4.92 | <0.001 | 0.90 | |||||||||
| Quadratic | 4.70 | <0.001 | 0.86 | 0.51 | 0.613 | 0.09 | |||||||
| Cubic | 5.25 | <0.001 | 0.96 | 0.41 | 0.683 | 0.08 | 1.11 | 0.277 | 0.20 | ||||
| Quartic | 4.00 | <0.001 | 0.73 | 1.27 | 0.215 | 0.23 | 0.51 | 0.615 | 0.09 | 1.00 | 0.327 | 0.18 | |
| Valence–zygomatic | Linear | 2.99 | 0.006 | 0.55 | |||||||||
| Quadratic | 3.48 | 0.002 | 0.64 | 0.32 | 0.752 | 0.06 | |||||||
| Cubic | 3.21 | 0.003 | 0.59 | 1.12 | 0.271 | 0.21 | 0.26 | 0.794 | 0.05 | ||||
| Quartic | 2.43 | 0.022 | 0.44 | 1.38 | 0.178 | 0.25 | 1.22 | 0.231 | 0.22 | 1.62 | 0.117 | 0.30 | |
| Valence–trapezius | Linear | 0.26 | 0.800 | 0.05 | |||||||||
| Quadratic | 0.39 | 0.698 | 0.07 | 1.36 | 0.185 | 0.25 | |||||||
| Cubic | 0.05 | 0.960 | 0.01 | 1.76 | 0.089 | 0.32 | 0.34 | 0.736 | 0.06 | ||||
| Quartic | 0.46 | 0.649 | 0.08 | 1.77 | 0.087 | 0.32 | 1.07 | 0.295 | 0.20 | 0.56 | 0.583 | 0.10 | |
| Arousal–EDA | Linear | 3.12 | 0.004 | 0.57 | |||||||||
| Quadratic | 2.48 | 0.019 | 0.45 | 0.77 | 0.447 | 0.14 | |||||||
| Cubic | 2.34 | 0.026 | 0.43 | 0.40 | 0.692 | 0.07 | 0.90 | 0.375 | 0.17 | ||||
| Quartic | 1.78 | 0.085 | 0.33 | 0.78 | 0.442 | 0.14 | 0.13 | 0.896 | 0.02 | 0.06 | 0.954 | 0.01 | |
| Arousal–fingertip | Linear | 2.83 | 0.008 | 0.52 | |||||||||
| Quadratic | 1.78 | 0.086 | 0.33 | 0.01 | 0.990 | 0.00 | |||||||
| Cubic | 0.94 | 0.354 | 0.17 | 0.72 | 0.475 | 0.13 | 0.31 | 0.758 | 0.06 | ||||
| Quartic | 0.47 | 0.640 | 0.09 | 1.32 | 0.197 | 0.24 | 0.91 | 0.370 | 0.17 | 2.55 | 0.016 | 0.47 | |
| Arousal–trapezius | Linear | 0.01 | 0.989 | 0.00 | |||||||||
| Quadratic | 0.37 | 0.717 | 0.07 | 1.24 | 0.224 | 0.23 | |||||||
| Cubic | 0.27 | 0.789 | 0.05 | 0.18 | 0.859 | 0.03 | 0.18 | 0.859 | 0.03 | ||||
| Quartic | 0.27 | 0.789 | 0.05 | 0.19 | 0.851 | 0.04 | 0.01 | 0.989 | 0.00 | 1.38 | 0.177 | 0.25 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, W.; Kochiyama, T. Exploration of Emotion Dynamics Sensing Using Trapezius EMG and Fingertip Temperature. Sensors 2022, 22, 6553. https://doi.org/10.3390/s22176553
Sato W, Kochiyama T. Exploration of Emotion Dynamics Sensing Using Trapezius EMG and Fingertip Temperature. Sensors. 2022; 22(17):6553. https://doi.org/10.3390/s22176553
Chicago/Turabian StyleSato, Wataru, and Takanori Kochiyama. 2022. "Exploration of Emotion Dynamics Sensing Using Trapezius EMG and Fingertip Temperature" Sensors 22, no. 17: 6553. https://doi.org/10.3390/s22176553
APA StyleSato, W., & Kochiyama, T. (2022). Exploration of Emotion Dynamics Sensing Using Trapezius EMG and Fingertip Temperature. Sensors, 22(17), 6553. https://doi.org/10.3390/s22176553







