Implementation of a Morphological Filter for Removing Spikes from the Epileptic Brain Signals to Improve Identification Ripples
Abstract
1. Introduction
2. Study Background
- 1.
- Non-invasive (first line):
- Video EEG;
- Neuro-psychology;
- Magnetic resonance imaging (MRI)/functional magnetic resonance imaging (fMRI).
- 2.
- Non-invasive (second line):
- Positron emission tomography (PET);
- Single photon emission computed tomography (SPECT);
- Magnetonecephalography (MEG).
- 3.
- Invasive (third line):
- Intracranial EEG.
3. Materials and Methods
3.1. Data Selection
3.2. Study Participants
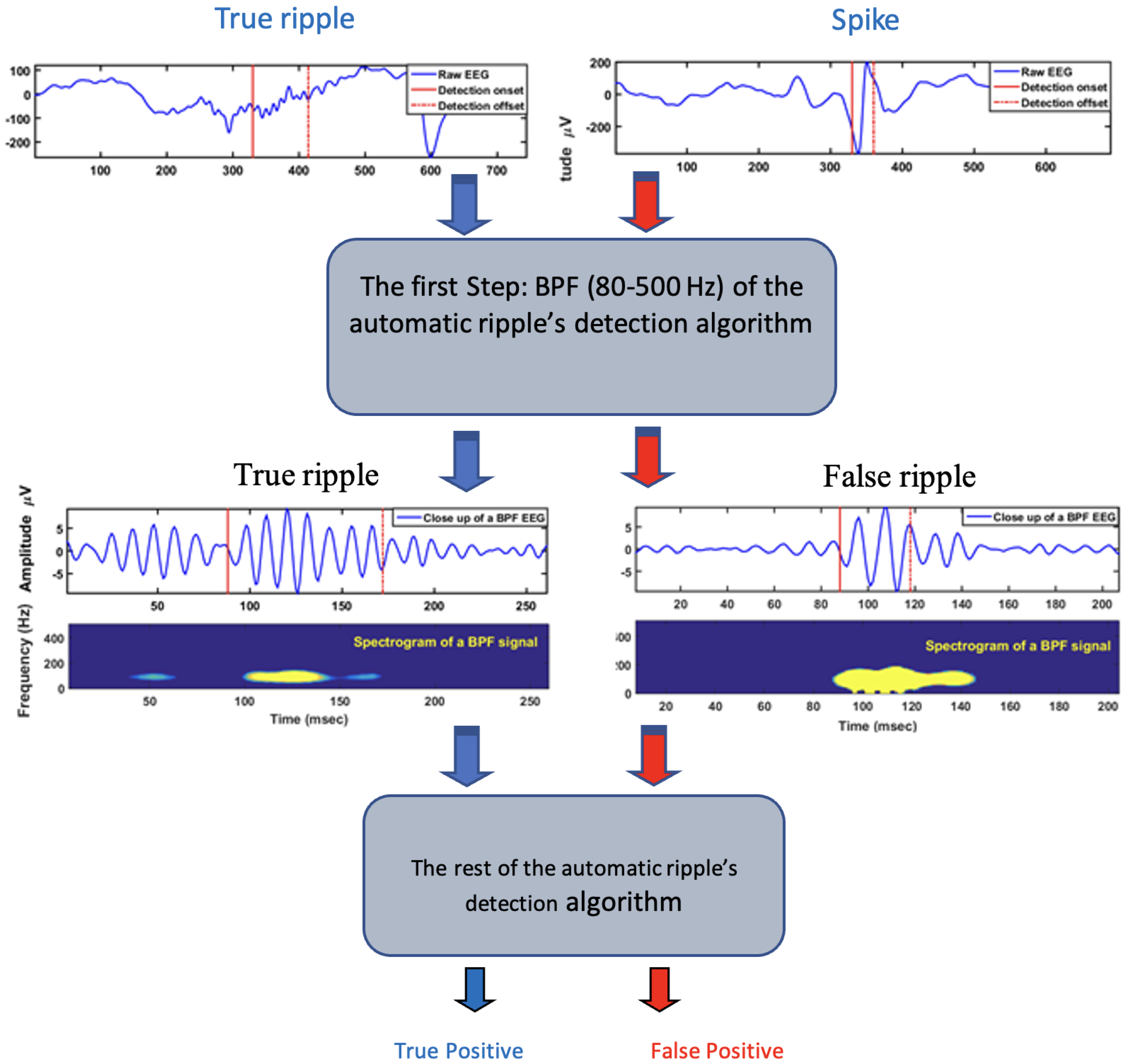
3.3. Method for Ripples and Spikes Identification
3.4. Optimal Threshold for Spikes Truncating Identification
- —the analyzed EEG signal;
- —the structuring element;
- —the reflection of structuring element;
- D—the domain of signal .
