Development and Applications of Compton Camera—A Review
Abstract
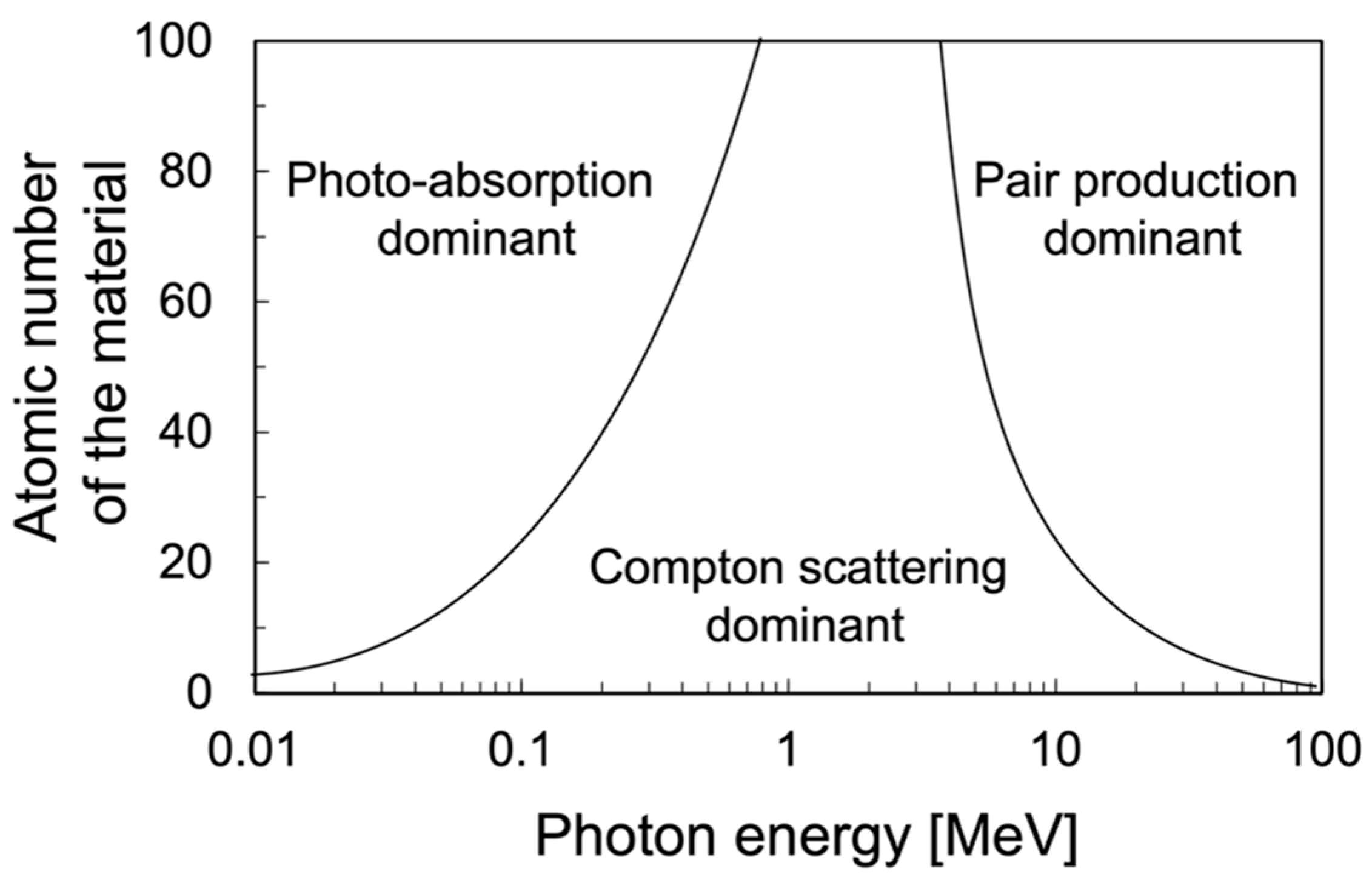
:1. Introduction
2. Types of Compton Cameras
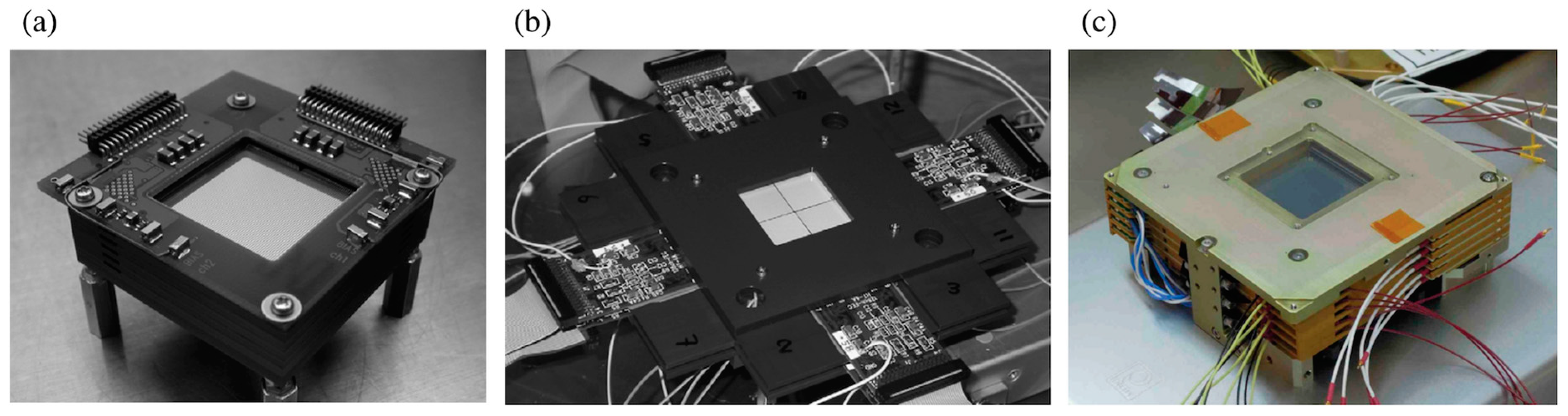
2.1. Si/CdTe Detector-Based Compton Cameras
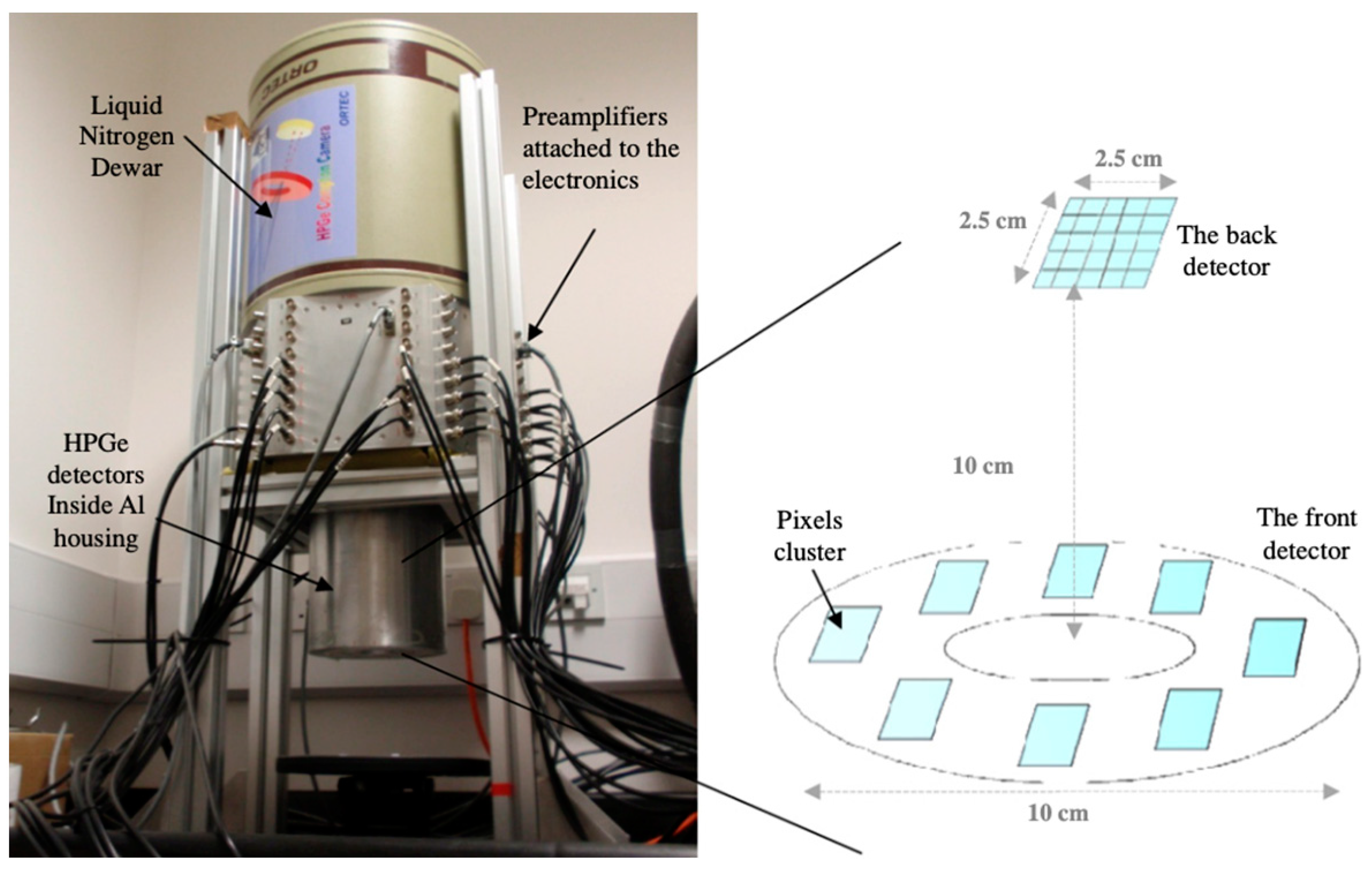
2.2. Ge Detector-Based Compton Cameras
2.3. Scintillator-Based Compton Cameras
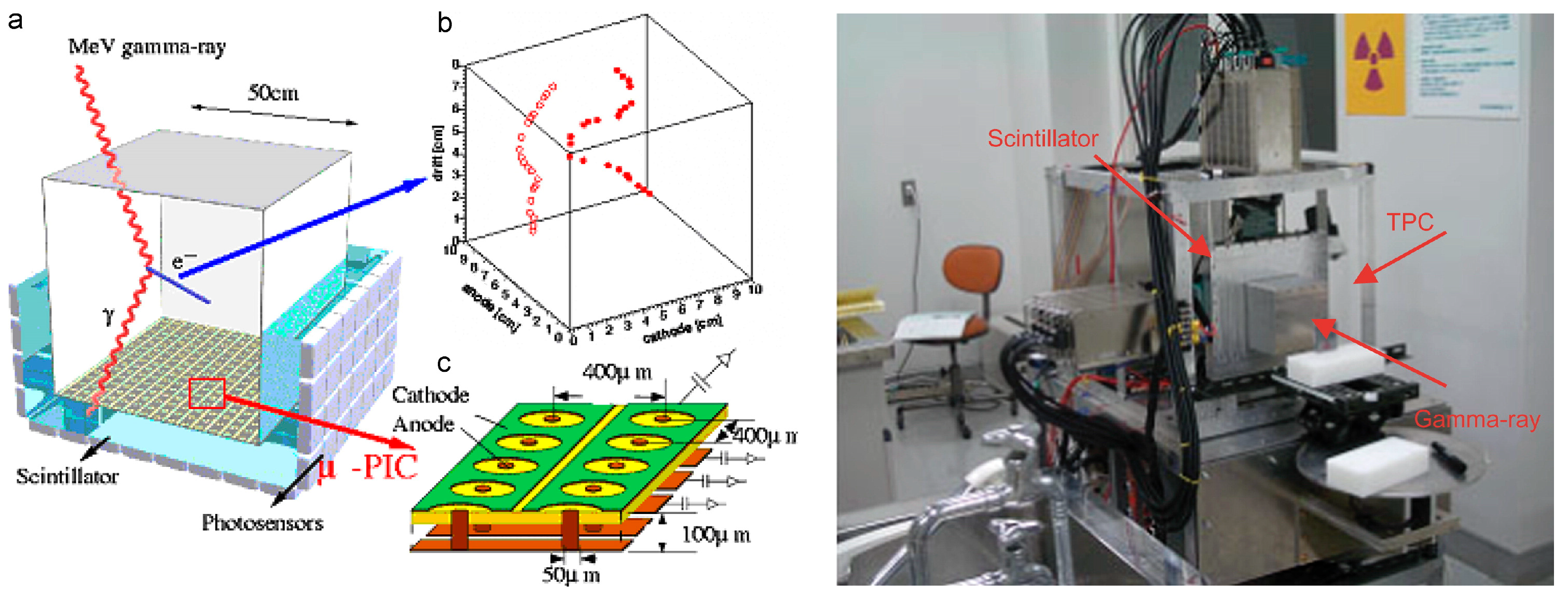
2.4. Electron-Tracking Compton Cameras
2.5. Other Compton Cameras
3. Applications of Compton Cameras
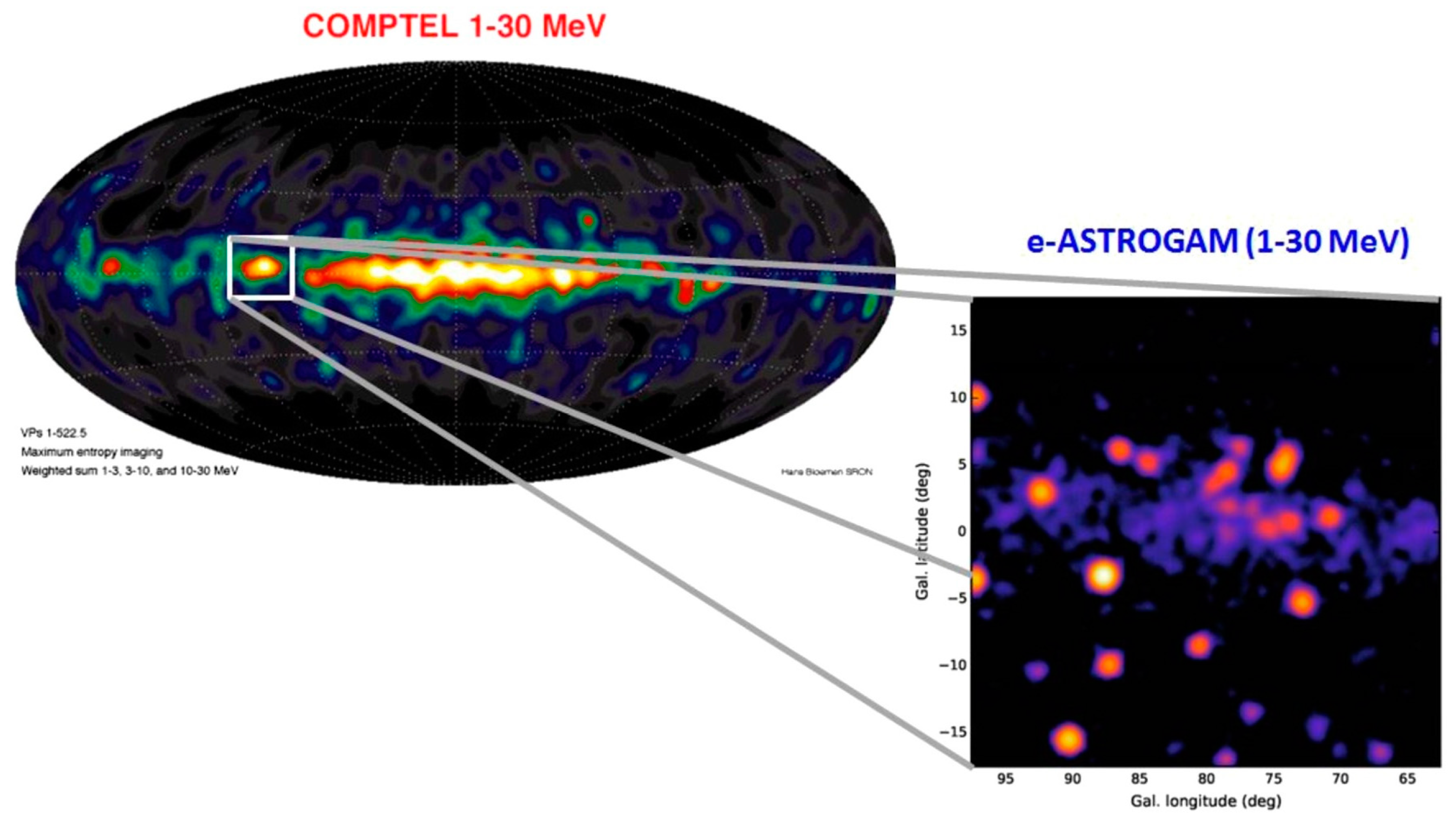
3.1. Astronomical Observations
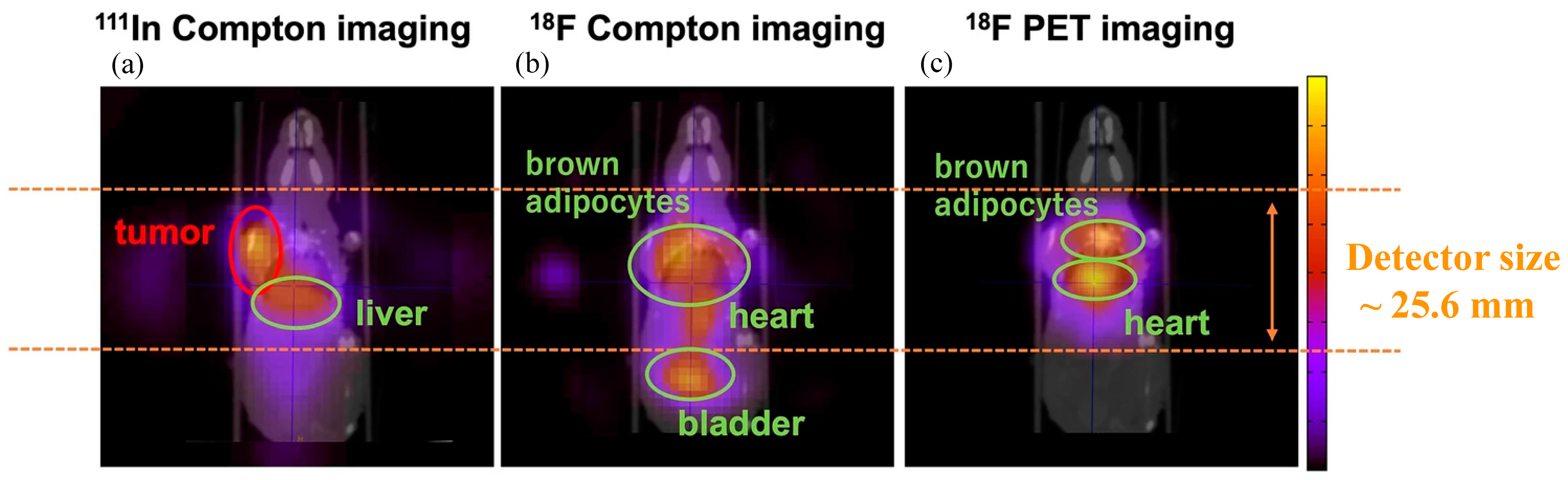
3.2. Nuclear Medical Imaging
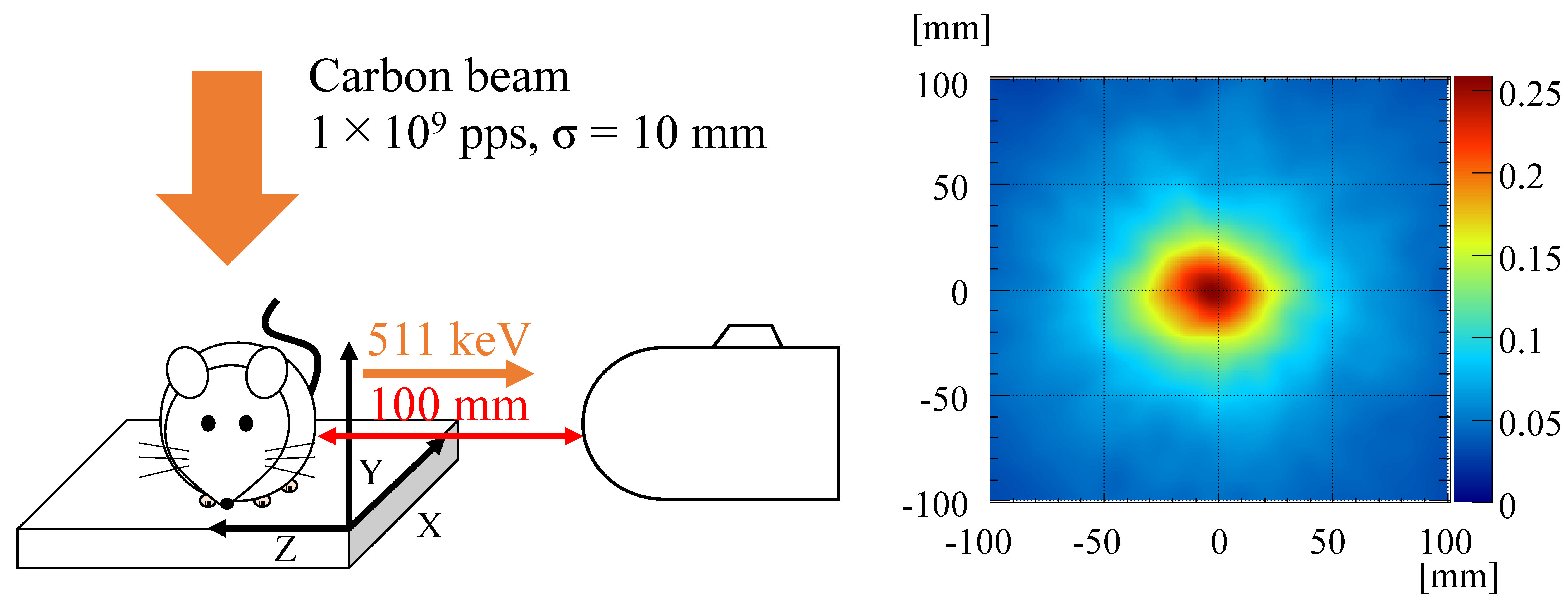
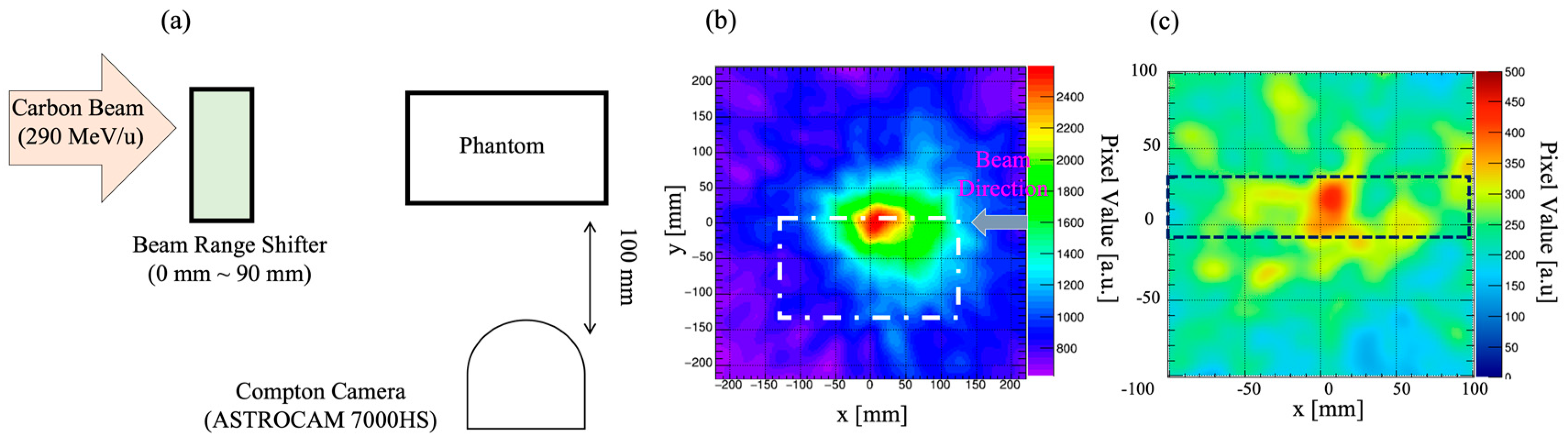
3.3. Beam-Range Monitoring in Particle Radiotherapy
3.4. Environmental Measurement
3.5. Other Specific Applications
4. Image Reconstruction Methods for Compton Imaging
4.1. Simple Back-Projection Methods
4.2. Filtered Back-Projection Methods
4.3. Expectation-Maximization Methods
4.4. Stochastic Origin Ensemble Methods
5. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Schoenfelder, V.; Hirner, A.; Schneider, K. A telescope for soft gamma astronomy. Nucl. Instrum. Methods 1973, 107, 385–394. [Google Scholar] [CrossRef]
- Herzo, D.; Koga, R.; Millard, W.A.; Moon, S.; Ryan, J.; Wilson, R.; Zych, A.D.; White, R.S. A large double scatter telescope for gamma rays and neutrons. Nucl. Instrum. Methods 1975, 123, 583. [Google Scholar] [CrossRef]
- Lockwood, J.A.; Hsieh, L.; Friling, L.; Chen, C.; Swartz, D. Atmospheric neutron and gamma ray fluxes and energy spectra. J. Geophys. Res. 1979, 84, 1402. [Google Scholar] [CrossRef]
- Schoenfelder, V.; Aarts, H.; Bennett, K.; de Boer, H.; Clear, J.; Collmar, W.; Connors, A.; Deerenberg, A.; Diehl, R.; von Dordrecht, A.; et al. Instrument description and performance of the imaging gamma-ray telescope COMPTEL aboard the Compton gamma-ray observatory. Astrophys. J. 1993, 86, 657. [Google Scholar] [CrossRef]
- Todd, R.; Nightingale, J.M.; Everett, D.B. A proposed γ camera. Nature 1974, 251, 132–134. [Google Scholar] [CrossRef]
- Takahashi, T.; Makishima, K.; Fukazawa, Y.; Kokubun, M.; Nakazawa, K.; Nomachi, M.; Tajima, H.; Tashiro, M.; Terada, Y. Hard x-ray and gamma-ray detectors for the NEXT mission. Astron. Rev. 2004, 48, 309–313. [Google Scholar]
- Takahashi, T.; Kokubun, M.; Mitsuda, K.; Kelly, R.L.; Ohashi, T.; Aharonian, F.; Akamatsu, H.; Akimoto, F.; Allen, S.W.; Anabuki, N.; et al. Hitomi (ASTRO-H) x-ray astronomy satellite. J. Astron. Telesc. Instrum. 2018, 4, 021402. [Google Scholar]
- Sato, Y.; Ozawa, S.; Terasaka, Y.; Kaburagi, M.; Tanifuji, Y.; Kawabata, K.; Miyamura, H.N.; Izumi, R.; Suzuki, T.; Torii, T. Remote radiation imaging system using a compact gamma-ray imager mounted on a multicopter drone. J. Nucl. Sci. Technol. 2018, 55, 90–96. [Google Scholar] [CrossRef]
- Kagaya, M.; Katagiri, H.; Enomoto, R.; Hanafusa, R.; Hosokawa, M.; Itoh, Y.; Muraishi, H.; Nakayama, K.; Satoh, K.; Takeda, T.; et al. Development of a low-cost-high-sensitivity Compton camera using CsI (Tl) scintillators (γI). Nucl. Instrum. Methods Phys. Res. A 2015, 804, 25–32. [Google Scholar] [CrossRef]
- Tomono, D.; Mizumoto, T.; Takada, A.; Komura, S.; Matsuoka, Y.; Mizumura, Y.; Oda, M.; Tanimori, T. First on-site true gamma-ray imaging-spectroscopy of contamination near Fukushima plant. Sci. Rep. 2017, 7, 41972. [Google Scholar] [CrossRef]
- Tumer, O.T.; Akyuz, A.; Bhattacharya, D.; Blair, S.C.; Case, G.L.; Dixon, D.D.; Liu, C.-J.; O’Neill, T.J.; Samimi, J.; White, R.S.; et al. The TIGRE instrument for 0.3–100 MeV gamma-ray astronomy. IEEE Trans. Nucl. Sci. 1995, 42, 907–916. [Google Scholar] [CrossRef]
- Choppin, G.R.; Liljenzin, J.-O.; Rydberg, J. (Eds.) Absorption of Nuclear Radiation. In Radiochemistry and Nuclear Chemistry, 3rd ed.; Butterworth-Heinemann: Oxford, UK, 2002; Chapter 6; pp. 123–165. ISBN 9780750674638. [Google Scholar] [CrossRef]
- Schoenfelder, V.; Graser, U.; Diehl, R. Properties and performance of the MPI balloon borne Compton telescope. Astron. Astrophys. 1982, 110, 138. [Google Scholar]
- Schoenfelder, V.; Bennett, K.; Bloemen, H.; Diehl, R.; Hermsen, W.; Lichti, G.; McConnell, M.; Ryan, J.; Strong, A.; Winkler, C. COMPTEL overview: Achievements and expectations. Astron. Astrophys. Suppl. Ser. 1996, 120, 13–21. [Google Scholar]
- Schoenfelder, V. The Universe in Gamma Rays; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Schoenfelder, V. Lessons learnt from COMPTEL for future telescopes. New Astron. Rev. 2004, 48, 193–198. [Google Scholar] [CrossRef]
