Identifying Demographic, Clinical, Muscular and Histological Factors Associated with Ultrasound Cervical Multifidus Measurement Errors in a Chronic Neck Pain Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Sample Size Calculation
2.4. Data Collection
2.4.1. Sociodemographic Data
2.4.2. Clinical Data
2.4.3. Cervical Multifidus Ultrasound Imaging
2.5. Statistical Analyses
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Whittaker, J.L.; Ellis, R.; Hodges, P.W.; OSullivan, C.; Hides, J.; Fernandez-Carnero, S.; Arias-Buria, J.L.; Teyhen, D.S.; Stokes, M.J. Imaging with ultrasound in physical therapy: What is the PT’s scope of practice? A competency-based educational model and training recommendations. Br. J. Sports Med. 2019, 53, 1447–1453. [Google Scholar] [CrossRef]
- Diep, D.; Chen, K.J.Q.; Kumbhare, D. Ultrasound-guided interventional procedures for myofascial trigger points: A systematic review. Reg. Anesth. Pain Med. 2021, 46, 73–80. [Google Scholar] [CrossRef]
- Valera-Calero, J.A.; Fernández-de-Las-Peñas, C.; Varol, U.; Ortega-Santiago, R.; Gallego-Sendarrubias, G.M.; Arias-Buría, J.L. Ultrasound Imaging as a Visual Biofeedback Tool in Rehabilitation: An Updated Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 7554. [Google Scholar] [CrossRef]
- Finnoff, J.T.; Ray, J.; Corrado, G.; Kerkhof, D.; Hill, J. Sports Ultrasound: Applications Beyond the Musculoskeletal System. Sports Health 2016, 8, 412–417. [Google Scholar] [CrossRef]
- Yelverton, C.; Wood, J.J.; Petersen, D.L.; Peterson, C. Changes in Vertebral Artery Blood Flow in Different Head Positions and Post-Cervical Manipulative Therapy. J. Manip. Physiol. Ther. 2020, 43, 144–151. [Google Scholar] [CrossRef]
- Dieterich, A.V.; Pickard, C.M.; Deshon, L.E.; Strauss, G.R.; Gibson, W.; Davey, P.; McKay, J. M-mode ultrasound used to detect the onset of deep muscle activity. J. Electromyogr. Kinesiol. 2015, 25, 224–231. [Google Scholar] [CrossRef]
- Valera-Calero, J.A.; Sánchez-Jorge, S.; Buffet-García, J.; Varol, U.; Gallego-Sendarrubias, G.M.; Álvarez-González, J. Is Shear-Wave Elastography a Clinical Severity Indicator of Myofascial Pain Syndrome? An Observational Study. J. Clin. Med. 2021, 10, 2895. [Google Scholar] [CrossRef] [PubMed]
