Noninvasive Non-Contact SpO2 Monitoring Using an Integrated Polarization-Sensing CMOS Imaging Sensor
Abstract
:1. Introduction
2. Methodology
2.1. Pulse Oximetry
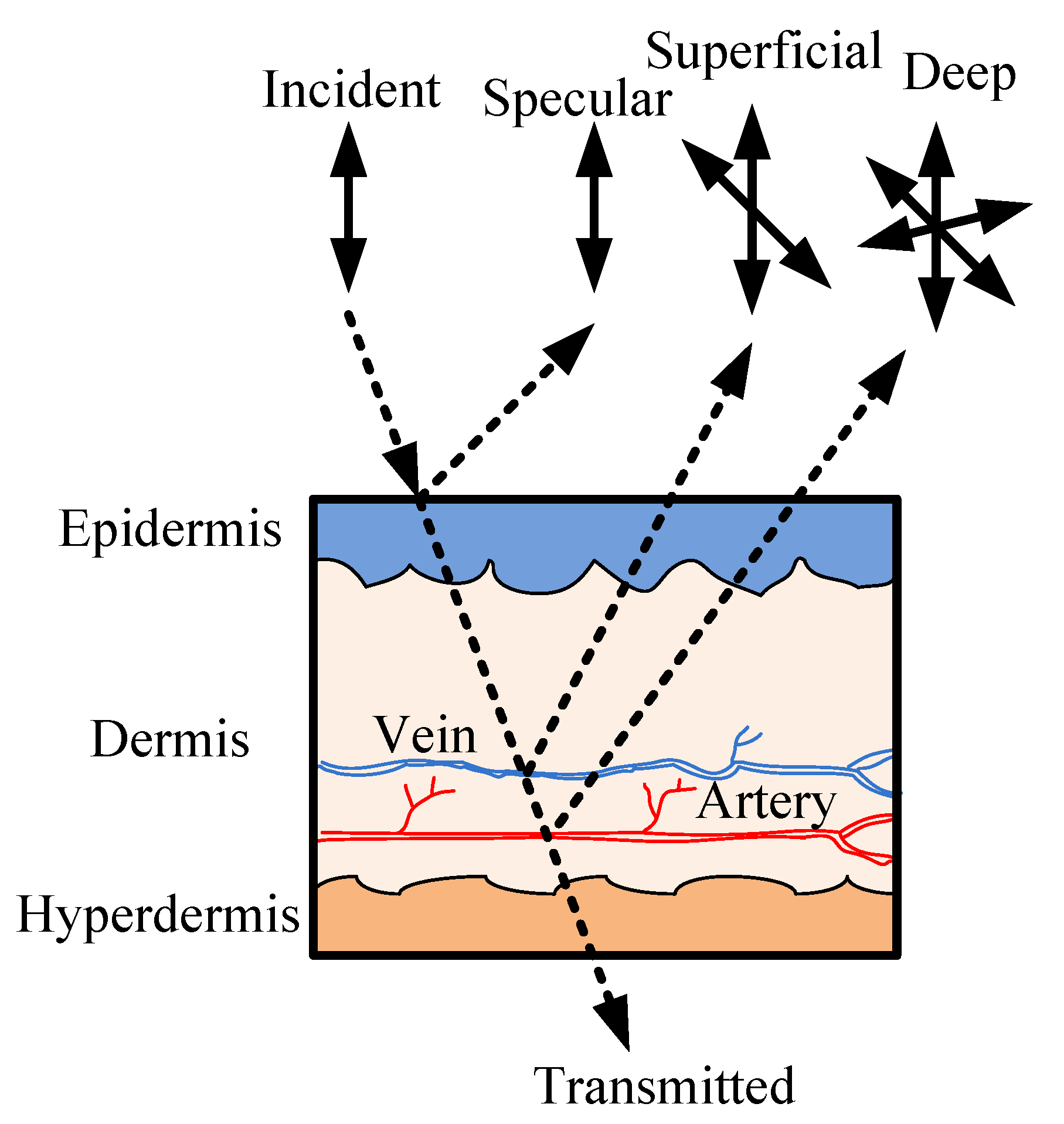
2.2. Optical Sectioning of the Skin on a Finger Tip
3. Experimental Set-Up
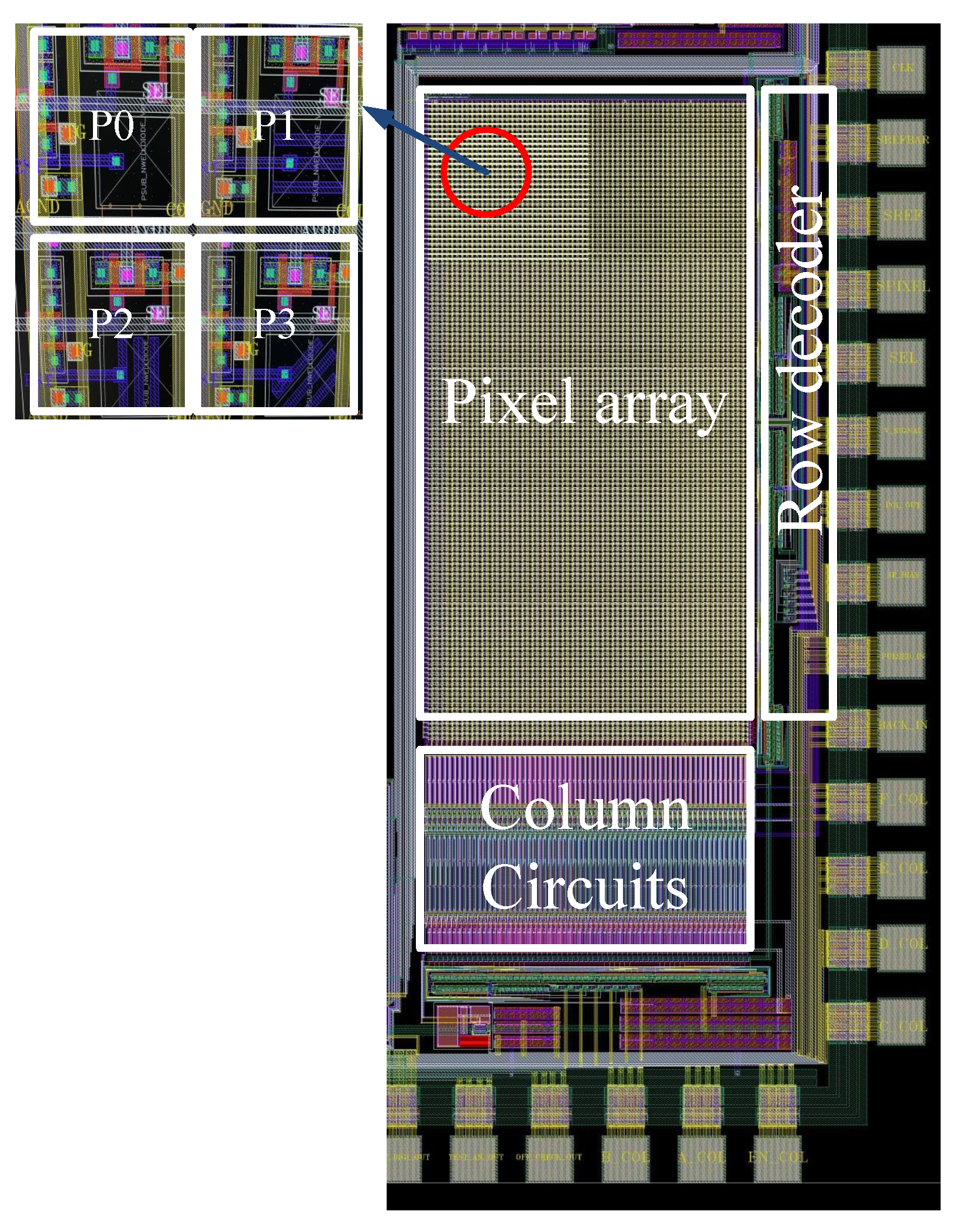
3.1. Custom Image Sensor with a Wire Grid Polarizer on Each Pixel
3.2. Data Collection
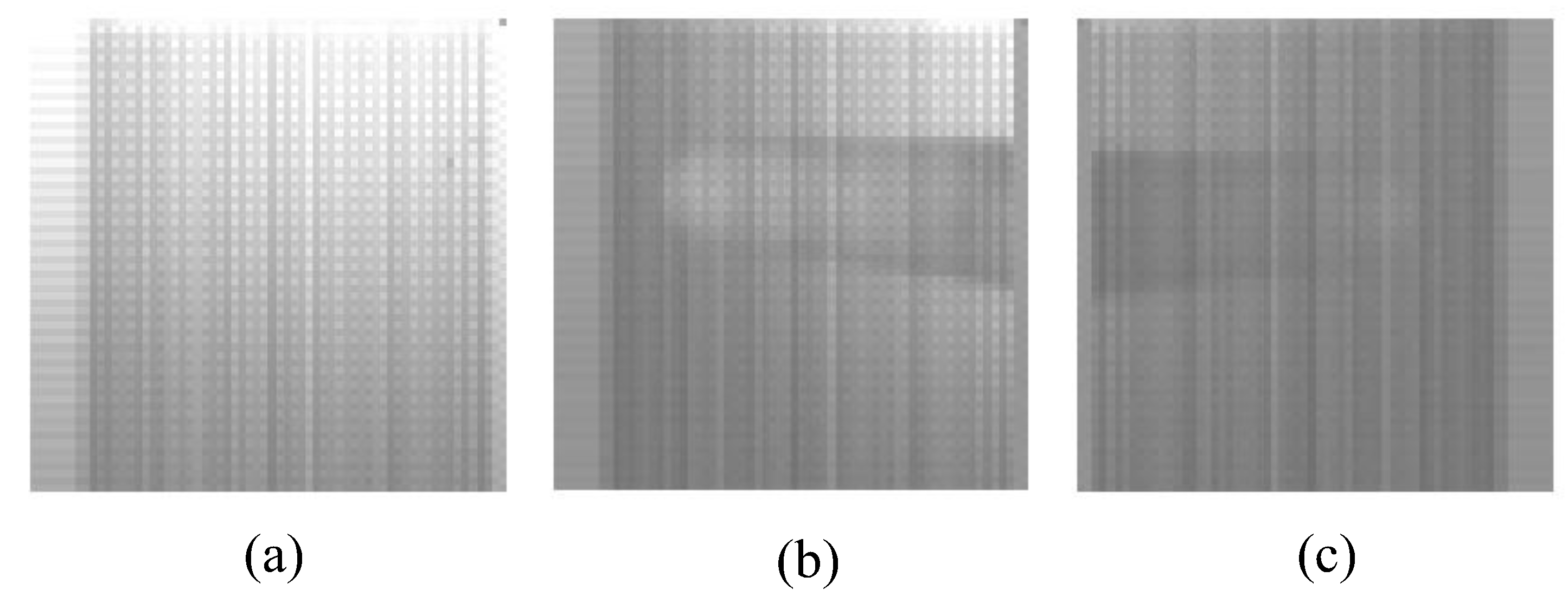
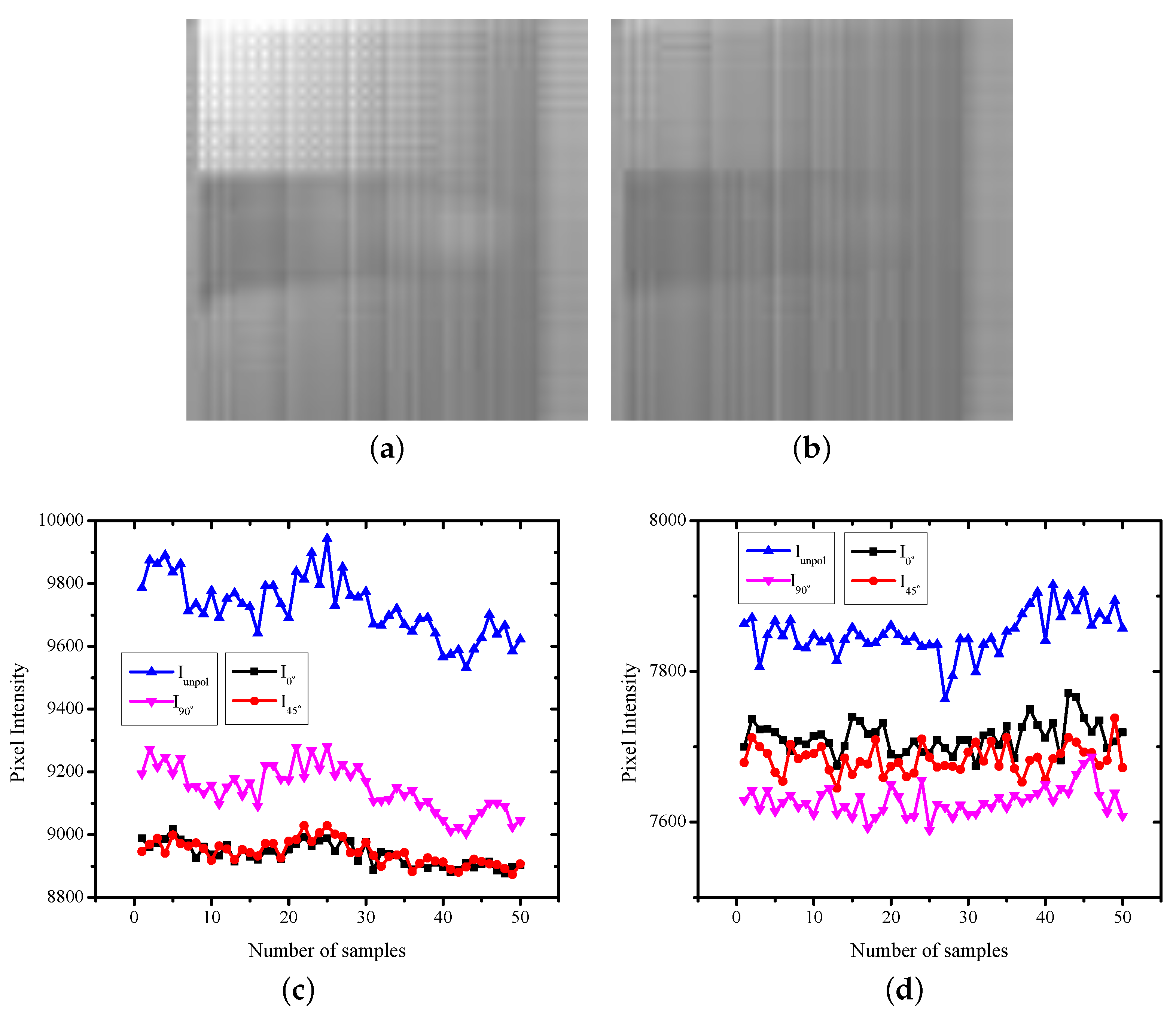
3.3. Data Analysis
4. Experiment Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Plueddemann, A.; Thompson, M.; Heneghan, C.; Price, C. Pulse oximetry in primary care: Primary care diagnostic technology update. Br. J. Gen. Pract. 2011, 61, 358–359. [Google Scholar] [CrossRef] [PubMed]
- Aoyagi, T.; Kishi, M.; Yamaguchi, K.; Watanabe, S. Improvement of the ear piece oximeter. Jpn. Soc. Med. Electron. Biol. Eng. 1974, 974, 90–91. [Google Scholar]
- Clayton, D.G.; Webb, R.K.; Ralston, A.C.; Duthie, D.; Runciman, W.B. Pulse oximeter probes. A comparison between finger, nose, ear and forehead probes under conditions of poor perfusion. Anaesthesia 1991, 46, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Branson, R.D.; Mannheimer, P.D. Forehead oximetry in critically ill patients: The case for a new monitoring site. Respir. Care Clin. N. Am. 2004, 10, 359–367. [Google Scholar] [CrossRef]
- Leitão, C.S.J.; Antunes, P.F.d.C.; Bastos, J.A.; Pinto, J.; André, P.S.d.B. Plastic Optical Fiber Sensor for Noninvasive Arterial Pulse Waveform Monitoring. IEEE Sens. J. 2015, 15, 14–18. [Google Scholar] [CrossRef]