- Mitani, T.; Tanaka, T.; Nakazawa, K.; Takahashi, T.; Takashima, T.; Tajima, H.; Nakamura, H.; Nomachi, M.; Nakamoto, T.; Fukazawa, Y. A prototype Si/CdTe Compton camera and the polarization measurement. IEEE Trans. Nucl. Sci. 2004, 51, 2432–2437. [Google Scholar] [CrossRef]
- Bandstra, M.S.; Bellm, E.C.; Boggs, S.E.; Perez-Becker, D.; Zoglauer, A.; Chang, H.-K.; Chiu, J.-L.; Liang, J.-S.; Chang, Y.-H.; Liu, Z.-K.; et al. Detection and imaging of the crab nebula with the nuclear Compton telescope. Astrophys. J. 2011, 738, 8–16. [Google Scholar] [CrossRef]
- Hirose, K. Fukushima Daiichi Nuclear Plant accident: Atmospheric and oceanic impacts over the five years. J. Environ. Radioact. 2016, 157, 113–130. [Google Scholar] [CrossRef]
- Wahl, C.G.; Kaye, W.; Wang, W.; Zhang, F.; Jaworski, J.; Boucher, Y.A.; King, A.; He, Z. Polaris-H measurements and performance. In Proceedings of the 2014 IEEE Nuclear Science Symposium and Medical Imaging Conference (NSS/MIC), Seattle, WA, USA, 8–15 November 2014; pp. 1–4. [Google Scholar] [CrossRef]
- Takahashi, T.; Takeda, S.; Watanabe, S.; Tajima, H. Visualization of radioactive substances with a Si/CdTe Compton Camera. In Proceedings of the 2012 IEEE Nuclear Science Symposium and Medical Imaging Conference Record (NSS/MIC), Anaheim, CA, USA, 29 October–3 November 2012; pp. 4199–4204. [Google Scholar] [CrossRef]
- Anger, H.O. Scintillation Camera. Rev. Sci. Instrum. 1958, 29. [Google Scholar] [CrossRef]
- Singh, M. An electronically collimated gamma camera for single photon emission computed tomography. Part I: Theoretical considerations and design criteria. Med. Phys. 1983, 10, 421–427. [Google Scholar]
- Singh, M.; Doria, D. An electronically collimated gamma camera for single photon emission computed tomography. Part II: Image reconstruction and preliminary experimental measurements. Med. Phys. 1983, 10, 428–435. [Google Scholar] [CrossRef]
- Martin, J.B.; Knoll, G.F.; Wehe, D.K.; Dogan, N.; Jordanov, V.; Petrick, N.; Singh, M. A ring Compton scatterer camera for imaging medium energy gamma rays. IEEE Trans. Nucl. Sci. 1993, 40, 972–978. [Google Scholar] [CrossRef]
- LeBlanc, J.W.; Clinthorne, N.H.; Hua, C.-H.; Nygard, E.; Rogers, W.L.; Wehe, D.K.; Weilhammer, P.; Wilderman, S.J. C-SPRINT: A prototype Compton camera system for low energy gamma ray imaging. IEEE Trans. Nucl. Sci. 1998, 45, 943–949. [Google Scholar] [CrossRef]
- LeBlanc, J.W.; Clinthorne, N.H.; Hua, C.-H.; Nygard, E.; Rogers, W.L.; Wehe, D.K.; Weilhammer, P.; Wilderman, S.J. Experimental results from the C=SPRINT prototype Compton camera. IEEE Trans. Nucl. Sci. 1999, 46, 201–204. [Google Scholar] [CrossRef]
- LeBlanc, J.W.; Clinthorne, N.H.; Hua, C.-H.; Rogers, W.L.; Wehe, D.K.; Wilderman, S.J. A Compton camera for nuclear medicine applications using 113mIn1. Nucl. Instrum. Methods A 1999, 422, 735–739. [Google Scholar] [CrossRef]
- Kamae, T.; Hanada, N.; Enomoto, R. Prototype design of multiple Compton gamma-ray camera. IEEE Nucl. Sci. Symp. 1987, 35, 352–355. [Google Scholar] [CrossRef]
- Dogan, N.; Wehe, D.K.; Knoll, G.F. Multiple Compton scattering gamma ray imaging camera. Nucl. Instrum. Methods Phys. Res. A 1990, 299, 501–506. [Google Scholar] [CrossRef]
- Wulf, E.A.; Phlips, B.F.; Johnson, W.N.; Kurfess, J.D.; Novikova, E.I. Thick silicon strip detector Compton imager. IEEE Trans. Nucl. Sci. 2004, 51, 1997–2003. [Google Scholar] [CrossRef]
- Kroeger, R.A.; Johnson, W.N.; Kurfess, J.D.; Phlips, B.F.; Wulf, E.A. Three-Compton telescope: Theory, simulations, and performance. IEEE Trans. Nucl. Sci. 2002, 49, 1887–1892. [Google Scholar] [CrossRef]
- Vetter, K.; Burks, M.; Cork, C.; Cunningham, M.; Chivers, D.; Hull, E.; Krings, T.; Manini, H.; Michailescu, L.; Nelson, K.; et al. High sensitivity compton imaging with position sensitive Si and Ge detectors. Nucl. Instrum. Methods Phys. Res. A 2007, 579, 363–366. [Google Scholar] [CrossRef]
- Kuhl, D.E.; Edwards, R.Q. Image separation radioisotope scanning. Radiology 1963, 80, 653–661. [Google Scholar] [CrossRef]
- Webb, S.; Broderick, M.; Flower, M.A. High resolution SPECT using divergent geometry. Br. J. Radiol. 1985, 58, 331–334. [Google Scholar] [CrossRef]
- Chesser, R.; Gemmell, H.G. The interfacing of a gamma camera to a DEC Gamma-11 data processing system for single photon emission tomography. Phys. Med. Biol. 1982, 27, 437–441. [Google Scholar] [CrossRef]
- Tanaka, E. Recent Progress on Single Photon and Positron Emission Tomography—From Detectors to Algorithms. IEEE Trans. Nucl. Sci. 1987, 34, 313–320. [Google Scholar] [CrossRef]