- Valera-Calero, J.A.; Ojedo-Martín, C.; Fernández-de-Las-Peñas, C.; Cleland, J.A.; Arias-Buría, J.L.; Hervás-Pérez, J.P. Reliability and Validity of Panoramic Ultrasound Imaging for Evaluating Muscular Quality and Morphology: A Systematic Review. Ultrasound Med. Biol. 2021, 47, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Valera-Calero, J.A.; Fernández-de-Las-Peñas, C.; Cleland, J.A.; Varol, U.; Ortega-Santiago, R.; Arias-Buría, J.L. Ultrasound assessment of deep cervical extensors morphology and quality in populations with whiplash associated disorders: An intra- and inter-examiner reliability study. Musculoskelet. Sci. Pract. 2022, 59, 102538. [Google Scholar] [CrossRef] [PubMed]
- Cloney, M.; Smith, A.C.; Coffey, T.; Paliwal, M.; Dhaher, Y.; Parrish, T.; Elliott, J.; Smith, Z.A. Fatty infiltration of the cervical multifidus musculature and their clinical correlates in spondylotic myelopathy. J. Clin. Neurosci. 2018, 57, 208–213. [Google Scholar] [CrossRef]
- Elliott, J.M.; Courtney, D.M.; Rademaker, A.; Pinto, D.; Sterling, M.M.; Parrish, T.B. The Rapid and Progressive Degeneration of the Cervical Multifidus in Whiplash: An MRI Study of Fatty Infiltration. Spine 2015, 40, E694–E700. [Google Scholar] [CrossRef] [PubMed]
- Uthaikhup, S.; Assapun, J.; Kothan, S.; Watcharasaksilp, K.; Elliott, J.M. Structural changes of the cervical muscles in elder women with cervicogenic headache. Musculoskelet. Sci. Pract. 2017, 29, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.; Jull, G.; Noteboom, J.T.; Darnell, R.; Galloway, G.; Gibbon, W.W. Fatty infiltration in the cervical extensor muscles in persistent whiplash-associated disorders: A magnetic resonance imaging analysis. Spine 2006, 31, E847–E855. [Google Scholar] [CrossRef]
- Valera-Calero, J.A.; Úbeda-D’Ocasar, E.; Caballero-Corella, M.; Fernández-de-Las-Peñas, C.; Sendarrubias, G.M.G.; Arias-Buría, J.L. Cervical Multifidus Morphology and Quality Are Not Associated with Clinical Variables in Women with Fibromyalgia: An Observational Study. Pain Med. 2022, 23, 1138–1143. [Google Scholar] [CrossRef] [PubMed]
- Schomacher, J.; Falla, D. Function and structure of the deep cervical extensor muscles in patients with neck pain. Man. Ther. 2013, 18, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Javanshir, K.; Amiri, M.; Mohseni-Bandpei, M.A.; Rezasoltani, A.; Fernández-de-las-Peñas, C. Ultrasonography of the cervical muscles: A critical review of the literature. J. Manip. Physiol. Ther. 2010, 33, 630–637. [Google Scholar] [CrossRef]