- (1)
- Read the raw signal and deal with each event in the data set (Figure 3):
- (2)
- In order to manifest the spike from the EEG background, the rectified first difference signal was computed as ; then the moving average filter with a suitable window size of 10 ms was used to smooth the signal (Figure 4).
- (3)
- Now it is necessary to apply the one-dimensional morphology filter. The following closing and opening filters were used:
- (a)
- To envelope the spike and background signal, a closing (dilation, then erosion) filter was applied with an appropriate 1 ms window size (Figure 5).
- (b)
- To truncate the enveloped spike from an appropriated level, an opening (erosion, then dilation) filter was used with an arbitrary value of 1 ms window size (Figure 6).
- (4)
- In this step, we sorted out all the truncated values of all events in the training set, then we selected the maximum value to set the initial threshold. As a result, most spikes (false positives) and very few ripples (true positives) were removed from the training set. Now to evaluate the performance of our technique, we measured the sensitivity (SE) and false detection rate (FDR) for all events in the new training set (events of ripples and few spikes) (Figure 7).
4. Results
- True positive (TP): spikes detected as spikes;
- False positive (FP): ripples detected as spikes;
- True negative (TN): ripples detected as ripples;
- False negative (FN): spikes detected as ripples.
5. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomas, G.P.; Jobst, B.C. Critical review of the responsive neurostimulator system for epilepsy. Med. Devices 2015, 8, 405. [Google Scholar]
- Batson, S.; Shankar, R.; Conry, J.; Boggs, J.; Radtke, R.; Mitchell, S.; Barion, F.; Murphy, J.; Danielson, V. Efficacy and safety of VNS therapy or continued medication management for treatment of adults with drug-resistant epilepsy: Systematic review and meta-analysis. J. Neurol. 2022, 269, 2874–2891. [Google Scholar] [CrossRef]
- Galanopoulou, A.S.; Buckmaster, P.S.; Staley, K.J.; Moshé, S.L.; Perucca, E.; Engel, J., Jr.; Löscher, W.; Noebels, J.L.; Pitkänen, A.; Stables, J.; et al. Identification of new epilepsy treatments: Issues in preclinical methodology. Epilepsia 2012, 53, 571–582. [Google Scholar] [CrossRef]
- World Health Organization; Global Campaign against Epilepsy; World Health Organization. Atlas: Epilepsy Care in The World; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- Moshé, S.L.; Perucca, E.; Ryvlin, P.; Tomson, T. Epilepsy: New advances. Lancet 2015, 385, 884–898. [Google Scholar] [CrossRef]
- Alotaiby, T.N.; Alshebeili, S.A.; Alshawi, T.; Ahmad, I.; El-Samie, A.; Fathi, E. EEG seizure detection and prediction algorithms: A survey. EURASIP J. Adv. Signal Process. 2014, 2014, 1–21. [Google Scholar] [CrossRef]
- Li, S.; Zhou, W.; Yuan, Q.; Geng, S.; Cai, D. Feature extraction and recognition of ictal EEG using EMD and SVM. Comput. Biol. Med. 2013, 43, 807–816. [Google Scholar] [CrossRef]
- Li, J.; Reiter-Campeau, S.; Namiranian, D.; Toffa, D.H.; Bouthillier, A.; Dubeau, F.; Nguyen, D.K. Insular Involvement in Cases of Epilepsy Surgery Failure. Brain Sci. 2022, 12, 125. [Google Scholar] [CrossRef]
- Thomson, L.; Fayed, N.; Sedarous, F.; Ronen, G.M. Life quality and health in adolescents and emerging adults with epilepsy during the years of transition: A scoping review. Dev. Med. Child Neurol. 2014, 56, 421–433. [Google Scholar] [CrossRef]
- Baker, G.A.; Jacoby, A.; Buck, D.; Stalgis, C.; Monnet, D. Quality of life of people with epilepsy: A European study. Epilepsia 1997, 38, 353–362. [Google Scholar] [CrossRef]
- Wang, M.; Perera, K.; Josephson, C.B.; Lamidi, M.; Lawal, O.A.; Awosoga, O.; Roach, P.; Patten, S.B.; Wiebe, S.; Sajobi, T.T. Association between antiseizure medications and quality of life in epilepsy: A mediation analysis. Epilepsia 2022, 63, 440–450. [Google Scholar] [CrossRef]