- Motomura, S.; Kanayama, Y.; Hiromura, M.; Fukuchi, T.; Ida, T.; Haba, H.; Watanabe, Y.; Enomoto, S. Improved imaging performance of a semiconductor Compton camera GREI makes for a new methodology to integrate bio-metal analysis and molecular imaging technology in living organisms. J. Anal. Atomic Spectrom. 2013, 28, 934–939. [Google Scholar] [CrossRef]
- Kauren, V.A.; Roel, V.H.; Stephen, V.; Christian, V. Review of SPECT collimator selection, optimizatiom, and fabrication for clinical and preclinical imaging. Med. Phys. 2015, 42, 4796–4813. [Google Scholar] [CrossRef]
- Carminati, M.; D’Adda, I.; Morahan, A.J.; Erlandsson, K.; Nagy, K.; Czeller, M.; Tolgyesi, B.; Nyitrai, Z.; Savi, A.; Mullekom, P.V.; et al. Clinical SiPM-Based MRI-Compatible SPECT: Preliminary Characterization. IEEE Trans. Radiat. Plasma Med. Sci. 2020, 4, 371–377. [Google Scholar] [CrossRef]
- Jongen, Y.; Stichelbaut, F. Verification of the Proton Beam Position in the Patient by the Prompt gamma rays Emission. In Proceedings of the 2003 39th Meeting of the Particle Therapy Co-Operative Group, San Francisco, CA, USA, 26–29 October 2003; Available online: https://www.ptcog.ch/archive/steering_committee_docs/ptcog39-2003/2003_PTCOG_39_Abstracts.pdf (accessed on 5 May 2022).
- Min, C.H.; Kim, C.H.; Youn, M.Y.; Kim, J.W. Prompt-gamma measurements for locating the dose falloff region in the proton therapy. Appl. Phys. Lett. 2006, 89, 183517. [Google Scholar] [CrossRef]
- Verburg, J.M.; Seco, J. Proton range verification through prompt gamma-ray spectroscopy. Phys. Med. Biol. 2014, 59, 7089–7106. [Google Scholar] [CrossRef]
- Golnik, C.; Hueso-González, F.; Müller, A.; Dendooven, P.; Enghardt, W.; Fiedler, F.; Kormoll, T.; Katja, R.; Petzoldt, J.; Wagner, A.; et al. Range assessment in particle therapy based on prompt γ-ray timing measurements. Phys. Med. Biol. 2014, 59, 5399–5422. [Google Scholar] [CrossRef]
- Zarifi, M.; Guatelli, S.; Bolst, D.; Hutton, B.; Rosenfeld, A.; Qi, Y. Characterization of prompt gamma-ray emission with respect to the Bragg peak for proton beam range verification: A Monte Carlo study. Phys. Med. 2017, 33, 197–206. [Google Scholar] [CrossRef]
- Testa, E.; Bajard, M.; Chevallier, M.; Dauvergne, D.; Foulher, F.L.; Freud, N.; Letang, J.M.; Poizat, J.C.; Ray, C.; Testa, M. Dose profile monitoring with carbon ions by means of prompt gamma measurements. Nucl. Instrum. Methods Phys. Res. B 2009, 267, 993–996. [Google Scholar] [CrossRef]
- Kurosawa, S.; Kubo, H.; Ueno, K.; Kabuki, S.; Iwaki, S.; Takahashi, M.; Taniue, K.; Higashi, N.; Miuchi, K.; Tanimori, T.; et al. Prompt gamma detection for range verification in proton therapy. Curr. Appl. Phys. 2012, 12, 364–368. [Google Scholar] [CrossRef]
- Andreo, P. On the clinical spatial resolution achievable with protons and heavier charged particle radiotherapy beams. Phys. Med. Biol. 2009, 54, N205. [Google Scholar] [CrossRef]
- Krimmer, J.; Dauvergne, D.; Létang, J.; Testa, E. Prompt-gamma monitoring in hadrontherapy: A review. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2018, 878, 58–73. [Google Scholar] [CrossRef]
- Han, L.; Rogers, W.L.; Huh, S.S.; Clinthorne, N. Statistical performance evaluation and comparison of a Compton medical imaging system and a collimated Anger camera for higher energy photon imaging. Phys. Med. Biol. 2008, 53, 7029–7045. [Google Scholar] [CrossRef]
- Fontana, M.; Dauvergne, D.; Letang, J.M.; Ley, J.-L.; Testa, E. Compton camera study for high efficiency SPECT and benchmark with anger system. Phys. Med. Biol. 2017, 62, 8794–8812. [Google Scholar] [CrossRef]
- Scheiber, C.; Chambron, J. CdTe detectors in medicine: A review of current applications and future perspectives. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 1992, 322, 604–614. [Google Scholar] [CrossRef]
- Abbaspour, S.; Mahmoudian, B.; Islamian, J.P. Cadmium Telluride Semicondoctor Detector for Improved Spatial and Energy Resolution Radioisotopic Imaging. World J. Nucl. Med. 2017, 16, 101–107. [Google Scholar] [CrossRef]
- Llosá, G. SiPM-based Compton cameras. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2019, 926, 148–152. [Google Scholar] [CrossRef]
- Tashima, H.; Yamaya, T. Compton imaging for medical applications. Radiol. Phys. Technol. 2022, 15, 187–205. [Google Scholar] [CrossRef]
- Takeda, S. Experimental Study of a Si/CdTe Semiconductor Compton Camera for the Next Generation of Gamma-Ray Astronomy. Ph.D. Thesis, University of Tokyo, Tokyo, Japan, 2009. Available online: https://member.ipmu.jp/takahashi_lab_UT/public_html/DownLoad/Takeda_dthesis.pdf (accessed on 26 March 2022).