- Purushotham, S.; Stephenson, R.S.; Sanderson, A.; Abichandani, D.; Greig, C.; Gardner, A.; Falla, D. Microscopic changes in the spinal extensor musculature in people with chronic spinal pain: A systematic review. Spine J. 2022, 22, 1205–1221. [Google Scholar] [CrossRef]
- Cohen, J.F.; Korevaar, D.A.; Altman, D.G.; Bruns, D.E.; Gatsonis, C.A.; Hooft, L.; Irwig, L.; Levine, D.; Reitsma, J.B.; De Vet, H.C.W.; et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: Explanation and elaboration. BMJ Open 2016, 6, e012799. [Google Scholar] [CrossRef]
- Childs, J.D.; Cleland, J.A.; Elliott, J.M.; Teyhen, D.S.; Wainner, R.S.; Whitman, J.M.; Sopky, B.J.; Godges, J.J.; Flynn, T.; Delitto, A.; et al. Neck pain: Clinical practice guidelines linked to the International Classification of Functioning, Disability, and Health from the Orthopedic Section of the American Physical Therapy Association. J. Orthop. Sports Phys. Ther. 2008, 38, A1–A34. [Google Scholar] [CrossRef]
- VanVoorhis, C.W.; Morgan, B.L. Understanding power and rules of thumb for determining sample sizes. Tutor. Quant. Methods Psychol. 2007, 3, 43–50. [Google Scholar] [CrossRef]
- Green, S.B. How Many Subjects Does It Take To Do A Regression Analysis. Multivar. Behav. Res. 1991, 26, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.J. A Primer of Multivariate Statistics, 3rd ed.; Psychology Press: Hove, UK, 2001. [Google Scholar] [CrossRef]
- Peterson, C.M.; Thomas, D.M.; Blackburn, G.L.; Heymsfield, S.B. Universal equation for estimating ideal body weight and body weight at any BMI. Am. J. Clin. Nutr. 2016, 103, 1197–1203, Erratum in Am. J. Clin. Nutr. 2017, 105, 772. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Hobden, E.; Stiell, I.G.; Wells, G.A. Clinically important change in the visual analog scale after adequate pain control. Acad. Emerg. Med. 2003, 10, 1128–1130. [Google Scholar] [CrossRef]
- Andrade Ortega, J.A.; Delgado Martínez, A.D.; Almécija Ruiz, R. Validation of the Spanish version of the Neck Disability Index. Spine 2010, 35, E114–E118. [Google Scholar] [CrossRef] [PubMed]
- Young, I.A.; Dunning, J.; Butts, R.; Mourad, F.; Cleland, J.A. Reliability, construct validity, and responsiveness of the neck disability index and numeric pain rating scale in patients with mechanical neck pain without upper extremity symptoms. Physiother. Theory Pract. 2019, 35, 1328–1335. [Google Scholar] [CrossRef]