- Asiri, S.; Al-Otaibi, A.; Al Hameed, M.; Hamhom, A.; Alenizi, A.; Eskandrani, A.; AlKhrisi, M.; Aldosari, M.M. Seizure-related injuries in people with epilepsy: A cohort study from Saudi Arabia. Epilepsia Open 2022, 7, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D. Sudden unexpected death in epilepsy. Curr. Opin. Neurol. 2022, 35, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.; Vajdic, C.M.; Reppermund, S.; Cvejic, R.C.; Srasuebkul, P.; Trollor, J. Mortality rate, risk factors, and causes of death in people with epilepsy and intellectual disability. Seizure 2022, 101, 75–82. [Google Scholar] [CrossRef]
- Wadhera, T. Brain network topology unraveling epilepsy and ASD Association: Automated EEG-based diagnostic model. Expert Syst. Appl. 2021, 186, 115762. [Google Scholar] [CrossRef]
- Adeli, H.; Ghosh-Dastidar, S. Automated EEG-Based Diagnosis of Neurological Disorders: Inventing the Future of Neurology; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Smith, S.J. EEG in the diagnosis, classification, and management of patients with epilepsy. J. Neurol. Neurosurg. Psychiatry 2005, 76, ii2–ii7. [Google Scholar] [CrossRef] [PubMed]
- Antoniades, A.; Spyrou, L.; Took, C.C.; Sanei, S. Deep learning for epileptic intracranial EEG data. In Proceedings of the 2016 IEEE 26th International Workshop on Machine Learning for Signal Processing (MLSP), Salerno, Italy, 13–16 September 2016; pp. 1–6. [Google Scholar]
- Pacia, S.V.; Ebersole, J.S. Intracranial EEG in temporal lobe epilepsy. J. Clin. Neurophysiol. 1999, 16, 399. [Google Scholar] [CrossRef] [PubMed]
- Jobst, B.C.; Bartolomei, F.; Diehl, B.; Frauscher, B.; Kahane, P.; Minotti, L.; Sharan, A.; Tardy, N.; Worrell, G.; Gotman, J. Intracranial EEG in the 21st Century. Epilepsy Curr. 2020, 20, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Nahum, L.; Gabriel, D.; Spinelli, L.; Momjian, S.; Seeck, M.; Michel, C.M.; Schnider, A. Rapid consolidation and the human hippocampus: Intracranial recordings confirm surface EEG. Hippocampus 2011, 21, 689–693. [Google Scholar] [CrossRef]
- Ponz, A.; Montant, M.; Liegeois-Chauvel, C.; Silva, C.; Braun, M.; Jacobs, A.M.; Ziegler, J.C. Emotion processing in words: A test of the neural re-use hypothesis using surface and intracranial EEG. Soc. Cogn. Affect. Neurosci. 2014, 9, 619–627. [Google Scholar] [CrossRef]
- Cimbalnik, J.; Dolezal, J.; Topçu, Ç.; Lech, M.; Marks, V.S.; Joseph, B.; Dobias, M.; Van Gompel, J.; Worrell, G.; Kucewicz, M. Intracranial electrophysiological recordings from the human brain during memory tasks with pupillometry. Sci. Data 2022, 9, 1–10. [Google Scholar] [CrossRef]
- Parvizi, J.; Kastner, S. Promises and limitations of human intracranial electroencephalography. Nat. Neurosci. 2018, 21, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Kawala-Sterniuk, A.; Browarska, N.; Al-Bakri, A.; Pelc, M.; Zygarlicki, J.; Sidikova, M.; Martinek, R.; Gorzelanczyk, E.J. Summary of over fifty years with brain-computer interfaces—a review. Brain Sci. 2021, 11, 43. [Google Scholar] [CrossRef] [PubMed]
- Lachaux, J.P.; Axmacher, N.; Mormann, F.; Halgren, E.; Crone, N.E. High-frequency neural activity and human cognition: Past, present and possible future of intracranial EEG research. Prog. Neurobiol. 2012, 98, 279–301. [Google Scholar] [CrossRef] [PubMed]
- Ung, H.; Baldassano, S.N.; Bink, H.; Krieger, A.M.; Williams, S.; Vitale, F.; Wu, C.; Freestone, D.; Nurse, E.; Leyde, K.; et al. Intracranial EEG fluctuates over months after implanting electrodes in human brain. J. Neural Eng. 2017, 14, 056011. [Google Scholar] [CrossRef]