- Joseph, G.; Joseph, A.; Titus, G.; Thomas, R.M.; Jose, D. Photoplethysmogram (PPG) signal analysis and wavelet de-noising. In Proceedings of the 2014 Annual International Conference on Emerging Research Areas: Magnetics, Machines and Drives (AICERA/iCMMD), Kottayam, India, 24–26 July 2014; pp. 1–5. [Google Scholar]
- Takano, C.; Ohta, Y. Heart rate measurement based on a time-lapse image. Med. Eng. Phys. 2007, 29, 853–857. [Google Scholar] [CrossRef]
- Lee, H.; Ko, H.; Lee, J. Reflectance pulse oximetry: Practical issues and limitations. ICT Express 2016, 2, 195–198. [Google Scholar] [CrossRef] [Green Version]
- Hernando, A.; Peláez, M.D.; Lozano, M.T.; Aiger, M.; Gil, E.; Lázaro, J. Finger and forehead PPG signal comparison for respiratory rate estimation based on pulse amplitude variability. In Proceedings of the Signal Processing Conference (EUSIPCO), Kos, Greece, 28 August–2 September 2017; pp. 2076–2080. [Google Scholar]
- Nilsson, L.; Goscinski, T.; Kalman, S.; Lindberg, L.G.; Johansson, A. Combined photoplethysmographic monitoring of respiration rate and pulse: A comparison between different measurement sites in spontaneously breathing subjects. ActaAnaesthesiol. Scand. 2007, 51, 1250–1257. [Google Scholar] [CrossRef]
- Yuan, H.; Memon, S.F.; Newe, T.; Lewis, E.; Leen, G. Motion artefact minimization from photoplethysmography based non-invasive hemoglobin sensor based on an envelope filtering algorithm. Measurement 2018, 115, 288–298. [Google Scholar] [CrossRef]