- Valera-Calero, J.A.; Sánchez-Jorge, S.; Álvarez-González, J.; Ortega-Santiago, R.; Cleland, J.A.; Fernández-De-Las-Peñas, C.; Arias-Buría, J.L. Intra-rater and inter-rater reliability of rehabilitative ultrasound imaging of cervical multifidus muscle in healthy people: Imaging capturing and imaging calculation. Musculoskelet. Sci. Pract. 2020, 48, 102158. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.P.; Tseng, W.Y.; Shau, Y.W.; Wang, C.L.; Wang, H.K.; Wang, S.F. Measurement of segmental cervical multifidus contraction by ultrasonography in asymptomatic adults. Man. Ther. 2007, 12, 286–294. [Google Scholar] [CrossRef]
- Valera-Calero, J.A.; Arias-Buría, J.L.; Fernández-de-Las-Peñas, C.; Cleland, J.A.; Gallego-Sendarrubias, G.M.; Cimadevilla-Fernández-Pola, E. Echo-intensity and fatty infiltration ultrasound imaging measurement of cervical multifidus and short rotators in healthy people: A reliability study. Musculoskelet. Sci. Pract. 2021, 53, 102335. [Google Scholar] [CrossRef]
- Valera-Calero, J.A.; Al-Buqain-Ortega, A.; Arias-Buría, J.L.; Fernández-de-Las-Peñas, C.; Varol, U.; Ortega-Santiago, R. Echo-intensity, fatty infiltration, and morphology ultrasound imaging assessment in healthy and whiplash associated disorders populations: An observational study. Eur. Spine J. 2021, 30, 3059–3067. [Google Scholar] [CrossRef]
- Henderson, A.R. Testing experimental data for univariate normality. Clin. Chim. Acta 2006, 366, 112–129. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.H. Biostatistics 104: Correlational analysis. Singap. Med. J. 2003, 44, 614–619. [Google Scholar]
- Stokes, M.; Hides, J.; Elliott, J.; Kiesel, K.; Hodges, P. Rehabilitative ultrasound imaging of the posterior paraspinal muscles. J. Orthop. Sports Phys. Ther. 2007, 37, 581–595. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.J.; Chai, H.M.; Wang, S.F. Reliability of thickness measurements of the dorsal muscles of the upper cervical spine: An ultrasonographic study. J. Orthop. Sports Phys. Ther. 2009, 39, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Amiri-Arimi, S.; Mohseni Bandpei, M.A.; Rezasoltani, A.; Javanshir, K.; Biglarian, A. Measurement of Cervical Multifidus and Longus Colli Muscle Dimensions in Patients with Cervical Radiculopathy and Healthy Controls Using Ultrasonography: A Reliability Study. PM&R 2019, 11, 236–242. [Google Scholar] [CrossRef]
