SWCNT Network-FET Device for Human Serum Albumin Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Substrates
2.2. SWCNT Thin Film Deposition
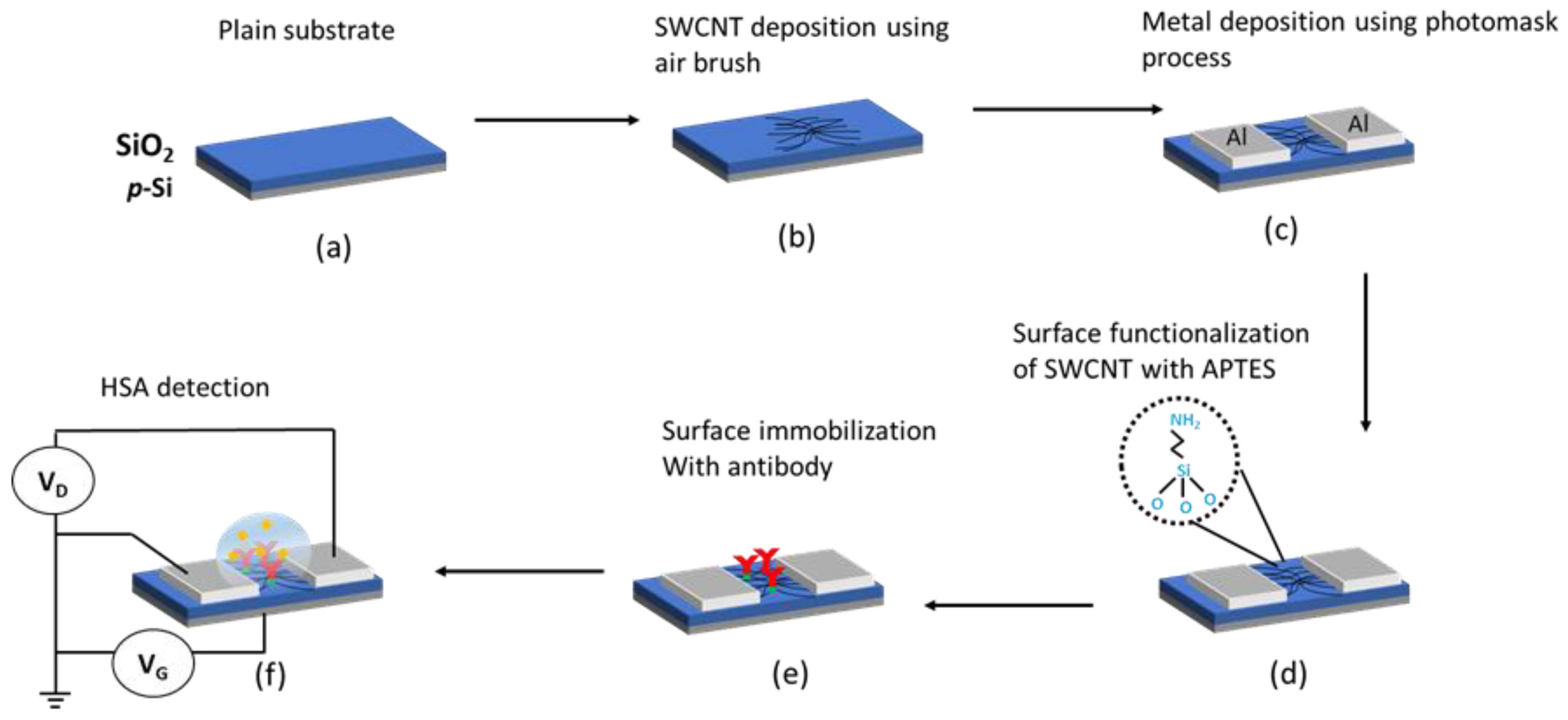
2.3. Fabrication of the SWCNT Network Device
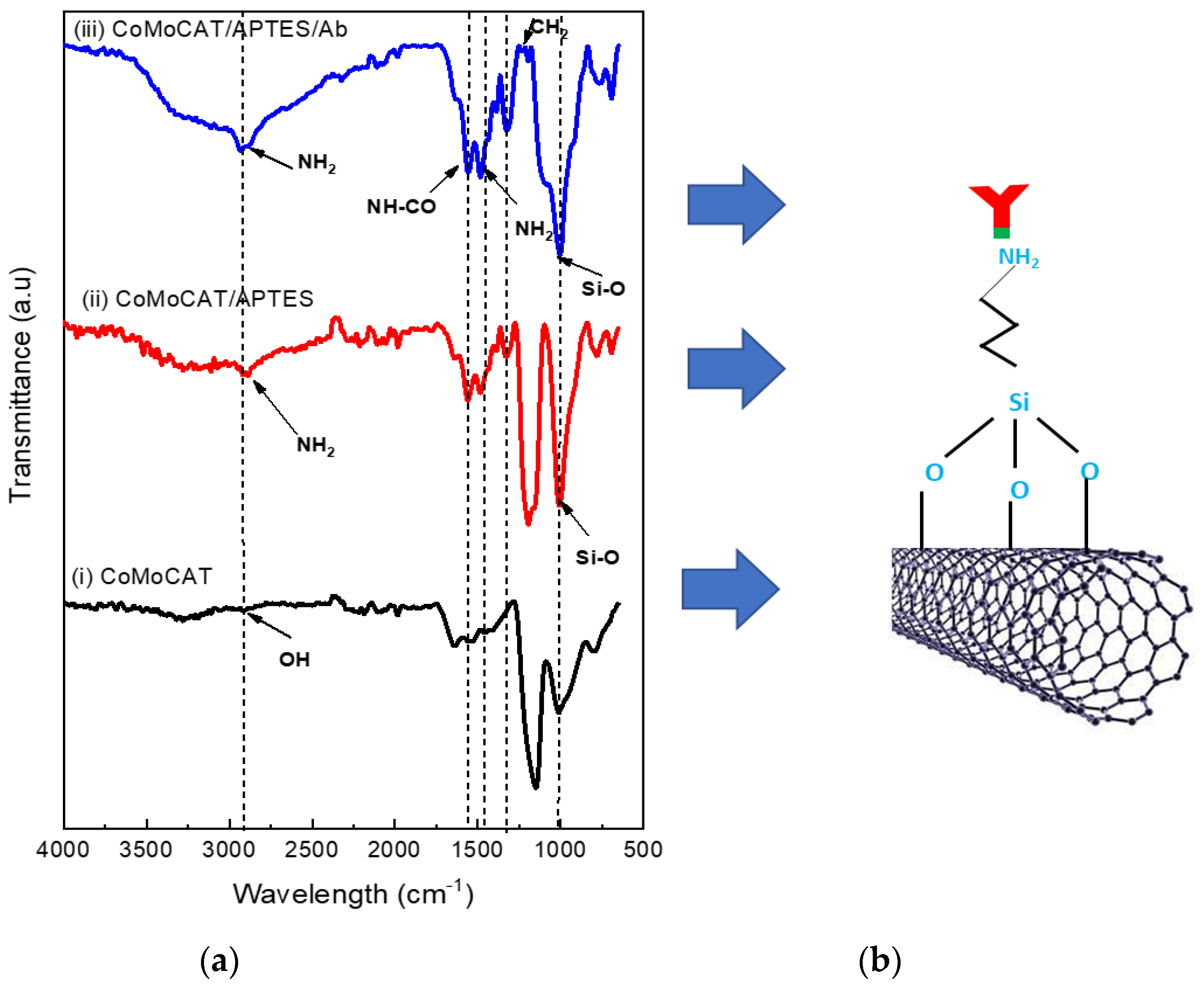
2.4. SWCNTs Surface Functionalisation, Detection and Characterisation
2.5. FET-Based Biosensor Electrical Characterisation
3. Results
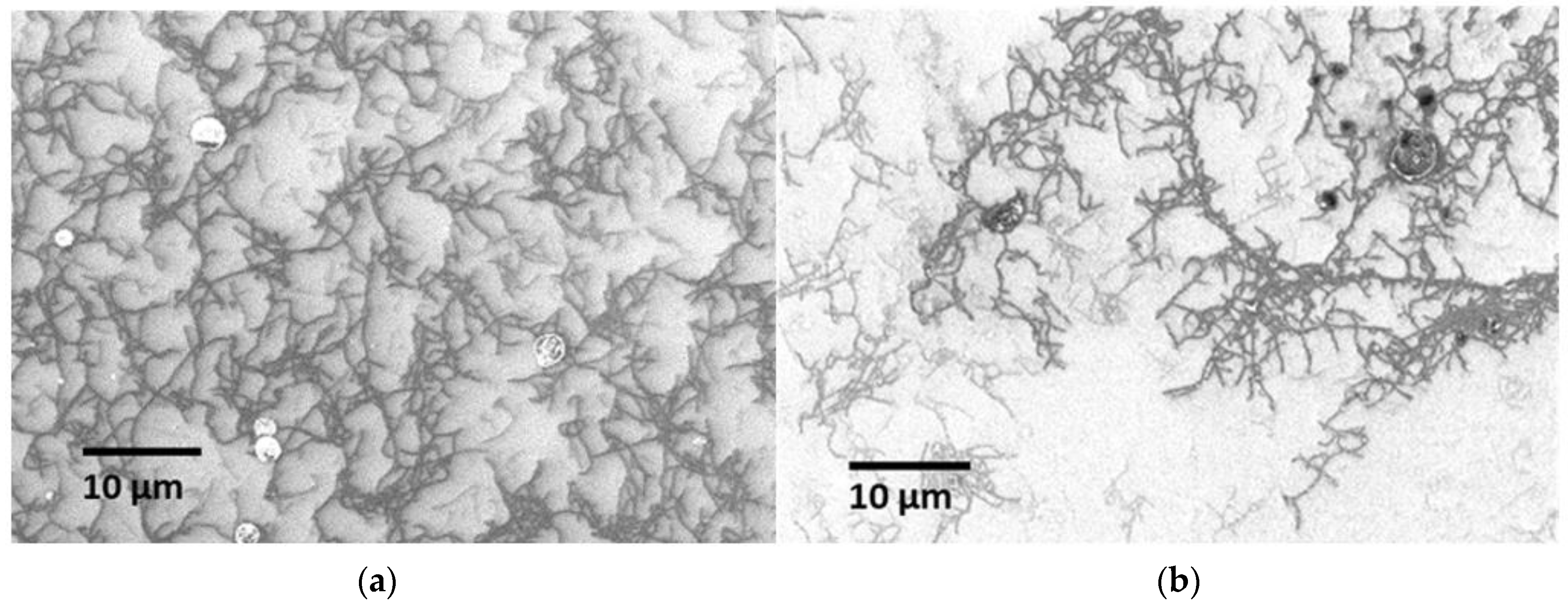
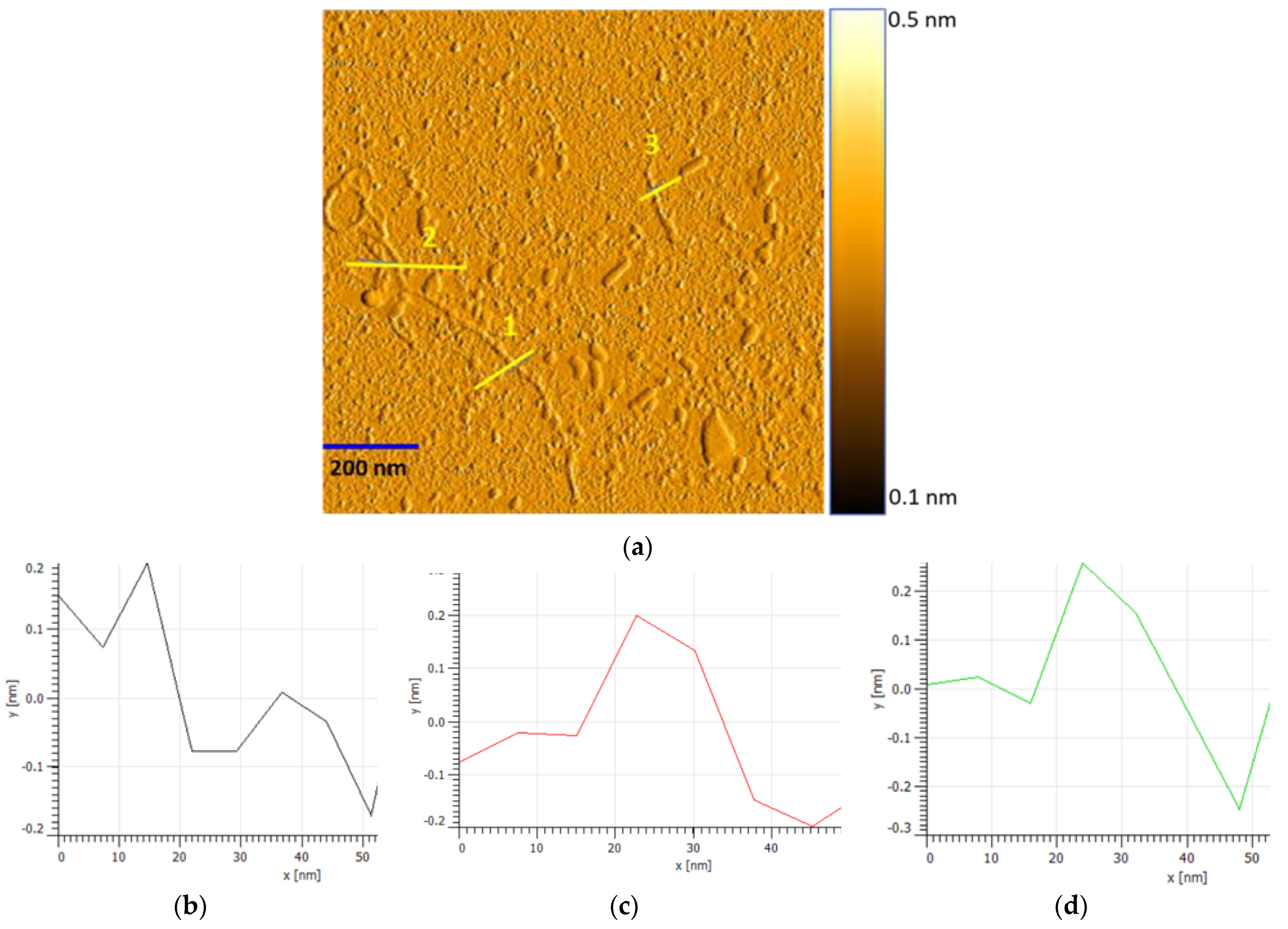
3.1. Thin Film Characterisation
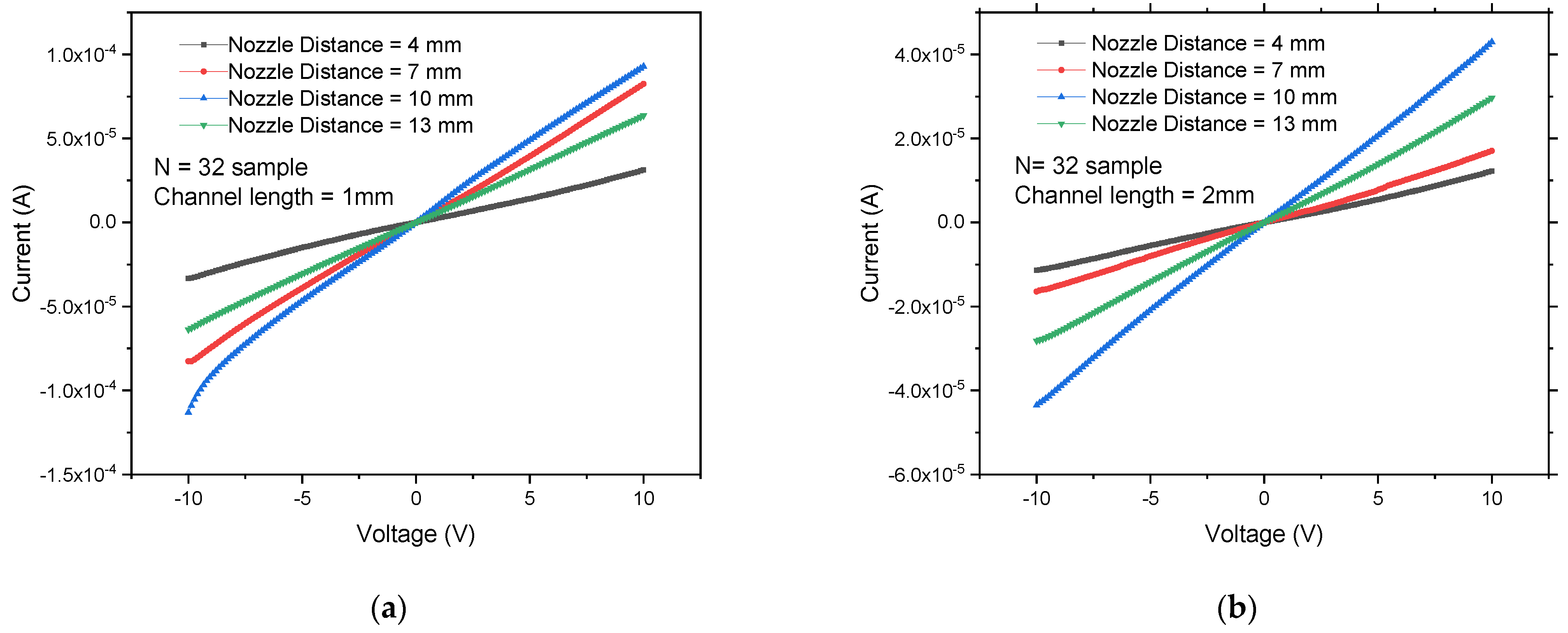
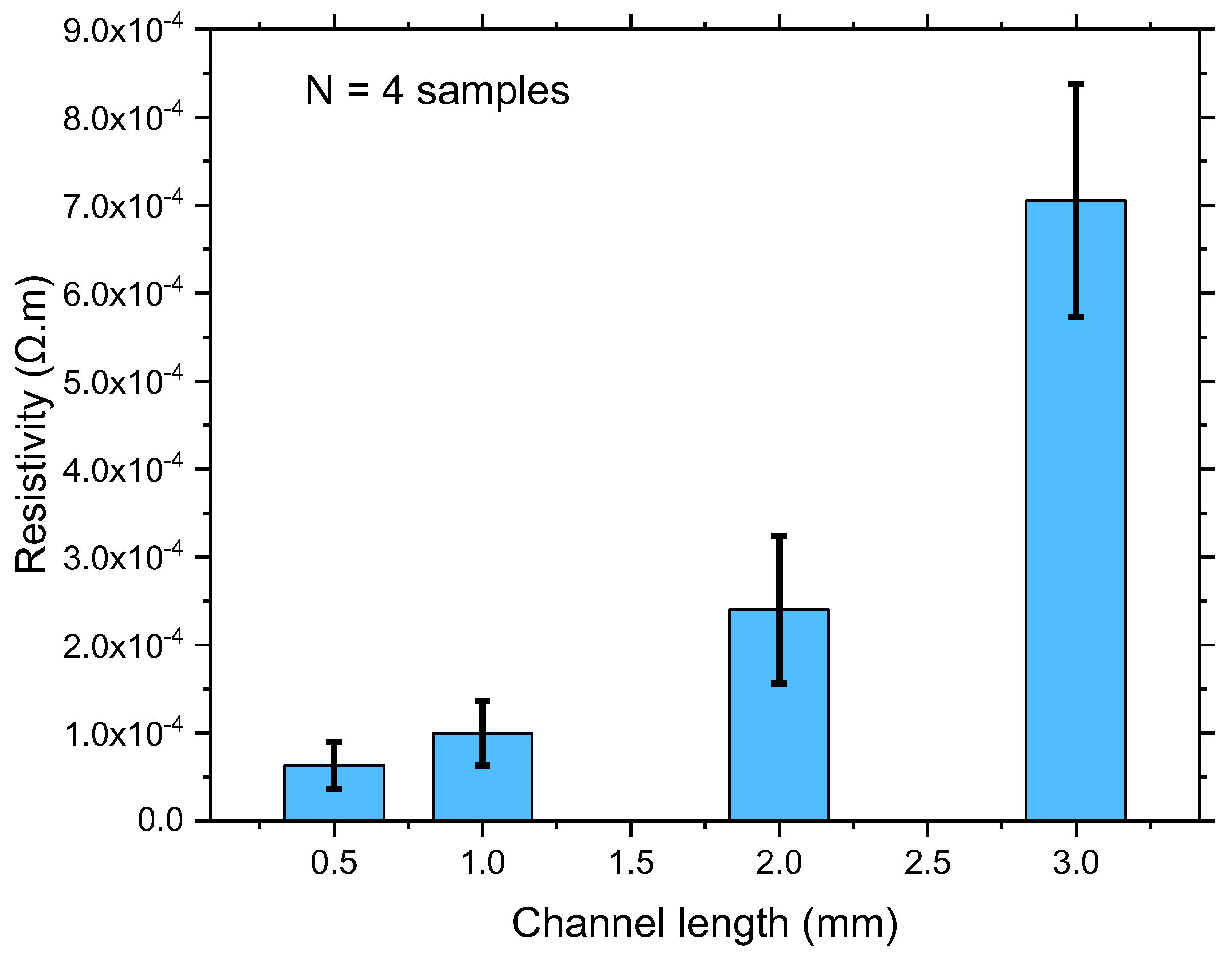
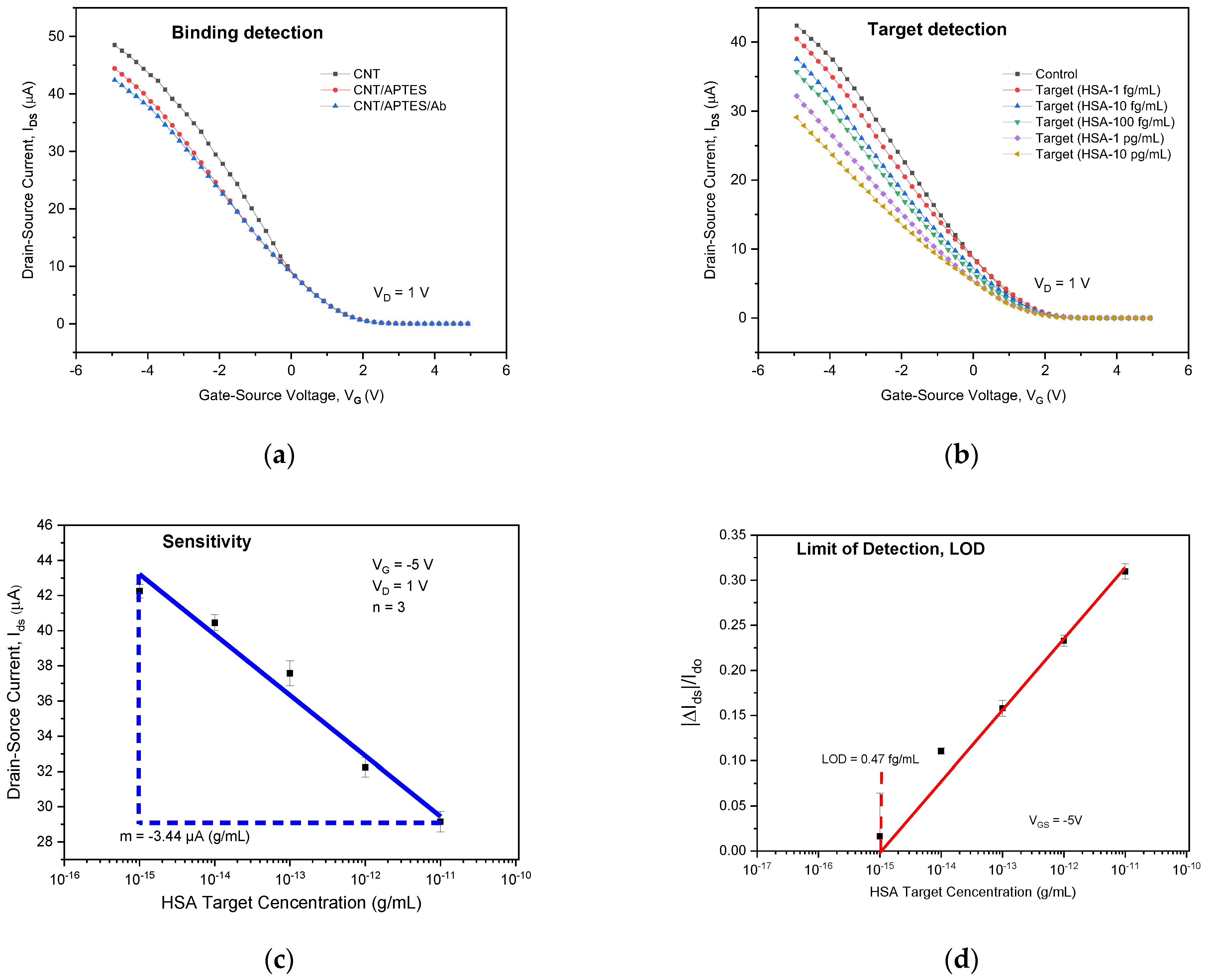
3.2. Electrical Characteristics of Network SWCNT-FET
3.3. Characterisation of Surface Functionalisation
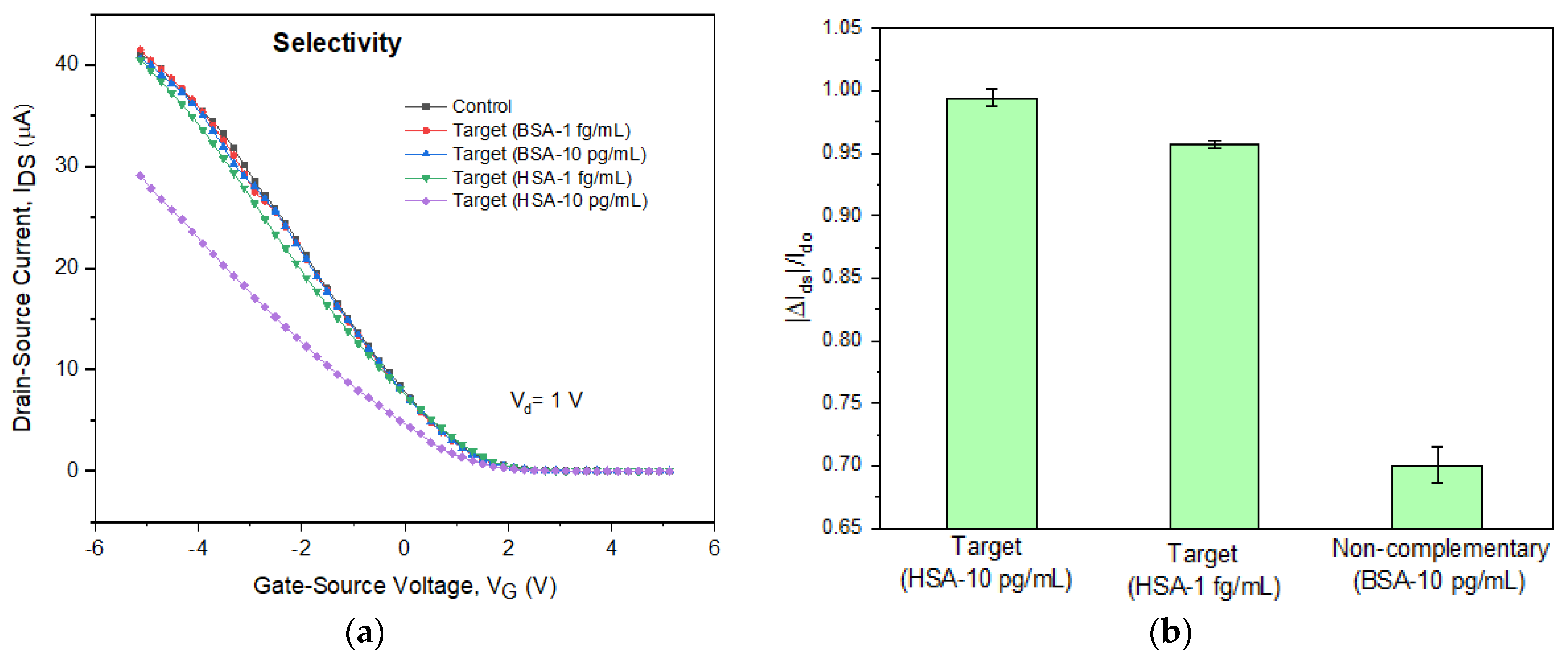
3.4. Detection of HSA
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hu, L.; Hecht, D.S.; Gruner, G. Percolation in transparent and conducting carbon nanotube networks. Nano Lett. 2004, 4, 2513–2517. [Google Scholar] [CrossRef]
- Theng, L.L.; Yahya, I.; Fariz, M.; Taib, M.; Mohamed, M.A. Electrical and Structural Characterization of Graphene Carbon Nanotubes Hybrids (GCH). Structures 2019, 12, 83–89. [Google Scholar]
- Mohamed, M.A.; Azam, M.A.; Shikoh, E.; Fujiwara, A. Fabrication and characterization of carbon nanotube field-effect transistors using ferromagnetic electrodes with different coercivities. Jpn. J. Appl. Phys. 2010, 49, 4–8. [Google Scholar] [CrossRef]