- Baud, M.O.; Schindler, K.; Rao, V.R. Under-sampling in epilepsy: Limitations of conventional EEG. Clin. Neurophysiol. Pract. 2021, 6, 41–49. [Google Scholar] [CrossRef]
- Jasper, H.H.; Carmichael, L. Electrical potentials from the intact human brain. Science 1935, 81, 51–53. [Google Scholar] [CrossRef]
- Reif, P.S.; Strzelczyk, A.; Rosenow, F. The history of invasive EEG evaluation in epilepsy patients. Seizure 2016, 41, 191–195. [Google Scholar] [CrossRef]
- Lachaux, J.P.; Rudrauf, D.; Kahane, P. Intracranial EEG and human brain mapping. J. Physiol. 2003, 97, 613–628. [Google Scholar] [CrossRef]
- McCarty, M.J.; Woolnough, O.; Mosher, J.C.; Seymour, J.; Tandon, N. The listening zone of human electrocorticographic field potential recordings. Eneuro 2022, 9. [Google Scholar] [CrossRef]
- Kwan, P.; Schachter, S.C.; Brodie, M.J. Drug-resistant epilepsy. N. Engl. J. Med. 2011, 365, 919–926. [Google Scholar] [CrossRef]
- Liu, J.t.; Liu, B.; Zhang, H. Surgical versus medical treatment of drug-resistant epilepsy: A systematic review and meta-analysis. Epilepsy Behav. 2018, 82, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.Y.; Panov, F. Identification and treatment of drug-resistant epilepsy. CONTINUUM Lifelong Learn. Neurol. 2019, 25, 362–380. [Google Scholar] [CrossRef] [PubMed]
- González, F.L.; Osorio, X.R.; Rein, A.G.N.; Martínez, M.C.; Fernández, J.S.; Haba, V.V.; Pedraza, A.D.; Cerdá, J.M. Drug-resistant epilepsy: Definition and treatment alternatives. Neurología 2015, 30, 439–446. [Google Scholar] [CrossRef]
- Ryvlin, P.; Rheims, S. Epilepsy surgery: Eligibility criteria and presurgical evaluation. Dialogues Clin. Neurosci. 2022, 10, 91–103. [Google Scholar] [CrossRef]
- Boon, P.; Raedt, R.; De Herdt, V.; Wyckhuys, T.; Vonck, K. Electrical stimulation for the treatment of epilepsy. Neurotherapeutics 2009, 6, 218–227. [Google Scholar] [CrossRef]
- Wu, Y.C.; Liao, Y.S.; Yeh, W.H.; Liang, S.F.; Shaw, F.Z. Directions of deep brain stimulation for epilepsy and Parkinson’s disease. Front. Neurosci. 2021, 15, 671. [Google Scholar] [CrossRef]
- Li, M.C.; Cook, M.J. Deep brain stimulation for drug-resistant epilepsy. Epilepsia 2018, 59, 273–290. [Google Scholar] [CrossRef]
- Hossain, P.S.F.; Shaikat, I.M.; George, F.P. Emotion Recognition Using Brian Signals Based on Time-Frequency Analysis and Supervised Learning Algorithm. Ph.D. Thesis, BRAC University, Dhaka, Bangladesh, 2018. [Google Scholar]
- Nunez, M.D.; Charupanit, K.; Sen-Gupta, I.; Lopour, B.A.; Lin, J.J. Beyond rates: Time-varying dynamics of high frequency oscillations as a biomarker of the seizure onset zone. J. Neural Eng. 2022, 19, 016034. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, J.; Liu, T.; Chen, F.; Chen, S.; Yuan, L.; Zhai, F.; Liang, S. Diagnostic value of high-frequency oscillations for the epileptogenic zone: A systematic review and meta-analysis. Seizure 2022, 99, 82–90. [Google Scholar] [CrossRef]
- Papadelis, C.; Perry, M.S. Localizing the epileptogenic zone with novel biomarkers. In Seminars in Pediatric Neurology; Elsevier: Amsterdam, The Netherlands, 2021; Volume 39, p. 100919. [Google Scholar]
- King-Stephens, D. The ambiguous nature of fast ripples in epilepsy surgery. Epilepsy Curr. 2019, 19, 91–92. [Google Scholar] [CrossRef]
- Kobayashi, K.; Shibata, T.; Tsuchiya, H.; Akiyama, T. Exclusion of the possibility of “false ripples” from ripple band high-frequency oscillations recorded from scalp electroencephalogram in children with epilepsy. Front. Hum. Neurosci. 2021, 15, 696882. [Google Scholar] [CrossRef] [PubMed]