- Zoglauer, A.; Kanbach, G. Doppler broadening as a lower limit to the angular resolution of next-generation Compton telescopes. In X-ray and Gamma-Ray Telescopes and Instruments for Astronomy; International Society for Optics and Photonics: Bellingham, WA, USA, 2003; Volume 4851, pp. 1303–1309. [Google Scholar]
- Tanaka, T. Recent achievements of the high resolution Schottky CdTe diode for γ-ray detectors. New Astron. Rev. 2004, 48, 309–313. [Google Scholar] [CrossRef]
- Oonuki, K.; Tanaka, T.; Watanabe, S.; Takeda, S.; Nakazawa, K.; Ushio, M.; Mitani, T.; Takahashi, T.; Tajima, H. A stacked CdTe pixel detector for a Compton camera. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2007, 573, 57–60. [Google Scholar] [CrossRef] [Green Version]
- Harkness, L.J.; Boston, A.J.; Boston, H.C.; Cooper, R.J.; Cresswell, J.R.; Grint, A.N.; Nolan, P.J.; Oxley, D.C.; Scraggs, D.P.; Beveridge, T.; et al. Optimisation of a dual head semiconductor Compton camera using Geant4. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2009, 604, 351–354. [Google Scholar] [CrossRef]
- Takahashi, T.; Nakazawa, K.; Kamae, T.; Tajima, H.; Fukazawa, Y.; Masaharu, N.; Kokubun, M. High resolution CdTe detectors for the next generation multi-Compton gamma-ray telescope. In X-ray and Gamma-Ray Telescopes and Instruments for Astronomy; SPIE: Bellingham, WA, USA, 2003; Volume 4851. [Google Scholar]
- Takahashi, T.; Kamae, T.; Makishima, K. Future hard X-ray and gamma-ray observations. ASP Conf. Ser. 2002, 251, 210–213. [Google Scholar]
- Watanabe, S.; Tanaka, T.; Nakazawa, K.; Mitani, T.; Oonuki, K.; Takahashi, T.; Takashima, T.; Tajima, H.; Fukazawa, Y.; Nomachi, M.; et al. A Si/CdTe semiconductor Compton camera. IEEE Trans. Nucl. Sci. 2005, 52, 2045–2051. [Google Scholar] [CrossRef]
- Takeda, S.; Ishikawa, S.; Odaka, H.; Watanabe, S.; Takahashi, T.; Nakazawa, K.; Tajima, H.; Kuroda, Y.; Onishi, M.; Fukazawa, Y.; et al. A new Si/CdTe semiconductor Compton camera developed for high-angular resolution. In Hard X-Ray and Gamma-Ray Detector Physics IX; SPIE: Bellingham, WA, USA, 2007; Volume 6706, pp. 187–197. [Google Scholar]
- Watanabe, S.; Takeda, S.; Ishikawa, S.; Odaka, H.; Ushio, M.; Tanaka, T.; Nakazawa, K.; Takahashi, T.; Tajima, H.; Fukazawa, Y.; et al. Development of semiconductor imaging detectors for a Si/CdTe Compton camera. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2007, 579, 871–877. [Google Scholar] [CrossRef]
- Takeda, S.; Ichinohe, Y.; Hagino, K.; Odaka, H.; Yuasa, T.; Ishikawa, S.; Fukuyama, T.; Saito, S.; Sato, T.; Sato, G.; et al. Applications and Imaging Techniques of a Si/CdTe Compton Gamma-Ray Camera. Phys. Procedia 2012, 37, 859–866. [Google Scholar] [CrossRef]
- Takahashi, T.; Awaki, A.; Dotani, T.; Fukazawa, Y.; Hayashida, K.; Kamae, T.; Kataoka, J.; Kawai, N.; Kitamoto, S.; Kohmura, T.; et al. Wide band X-ray Imager (WXI) and Soft Gamma-ray Detector (SGD) for the NeXT Mission. In UV And Gamma-Ray Space Telescope Systems; SPIE: Bellingham, WA, USA, 2004; Volume 5488, pp. 549–560. [Google Scholar] [CrossRef]
- Tajima, H.; Madejski, G.; Mitani, T.; Tanaka, T.; Nakamura, H.; Nakazawa, K.; Takahashi, T.; Fukazawa, Y.; Kamae, T.; Kokubun, M.; et al. Gamma-ray polarimetry with Compton telescope. In UV and Gamma-Ray Space Telescope Systems; SPIE: Bellingham, WA, USA, 2004. [Google Scholar] [CrossRef]
- Takahashi, T.; Kelly, R.; Mitsuda, K.; Kunieda, H.; Petre, R.; White, N.; Dotani, T.; Fujimoto, R.; Fukazawa, Y.; Hayashida, K.; et al. The NeXT Mission. In Proceedings of the SPIE Space Telescopes and Instrumentation, Marseille, France, 15 July 2008. [Google Scholar]
- Von Ballmoos, P.; Alvarez, J.; Barriere, N.; Boggs, S.; Bykov, A.; Del Cura Velayos, J.M.; Frontera, F.; Hanlon, L.; Hermanz, M.; Hinglais, E.; et al. The DUAL mission concept. In UV, X-Ray, and Gamma-Ray Space Instrumentation for Astronomy XVII; SPIE: Bellingham, WA, USA, 2011. [Google Scholar] [CrossRef]
- Watanabe, S.; Ishikawa, S.; Aono, H.; Takeda, S.; Odaka, H.; Kokubun, M.; Takahashi, T.; Nakazawa, K.; Tajima, H.; Onishi, M.; et al. High energy resolution hard x-ray and gamma-ray imagers using CdTe diode devices. IEEE Trans. Nucl. Sci. 2009, 56, 777–782. [Google Scholar] [CrossRef]
- Tashiro, M.; Kamae, T.; Makishima, K.; Takahashi, T.; Murakami, Y.; Fukazawa, Y.; Kokubun, M.; Nakazawa, K.; Nomachi, M.; Yoshida, A.; et al. Performance of the ASTRO-E hard x-ray detector. IEEE Trans. Nucl. Sci. 2002, 49, 1893–1897. [Google Scholar] [CrossRef]
- Kokubun, M.; Abe, K.; Ezoe, Y.; Fukazawa, S.; Hong, H.; Inoue, T.; Itoh, T.; Kamae, D.; Kasama, M.; Kawaharada, N.; et al. Improvements of the Astro-E2 hard X-ray detector (HXD-II). IEEE Trans. Nucl. Sci. 2004, 51, 1991–1996. [Google Scholar] [CrossRef]
- Mitsuda, K.; Bautz, M.; Inoue, H.; Kelly, R.L.; Koyama, K.; Kunieda, H.; Makishima, K.; Ogawara, Y.; Petre, R.; Takahashi, T.; et al. The x-ray observatory Suzaku. Publ. Astron. Soc. Jpn. 2007, 59, S1–S8. [Google Scholar] [CrossRef]
- Tsuruda, T. Nuclear Power Plant Explosions at Fukushima-Daiichi. Procedia Eng. 2013, 62, 71–77. [Google Scholar] [CrossRef]
- Matsuura, D.; Genba, K.; Kuroda, Y.; Ikebuchi, H.; Tomonaka, T. ‘ASTROCAM 7000HS’ radioactive substance visualization camera. Mitsubishi Heavy Ind. Tech. Rev. 2014, 51, 68–75. [Google Scholar]
- Takahashi, T.; Watanabe, S.; Takeda, S.; Ichinohe, Y.; Tajima, H.; Kuroda, Y.; Ikebuchi, H.; Genba, K.; Matsuura, D. To uncover hotspots of radiation with a Si/CdTe Compton camera. Available online: https://member.ipmu.jp/takahashi_lab_UT/public_html-JAXA-IPMU/DownLoad/Hiroshima2013_public.pdf (accessed on 1 August 2021).
- Takeda, S.; Harayama, A.; Ichinohe, Y.; Odaka, H.; Watanabe, S.; Takahashi, T.; Tajima, H.; Genba, K.; Matsuura, D.; Ikebuchi, H.; et al. A portable Si/CdTe Compton camera and its applications to the visualization of radioactive substances. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2015, 787, 207–211. [Google Scholar] [CrossRef]
- Sakai, M.; Kubota, Y.; Parajuli, R.K.; Kikuchi, M.; Arakawa, K.; Nakano, T. Compton imaging with 99mTc for human imaging. Sci. Rep. 2019, 9, 12906. [Google Scholar] [CrossRef] [PubMed]
- Sakai, M.; Parajuli, R.K.; Kikuchi, M.; Yamaguchi, M.; Nagao, Y.; Kawachi, N.; Arakawa, K.; Nakano, T. Effect of number of views on cross-sectional Compton imaging: A fundamental study with backprojection. Phys. Med. 2018, 56, 1–9. [Google Scholar] [CrossRef]
- Sakai, M.; Parajuli, R.K.; Kubota, Y.; Kubo, N.; Kikuchi, M.; Arakawa, K.; Nakano, T. Improved iterative reconstruction method for Compton imaging using median filter. PLoS ONE 2020, 15, e0229366. [Google Scholar] [CrossRef]
- Sakai, M.; Parajuli, R.K.; Kubota, Y.; Kubo, N.; Yamaguchi, M.; Nagao, Y.; Kawachi, N.; Kikuchi, M.; Arakawa, K.; Tashiro, M. Crosstalk reduction using a dual energy window scatter correction in Compton imaging. Sensors 2020, 20, 2453. [Google Scholar] [CrossRef]
- Sakai, M.; Yamaguchi, M.; Nagao, Y.; Kawachi, N.; Kikuchi, M.; Torikai, K.; Kamiya, T.; Takeda, S.; Watanabe, S.; Takahashi, T. In vivo simultaneous imaging with 99mTc and18F using a Compton camera. Phys. Med. Biol. 2018, 63, 205006. [Google Scholar] [CrossRef]
- Nakano, T.; Sakai, M.; Torikai, K.; Suzuki, Y.; Takeda, S.; Noda, S.; Yamaguchi, M.; Nagao, Y.; Kikuchi, M.; Odaka, H. Imaging of 99mTc-DMSA and 18F-FDG in humans using a Si/CdTe Compton camera. Phys. Med. Biol. 2020, 65, 05LT01. [Google Scholar] [CrossRef]
- Parajuli, R.; Sakai, M.; Wataru, K.; Torikai, K.; Kikuchi, M.; Arakawa, K.; Torikoshi, M.; Nakano, T. Annihilation gamma imaging for carbon ion beam range monitoring using Si/CdTe Compton camera. Phys. Med. Biol. 2019, 64, 055003. [Google Scholar] [CrossRef]
- Parajuli, R.K.; Sakai, M.; Arakawa, K.; Kubota, Y.; Kubo, N.; Tashiro, M. Carbon range verification with 718 keV Compton imaging. Sci. Rep. 2021, 11, 21696. [Google Scholar] [CrossRef]
- Shiba, S.; Parajuli, R.K.; Sakai, M.; Oike, T.; Ohno, T.; Nakano, T. Use of a Si/CdTe Compton Camera for in vivo real-time monitoring of annihilation gamma rays generated by carbon ion beam irradiation. Front. Oncol. 2020, 10, 635. [Google Scholar] [CrossRef]
- Turecek, D.; Jakubek, J.; Trojanova, E.; Sefc, L. Compton camera based on Timepix3 technology. J. Instrum. 2018, 13, C11022. [Google Scholar] [CrossRef]
- Turecek, D.; Jakubek, J.; Trojanova, E.; Sefc, L. Single layer Compton camera based on Timepix3 technology. J. Instrum. 2020, 15, C01014. [Google Scholar] [CrossRef]
- Tomita, H.; Mukai, A.; Kanamori, K.; Shimazoe, K.; Woo, H.; Tamura, Y.; Hara, S.; Terabayashi, R.; Uenomachi, M.; Nurrachman, A.; et al. Gamma-ray Source Identification by a Vehicle-mounted 4π Compton Imager. In Proceedings of the 2020 IEEE/SICE International Symposium on System Integration (SII), Honolulu, HI, USA, 12–15 January 2020; pp. 18–21. [Google Scholar] [CrossRef]
- Uche, C.Z.; Round, W.H.; Cree, M.J. A Monte Carlo evaluation of three Compton camera absorbers. Australas. Phys. Eng. Sci. Med. 2011, 34, 351–360. [Google Scholar] [CrossRef]