- Hartmann, V.; Liu, H.; Chen, F.; Qiu, Q.; Hughes, S.; Zheng, D. Quantitative Comparison of Photoplethysmographic Waveform Characteristics: Effect of Measurement Site. Front. Physiol. 2019, 10, 198. [Google Scholar] [CrossRef]
- Sun, Y.; Thakor, N. Photoplethysmography revisited: From contact to noncontact, from point to imaging. IEEE Trans. Biomed. Eng. 2016, 63, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Alian, A.A.; Shelley, K.H. Photoplethysmography: Analysis of the pulse oximeter waveform. In Monitoring Technologies in Acute Care Environments; Ehrenfeld, J., Cannesson, M., Eds.; Springer: New York, NY, USA, 2014; pp. 165–178. [Google Scholar]
- Sun, Z.; He, Q.; Li, Y.; Wang, W.; Wang, R.K. Robust non-contact peripheral oxygenation saturation measurement using smart phone-enabled imaging photoplethysmography. Biomed. Opt. Express 2011, 12, 1746–1760. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, K.; Ward, T.; Markham, C. Noncontact simultaneous dual wavelength photoplethysmography: A further step toward non-contact pulse oximetry. Rev. Sci. Instrum. 2007, 78, 044304. [Google Scholar] [CrossRef] [PubMed]
- Poh, M.Z.; McDuff, D.J.; Picard, R.W. Non-contact, automated cardiac pulse measurements using video imaging and blind source separation. Opt. Express 2010, 18, 10762–10774. [Google Scholar] [CrossRef]