- Øverås, C.K.; Myhrvold, B.L.; Røsok, G.; Magnesen, E. Musculoskeletal diagnostic ultrasound imaging for thickness measurement of four principal muscles of the cervical spine—A reliability and agreement study. Chiropr. Man. Therap. 2017, 25, 2. [Google Scholar] [CrossRef]
- Kristjansson, E. Reliability of ultrasonography for the cervical multifidus muscle in asymptomatic and symptomatic subjects. Man. Ther. 2004, 9, 83–88. [Google Scholar] [CrossRef]
- Nishihara, K.; Kawai, H.; Hayashi, H.; Naruse, H.; Kimura, A.; Gomi, T.; Hoshi, F. Frequency analysis of ultrasonic echo intensities of the skeletal muscle in elderly and young individuals. Clin. Interv. Aging 2014, 9, 1471–1478. [Google Scholar] [CrossRef]
- Narici, M.; McPhee, J.; Conte, M.; Franchi, M.V.; Mitchell, K.; Tagliaferri, S.; Monti, E.; Marcolin, G.; Atherton, P.J.; Smith, K.; et al. Age-related alterations in muscle architecture are a signature of sarcopenia: The ultrasound sarcopenia index. J. Cachexia Sarcopenia Muscle 2021, 12, 973–982. [Google Scholar] [CrossRef]
- Hioki, M.; Kanehira, N.; Koike, T.; Saito, A.; Shimaoka, K.; Sakakibara, H.; Oshida, Y.; Akima, H. Age-related changes in muscle volume and intramuscular fat content in quadriceps femoris and hamstrings. Exp. Gerontol. 2020, 132, 110834. [Google Scholar] [CrossRef]
- Fukumoto, Y.; Ikezoe, T.; Yamada, Y.; Tsukagoshi, R.; Nakamura, M.; Takagi, Y.; Kimura, M.; Ichihashi, N. Age-Related Ultrasound Changes in Muscle Quantity and Quality in Women. Ultrasound Med. Biol. 2015, 41, 3013–3017. [Google Scholar] [CrossRef] [PubMed]
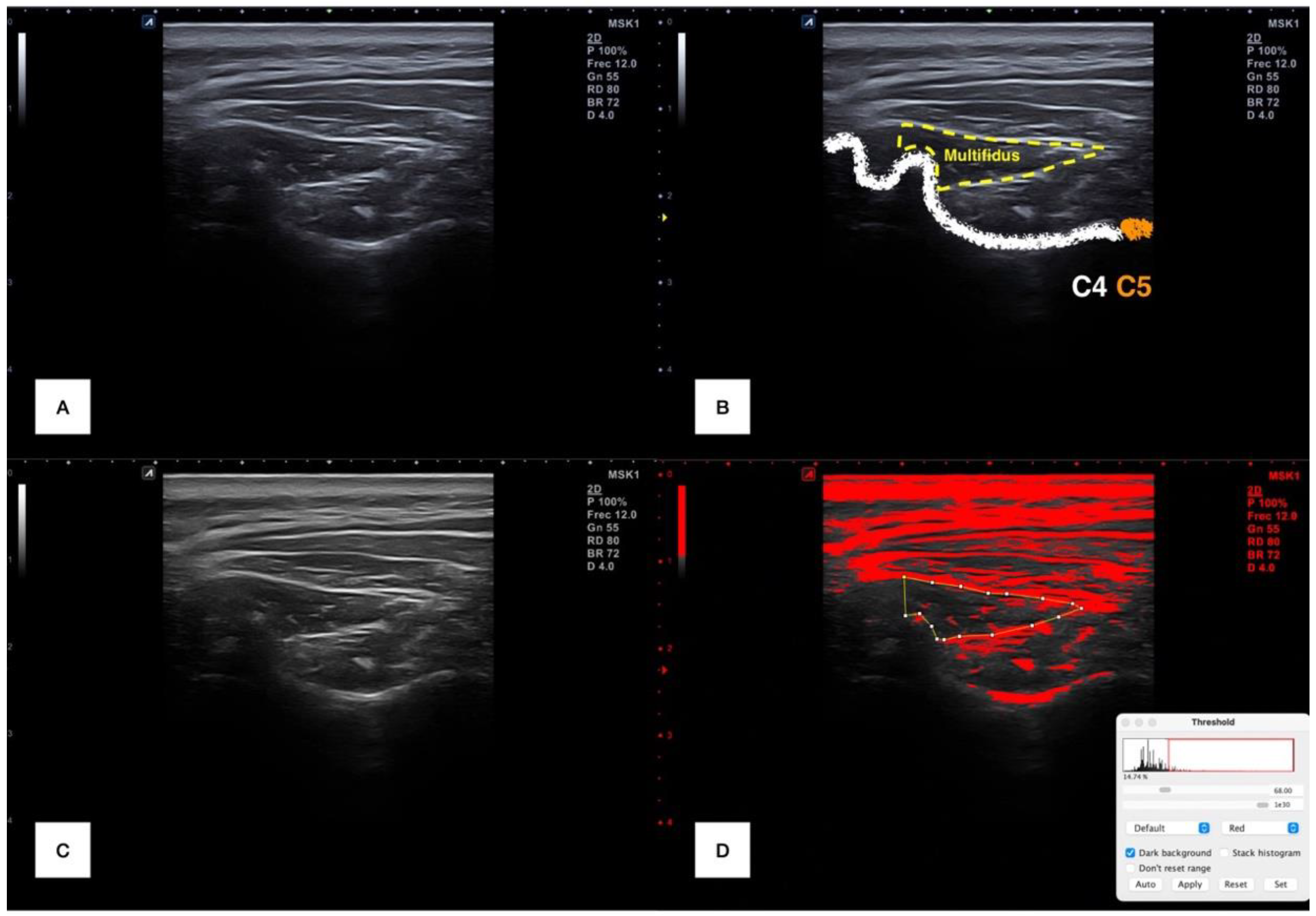
| Variables | Gender | Side | ||||
|---|---|---|---|---|---|---|
| Males (n = 58) | Females (n = 68) | Difference | Left (n = 126) | Right (n = 126) | Difference | |
| Sociodemographic Characteristics | ||||||
| Age (years) | 36.4 ± 11.3 | 37.9 ± 15.7 | 1.5 (−5.8; 7.2) | - | - | - |
| Height (m) | 1.80 ± 0.06 | 1.64 ± 0.06 | 0.16 (0.14;0.18) * | - | - | - |
| Weight (kg) | 78.1 ± 9.3 | 63.5 ± 11.8 | 14.5 (11.2;17.8) * | - | - | - |
| Body Mass Index (kg/m2) | 24.1 ± 3.1 | 23.6 ± 4.4 | 0.5 (−0.8;1.7) | - | - | - |
| Clinical Characteristics | ||||||