- Uche, C.Z.; Round, W.H.; Cree, M.J. Evaluation of detector material and radiation source position on Compton camera’s ability for multitracer imaging. Australas. Phys. Eng. Sci. Med. 2012, 35, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, M.; Wulf, E.A.; Phlips, B.; Krawczynski, H.; Martin, J.; Dowknott, P. Compton imaging with thick Si and CZT detectors. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2012, 682, 79–84. [Google Scholar] [CrossRef]
- Lee, Y. Preliminary evaluation of dual-head Compton camera with Si/CZT material for breast cancer detection: Monte Carlo simulation study. Optik 2020, 202, 163519. [Google Scholar] [CrossRef]
- Milbrath, B.D.; Peurrung, A.J.; Bliss, M.; Weber, W.J. Radiation detector materials: An overview. J. Mater. Res. 2008, 23, 2561–2581. [Google Scholar] [CrossRef]
- Lintereur, A.T.; Qiu, W.; Nino, J.C.; Baciak, J.E. Iodine based compound semiconductors for room temperature gamma-ray spectroscopy. Proc. SPIE Optics and Photonics in Global Homeland Security IV; SPIE: Bellingham, WA, USA, 2008; Volume 6945, pp. 11–20. [Google Scholar]
- Pennicard, D.; Pirard, B.; Tolbanov, O.; Iniewski, K. Semiconductor materials for x-ray detectors. MRS Bull. 2017, 42, 445–450. [Google Scholar] [CrossRef]
- Omer, M.; Shizuma, T.; Hajima, R.; Koizumi, M. Calculating off-axis efficiency of coaxial HPGe detectors by Monte Carlo simulation. Radiat. Phys. Chem. 2022, 198, 110241. [Google Scholar] [CrossRef]
- Lee, I.Y. The GAMMASPHERE. Nucl. Phys. A 1990, 520, c641–c655. [Google Scholar] [CrossRef]
- Simpson, J. The Euroball Spectrometer. Z. Phys. A 1997, 358, 139–143. [Google Scholar] [CrossRef]
- Simpson, J. The AGATA Project. J. Phys. Conf. Ser. 2006, 41, 006. [Google Scholar] [CrossRef]
- Lee, I.Y.; Clark, R.M.; Cromaz, M.; Deleplanque, M.A.; Descovich, M.; Diamond, R.M.; Fallon, P.; Macchiavelli, A.O.; Stephens, F.S.; Ward, D. GRETINA: A gamma ray energy tracking array. Nucl. Phys. A 2004, 746, 255. [Google Scholar] [CrossRef]
- Cooper, R.J.; Boston, A.J.; Boston, H.C.; Cresswell, J.R.; Grint, A.N.; Mather, A.R.; Nolan, P.J.; Scraggs, D.P.; Turk, G.; Hall, C.J.; et al. SmartPET: Applying HPGe and pulse shape analysis to small-animal PET. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2007, 579, 313–317. [Google Scholar] [CrossRef]
- Boston, H.C.; Gillam, J.; Boston, A.J.; Cooper, R.J.; Cresswell, J.; Grint, A.N.; Mather, A.R.; Nolan, P.J.; Scraggs, D.P.; Turk, G.; et al. Orthogonal strip HPGe planar SmartPET detectors in Compton configuration. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2007, 580, 929–933. [Google Scholar] [CrossRef]
- Takeda, S.; Fukuchi, T.; Kanayama, Y.; Motomura, S.; Hiromura, M.; Takahashi, T.; Enomoto, S. Millimeter-order imaging technique from 100 keV to MeV based on germanium Compton camera. In Hard X-Ray, Gamma-Ray, and Neutron Detector Physics XII; SPIE: Bellingham, WA, USA, 2010; Volume 7805, p. 780515. [Google Scholar] [CrossRef]
- Motomura, S.; Enomoto, S.; Haba, H.; Igarashi, K.; Gono, Y.; Yano, Y. Gamma-Ray Compton Imaging of Multitracer in Biological Samples Using Strip Germanium Telescope. IEEE Trans. Nucl. Sci. 2007, 54, 710–717. [Google Scholar] [CrossRef]
- Motomura, S.; Kanayama, Y.; Haba, H.; Watanabe, Y.; Enomoto, S. Multiple molecular simultaneous imaging in live mouse using semiconductor Compton camera. J. Anal. At. Spectrom. 2008, 23, 1089–1092. [Google Scholar] [CrossRef]
- Munekane, M.; Motomura, S.; Kamino, S.; Ueda, M.; Haba, H.; Yoshikawa, Y.; Yasui, H.; Hiromura, M.; Enomoto, S. Visualization of biodistribution of Zn complex with antidiabetic activity using semiconductor Compton camera GREI. Biochem. Biophys. Rep. 2016, 5, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Alnaaimi, M.A.; Royle, G.J.; Ghoggali, W.; Banoqitah, E.; Cullum, I.; Speller, R.D. Performance evaluation of a pixelated Ge Compton Camera. Phys. Med. Biol. 2011, 56, 3473. [Google Scholar] [CrossRef] [PubMed]
- Knoll, G.F. Radiation Detection and Measurement, 4th ed.; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2010; ISBN 978-0-470-13148-0. [Google Scholar]
- Zschornack, G. Handbook of X-Ray Data; Springer: Berlin/Heidelberg, Germany, 2007; ISBN 978-3-540-28618-9. [Google Scholar]
- Hofstadter, R. The Detection of Gamma-Rays with Thallium-Activated Sodium Iodide Crystals. Phys. Rev. 1949, 75, 796–810. [Google Scholar] [CrossRef]
- Watanabe, T.; Enomoto, R.; Muraishi, H.; Katagiri, H.; Kagaya, M.; Fukushi, M.; Kano, D.; Satoh, W.; Takeda, T.; Tanaka, M.M.; et al. Development of an omnidirectional gamma-ray imaging compton camera for low-radiation-level environmental monitoring. Jpn. J. Appl. Phys. 2018, 57, 026401. [Google Scholar] [CrossRef]
- Katagiri, H.; Satoh, W.; Enomoto, R.; Wakamatsu, R.; Watanabe, T.; Muraishi, H.; Kagaya, M.; Tanaka, S.; Wada, K.; Tanaka, M.; et al. Development of an all-sky gamma-ray Compton camera based on scintillators for high-dose environments. J. Nucl. Sci. Technol. 2018, 55, 1172–1179. [Google Scholar] [CrossRef]
- Muraishi, H.; Enomoto, R.; Katagiri, H.; Kagaya, M.; Watanabe, T.; Narita, N.; Kano, D. Visualization of low-level gamma radiation sources using a low-cost, high-sensitivity, omnidirectional compton camera. J. Vis. Exp. 2020, 155, e60463. [Google Scholar] [CrossRef]
- Kamada, K.; Endo, T.; Tsutumi, K.; Pejchal, J.; Nikl, M. Composition Engineering in Cerium-Doped (Lu, Gd)3(Ga, Al)5O12. Cryst. Growth Des. 2011, 3, 4484–4490. [Google Scholar] [CrossRef]
- Kamada, K.; Shoji, Y.; Kochurikhin, V.V.; Okumura, S.; Yamamoto, S.; Nagura, A.; Yeom, J.Y.; Kurosawa, S.; Yokota, Y.; Ohashi, Y.; et al. Growth and scintillation properties of 3 inch diameter Ce doped Gd3Ga3Al2O12 scintillation single crystal. J. Cryst. Growth 2016, 452, 81–84. [Google Scholar] [CrossRef]
- Kataoka, J.; Kishimoto, A.; Nishiyama, T.; Fujita, T.; Takeuchi, K.; Kato, T.; Nakamori, T.; Ohsuka, S.; Nakamura, S.; Hirayanagi, M.; et al. Handy Compton camera using 3D position-sensitive scintillators coupled with large-area monolithic MPPC arrays. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2013, 732, 403–407. [Google Scholar] [CrossRef]
- Kishimoto, A.; Kataoka, J.; Nishiyama, T.; Fujita, T. Performance and field tests of a handheld Compton camera using 3-D position-sensitive scintillators coupled to multi-pixel photon counter arrays. J. Instrum. 2014, 9, P11025. [Google Scholar] [CrossRef]
- Takeuchi, K.; Kataoka, J.; Nishiyama, T.; Fujita, T.; Kishimoto, A.; Ohsuka, S.; Nakamura, S.; Adachi, S.; Hirayanagi, M.; Uchiyama, T.; et al. “Stereo Compton cameras” for the 3-D localization of radioisotopes. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2014, 765, 187–191. [Google Scholar] [CrossRef]
- Kataoka, J.; Kishimoto, A.; Fujita, T.; Nishiyama, T.; Kurei, Y.; Tsujikawa, T.; Oshima, T.; Taya, T.; Iwamoto, Y.; Ogata, H.; et al. Recent progress of MPPC-based scintillation detectors in high precision X-ray and gamma-ray imaging. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2015, 784, 248–254. [Google Scholar] [CrossRef]
- Taya, T.; Kataoka, J.; Kishimoto, A.; Iwamoto, Y.; Koide, A.; Nishio, T.; Kabuki, S.; Inaniwa, T. First demonstration of real-time gamma imaging by using a handheld Compton camera for particle therapy. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2016, 831, 355–361. [Google Scholar] [CrossRef]
- Mochizuki, S.; Kataoka, J.; Koide, A.; Fujieda, K.; Maruhashi, T.; Kurihara, T.; Sueoka, K.; Tagawa, L.; Yoneyama, M.; Inaniwa, T. High-precision compton imaging of 4.4 MeV prompt gamma-ray toward an on-line monitor for proton therapy. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2019, 936, 43–45. [Google Scholar] [CrossRef]
- Fujieda, K.; Kataoka, J.; Mochizuki, S.; Tagawa, L.; Sato, S.; Tanaka, R.; Matsunaga, K.; Kamiya, T.; Watabe, T.; Kato, H.; et al. First demonstration of portable Compton camera to visualize 223-Ra concentration for radionuclide therapy. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2020, 958, 162802. [Google Scholar] [CrossRef]
- Ogane, K.; Uenomachi, M.; Shimazoe, K.; Takahashi, M.; Takahashi, H.; Seto, Y.; Momose, T. Simultaneous measurements of single gamma ray of 131I and annihilation radiation of 18F with Compton PET hybrid camera. Appl. Radiat. Isot. 2021, 176, 109864. [Google Scholar] [CrossRef]
- Takahashi, T.; Kawarabayashi, J.; Tomita, H.; Iguchi, T.; Takada, E. Development of omnidirectional gamma-imager with stacked scintillators. In Proceedings of the 2013 3rd International Conference on Advancements in Nuclear Instrumentation, Measurement Methods and their Applications (ANIMMA), Marseille, France, 23–27 June 2013; pp. 1–4. [Google Scholar] [CrossRef]
- Bloser, P.F.; Hunter, S.D.; Ryan, J.M.; McConnell, M.L.; Miller, R.S.; Jackson, T.N.; Bai, B.; Jung, S. Applications of Gas Imaging micro-well detectors to an advanced Compton telescope. New Astron. Rev. 2004, 48, 299–303. [Google Scholar] [CrossRef]
- Tanaka, A.; Hattori, K.; Kubo, H.; Miuchi, K.; Nagayoshi, T.; Nishimura, H.; Okada, Y.; Orito, R.; Sekiya, H.; Tada, A.; et al. Development of an advanced Compton camera with gaseous TPC and scintillator. Nucl. Instr. Methods A 2005, 546, 258–262. [Google Scholar]