- Zweiphenning, W.J.; von Ellenrieder, N.; Dubeau, F.; Martineau, L.; Minotti, L.; Hall, J.A.; Chabardes, S.; Dudley, R.; Kahane, P.; Gotman, J.; et al. Correcting for physiological ripples improves epileptic focus identification and outcome prediction. Epilepsia 2022, 63, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Van Mierlo, P.; Vorderwülbecke, B.J.; Staljanssens, W.; Seeck, M.; Vulliémoz, S. Ictal EEG source localization in focal epilepsy: Review and future perspectives. Clin. Neurophysiol. 2020, 131, 2600–2616. [Google Scholar] [CrossRef] [PubMed]
- Baroumand, A.G.; Arbune, A.A.; Strobbe, G.; Keereman, V.; Pinborg, L.H.; Fabricius, M.; Rubboli, G.; Madsen, C.G.; Jespersen, B.; Brennum, J.; et al. Automated ictal eeg source imaging: A retrospective, blinded clinical validation study. Clin. Neurophysiol. 2021, 141, 119–125. [Google Scholar] [CrossRef]
- Vespa, S.; Baroumand, A.G.; Santos, S.F.; Vrielynck, P.; De Tourtchaninoff, M.; Feys, O.; Strobbe, G.; Raftopoulos, C.; van Mierlo, P.; El Tahry, R. Ictal EEG source imaging and connectivity to localize the seizure onset zone in extratemporal lobe epilepsy. Seizure 2020, 78, 18–30. [Google Scholar] [CrossRef] [PubMed]
- LeVan, P.; Urrestarazu, E.; Gotman, J. A system for automatic artifact removal in ictal scalp EEG based on independent component analysis and Bayesian classification. Clin. Neurophysiol. 2006, 117, 912–927. [Google Scholar] [CrossRef] [PubMed]
- Pillai, J.; Sperling, M.R. Interictal EEG and the diagnosis of epilepsy. Epilepsia 2006, 47, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Le Van Quyen, M.; Martinerie, J.; Adam, C.; Varela, F.J. Nonlinear analyses of interictal EEG map the brain interdependences in human focal epilepsy. Phys. D Nonlinear Phenom. 1999, 127, 250–266. [Google Scholar] [CrossRef]
- Lee, S.K.; Kim, J.Y.; Hong, K.S.; Nam, H.W.; Park, S.H.; Chung, C.K. The clinical usefulness of ictal surface EEG in neocortical epilepsy. Epilepsia 2000, 41, 1450–1455. [Google Scholar] [CrossRef]
- Thamcharoenvipas, T.; Takahashi, Y.; Kimura, N.; Matsuda, K.; Usui, N. Localizing and Lateralizing Value of Seizure Onset Pattern on Surface EEG in FCD Type II. Pediatr. Neurol. 2022, 129, 48–54. [Google Scholar] [CrossRef]
- Foldvary, N.; Klem, G.; Hammel, J.; Bingaman, W.; Najm, I.; Lüders, H. The localizing value of ictal EEG in focal epilepsy. Neurology 2001, 57, 2022–2028. [Google Scholar] [CrossRef] [PubMed]
- Ebersole, J.S.; Pacia, S.V. Localization of temporal lobe foci by ictal EEG patterns. Epilepsia 1996, 37, 386–399. [Google Scholar] [CrossRef] [PubMed]
- Walczak, T.S.; Radtke, R.A.; Lewis, D.V. Accuracy and interobserver reliability of scalp ictal EEG. Neurology 1992, 42, 2279. [Google Scholar] [CrossRef] [PubMed]
- Helmstaedter, C.; Kurthen, M.; Lux, S.; Reuber, M.; Elger, C.E. Chronic epilepsy and cognition: A longitudinal study in temporal lobe epilepsy. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 2003, 54, 425–432. [Google Scholar] [CrossRef]
- Taft, C.; Sager Magnusson, E.; Ekstedt, G.; Malmgren, K. Health-related quality of life, mood, and patient satisfaction after epilepsy surgery in Sweden—A prospective controlled observational study. Epilepsia 2014, 55, 878–885. [Google Scholar] [CrossRef]
- Zentner, J.; Hufnagel, A.; Ostertun, B.; Wolf, H.K.; Behrens, E.; Campos, M.G.; Solymosi, L.; Elger, C.E.; Wiestler, O.D.; Schramm, J. Surgical treatment of extratemporal epilepsy: Clinical, radiologic, and histopathologic findings in 60 patients. Epilepsia 1996, 37, 1072–1080. [Google Scholar] [CrossRef]
- Liu, S.Y.; Yang, X.L.; Chen, B.; Hou, Z.; An, N.; Yang, M.H.; Yang, H. Clinical outcomes and quality of life following surgical treatment for refractory epilepsy: A systematic review and meta-analysis. Medicine 2015, 94, e500. [Google Scholar] [CrossRef]
- Ben-Menachem, E.; Sander, J.W.; Privitera, M.; Gilliam, F. Measuring outcomes of treatment with antiepileptic drugs in clinical trials. Epilepsy Behav. 2010, 18, 24–30. [Google Scholar] [CrossRef]