- Tran, T.T.; Mulchandani, A. Carbon nanotubes and graphene nano field-effect transistor-based biosensors. TrAC Trends Anal. Chem. 2016, 79, 222–232. [Google Scholar] [CrossRef] [Green Version]
- Karim, S.S.A.; Dee, C.F.; Majlis, B.Y.; Mohamed, M.A. Recent progress on fabrication of zinc oxide nanorod-based field effect transistor biosensors. Sains Malays. 2019, 48, 1301–1310. [Google Scholar] [CrossRef]
- Tung, N.T.; Tue, P.T.; Thi Ngoc Lien, T.; Ohno, Y.; Maehashi, K.; Matsumoto, K.; Nishigaki, K.; Biyani, M.; Takamura, Y. Peptide aptamer-modified single-walled carbon nanotube-based transistors for high-performance biosensors. Sci. Rep. 2017, 7, 17881. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Guo, X.; Wang, J.; Guo, J.; Zhang, Y.; Zhang, W.; Sang, S. High sensitivity detection of human serum albumin using a novel magnetoelastic immunosensor. J. Mater. Sci. 2019, 54, 9679–9688. [Google Scholar] [CrossRef]
- Gruner, G. Carbon nanotube transistors for biosensing applications. Anal. Bioanal. Chem. 2006, 384, 322–335. [Google Scholar] [CrossRef]
- Cieplak, M.; Szwabinska, K.; Sosnowska, M.; Chandra, B.K.C.; Borowicz, P.; Noworyta, K.; D’Souza, F.; Kutner, W. Selective electrochemical sensing of human serum albumin by semi-covalent molecular imprinting. Biosens. Bioelectron. 2015, 74, 960–966. [Google Scholar] [CrossRef] [Green Version]
- Erickson, H.P. Size and shape of protein molecules at the nanometer level determined by sedimentation, gel filtration, and electron microscopy. Biol. Proced. Online 2009, 11, 32–51. [Google Scholar] [CrossRef] [Green Version]
- Peters, T., Jr. Foreword. In All about Albumin; Academic Press: Cambridge, MA, USA, 1995. [Google Scholar]