- Kabuki, S.; Hattori, K.; Kohara, R.; Kunieda, E.; Kubo, A.; Kubo, H.; Miuchi, K.; Nakahara, T.; Nagayoshi, T.; Nishimura, H.; et al. Development of an Electron Tracking Compton Camera Using Micro Pixel Gas Chamber for Medical Imaging. Nucl. Instr. Methods A 2007, 580, 1031–1035. [Google Scholar] [CrossRef] [Green Version]
- Muichi, K.; Kubo, H.; Nagayoshi, T.; Okada, Y.; Orito, R.; Takada, A.; Takeda, A.; Tanimori, T.; Ueno, M.; Bouianov, O.; et al. Performance and applications of a µ-TPC. Nucl. Instr. Methods A 2004, 535, 236–241. [Google Scholar]
- Kurosawa, S.; Kubo, H.; Hattori, K.; Ida, C.; Iwaki, S.; Kabuki, S.; Kubo, A.; Kunieda, E.; Miuchi, k.; Nakahara, T.; et al. Development of an 8 × 8 array of LaBr3(Ce) scintillator pixels for a gaseous Compton gamma-ray camera. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2010, 623, 249–251. [Google Scholar] [CrossRef]
- Kabuki, S.; Kimura, H.; Amano, H.; Nakamoto, Y.; Kubo, H.; Miuchi, K.; Kurosawa, S.; Takahashi, M.; Kawashima, H.; Ueda, M.; et al. Electron-tracking Compton gamma-ray camera for small animal and phantom imaging. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2010, 623, 606–607. [Google Scholar] [CrossRef]
- Tanimori, T.; Kubo, H.; Takada, A.; Iwaki, S.; Komura, S.; Kurosawa, S.; Matsuoka, Y.; Miuchi, K.; Miyamoto, S.; Mizumoto, T. An electron-tracking telescope for a survey of the deep universe by MeV gamma-rays. Astrophys. J. 2015, 810, 28. [Google Scholar] [CrossRef]
- Mizutomo, T.; Tomono, D.; Takada, A.; Tanimori, T.; Komura, S.; Kubo, H.; Matsuoka, Y.; Mizumura, Y.; Nakamura, K.; Nakamura, S.; et al. A performance study of an electron-tracking Compton camera with a compact system for environmental gamma-ray observation. J. Instrum. 2015, 10, C06003. [Google Scholar]
- Vetter, K.; Chivers, D.; Plimley, B.; Coffer, A.; Aucott, T.; Looker, Q. First demonstration of electron-tracking based Compton imaging in solid-state detectors. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2011, 652, 599–601. [Google Scholar] [CrossRef]
- Yoneda, H.; Saito, S.; Watanabe, S.; Ikeda, H.; Takahashi, T. Development of Si-CMOS hybrid detectors towards electron tracking based Compton imaging in semiconductor detectors. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2018, 912, 269–273. [Google Scholar] [CrossRef]
- Plimley, B.; Chivers, D.; Coffer, A.; Vetter, K. Experimental Benchmark of Electron Trajectory Reconstruction Algorithm for Advanced Compton Imaging. IEEE Trans. Nucl. Sci. 2013, 60, 2308–2313. [Google Scholar] [CrossRef]
- Wen, J.; Zheng, X.; Gao, H.; Zeng, M.; Zhang, Y.; Yu, M.; Wu, Y.; Cang, J.; Ma, G.; Zhao, Z. Optimization of Timepix3-based conventional Compton camera using electron track algorithm. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2022, 1021, 165954. [Google Scholar] [CrossRef]
- Yoshihara, Y.; Shimazoe, K.; Mizumachi, Y.; Takahashi, H.; Kamada, K.; Takeda, A.; Tsuru, T.; Arai, Y. Development of electron-tracking Compton imaging system with 30-μm SOI pixel sensor. J. Instrum. 2017, 12, C01045. [Google Scholar] [CrossRef]
- Katagiri, H.; Narita, N.; Enomoto, R.; Muraishi, H.; Kano, D.; Watanabe, T.; Wakamatsu, R.; Kagaya, M.; Tanaka, M.M. Development of an omnidirectional Compton camera using CaF2(Eu) scintillators to visualize gamma rays with energy below 250 keV for radioactive environmental monitoring in nuclear medicine facilities. Nucl. Instr. Methods Phys. A 2021, 996, 165133. [Google Scholar] [CrossRef]
- Kasper, J.; Rusiecka, K.; Hetzel, R.; Kozani, M.K.; Lalik, R.; Magiera, A.; Stahl, A.; Wrońska, A. The SiFi-CC project—Feasibility study of a scintillation-fiber-based Compton camera for proton therapy monitoring. Phys. Med. 2020, 76, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, L.; Borja-Lloret, M.; Etxebeste, A.; Muñoz, E.; Oliver, J.F.; Ros, A.; Roser, J.; Senra, C.; Viegas, R.; Llosá, G. Performance evaluation of MACACO II Compton camera. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2021, 1014, 165702. [Google Scholar] [CrossRef]
- McCleskey, M.; Kaye, W.; Mackin, D.S.; Beddar, S.; He, Z.; Polf, J.C. Evaluation of a multistage CdZnTe Compton camera for prompt γ imaging for proton therapy. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2015, 785, 163–169. [Google Scholar] [CrossRef]
- Bolotnikov, A.E.; Camarda, G.S.; de Geronimo, G.; Fried, J.; Hodges, D.; Hossain, A.; Kim, K.; Mahler, G.; Giraldo, L.O.; Vernon, E.; et al. A 4 × 4 array module of position-sensitive virtual Frisch-grid CdZnTe detectors for gamma-ray imaging spectrometers. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2020, 954, 161036. [Google Scholar] [CrossRef]
- Tanimori, T.; Ikeno, M.; Kubo, H.; Miuchi, K.; Kabuki, S.; Parker, J.D.; Kishimoto, Y.; Komura, S.; Kurosawa, S.; Iwaki, S.; et al. Development of electron tracking Compton camera for both balloon and future satellite experiments for MeV gamma-ray astronomy. In Space Telescopes and Instrumentation 2012: Ultraviolet to Gamma Ray; SPIE: Bellingham, WA, USA, 2012; Volume 8443. [Google Scholar] [CrossRef]
- Mizumura, Y.; Tanimori, T.; Kubo, H.; Takada, A.; Parker, J.D.; Mizumoto, T.; Sonoda, S.; Tomono, D.; Sawano, T.; Nakamura, K. Development of a 30 cm-cube Electron-Tracking Compton camera for the SMILE-II Experiment. J. Instrum. 2014, 9, C0504. [Google Scholar] [CrossRef]
- Schönfelder, V.; Bennett, K.; Blom, J.J.; Bloemen, H.; Collmar, W.; Connors, A.; Diehl, R.; Hermsen, W.; Iyudin, A.; Kippen, R.M.; et al. The first COMPTEL source catalogue. Astron. Astrophys. Suppl. Ser. 2000, 143, 145–179. [Google Scholar] [CrossRef]
- Takada, A.; Kubo, H.; Nishimura, H.; Ueno, K.; Hattori, K.; Kabuki, S.; Kurosawa, S.; Miuchi, K.; Mizuta, E.; Nagayoshi, T.; et al. Observation of diffuse cosmic and atmospheric gamma rays at balloon altitudes with an electron-tracking Compton camera. Astrophys. J. 2011, 733, 13. [Google Scholar] [CrossRef]
- Hamaguchi, K.; Tanimori, T.; Takada, T.; Beacom, J.F.; Gunji, S.; Mori, M.; Nakamori, T.; Shrader, C.R.; Smith, D.M.; Tamagawa, T.; et al. A space-based all-sky MeV gamma ray survey with the electron tracking Compton camera. arXiv 2019, arXiv:1907.06658. [Google Scholar]
- Takada, A.; Takemura, T.; Yoshikawa, K.; Mizumura, Y.; Ikeda, T.; Nakamura, Y.; Onozaka, T.; Abe, M.; Hamaguchi, K.; Kubo, H.; et al. First observation of the MeV gamma-ray universe with bijective imaging spectroscopy using the electron-tracking Compton telescope on board SMILE-2+. Astrophys. J. 2022, 930, 6. [Google Scholar] [CrossRef]
- De Angelis, A.; Tatischeff, V.; Grenier, I.A.; McEnery, J.; Mallamaci, M.; Tavani, M.; Oberlack, U.; Hanlon, L.; Walter, R.; Argan, A.; et al. Science with e-ASTROGAM: A space mission for MeV–GeV gamma-ray astrophysics. J. High Energy Astrophys. 2018, 19, 1–106. [Google Scholar] [CrossRef]
- Singh, M.; Brechner, R.R. Experimental test-object study of electronically collimated SPECT. J. Nucl. Med. 1990, 31, 178–186. [Google Scholar]
- Kabuki, S.; Hattori, K.; Kawashima, H.; Kimura, H.; Kohara, R.; Kubo, A.; Kurosawa, S.; Kunieda, E.; Miuchi, K.; Miyazaki, O.; et al. Diagnostic approach of using an electron tracking compton gamma-ray camera based on small animal and phantom experiments. In Proceedings of the 2007 IEEE Nuclear Science Symposium Conference Record, Honolulu, HI, USA, 26 October–3 November 2007; pp. 3395–3399. [Google Scholar] [CrossRef]
- Takeda, S.; Odaka, H.; Ishikawa, S.; Watanabe, S.; Aono, H.; Takahashi, T.; Kanayama, Y.; Hiromura, M.; Enomoto, S. Demonstration of in-vivo Multi-Probe Tracker Based on a Si/CdTe Semiconductor Compton Camera. IEEE Trans. Nucl. Sci. 2012, 59, 70–76. [Google Scholar] [CrossRef]
- Kishimoto, A.; Kataoka, J.; Taya, T.; Tagawa, L.; Mochizuki, S.; Ohsuka, S.; Nagao, Y.; Kurita, K.; Yamaguchi, M.; Kawachi, N.; et al. First demonstration of multi-color 3-D in vivo imaging using ultra-compact Compton camera. Sci. Rep. 2017, 7, 2110. [Google Scholar] [CrossRef]
- Uenomachi, M.; Takahashi, M.; Shimazoe, K.; Takahashi, H.; Kamada, K.; Orita, T.; Ogane, K.; Tsuji, A.B. Simultaneous in vivo imaging with PET and SPECT tracers using a Compton-PET hybrid camera. Sci. Rep. 2021, 11, 17933. [Google Scholar] [CrossRef]
- Suzuki, Y.; Yamaguchi, M.; Odaka, H.; Shimada, H.; Yoshida, Y.; Torikai, K.; Satoh, T.; Arakawa, K.; Kawachi, N.; Watanabe, S.; et al. Three-dimensional and multienergy gamma-ray simultaneous imaging by using a Si/CdTe Compton camera. Radiology 2013, 267, 941–947. [Google Scholar] [CrossRef]
- Shimazoe, K.; Yoshina, M.; Ohshima, Y.; Uenomachi, M.; Oogane, K.; Orita, T.; Takahashi, H.; Kamada, K.; Yoshikawa, A.; Takahashi, M. Development of simultaneous PET and Compton Imaging using GAGG-SiPM based pixel detector. Nucl. Instrum. Methods Phys. A 2020, 954, 161499. [Google Scholar] [CrossRef]
- Yoshida, E.; Tashima, H.; Nagatsu, K.; Tsuji, A.B.; Kamada, K.; Parodi, K.; Yamaya, T. Whole gamma imaging: A new concept of PET combined with Compton Imaging. Phys. Med. Biol. 2020, 65, 125013. [Google Scholar] [CrossRef]
- Grignon, C.; Barbet, J.; Bardiès, M.; Carlier, T.; Chatal, J.F.; Couturier, O.; Cussonneau, J.P.; Faivre, A.; Ferrer, L.; Girault, S.; et al. Nuclear medical imaging using β+γ coincidences from 44Sc radio-nuclide with liquid xenon as detection medium. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2007, 571, 142–145. [Google Scholar] [CrossRef]