- Zijlmans, M.; Jiruska, P.; Zelmann, R.; Leijten, F.S.; Jefferys, J.G.; Gotman, J. High-frequency oscillations as a new biomarker in epilepsy. Ann. Neurol. 2012, 71, 169–178. [Google Scholar] [CrossRef]
- Bragin, A.; Engel, J., Jr.; Staba, R.J. High-frequency oscillations in epileptic brain. Curr. Opin. Neurol. 2010, 23, 151. [Google Scholar] [CrossRef]
- Staba, R.J.; Bragin, A. High-frequency oscillations and other electrophysiological biomarkers of epilepsy: Underlying mechanisms. Biomarkers Med. 2011, 5, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Pail, M.; Cimbálník, J.; Roman, R.; Daniel, P.; Shaw, D.J.; Chrastina, J.; Brázdil, M. High frequency oscillations in epileptic and non-epileptic human hippocampus during a cognitive task. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Thomschewski, A.; Hincapié, A.S.; Frauscher, B. Localization of the epileptogenic zone using high frequency oscillations. Front. Neurol. 2019, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Saeid, S.; Chambers, J. EEG Signal Processing; John Willey & Sons: Chichester, UK, 2007. [Google Scholar]
- Gloor, P. Contributions of electroencephalography and electrocorticography to the neurosurgical treatment of the epilepsies. In Neurosurgical Management of the Epilepsies; Raven Press: New York, NY, USA, 1975; pp. 59–105. [Google Scholar]
- Staba, R.J.; Wilson, C.L.; Bragin, A.; Fried, I.; Engel Jr, J. Quantitative analysis of high-frequency oscillations (80–500 Hz) recorded in human epileptic hippocampus and entorhinal cortex. J. Neurophysiol. 2002, 88, 1743–1752. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.; Staba, R.; Asano, E.; Otsubo, H.; Wu, J.; Zijlmans, M.; Mohamed, I.; Kahane, P.; Dubeau, F.; Navarro, V.; et al. High-frequency oscillations (HFOs) in clinical epilepsy. Prog. Neurobiol. 2012, 98, 302–315. [Google Scholar] [CrossRef] [PubMed]
- Gulyás, A.I.; Freund, T.T. Generation of physiological and pathological high frequency oscillations: The role of perisomatic inhibition in sharp-wave ripple and interictal spike generation. Curr. Opin. Neurobiol. 2015, 31, 26–32. [Google Scholar] [CrossRef]
- Bragin, A.; Engel Jr, J.; Wilson, C.L.; Fried, I.; Mathern, G.W. Hippocampal and entorhinal cortex high-frequency oscillations (100–500 Hz) in human epileptic brain and in kainic acid-treated rats with chronic seizures. Epilepsia 1999, 40, 127–137. [Google Scholar] [CrossRef]
- Jacobs, J.; LeVan, P.; Chander, R.; Hall, J.; Dubeau, F.; Gotman, J. Interictal high-frequency oscillations (80–500 Hz) are an indicator of seizure onset areas independent of spikes in the human epileptic brain. Epilepsia 2008, 49, 1893–1907. [Google Scholar] [CrossRef]
- Ochi, A.; Otsubo, H.; Donner, E.J.; Elliott, I.; Iwata, R.; Funaki, T.; Akizuki, Y.; Akiyama, T.; Imai, K.; Rutka, J.T.; et al. Dynamic changes of ictal high-frequency oscillations in neocortical epilepsy: Using multiple band frequency analysis. Epilepsia 2007, 48, 286–296. [Google Scholar] [CrossRef]
- Dimakopoulos, V.; Mégevand, P.; Boran, E.; Momjian, S.; Seeck, M.; Vulliémoz, S.; Sarnthein, J. Blinded study: Prospectively defined high-frequency oscillations predict seizure outcome in individual patients. Brain Commun. 2021, 3, fcab209. [Google Scholar] [CrossRef]
- Ahmed, R.; Otsubo, H.; Snead III, C.; Donner, E.; Widjaja, E.; Ochi, A.; Drake, J.M.; Rutka, J.T. Diagnostic evaluation and surgical management of pediatric insular epilepsy utilizing magnetoencephalography and invasive EEG monitoring. Epilepsy Res. 2018, 140, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Valenca, L.; Dubeau, F.; Mari, F.; Zelmann, R.; Gotman, J. Interictal scalp fast oscillations as a marker of the seizure onset zone. Neurology 2011, 77, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Goldenholz, D.M.; Gotman, J.; Seyal, M.; Bateman, L.M.; Andrade-Valenca, L.; Zelmann, R.; Dubeau, F. Interictal Scalp Fast Oscillations as a Marker of the Seizure Onset ZoneAuthor Response. Neurology 2012, 78, 224–225. [Google Scholar] [CrossRef] [PubMed]