- Smales, C.M.; James, D.C. Therapeutic Proteins; Humana Press: Totowa, NJ, USA, 2005; ISBN 1588293904. [Google Scholar]
- Milik, A.; Hrynkiewicz, E. On translation of LD, IL and SFC given according to IEC-61131 for hardware synthesis of reconfigurable logic controller. IFAC Proc. Vol. 2014, 19, 4477–4483. [Google Scholar] [CrossRef]
- Bachmann, L.M.; Nilsson, G.; Bruns, D.E.; McQueen, M.J.; Lieske, J.C.; Zakowski, J.J.; Miller, W.G. State of the art for measurement of urine albumin: Comparison of routine measurement procedures to isotope dilution tandem mass spectrometry. Clin. Chem. 2014, 60, 471–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.; Kim, T.H. Determination of human serum albumin using a single-walled carbon nanotube-FET modified with bromocresol green. Microchim. Acta 2016, 183, 1513–1518. [Google Scholar] [CrossRef]
- Hong, T.; Wang, T.; Xu, Y.-Q. Direct measurement of π coupling at the single molecule level using a carbon nanotube force sensor. Nano Lett. 2018, 18, 7883–7888. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Smit, J.H.; Prusty, D.K.; Musser, A.J.; Tombros, N.; Lee, P.C.W.; Herrmann, A.; Kwak, M. Ultrasensitive Detection of Oligonucleotides: Single-Walled Carbon Nanotube Transistor Assembled by DNA Block Copolymer. J. Nanosci. Nanotechnol. 2017, 17, 5175–5180. [Google Scholar] [CrossRef]
- Sun, Z.; Hong, C.; Gao, K.; Qiao, X.; Pan, B.G. Electrochemical immunosensor for alpha-fetoprotein based on prussian blue-carbon nanotube@polydopamine. Micro Nano Lett. 2017, 13, 58–62. [Google Scholar] [CrossRef]