| Pain intensity (0–10) | 4.5 ± 1.1 | 5.1 ± 1.2 | 0.5 (−0.1;1.0) | - | - | - |
| Pain duration (months) | 4.6 ± 1.2 | 5.6 ± 3.4 | 1.0 (0.2;2.2) | - | - | - |
| NDI (0–100) | 29.1 ± 10.2 | 30.0 ± 12.6 | 0.8 (7.9;9.2) | - | - | - |
| Cervical Multifidus Characteristics | ||||||
| Area (mm2) | 105.7 ± 20.8 | 77.5 ± 19.6 | 28.2 (25.1;36.6) * | 91.9 ± 26.8 | 89.1 ± 21.0 | 2.8 (−2.7;7.5) |
| Perimeter (mm) | 50.6 ± 4.7 | 41.0 ± 5.5 | 9.6 (7.7;11.7) * | 45.6 ± 6.8 | 45.2 ± 6.3 | 0.4 (−1.6;2.4) |
| Echo Intensity (0–255) | 42.3 ± 12.5 | 53.4 ± 13.6 | 11.1 (7.9;16.3) * | 48.3 ± 13.1 | 48.3 ± 15.9 | 0.8 (−3.6;5.2) |
| Infiltration Percentage (%) | 24.9 ± 6.4 | 26.4 ± 10.2 | 1.5 (−1.9;5.2) | 25.9 ± 9.7 | 25.5 ± 9.6 | 0.2 (−2.6;3.3) |
| Variables | Mean | Examiners | Absolute Error | ICC3,2 (95% CI) | SEM | MDC | |
|---|---|---|---|---|---|---|---|
| A | B | ||||||
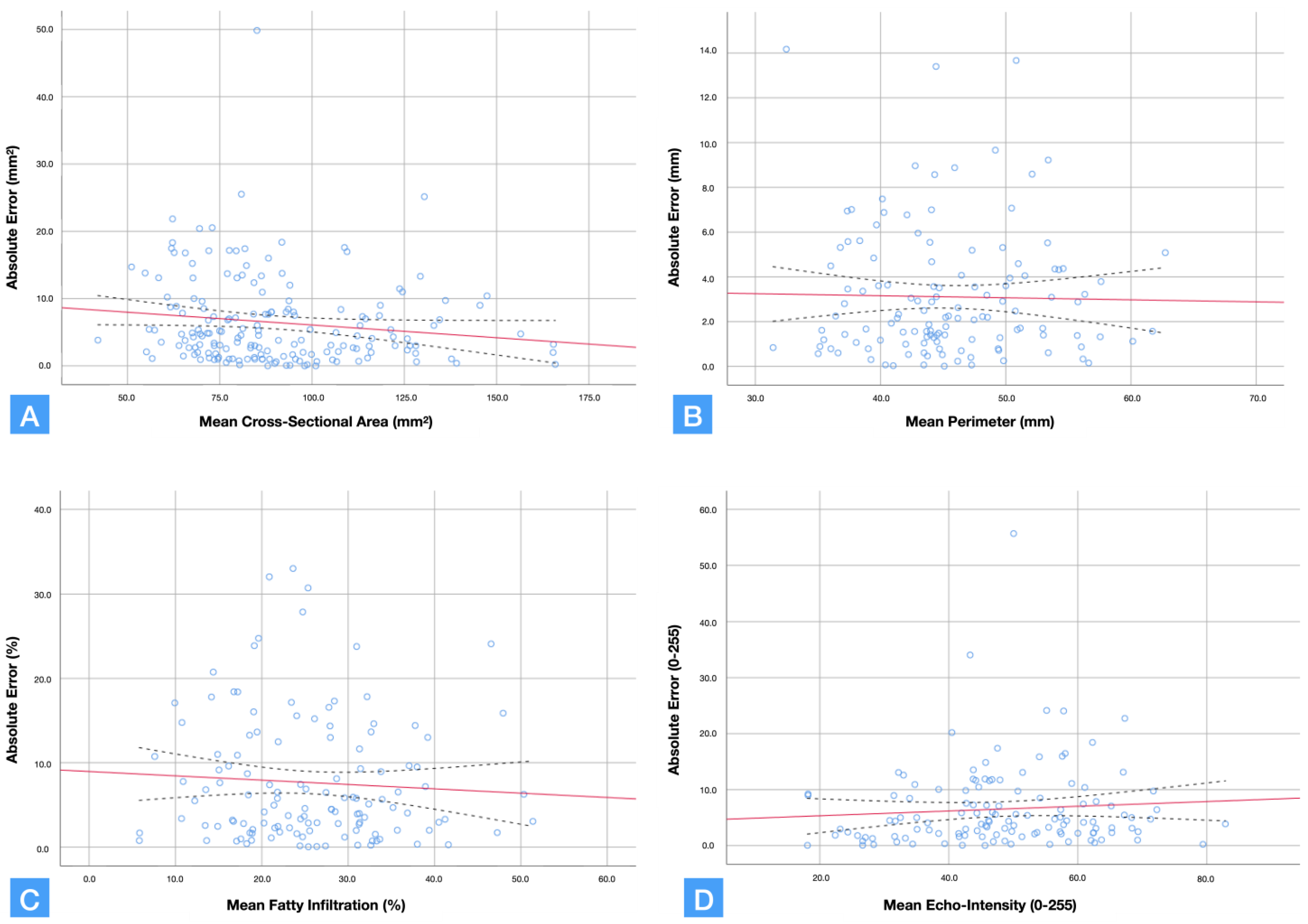
| Cross-sectional area (mm2) | 90.5± 24.2 | 89.7 ± 24.4 | 91.2 ± 25.0 | 6.5 ± 6.4 | 0.965 (0.953; 0.975) | 4.5 | 12.5 |
| Perimeter (mm) | 45.4 ± 6.4 | 44.9 ± 6.5 | 45.9 ± 6.9 | 3.1 ± 2.9 | 0.898 (0.855; 0.928) | 2.0 | 5.6 |
| Mean Echo Intensity (0–255) | 48.3 ± 13.6 | 49.1 ± 14.1 | 47.5 ± 14.7 | 6.3 ± 6.1 | 0.882 (0.832; 0.917) | 4.7 | 12.9 |
| Infiltration Percentage (%) | 25.7 ± 9.3 | 28.5 ± 9.9 | 22.9 ± 10.8 | 7.6 ± 7.4 | 0.758 (0.655; 0.830) | 4.6 | 12.7 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age | ||||||||||||||
| 2. Weight | n.s. | |||||||||||||
| 3. Height | −0.492 ** | 0.486 ** | ||||||||||||
| 4. BMI | 0.352 ** | 0.800 ** | −0.175 * | |||||||||||
| 5. NDI | n.s. | n.s. | n.s. | n.s. | ||||||||||
| 6. Pain intensity | n.s. | n.s. | n.s. | n.s. | 0.295 * | |||||||||
| 7. Pain duration | 0.417 ** | n.s. | n.s. | n.s. | 0.308 * | n.s. | ||||||||
| 8. Mean Area | −0.495 ** | 0.354 ** | 0.530 ** | n.s. | 0.282 * | n.s. | n.s. | |||||||
| 9. Mean Perimeter | −0.523 ** | 0.219 ** | 0.520 ** | −0.189 * | n.s. | n.s. | n.s. | 0.835 ** | ||||||
| 10. Mean EI | n.s. | −0.453 ** | −0.243 ** | −0.324 * | n.s. | n.s. | n.s. | −0.324 ** | −0.197 * | |||||
| 11. Mean Fatty infiltration | n.s. | −0.332 ** | n.s. | −0.301 ** | n.s. | n.s. | n.s. | n.s. | n.s. | 0.681 ** | ||||
| 12. Area Error | 0.336 ** | n.s. | −0.234 ** | 0.173 * | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |||
| 13. Perimeter Error | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | 0.205 * | ||