- Kolstein, M.; Chmeissani, M. Using triple gamma coincidences with a pixelated semiconductor Compton-PET scanner: A simulation study. J. Instrum. 2016, 11, C01039. [Google Scholar] [CrossRef]
- Sitarz, M.; Cussonneau, J.P.; Matulewicz, T.; Haddad, F. Radionuclide candidates for β+γ coincidence PET: An overview. Appl. Radiat. Isot. 2020, 155, 108898. [Google Scholar] [CrossRef]
- Kishimoto, A.; Kataoka, J.; Koide, A.; Sueoka, K.; Iwamoto, Y.; Taya, T.; Ohsuka, S. Development of compact scintillator-based high-resolution Compton camera for molecular imaging. Nucl. Instr. Methods Phys. A 2017, 845, 656–659. [Google Scholar] [CrossRef]
- Omata, A.; Kataoka, J.; Fujieda, K.; Sato, S.; Kuriyama, E.; Kato, H.; Toyoshima, A.; Teramoto, T.; Ooe, K.; Liu, Y.; et al. Performance demonstration of a hybrid Compton camera with an active pinhole for wide-band X-ray and gamma-ray imaging. Sci. Rep. 2020, 10, 14064. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.-H.; Kim, J.-W. Monte Carlo design study of a gamma detector system to locate distal dose falloff in proton therapy. IEEE Trans. Nucl. Sci. 2009, 56, 46–50. [Google Scholar] [CrossRef]
- Kabuki, S.; Ueno, K.; Kurosawa, S.; Iwaki, S.; Kubo, H.; Miuchi, K.; Fuji, Y.; Kim, D.; Kim, J.; Kohara, R.; et al. Study on the use of electron-tracking Compton gamma-ray camera to monitor the therapeutic proton dose distribution in real time. In Proceedings of the 2009 IEEE Nuclear Science Symposium Conference Record (NSS/MIC), Orlando, FL, USA, 24 October–1 November 2009; pp. 2437–2440. [Google Scholar]
- Polf, J.C.; Avery, S.; Mackin, D.S.; Beddar, S. Imaging of prompt gamma rays emitted during delivery of clinical proton beams with a Compton camera: Feasibility studies for range verification. Phys. Med. Biol. 2015, 60, 7085–7099. [Google Scholar] [CrossRef]
- Fontana, M.; Ley, J.-L.; Dauvergne, D.; Freud, N.; Krimmer, J.; Letang, J.M.; Maxim, V.; Richard, M.-H.; Rinaldi, I.; Testa, E. Monitoring Ion Beam Therapy with a Compton Camera: Simulation Studies of the Clinical Feasibility. IEEE Trans. Radiat. Plasma Med. Sci. 2020, 4, 218–232. [Google Scholar] [CrossRef]
- Parodi, K.; Paganetti, H.; Shih, H.A.; Michaud, S.; Loeffler, J.S.; Delaney, T.F.; Liebsch, N.J.; Munzenrider, J.E.; Fischman, A.J.; Knopf, A.; et al. Patient study of in vivo verification of beam delivery and range, using positron emission tomography and computed tomography imaging after proton therapy. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 920–934. [Google Scholar] [CrossRef]
- Bongrand, A.; Busato, E.; Force, P.; Martin, F.; Montarou, G. Use of short-lived positron emitters for in-beam and real-time β+ range monitoring in proton therapy. Phys. Med. 2020, 69, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Draeger, E.; Makin, D.; Peterson, S.; Chen, H.; Avery, S.; Beddar, S.; Polf, J.C. 3D prompt gamma imaging for proton beam range verification. Phys. Med. Biol. 2018, 63, 035019. [Google Scholar] [CrossRef]
- Golnik, C.; Bemmerer, D.; Enghardt, W.; Fiedler, F.; Hueso-Gonzalez, F.; Pausch, G.; Romer, K.; Rohling, H.; Schone, S.; Wagner, L.; et al. Tests of Compton imaging prototype in a monoenergetic 4.44 MeV photon field—A benchmark setup for prompt gamma-ray imaging. J. Instrum. 2016, 11, P06009. [Google Scholar] [CrossRef]
- Hueso-González, F.; Pausch, G.; Petzoldt, J.; Römer, K.E.; Enghardt, W. Prompt Gamma Rays Detected with a BGO Block Compton Camera Reveal Range Deviations of Therapeutic Proton Beams. IEEE Trans. Radiat. Plasma Med. Sci. 2017, 1, 76–86. [Google Scholar] [CrossRef]
- International Atomic Energy Agency. Management of Naturally Occurring Radioactive Material (NORM) in Industry; Proceedings Series; International Atomic Energy Agency, IAEA: Vienna, Austria, 2022. [Google Scholar]
- Matsunaga, H.; Kashiwazaki, Y.; Orita, M.; Taira, Y.; Takamura, N. Risk perception of internal and external radiation exposure among administration staff affected by the Fukushima Daiichi Nuclear Power Plant accident. J. Environ. Radioact. 2022, 248, 106869. [Google Scholar] [CrossRef]
- Carminati, M.; Di Vita, D.; Morandi, G.; D’Adda, I.; Fiorini, C. Handheld Magnetic-Compliant Gamma-Ray Spectrometer for Environmental Monitoring and Scrap Metal Screening. Sensors 2022, 22, 1412. [Google Scholar] [CrossRef]
- Tomono, D.; Tanimori, T.; Kubo, H.; Takeda, A.; Mizumoto, T.; Mizumura, Y.; Sawano, T.; Matsuoka, Y.; komura, S.; Nakamura, S.; et al. First application to environmental gamma-ray imaging with an electron tracking compton camera. In Proceedings of the 2013 IEEE Nuclear Science Symposium and Medical Imaging Conference (2013 NSS/MIC), Seoul, Korea, 27 October–2 November 2013; pp. 1–5. [Google Scholar] [CrossRef]
- Buonanno, L.; di Vita, D.; Carminati, M.; Fiorini, C. A Directional Gamma-Ray Spectrometer with Microcontroller-Embedded Machine Learning. IEEE J. Emerg. Sel. Top. Circuits Syst. 2020, 10, 433–443. [Google Scholar] [CrossRef]
- Jiang, J.; Shimazoe, K.; Nakamura, Y.; Takahashi, H.; Shikaze, Y.; Nishizawa, Y.; Yoshida, M.; Sanada, Y.; Torii, T.; Yoshino, M.; et al. A prototype of aerial radiation monitoring system using an unmanned helicopter mounting a GAGG scintillator Compton camera. J. Nucl. Sci. Technol. 2016, 53, 1067–1075. [Google Scholar] [CrossRef]
- Shikaze, Y.; Nishizawa, Y.; Sanada, Y.; Torii, T.; Jiang, J.; Shimazoe, K.; Takahashi, H.; Yoshino, M.; Ito, S.; Endo, T.; et al. Field test around Fukushima Daiichi nuclear power plant site using improved Ce:Gd3(Al,Ga)5O12 scintillator Compton camera mounted on an unmanned helicopter. J. Nucl. Sci. Technol. 2016, 53, 1907–1918. [Google Scholar] [CrossRef]
- Sato, Y.; Kawabata, K.; Ozawa, S.; Izumi, R.; Kaburagi, M.; Tanifuji, Y.; Terasaka, Y.; Miyamura, H.N.; Kawamura, T.; Suzuki, T.; et al. Radiation imaging system using a Compact Gamma-ray Imager mounted on a remotely operated machine. IFAC Pap. Online 2017, 50, 1062–1066. [Google Scholar] [CrossRef]
- Sato, Y.; Terasaka, Y.; Utsugi, W.; Kikuchi, H.; Kiyooka, H.; Torii, T. Radiation imaging using a compact Compton camera mounted on a crawler robot inside reactor buildings of Fukushima Daiichi Nuclear Power Station. J. Nucl. Sci. Technol. 2019, 56, 801–808. [Google Scholar] [CrossRef]
- Sato, Y.; Terasaka, Y. Radiation imaging using an integrated Radiation Imaging System based on a compact Compton camera under unit 1/2 exhaust stack of Fukushima Daiichi Nuclear Power Station. J. Nucl. Sci. Technol. 2022, 59, 677–687. [Google Scholar] [CrossRef]
- Al Hamrashdi, H.; Monk, S.D.; Cheneler, D. Passive Gamma-Ray and Neutron Imaging Systems for National Security and Nuclear Non-Proliferation in Controlled and Uncontrolled Detection Areas: Review of Past and Current Status. Sensors 2019, 19, 2638. [Google Scholar] [CrossRef]
- Hoover, A.S.; Kippen, R.M.; Sullivan, J.P.; Rawool-Sullivan, M.W.; Baird, W.; Sorensen, E.B. The LANL prototype Compton gamma-ray imager: Design and image reconstruction techniques. In Proceedings of the IEEE Symposium Conference Record Nuclear Science, Rome, Italy, 16–22 October 2004; pp. 1630–1634. [Google Scholar] [CrossRef]
- Niedermayr, T.; Vetter, K.; Mihailescu, L.; Schmid, G.J.; Beckedahl, D.; Blair, J.; Kammeraad, J. Gamma-ray imaging with a coaxial HPGe detector. Nucl. Instrum. Methods Phys. Res. A 2005, 553, 501–511. [Google Scholar] [CrossRef]
- Sweeney, A.; Boston, A.J.; Boston, H.C.; Cresswell, J.P.; Dormand, J.; Ellis, M.; Harkness, L.J.; Jones, M.; Judson, D.S.; Nolan, P.J.; et al. Compton imaging with a planar semiconductor system using pulse shape analysis. In Chemical, Biological, Radiological, Nuclear, and Explosives (CBRNE) Sensing XII; SPIE: Bellingham, WA, USA, 2011; Volume 8018, p. 80180J-1. [Google Scholar]
- Poitrasson-Rivière, A.; Hamel, M.C.; Ide, K.; Polack, J.K.; McMillan, K.L.; Clarke, S.D.; Flaska, M.; Pozzi, S.A.; Pausch, G.; Herbach, C.M.; et al. Large-scale Compton-camera simulations, validation experiments, and image reconstruction. In Proceedings of the 2011 IEEE Nuclear Science Symposium Conference Record, Valencia, Spain, 23–29 October 2011; pp. 212–215. [Google Scholar] [CrossRef]
- Kei, G.; Matsumura, D.; Sekino, H. Development of technology for NORM visualization. J. Jpn. Assoc. Pet. Technol. 2015, 80, 219–227. [Google Scholar] [CrossRef]
- Japan Pavilion. Globe 2014. Available online: https://www.jetro.go.jp/ext_images/canada/pdf/jogmec.pdf (accessed on 26 March 2022).
- Rohe, R.C.; Sharfi, M.M.; Kecevar, K.A.; Valentine, J.D.; Bonnerave, C. The spatially-variant back-projection point kernel function of an energy-subtraction Compton scatter camera for medical imaging. In Proceedings of the 1996 IEEE Nuclear Science Symposium Conference Record, Anaheim, CA, USA, 2–9 November 1996; Volume 2, pp. 1260–1264. [Google Scholar] [CrossRef]
- Wilderman, S.J.; Rogers, W.L.; Knoll, G.F.; Engdahl, J.C. Fast algorithm for list mode back-projection of Compton scatter camera data. IEEE Trans. Nucl. Sci. 1998, 45, 957–962. [Google Scholar] [CrossRef]
- Haefner, A.; Gunter, D.; Barnowski, R.; Vetter, K. A Filtered Back-Projection Algorithm for 4π Compton Camera Data. IEEE Trans. Nucl. Sci. 2015, 62, 1911–1917. [Google Scholar] [CrossRef]