- Lee, C.S.; Ju, Y.; Kim, J.; Kim, T.H. Electrochemical functionalization of single-walled carbon nanotubes with amine-terminated dendrimers encapsulating Pt nanoparticles: Toward facile field-effect transistor-based sensing platforms. Sens. Actuators B Chem. 2018, 275, 367–372. [Google Scholar] [CrossRef]
- Zamzami, M.A.; Rabbani, G.; Ahmad, A.; Basalah, A.A.; Al-Sabban, W.H.; Nate Ahn, S.; Choudhry, H. Carbon nanotube field-effect transistor (CNT-FET)-based biosensor for rapid detection of SARS-CoV-2 (COVID-19) surface spike protein S1. Bioelectrochemistry 2022, 143, 107982. [Google Scholar] [CrossRef]
- Ervin, M.H.; Miller, B.S. Air Brush Fabricated Carbon Nanotube Supercapacitor Electrodes; Defense Technical Information Center: Fort Belvoir, VA, USA, 2010. [Google Scholar]
- Nur Nasyifa, M.M.; Ruslinda, A.R.; Halim, N.H.A.; Abidin, A.S.Z.; Faudzi, F.N.M.; Ahmad, N.A.; Lockman, Z.; Rezek, B.; Kromka, A.; Gopinath, S.C.B. Immuno-probed graphene nanoplatelets on electrolyte-gated field-effect transistor for stable cortisol quantification in serum. J. Taiwan Inst. Chem. Eng. 2020, 117, 10–18. [Google Scholar] [CrossRef]
- Kim, K.; Park, C.; Kwon, D.; Kim, D.; Meyyappan, M.; Jeon, S.; Lee, J.S. Silicon nanowire biosensors for detection of cardiac troponin I (cTnI) with high sensitivity. Biosens. Bioelectron. 2016, 77, 695–701. [Google Scholar] [CrossRef]
- Yahya, I.; Kunaraj, A.; Mustaza, S.M.; Clowes, S.; Silva, S.R.P. Methods for Estimating Composition of Single Walled Carbon Nanotubes Based on Electronic Type. Mater. Sci. Forum 2022, 1055, 77–86. [Google Scholar] [CrossRef]