| 14. Mean EI Error | 0.382 ** | n.s. | −0.287 ** | n.s. | n.s. | n.s. | n.s. | −0.265 ** | −0.308 ** | n.s. | n.s. | 0.349 ** | 0.248 ** | |
| 15. Fatty infiltration Error | 0.322 ** | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | −0.291 ** | −0.420 ** | n.s. | n.s. | n.s. | n.s. | n.s. |
| Predictor Outcome | B | SE B | 95% CI | β | t | P | |
|---|---|---|---|---|---|---|---|
| Cross-sectional area error | Step 1 Age | 0.138 | 0.029 | (0.080; 0.195) | 0.336 | 4.710 | <0.001 |
| Mean echo-intensity error | Step 1 Age | 0.171 | 0.037 | (0.097; 0.245) | 0.382 | 4.601 | <0.001 |
| Fatty infiltration estimation error | Step 1 Age | 0.146 | 0.039 | (0.070;0.222) | 0.322 | 3.785 | <0.001 |
| Step 2 Age Mean cross-sectional area | 0.109 -0.063 | 0.043 0.032 | (0.025;0.193) (−0.126;0.000) | 0.240 −0.184 | 2.559 −1.966 | 0.012 0.047 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valera-Calero, J.A.; Navarro-Santana, M.J.; Plaza-Manzano, G.; Fernández-de-las-Peñas, C.; Ortega-Santiago, R. Identifying Demographic, Clinical, Muscular and Histological Factors Associated with Ultrasound Cervical Multifidus Measurement Errors in a Chronic Neck Pain Population. Sensors 2022, 22, 8344. https://doi.org/10.3390/s22218344
Valera-Calero JA, Navarro-Santana MJ, Plaza-Manzano G, Fernández-de-las-Peñas C, Ortega-Santiago R. Identifying Demographic, Clinical, Muscular and Histological Factors Associated with Ultrasound Cervical Multifidus Measurement Errors in a Chronic Neck Pain Population. Sensors. 2022; 22(21):8344. https://doi.org/10.3390/s22218344
Chicago/Turabian StyleValera-Calero, Juan Antonio, Marcos José Navarro-Santana, Gustavo Plaza-Manzano, César Fernández-de-las-Peñas, and Ricardo Ortega-Santiago. 2022. "Identifying Demographic, Clinical, Muscular and Histological Factors Associated with Ultrasound Cervical Multifidus Measurement Errors in a Chronic Neck Pain Population" Sensors 22, no. 21: 8344. https://doi.org/10.3390/s22218344
APA StyleValera-Calero, J. A., Navarro-Santana, M. J., Plaza-Manzano, G., Fernández-de-las-Peñas, C., & Ortega-Santiago, R. (2022). Identifying Demographic, Clinical, Muscular and Histological Factors Associated with Ultrasound Cervical Multifidus Measurement Errors in a Chronic Neck Pain Population. Sensors, 22(21), 8344. https://doi.org/10.3390/s22218344