- Mundy, D.W.; Herman, M.G. An accelerated threshold-based back-projection algorithm for Compton camera image reconstruction. Med. Phys. 2011, 38, 15–22. [Google Scholar] [CrossRef]
- Lee, H.; Lee, T.; Lee, W. Adaption of Filtered Back-Projection to Compton Imaging with Non-Uniform Azimuthal Geometry. J. Korean Phys. Soc. 2016, 68, 1156–1164. [Google Scholar] [CrossRef]
- Basko, R.; Zeng, G.L.; Gullberg, G.T. Analytical reconstruction formula for one-dimensional Compton camera. IEEE Trans. Nucl. Sci. 1997, 44, 1342–1346. [Google Scholar] [CrossRef]
- Basko, R.; Gullberg, G.T.; Zeng, G.L. Using two one-dimensional Compton cameras of finite extent for transaxial tomography. J. Nucl. Med. 1997, 38, 1850. [Google Scholar]
- Basko, R.; Zeng, G.L.; Gullberg, G.T. Fully three-dimensional image reconstruction from “V”-projections acquired by Compton camera with three vertex electronic collimation. In Proceedings of the 1997 IEEE Nuclear Science Symposium Conference Record, Albuquerque, NM, USA, 9–15 November 1997; Volume 2, pp. 1077–1081. [Google Scholar] [CrossRef]
- Parra, L.C. Reconstruction of cone-beam projections from Compton scattered data. IEEE Trans. Nucl. Sci. 2000, 47, 1543–1550. [Google Scholar] [CrossRef]
- Tomitani, T.; Hirasawa, M. Image reconstruction from limited angle Compton camera data. Phys. Med. Biol. 2002, 47, 2129. [Google Scholar] [CrossRef]
- Hirasawa, M.; Tomitani, T. An analytical image reconstruction algorithm to compensate for scattering angle broadening in Compton cameras. Phys. Med. Biol. 2003, 48, 1009. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; He, Z. Filtered Back-Projection in4piCompton Imaging with a Single 3D Position Sensitive CdZnTe Detector. IEEE Trans. Nucl. Sci. 2006, 53, 2787–2796. [Google Scholar] [CrossRef]
- Shy, D.; Chen, Z.; Fessler, J.A.; He, Z. Filtered Backprojection in Compton Imaging Using a Spherical Harmonic Wiener Filter with Pixelated CdZnTe. IEEE Trans. Nucl. Sci. 2021, 68, 211–219. [Google Scholar] [CrossRef]
- Shepp, L.A.; Vardi, Y. Maximum Likelihood Reconstruction for Emission Tomography. IEEE Trans. Med. Imaging 1982, 1, 113–122. [Google Scholar] [CrossRef]
- Wilderman, S.J.; Clinthorne, N.H.; Fessler, J.A.; Rogers, W.L. List-mode maximum likelihood reconstruction of Compton scatter camera images in nuclear medicine. In Proceedings of the 1998 IEEE Nuclear Science Symposium Conference Record, IEEE Nuclear Science Symposium and Medical Imaging Conference (Cat. No.98CH36255), Toronto, ON, Canada, 8–14 November 1998; Volume 3, pp. 1716–1720. [Google Scholar] [CrossRef]
- Ikeda, S.; Odaka, H.; Uemura, M.; Takahashi, T.; Watanabe, S.; Takeda, S. Bin mode estimation methods for Compton camera imaging. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2014, 760, 46–56. [Google Scholar] [CrossRef]
- Hudson, H.M.; Larkin, R.S. Accelerated image reconstruction using ordered subsets of projection data. IEEE Trans. Med. Imaging 1994, 13, 601–609. [Google Scholar] [CrossRef]
- Kim, S.M.; Lee, J.S.; Lee, C.S.; Kim, C.H.; Lee, M.C.; Lee, D.S.; Lee, S.-J. Fully three-dimensional OSEM-based image reconstruction for Compton imaging using optimized ordering schemes. Phys. Med. Biol. 2010, 55, 5007–5027. [Google Scholar] [CrossRef] [PubMed]
- Tornga, S.R.; Sullivan, M.W.R.; Sullivan, J.P. Three-Dimensional Compton Imaging Using List-Mode Maximum Likelihood Expectation Maximization. IEEE Trans. Nucl. Sci. 2009, 56, 1372–1376. [Google Scholar] [CrossRef]
- Feng, Y.; Etxebeste, A.; Létang, J.M.; Sarrut, D.; Maxim, V. Total variation and point spread function priors for MLEM reconstruction in Compton camera imaging. In Proceedings of the 2018 IEEE Nuclear Science Symposium and Medical Imaging Conference Proceedings (NSS/MIC), Sydney, NSW, Australia, 10–17 November 2018; pp. 1–3. [Google Scholar] [CrossRef]
- Daniel, G.; Limousin, O.; Maier, D.; Meuris, A.; Carrel, F. Compton imaging reconstruction methods: A comparative performance study of direct back-projection, SOE, a new Bayesian algorithm and a new Compton inversion method applied to real data with caliste. EPJ Web Conf. 2020, 225, 06006. [Google Scholar] [CrossRef]
- Kohlhase, N.; Wegener, T.; Schaar, M.; Bolke, A.; Etxebeste, A.; Sarrut, D.; Rafecas, M. Capability of MLEM and OE to detect range shifts with a Compton camera in particle therapy. IEEE Trans. Radiat. Plasma Med. Sci. 2020, 4, 233–242. [Google Scholar] [CrossRef]
- Feng, Y.; Létang, J.M.; Sarrut, D.; Maxim, V. Influence of Doppler broadening model accuracy in Compton camera list-mode MLEM reconstruction. Inverse Probl. Sci. Eng. 2021, 29, 3509–3529. [Google Scholar] [CrossRef]
- Yabu, G.; Yoneda, H.; Orita, T.; Takeda, S.; Caradonna, P.; Takahashi, T.; Watanabe, S.; Moriyama, F. Tomographic Imaging by a Si/CdTe Compton Camera for ¹¹¹In and ¹3¹I Radionuclides. IEEE Trans. Radiat. Plasma Med. Sci. 2022, 6, 592–600. [Google Scholar] [CrossRef]
- Yao, Z.; Xiao, Y.; Wang, B.; Liu, Y.; Hou, Q.; Lu, L.; Chen, Z. Study of 3D fast Compton camera image reconstruction method by algebraic spatial sampling. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2020, 954, 161345. [Google Scholar] [CrossRef]
- Green, P.J. On Use of the EM for Penalized Likelihood Estimation. J. R. Stat. Soc. Ser. B (Methodol.) 1990, 52, 443–452. [Google Scholar]
- Andreyev, A.; Sitek, A.; Celler, A. Stochastic image reconstruction method for Compton camera. In Proceedings of the 2009 IEEE Nuclear Science Symposium Conference Record (NSS/MIC), Orlando, FL, USA, 24 October–1 November 2009. [Google Scholar]
- Andreyev, A.; Sitek, A.; Celler, A. Fast image reconstruction for Compton camera using stochastic origin ensemble approach. Med. Phys. 2010, 38, 429–438. [Google Scholar] [CrossRef]
- Andreyev, A.; Celler, A.; Ozsahin, I.; Sitek, A. Resolution recovery for Compton camera using origin ensemble algorithm. Med. Phys. 2016, 43 Pt 1, 4866–4876. [Google Scholar] [CrossRef]
- Harayama, A.; Takeda, S.; Sato, G.; Ikeda, H.; Watanabe, S.; Takahashi, T. Development of an ASIC for Si/CdTe detectors in a radioactive substance visualizing system. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2014, 765, 223–226. [Google Scholar] [CrossRef] [Green Version]
- Iyomoto, N.; Kurume, Y.; Kuroiwa, T.; Asagawa, S.; Tsuruta, T.; Nishida, Y.; Hamammura, Y.; Maehata, K.; Hayashi, T.; Muramatsu, H.; et al. Development of Gamma-Ray Position-Sensitive Transition-Edge Sensor Microcalorimeters. J. Low Temp. Phys. 2020, 200, 233–238. [Google Scholar] [CrossRef]
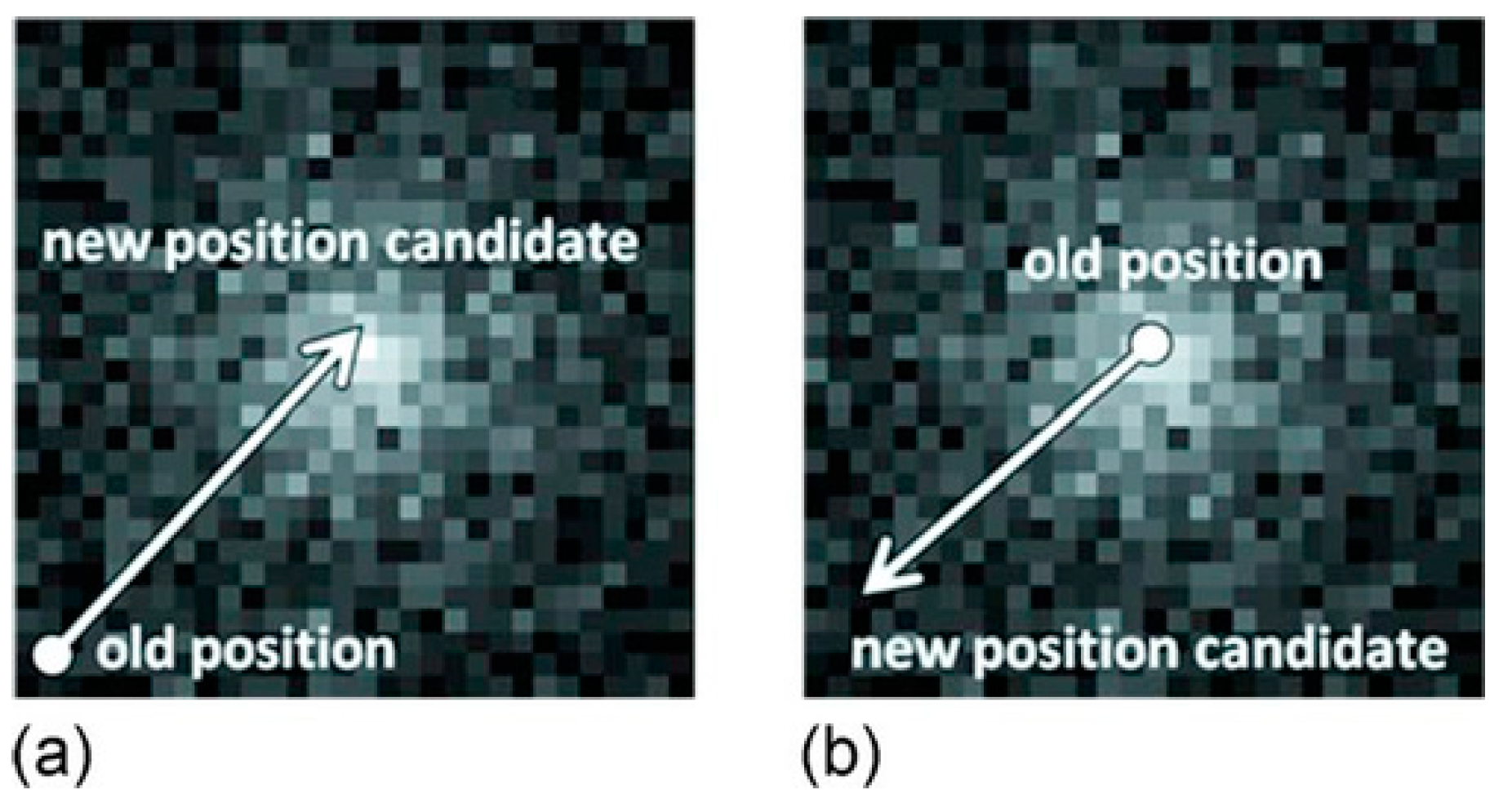
- Sakai, M.; Yamazaki, H.; Tashiro, M.; Sakurai, H. Position dependency analysis of point spread function in Compton imaging. In Proceedings of the International Conference on Technology and Social Science (ICTSS 2020), Kiryu, Japan, 2–4 December 2020; Available online: http://conf.e-jikei.org/ICTSS/2020/ (accessed on 15 January 2022).




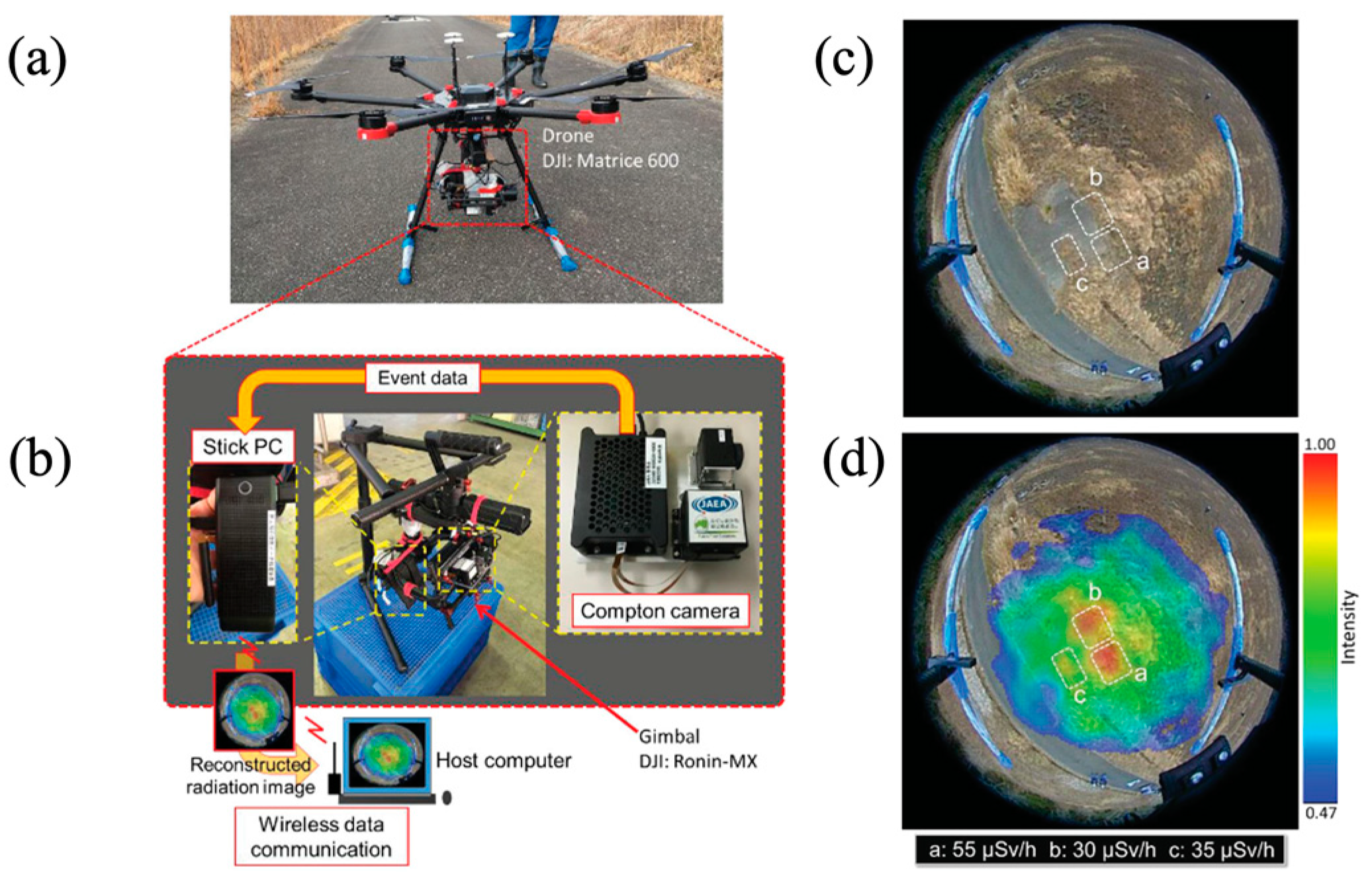
| Leakage Isotopes from FDNP | Emission Amount [PBq] | Disintegration | Decay Time |
|---|---|---|---|
| 137Cs | 6.1~35.9 | β− | 30 years |
| 134Cs | 11.8~18 | β− | 2.1 years |
| 85Kr | 44 | β− | 10.76 years |
| 129I | 5.5 × 10−5~5.5 × 10−6 | β− | 1.57 × 107 years |
| 131I | 65~380 | β− | 8 days |
| 133Xe | 11,400~15,000 | α β− | 5.245 days |
| Semiconductor Detector | Density [g/cm3] | Atomic Number [Z] | Band Gap Energy [eV] | Ionization Potential (∊) [eV] |
|---|---|---|---|---|
| Si | 2.33 | 14 | 1.12 | 3.6 |
| CdTe | 5.58 | 48, 52 | 1.44 | 4.43 |
| Ge | 5.33 | 32 | 0.67 | 2.9 |
| CdZnTe | 5.81 | 48, 30, 52 | 1.6 | 1.6 |
| HgI2 | 6.40 | 80, 53 | 2.13 | 4.2 |
| GaAs | 5.32 | 31, 33 | 1.42 | 4.3 |
| Scintillators Detectors | Density [g/cm3] | Light Yield [photon/MeV] | Decay Time [ns] | Peak Emissions [nm] | Atomic Number (Z) [eV] |
|---|---|---|---|---|---|
| NaI(Tl) | 3.7 | 45,000 | 230 | 415 | 51 |
| CsI(Tl) | 4.5 | 56,000 | 1000 | 530 | 54 |
| Ce:GAGG | 6.6 | 57,000 | 88 (91%) + 258 (9%) | 520 | 54.4 |
| CaF2(Eu) | 3.18 | 24,000 | 940 | 435 | 54 |
| BGO | 7.13 | 8000 | 300 | 480 | 74 |
| Ce:LaBr3 | 5.1 | 75,000 | 30 | 375 | 46.9 |
| Ce:LSO | 7.35 | 25,000 | 42 | 435 | 66 |
| Radio Isotopes | Half-Life Time | Decay Type | Energy [keV] |
|---|---|---|---|
| 111In | 2.83 days | EC | 171, 245 |
| 123I | 13.2 hrs | EC | 159 |
| 99Tc | 6.0 hrs | EC | 141 |
| 18F | 108 min | An. (β+) | 511 |
| 67Ga | 78.3 hrs | EC | 93, 184, 296, 388 |
| 85Sr | 64.8 days | EC | 514 |
| 64Cu | 12.7 hrs | EC | 579, 653, 1350 |
| 131I | 8.04 days | EC | 364 |
| 65Zn | 244 days | EC | 1116 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parajuli, R.K.; Sakai, M.; Parajuli, R.; Tashiro, M. Development and Applications of Compton Camera—A Review. Sensors 2022, 22, 7374. https://doi.org/10.3390/s22197374
Parajuli RK, Sakai M, Parajuli R, Tashiro M. Development and Applications of Compton Camera—A Review. Sensors. 2022; 22(19):7374. https://doi.org/10.3390/s22197374
Chicago/Turabian StyleParajuli, Raj Kumar, Makoto Sakai, Ramila Parajuli, and Mutsumi Tashiro. 2022. "Development and Applications of Compton Camera—A Review" Sensors 22, no. 19: 7374. https://doi.org/10.3390/s22197374