- Shimoni, A.; Azoubel, S.; Magdassi, S. Inkjet printing of flexible high-performance carbon nanotube transparent conductive films by “coffee ring effect”. Nanoscale 2014, 6, 11084–11089. [Google Scholar] [CrossRef] [PubMed]
- Polikarpov, Y.A.; Romashkin, A.V.; Struchkov, N.S.; Levin, D.D. High uniform carbon nanotube thin films spray deposition on substrates with patterned structures having height difference. In Proceedings of the 2019 IEEE Conference of Russian Young Researchers in Electrical and Electronic Engineering (EIConRus), Saint Petersburg, Russia, 28–31 January 2019; Volume 0184, pp. 1980–1985. [Google Scholar] [CrossRef]
- Abdelhalim, A.; Abdellah, A.; Scarpa, G.; Lugli, P. Fabrication of carbon nanotube thin films on flexible substrates by spray deposition and transfer printing. Carbon N. Y. 2013, 61, 72–79. [Google Scholar] [CrossRef]
- Yahya, I.; Bonaccorso, F.; Clowes, S.K.; Ferrari, A.C.; Silva, S.R.P. Temperature dependent separation of metallic and semiconducting carbon nanotubes using gel agarose chromatography. Carbon N. Y. 2015, 93, 574–594. [Google Scholar] [CrossRef] [Green Version]
- Yahya, I.; Mustaza, S.M.; Abdullah, H. Carbon Nanotube-Activated Thin Film Transparent Conductor Applications; Intech Open: London, UK, 2018; Volume 1, p. 13. [Google Scholar]
- Seager, C.H.; Pike, G.E. Percolation and conductivity: A computer study: II. Phys. Rev. B 1974, 10, 1435–1446. [Google Scholar] [CrossRef]