- Kong, L.; Zhao, Y.; Dong, L.; Jian, Y.; Jin, X.; Li, B.; Feng, Y.; Liu, M.; Liu, X.; Wu, H. Non-contact detection of oxygen saturation based on visible light imaging device using ambient light. Optics Express 2013, 21, 17464–17471. [Google Scholar] [CrossRef] [PubMed]
- Verkruysse, W.; Svaasand, L.O.; Nelson, J.S. Remote plethysmographic imaging using ambient light. Opt Express 2008, 16, 21434–21445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.Y.; Wang, J.J.; Lin, K.Y.; Chang, H.H.; Wu, H.K.; Chen, Y.S.; Lee, S.Y. Image Sensor-Based Heart Rate Evaluation From Face Reflectance Using Hilbert–Huang Transform. IEEE Sens. J. 2015, 15, 618–627. [Google Scholar] [CrossRef]
- de Haan, G.; van Leest, A. Improved motion robustness of remote-PPG by using the blood volume pulse signature. Physiol. Meas. 2014, 3, 1913–1926. [Google Scholar] [CrossRef] [Green Version]
- Jakachira, R.; Diouf, M.; Toussaint, C.K.C., Jr. Rapid blood-oxygenation-saturation measurement using radially polarized light from light-emitting diodes. In Proceedings of the SPIE 11956, Biophotonics in Exercise Science, Sports Medicine, Health Monitoring Technologies, and Wearables III, San Francisco, CA, USA, 22 January–28 February 2022; p. 119560B. [Google Scholar]
- Arimoto, H. Multispectral polarization imaging for observing blood oxygen saturation in skin tissue. Soc. Appl. Spectrosc. 2006, 60, 459–464. [Google Scholar] [CrossRef]
- Mishra, D.; Priyadarshini, N.; Chakraborty, S.; Sarkar, M. Blood Oxygen Saturation Measurement using Polarized Images. IEEE Sens. J. 2017, 17, 3900–3908. [Google Scholar] [CrossRef]