- Al-Bakri, A.F.; Yaghouby, F.; Besio, W.; Ding, L.; Modur, P.; Sunderam, S. Effect of Vigilance Changes on the Incidence of High Frequency Oscillations in the Epileptic Brain. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 991–994. [Google Scholar]
- Li, A.; Chennuri, B.; Subramanian, S.; Yaffe, R.; Gliske, S.; Stacey, W.; Norton, R.; Jordan, A.; Zaghloul, K.A.; Inati, S.K.; et al. Using network analysis to localize the epileptogenic zone from invasive EEG recordings in intractable focal epilepsy. Netw. Neurosci. 2018, 2, 218–240. [Google Scholar] [CrossRef]
- Tassi, L.; Jayakar, P.; Pieper, T.; Kahane, P. 6. Intracranial and electrical EEG stimulation recordings. Pediatr. Epilepsy Surg. 2016, 61. [Google Scholar]
- Graef, A.; Flamm, C.; Pirker, S.; Baumgartner, C.; Deistler, M.; Matz, G. Automatic ictal HFO detection for determination of initial seizure spread. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 2096–2099. [Google Scholar]
- Wong, S.M.; Arski, O.N.; Workewych, A.M.; Donner, E.; Ochi, A.; Otsubo, H.; Snead III, O.C.; Ibrahim, G.M. Detection of high-frequency oscillations in electroencephalography: A scoping review and an adaptable open-source framework. Seizure 2021, 84, 23–33. [Google Scholar] [CrossRef]
- Cimbálník, J.; Hewitt, A.; Worrell, G.; Stead, M. The CS algorithm: A novel method for high frequency oscillation detection in EEG. J. Neurosci. Methods 2018, 293, 6–16. [Google Scholar] [CrossRef]
- Gardner, A.B.; Worrell, G.A.; Marsh, E.; Dlugos, D.; Litt, B. Human and automated detection of high-frequency oscillations in clinical intracranial EEG recordings. Clin. Neurophysiol. 2007, 118, 1134–1143. [Google Scholar] [CrossRef]
- Gliske, S.V.; Irwin, Z.T.; Davis, K.A.; Sahaya, K.; Chestek, C.; Stacey, W.C. Universal automated high frequency oscillation detector for real-time, long term EEG. Clin. Neurophysiol. 2016, 127, 1057–1066. [Google Scholar] [CrossRef]
- Wu, M.; Qin, H.; Wan, X.; Du, Y. HFO detection in epilepsy: A stacked denoising autoencoder and sample weight adjusting factors-based method. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 1965–1976. [Google Scholar] [CrossRef]
- Worrell, G.A.; Parish, L.; Cranstoun, S.D.; Jonas, R.; Baltuch, G.; Litt, B. High-frequency oscillations and seizure generation in neocortical epilepsy. Brain 2004, 127, 1496–1506. [Google Scholar] [CrossRef] [PubMed]
- Crépon, B.; Navarro, V.; Hasboun, D.; Clemenceau, S.; Martinerie, J.; Baulac, M.; Adam, C.; Le Van Quyen, M. Mapping interictal oscillations greater than 200 Hz recorded with intracranial macroelectrodes in human epilepsy. Brain 2010, 133, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Chaibi, S.; Sakka, Z.; Lajnef, T.; Samet, M.; Kachouri, A. Automated detection and classification of high frequency oscillations (HFOs) in human intracereberal EEG. Biomed. Signal Process. Control 2013, 8, 927–934. [Google Scholar] [CrossRef]
- Gliske, S.V.; Stacey, W.C.; Moon, K.R.; Hero, A.O. The intrinsic value of HFO features as a biomarker of epileptic activity. In Proceedings of the 2016 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), Shanghai, China, 20–25 March 2016; pp. 6290–6294. [Google Scholar]
- Wagenaar, J.B.; Worrell, G.A.; Ives, Z.; Dümpelmann, M.; Litt, B.; Schulze-Bonhage, A. Collaborating and sharing data in epilepsy research. J. Clin. Neurophysiol. Off. Publ. Am. Electroencephalogr. Soc. 2015, 32, 235. [Google Scholar] [CrossRef] [PubMed]