- Yang, B.J.; Cho, K.J.; Kim, G.M.; Lee, H.K. Effect of CNT Agglomeration on the Electrical Conductivity and Percolation Threshold of Nanocomposites: A Micromechanics-based Approach. Comput. Model. Eng. Sci. 2014, 103, 343–365. [Google Scholar]
- Unalan, H.E.; Fanchini, G.; Kanwal, A.; Du Pasquier, A.; Chhowalla, M. Design criteria for transparent single-wall carbon nanotube thin-film transistors. Nano Lett. 2006, 6, 677–682. [Google Scholar] [CrossRef]
- Jung, S.; Hauert, R.; Haluska, M.; Roman, C.; Hierold, C. Understanding and improving carbon nanotube-electrode contact in bottom-contacted nanotube gas sensors. Sens. Actuators B Chem. 2021, 331, 129406. [Google Scholar] [CrossRef]
- An, L.; Yang, X.; Chang, C. On Contact Resistance of Carbon Nanotubes. Int. J. Theor. Appl. Nanotechnol. 2013, 1, 30–40. [Google Scholar] [CrossRef] [Green Version]
- De Andrade, M.J.; Lima, M.D.; Skákalová, V.; Bergmann, C.P.; Roth, S. Electrical properties of transparent carbon nanotube networks prepared through different techniques. Phys. Status Solidi Rapid Res. Lett. 2007, 1, 178–180. [Google Scholar] [CrossRef]
- Slattery, A.D.; Shearer, C.J.; Shapter, J.G.; Blanch, A.J.; Quinton, J.S.; Gibson, C.T. Improved application of carbon nanotube atomic force microscopy probes using peakforce tapping mode. Nanomaterials 2018, 8, 807. [Google Scholar] [CrossRef]
- Appenzeller, J.; Knoch, J.; Radosavljević, M.; Avouris, P. Multimode transport in schottky-barrier carbon-nanotube field-effect transistors. Phys. Rev. Lett. 2004, 92, 226802. [Google Scholar] [CrossRef]