- Mishra, D.; Gogna, G.; Barsaiyan, A.; Sarkar, M. Blood pulsation measurement using linearly polarized light. IEEE Sens. J. 2015, 15, 4488–4495. [Google Scholar] [CrossRef]
- Chakraborty, S.; Mishra, D.; Sarkar, M. PSPPG: Polarization Sensitive Photo-Plethysmography. Eurosensors Conf. (Procedia Eng.) 2016, 168, 1275–1278. [Google Scholar] [CrossRef]
- Friebel, M.; Roggan, A.; Mueller, G.J.; Meinke, M.C. Determination of optical properties of human blood in the spectral range 250 to 1100 nm using Monte Carlo simulations with hematocrit-dependent effective scattering phase functions. J. Biomed. Opt. 2006, 11, 034021. [Google Scholar] [CrossRef] [PubMed]
- Kamshilin, A.A.; Nippolainen, E.; Sidorov, I.S.; Vasilev, P.V.; Erofeev, N.P.; Podolian, N.P.; Romashko, R.V. A new look at the essence of the imaging photoplethysmography. Sci. Rep. 2015, 5, 10494. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, M.; Segundo, D.S.; van Hoof, C.; Theuwissen, A.J.P. Integrated polarization analyzing CMOS image sensor for real time material classication. IEEE Sens. J. 2011, 11, 1692–1703. [Google Scholar] [CrossRef]
- Sarkar, M.; Segundo, D.S.; van Hoof, C.; Theuwissen, A.J.P. A Biologically Inspired CMOS Image Sensor for Fast Motion and Polarization Detection. IEEE Sens. J. 2012, 13, 1065–1073. [Google Scholar] [CrossRef]
- Choudhary, T.R.; Ball, D.; Ramos, J.F.; McNaught, A.I.; Harvey, A.R. Assessment of Acute Mild Hypoxia on Retinal Oxygen Saturation Using Snapshot Retinal Oximetry Assessment of Acute Mild Hypoxia on Retinal OS. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7538–7543. [Google Scholar] [CrossRef]
