Evaluation of an AI-Based TB AFB Smear Screening System for Laboratory Diagnosis on Routine Practice
Abstract
:1. Introduction
2. Materials and Methods
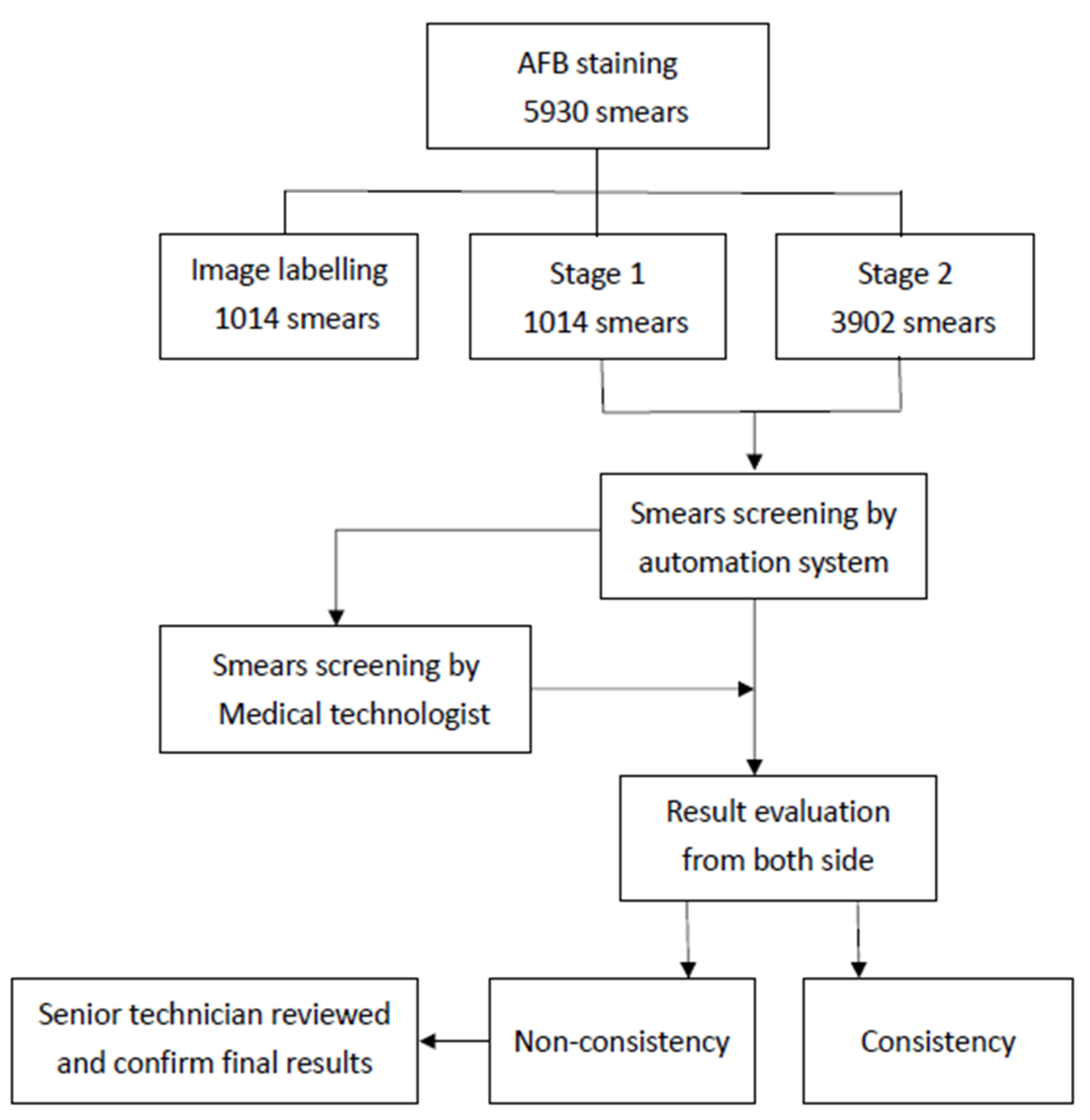
2.1. Study Design
2.2. Procedures
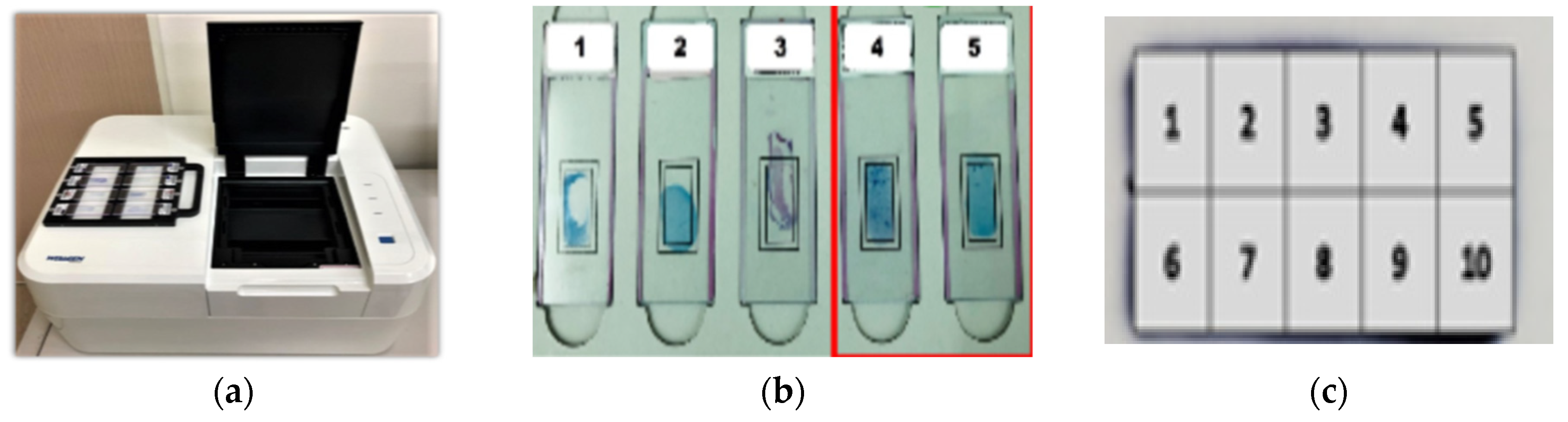
2.3. AI-Based Automatic TB Detection Device
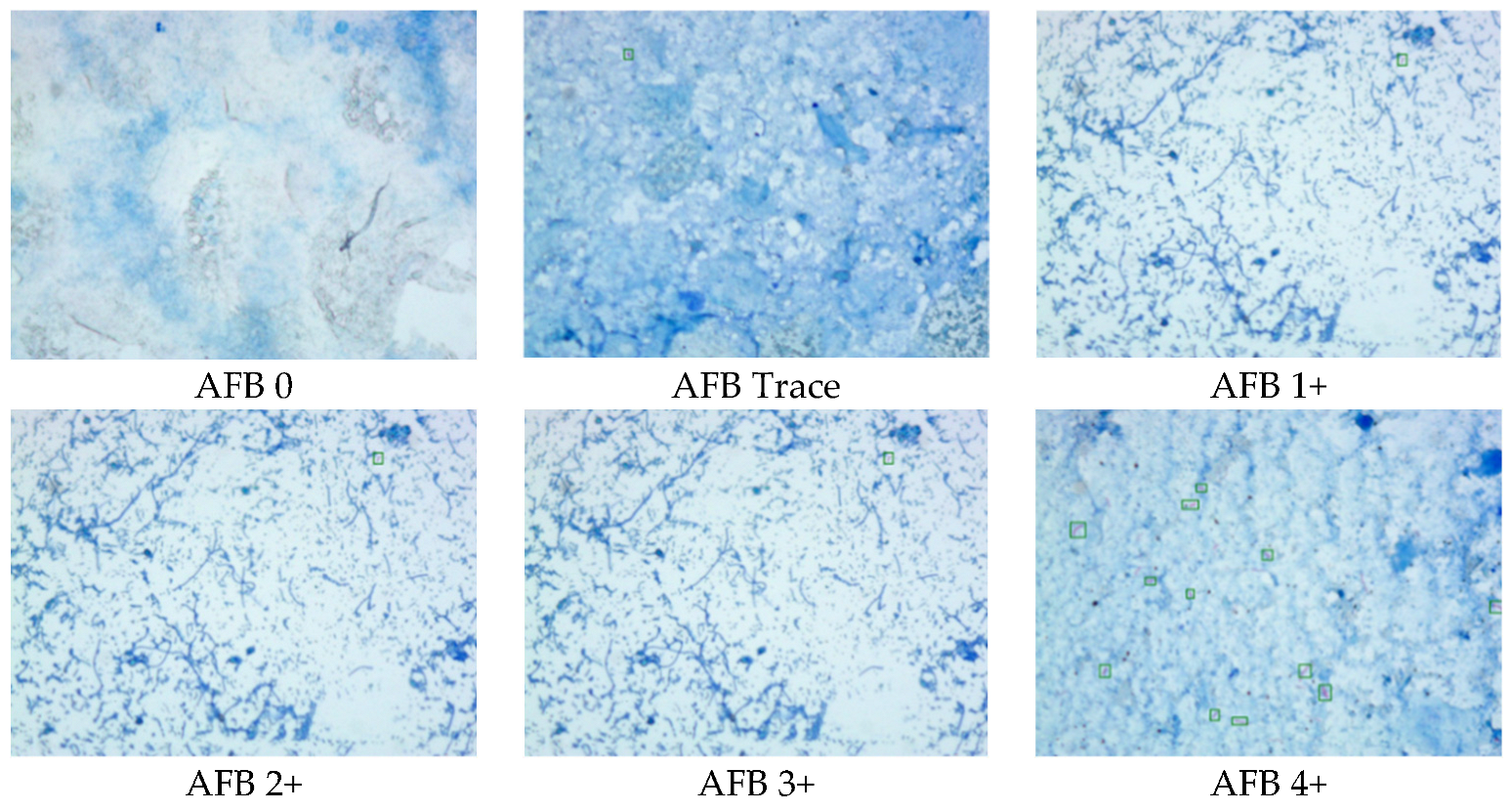
2.4. Data Interpretation
3. Results
3.1. The Performance of the Automation System
3.2. The Consistency between the Automated System and Manual Microscopy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2020; World Health Organization: Geneva, Switzerland, 2020.
- Reid, M.J.A.; Arinaminpathy, N.; Bloom, A.; Bloom, B.R.; Boehme, C.; Chaisson, R.; Chin, D.P.; Churchyard, G.; Cox, H.; Ditiu, L.; et al. Building a tuberculosis-free world: The Lancet Commission on tuberculosis. Lancet 2019, 393, 1331–1384. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Global Tuberculosis Report 2021; World Health Organization: Geneva, Switzerland, 2021.
- Taiwan CDC. Taiwan Tuberculosis Control Repor 2019; Taiwan CDC (Centers for Disease Control): Taipei, Taiwan, 2019.