- Hecht, D.; Grüner, G. Solution Cast Films of Carbon Nanotubes for Transparent Conductors and Thin Film Transistors. In Flexible Electronics; Springer: Boston, MA, USA, 2009; ISBN 9780387743639. [Google Scholar]
- Kabir, M.A.; Nandy, T.; Aminul Haque, M.; Dutta, A.; Mahmood, Z.H. Performance Analysis of Cntfet and Mosfet Focusing Channel Length, Carrier Mobility and Ballistic Conduction in High Speed Switching. Int. J. Adv. Mater. Sci. Eng. 2014, 3, 1–11. [Google Scholar] [CrossRef]
- Ortiz-Conde, A.; García-Sánchez, F.J.; Muci, J.; Terán Barrios, A.; Liou, J.J.; Ho, C.S. Revisiting MOSFET threshold voltage extraction methods. Microelectron. Reliab. 2013, 53, 90–104. [Google Scholar] [CrossRef]
- Islam, A.E. Variability and reliability of single-walled carbon nanotube field effect transistors. Electronics 2013, 2, 332–367. [Google Scholar] [CrossRef]
- Majoul, N.; Aouida, S.; Bessaïs, B. Progress of porous silicon APTES-functionalization by FTIR investigations. Appl. Surf. Sci. 2015, 331, 388–391. [Google Scholar] [CrossRef]
- Myndrul, V.; Coy, E.; Bechelany, M.; Iatsunskyi, I. Photoluminescence label-free immunosensor for the detection of Aflatoxin B1 using polyacrylonitrile/zinc oxide nanofibers. Mater. Sci. Eng. C 2021, 118, 111401. [Google Scholar] [CrossRef] [PubMed]
- Siti, S.S.; Nadzirah, S.; Kazmi, J.; Rahim, R.A.; Dee, C.F.; Hamzah, A.A.; Mohamed, M.A. Zinc oxide nanorods-based immuno-field-effect transistor for human serum albumin detection. J. Mater. Sci. 2021, 56, 15344–15353. [Google Scholar] [CrossRef]