- MacKenzie, L.E.; Choudhary, T.R.; McNaught, A.I.; Harvey, A.R. In vivo oximetry of human bulbar conjunctival and episcleral microvasculature using snapshot multispectral imaging. Exp. Eye Res. 2016, 149, 48–58. [Google Scholar] [CrossRef]
- Liang, Y.; Elgendi, M.; Chen, Z.; Ward, R. An optimal filter for short photoplethysmogram signals. Sci. Data 2018, 5, 180076. [Google Scholar] [CrossRef] [Green Version]
- Allen, J.; Murray, A. Effects of filtering on multisite photoplethysmography pulse waveform characteristics. In Proceedings of the Computers in Cardiology, Chicago, IL, USA, 19–22 September 2004; pp. 485–488. [Google Scholar]
- Liu, H.; Alle, J.; Khalid, S.G.; Che, F.; Zheng, D. Filtering-induced time shifts in photoplethysmography pulse features measured at different body sites: The importance of filter definition and standardization. Physiol. Meas. 2021, 42, 074001. [Google Scholar] [CrossRef]
- Hamilton, P.; Curley, M.; Aimi, R.; Sae-Hau, C. Comparison of Methods for Adaptive Removal of Motion Artifact. In Proceedings of the Computers in Cardiology, Cambridge, MA, USA, 24–27 September 2000; pp. 383–386. [Google Scholar]

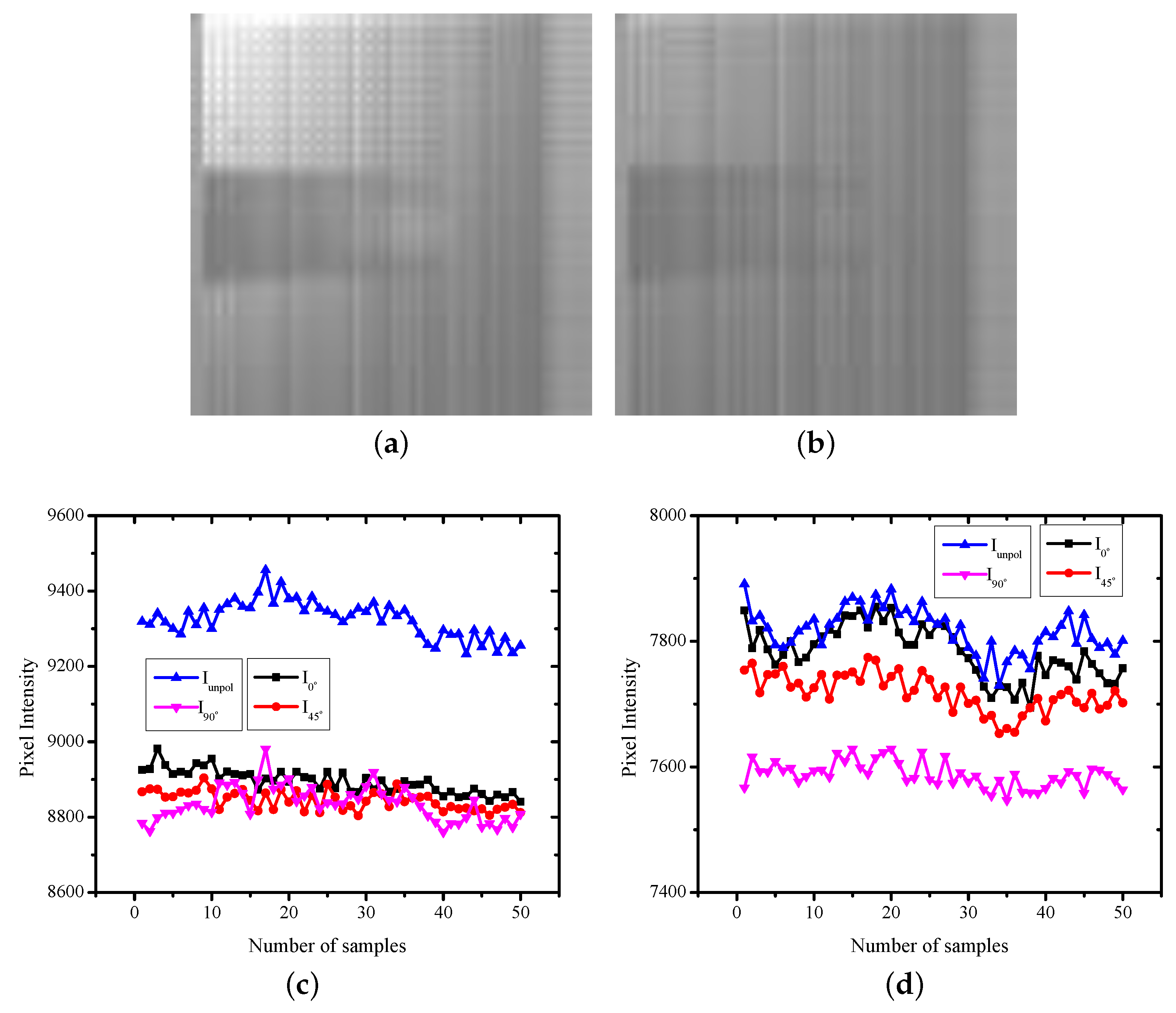
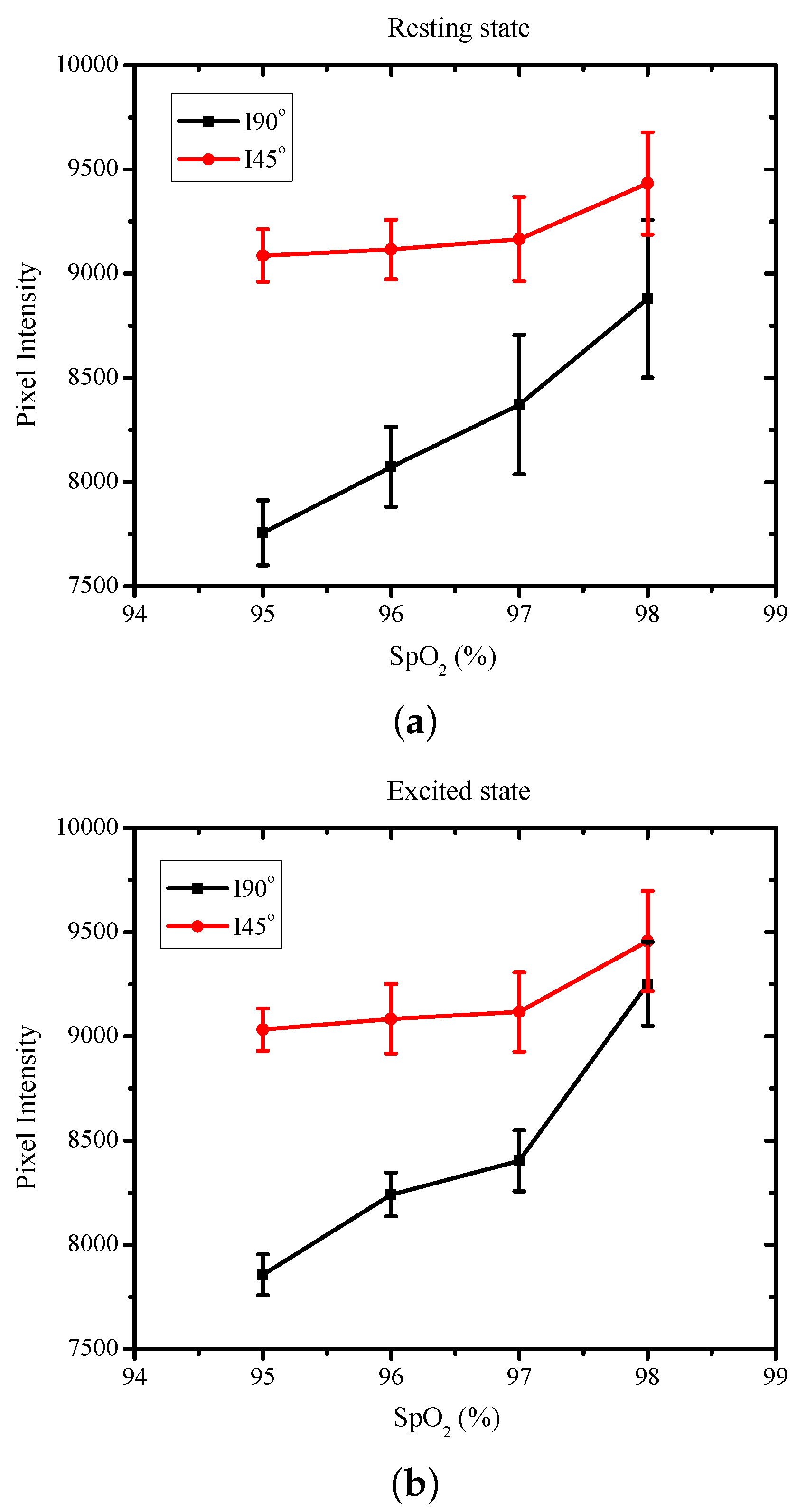
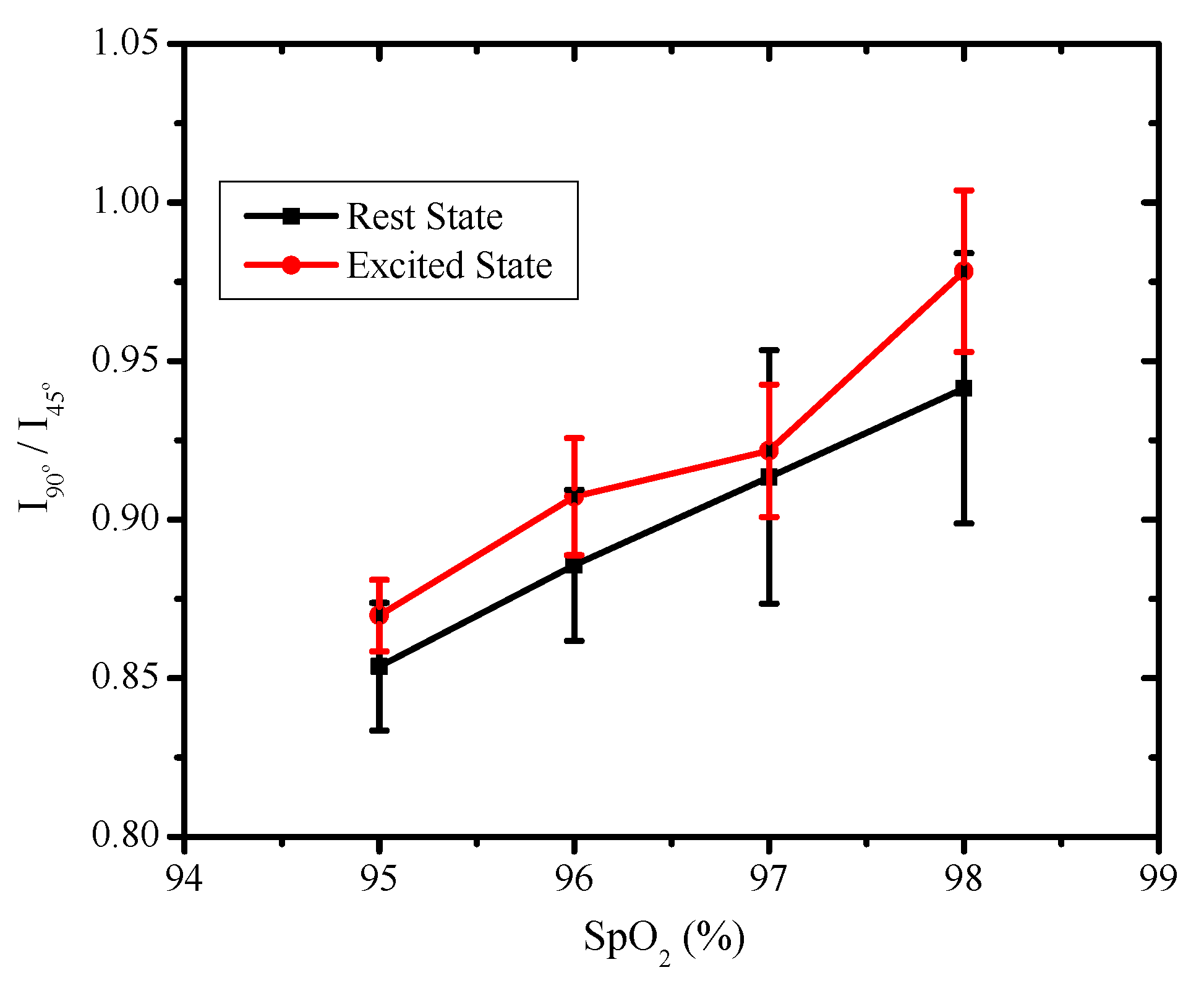
| H.R. | H.R. | SpO | ||
|---|---|---|---|---|
| (Resting) | (Excited) | (Resting) | (Excited) | |
| (I) | (II) | (III) | (IV) | (V) |
| 65 | 85 | 95 | 0.853 | 0.869 |