- Misiūnas, A.V.M.; Meškauskas, T.; Samaitienė, R. Algorithm for automatic EEG classification according to the epilepsy type: Benign focal childhood epilepsy and structural focal epilepsy. Biomed. Signal Process. Control 2019, 48, 118–127. [Google Scholar] [CrossRef]
- Sharmila, A. Epilepsy detection from EEG signals: A review. J. Med Eng. Technol. 2018, 42, 368–380. [Google Scholar] [CrossRef]
- Misiūnas, A.V.M.; Meškauskas, T.; Juozapavičius, A. On the implementation and improvement of automatic EEG spike detection algorithm. Liet. Mat. Rinkinys. Ser. A 2015, 56, 60–65. [Google Scholar] [CrossRef]
- Jankowski, M. Erosion, dilation and related operators. In Proceedings of the 8th International Mathematica Symposium, Kuressaare, Estonia, 3–5 July 2006; pp. 1–10. [Google Scholar]







| No. | Subject ID: with 5 [kHZ] Fs | Location | Age | Gender | Data Length | Seizure History | No. of Channels | No. of Seizures |
|---|---|---|---|---|---|---|---|---|
| 1 | I001_P001_D01 | Unknown | NA | M | 5 days and 4 h | Unknown | 62 | 4 |
| 2 | I001_P002_D01 | Left Temporal Lobe | NA | F | 5 days and 9 h | Partial/Complex | 15 | 2 |
| 3 | I001_P005_D01 | Temporal Lobe | NA | M | 1 day and 11 h | Partial/Complex | 36 | 1 |
| 4 | I001_P010_D01 | Temporal Lobe | NA | F | 4 days | Unknown | 56 | 10 |
| 5 | I001_P013_D01 | Occipital and Parietal Lobes | NA | F | 3 days and 13 h | Unknown | 72 | 5 |
| 6 | I001_P034_D01 | Temporal and Frontal Lobes | 35 | F | 1 day and 8 h | Partial/Complex | 47 | 15 |
| 7 | Study 036 | Temporal Lobe | NA | M | 4 day and 14 h | Partial/Simple | 96 | 4 |
| 8 | Study 40 | Parietal Lobe | 32 | M | 2 days and 23 h | Partial/Simple/ Complex | 116 | 7 |
| Part A | # of All Candidate Events | # of True Ripples | # of Sharp Transients | # of True Spikes | |||
|---|---|---|---|---|---|---|---|
| 136 | 113 | 2 | 21 | ||||
| Part B | Window Size of the Filter [ms] | TP | FP | # of Detectors (TP + FP) | FN | Sensitivity % | FDR % |
| 1 | 1 | 9 | 5 | 14 | 12 | 43 | 36 |
| 2 | 2 | 13 | 7 | 19 | 8 | 62 | 32 |
| 3 | 3 | 15 | 9 | 24 | 6 | 72 | 38 |
| 4 | 3.4 | 16 | 9 | 25 | 5 | 77 | 36 |
| 5 | 4 | 17 | 9 | 26 | 4 | 81 | 35 |
| 6 | 4.6 | 17 | 10 | 27 | 4 | 81 | 39 |
| 7 | 5 | 17 | 11 | 28 | 4 | 81 | 40 |
| 8 | 5.4 | 18 | 12 | 30 | 3 | 86 | 40 |
| 9 | 6 | 18 | 16 | 34 | 3 | 86 | 47 |
| 10 | 7 | 18 | 18 | 36 | 3 | 86 | 50 |
| 11 | 8 | 18 | 19 | 37 | 3 | 86 | 53 |
| Part A | # of All Candidate Events | # of True Ripples | # of Sharp Transients | # of True Spikes | |||
|---|---|---|---|---|---|---|---|
| 4 | 2 | 0 | 2 | ||||
| Part B | Window Size of the Filter [ms] | TP | FP | # of Detectors (TP + FP) | FN | Sensitivity % | FDR % |
| 4 ms window size | 2 | 2 | 4 | 0 | 100 | 50 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Bakri, A.F.; Martinek, R.; Pelc, M.; Zygarlicki, J.; Kawala-Sterniuk, A. Implementation of a Morphological Filter for Removing Spikes from the Epileptic Brain Signals to Improve Identification Ripples. Sensors 2022, 22, 7522. https://doi.org/10.3390/s22197522
Al-Bakri AF, Martinek R, Pelc M, Zygarlicki J, Kawala-Sterniuk A. Implementation of a Morphological Filter for Removing Spikes from the Epileptic Brain Signals to Improve Identification Ripples. Sensors. 2022; 22(19):7522. https://doi.org/10.3390/s22197522
Chicago/Turabian StyleAl-Bakri, Amir F., Radek Martinek, Mariusz Pelc, Jarosław Zygarlicki, and Aleksandra Kawala-Sterniuk. 2022. "Implementation of a Morphological Filter for Removing Spikes from the Epileptic Brain Signals to Improve Identification Ripples" Sensors 22, no. 19: 7522. https://doi.org/10.3390/s22197522
APA StyleAl-Bakri, A. F., Martinek, R., Pelc, M., Zygarlicki, J., & Kawala-Sterniuk, A. (2022). Implementation of a Morphological Filter for Removing Spikes from the Epileptic Brain Signals to Improve Identification Ripples. Sensors, 22(19), 7522. https://doi.org/10.3390/s22197522