- Xu, W.; Wei, Y.; Xing, D.; Chen, Q.; Luo, S. Quantification of human serum albumin by highly sensitive chemiluminescence method. In Proceedings of the 2007 IEEE/ICME International Conference on Complex Medical Engineering, Beijing, China, 23–27 May 2007; pp. 1694–1697. [Google Scholar] [CrossRef]



| Sensing Technique | Linear Range | Detection Limit | Reference |
|---|---|---|---|
| Chemosensor based on semi-covalent imprinting | 0.8 μg/mL–20 μg/mL | 16.5 ng/mL | [9] |
| Chemosensor based on semi-covalent imprinting | 4 μg/mL–800 μg/mL | 800 ng/mL | [9] |
| Wireless magnetoelastic (ME) immunosensor based on Au nanoparticle | 0.01 μg/mL−100 μg/mL | 0.01 μg/mL | [7] |
| CNT-FET | 0.07 μg/mL–70 μg/mL | 18.6 ng/mL | [15] |
| Chemiluminescence intensity | 0.05 μM/L–1.5 μM/L | 17.32 ng/mL | [45] |
| ZnO NRs-FET | 0.01 μg/mL–100 μg/mL | 9.81 ng/mL | [44] |
| CNT-FET based on the SWCNT network | 1 fg/mL–10 pg/mL | 0.47 fg/mL | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yahya, I.; Hassan, M.A.; Maidin, N.N.M.; Mohamed, M.A. SWCNT Network-FET Device for Human Serum Albumin Detection. Sensors 2022, 22, 8212. https://doi.org/10.3390/s22218212
Yahya I, Hassan MA, Maidin NNM, Mohamed MA. SWCNT Network-FET Device for Human Serum Albumin Detection. Sensors. 2022; 22(21):8212. https://doi.org/10.3390/s22218212
Chicago/Turabian StyleYahya, Iskandar, Muhamad Azuddin Hassan, Nur Nasyifa Mohd Maidin, and Mohd Ambri Mohamed. 2022. "SWCNT Network-FET Device for Human Serum Albumin Detection" Sensors 22, no. 21: 8212. https://doi.org/10.3390/s22218212