| 68 | 93 | 96 | 0.885 | 0.907 |
| 74 | 109 | 97 | 0.913 | 0.921 |
| 82 | 114 | 98 | 0.941 | 0.978 |
| [22] | [15] | [24] | Proposed Work | |
|---|---|---|---|---|
| Algorithm used | Radially polarized light from LED and an external camera system used for SpO measurements | Smartphone camera is used for capturing reflectance image while flashlight is used as illumination | Ratio of phase information from deep and superficial backscattering is used to estimate SpO | In-pixel wire grid polarizers are used to compute the ratio of phase information from deep and superficial backscattering |
| Light sources or wavelength used | One | One | One | One |
| Optical test bench | Complex due to use of collimated optics along with external polarizers | Simple, with no optical test bench used. Does not use phase information. | Complex due to use of polarizer and analyzer with manual varying of the transmission axis of the filters | Simple, with no manual variations of the transmission axis of the filter required |
| Sampling time | 16 s | 2 s | 3.4 s | 1 s |
| Computational complexity | Simple. The mean intensity at different polarization states is used to compute the ratio of polarization states. | Complex. Ratio of the green and red channel of the mobile phone camera is used along with appropriate weights in the calibration. | Simple. The ratio of intensities for two phases is used with normalization. | Simple. The ratio of intensities for two phases is used with normalization. |
| Ambient noise reduction | The polarizer filters in the optical test bench reduce specular reflection | The polarizer (near flashlight) and analyzer (near camera) were orthogonally oriented to minimize the specular reflection | The external polarizer filter near the camera reduced the specular reflection | The in-pixel embedded polarizer reduces the specular reflection |
| Accuracy | Not provided | 2–3% | The ratio of intensities of two phases is shown to be linear | The ratio of intensities of two phases is shown to be linear |
| Method used to vary oxygen saturation | Normal breathing conditions while sitting down were used for measurements | Medical grade manual pressure cuff was used to vary the oxygen saturation | The subject was asked to hold his or her breath for some time | The subject was asked to perform physical activity for 10 min |
| Number of test subjects | 6 | 5 | 15 | 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarkar, M.; Assaad, M. Noninvasive Non-Contact SpO2 Monitoring Using an Integrated Polarization-Sensing CMOS Imaging Sensor. Sensors 2022, 22, 7796. https://doi.org/10.3390/s22207796
Sarkar M, Assaad M. Noninvasive Non-Contact SpO2 Monitoring Using an Integrated Polarization-Sensing CMOS Imaging Sensor. Sensors. 2022; 22(20):7796. https://doi.org/10.3390/s22207796
Chicago/Turabian StyleSarkar, Mukul, and Maher Assaad. 2022. "Noninvasive Non-Contact SpO2 Monitoring Using an Integrated Polarization-Sensing CMOS Imaging Sensor" Sensors 22, no. 20: 7796. https://doi.org/10.3390/s22207796