- Campelo, T.A.; Cardoso de Sousa, P.R.; Nogueira, L.L.; Frota, C.C.; Zuquim Antas, P.R. Revisiting the methods for detecting Mycobacterium tuberculosis: What has the new millennium brought thus far? Access Microbiol. 2021, 3, 000245. [Google Scholar] [CrossRef] [PubMed]
- Steingart, K.R.; Henry, M.; Ng, V.; Hopewell, P.C.; Ramsay, A.; Cunningham, J.; Urbanczik, R.; Perkins, M.; Aziz, M.A.; Pai, M. Fluorescence versus conventional sputum smear microscopy for tuberculosis: A systematic review. Lancet Infect. Dis. 2006, 6, 570–581. [Google Scholar] [CrossRef]
- Islam, M.R.; Khatun, R.; Uddin, M.K.; Khan, M.S.; Rahman, M.T.; Ahmed, T.; Banu, S. Yield of two consecutive sputum specimens for the effective diagnosis of pulmonary tuberculosis. PLoS ONE 2013, 8, e67678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngabonziza, J.C.; Ssengooba, W.; Mutua, F.; Torrea, G.; Dushime, A.; Gasana, M.; Andre, E.; Uwamungu, S.; Nyaruhirira, A.U.; Mwaengo, D.; et al. Diagnostic performance of smear microscopy and incremental yield of Xpert in detection of pulmonary tuberculosis in Rwanda. BMC Infect. Dis. 2016, 16, 660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, J.J.; Chihota, V.N.; van der Meulen, M.; Fourie, P.B.; Fielding, K.L.; Grant, A.D.; Dorman, S.E.; Churchyard, G.J. “Proof-of-concept” evaluation of an automated sputum smear microscopy system for tuberculosis diagnosis. PLoS ONE 2012, 7, e50173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Garnier, S.; Sheen, P.; Zimic, M. Automatic diagnostics of tuberculosis using convolutional neural networks analysis of MODS digital images. PLoS ONE 2019, 14, e0212094. [Google Scholar] [CrossRef] [Green Version]
- Panicker, R.O.; Soman, B.; Saini, G.; Rajan, J. A Review of Automatic Methods Based on Image Processing Techniques for Tuberculosis Detection from Microscopic Sputum Smear Images. J. Med. Syst. 2016, 40, 17. [Google Scholar] [CrossRef] [PubMed]
- Zingue, D.; Weber, P.; Soltani, F.; Raoult, D.; Drancourt, M. Automatic microscopic detection of mycobacteria in sputum: A proof-of-concept. Sci. Rep. 2018, 8, 11308. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Y.; Ba, X.; Hou, A.; Zhang, K.; Chen, L.; Li, T. Automatic detection of mycobacterium tuberculosis using artificial intelligence. J. Thorac. Dis. 2018, 10, 1936–1940. [Google Scholar] [CrossRef] [PubMed]
- Taiwan Society of Laboratory Medicine. Concentred Smear Acid-Fast Stain Operation Guideline; Taiwan Society of Laboratory Medicine: Taipei, Taiwan, 2018; Volume TSLM-OP-T02(1). [Google Scholar]
- Smart Medical Microscope, Wellgen Medical Co., Ltd. Available online: https://www.wellgen.info/en-pas (accessed on 29 June 2022).
- US Food and Drug Administration. What is Digital Health? US Food and Drug Administration: Silver Spring, MD, USA, 2020.
- Paranjape, K.; Schinkel, M.; Hammer, R.D.; Schouten, B.; Nannan Panday, R.S.; Elbers, P.W.G.; Kramer, M.H.H.; Nanayakkara, P. The Value of Artificial Intelligence in Laboratory Medicine. Am. J. Clin. Pathol. 2021, 155, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Rudnitskaya, A.; Marini, F.; Tonacci, A.; Scafile, A.; Billeci, L.; Sansone, F. Electronic Nose and Tongue for Assessing Human Microbiota. Chemosensors 2022, 10, 85. [Google Scholar] [CrossRef]



| Stage 1 | Stage 2 | Included Cases with Adequate Smear | |
|---|---|---|---|
| Period | 19 December 2018– 28 March 2019 | 29 March 2019– 31 December 2019 | |
| True positive | 75 | 460 | 406 |
| True negative | 851 | 3197 | 2634 |
| False positive | 50 | 111 | 85 |
| False negative | 38 | 134 | 68 |
| Total cases | 1014 | 3902 | 3193 |
| Sensitivity | 60.0% | 77.4% | 85.7% |
| Specificity | 91.3% | 96.6% | 96.9% |
| Accuracy | 95.7% | 93.7% | 95.2% |
| No. of Cases | Percentage (%) | |
|---|---|---|
| Incomplete stain | 325 | 45.9% |
| Smear too thin | 110 | 15.5% |
| Smear dropped off | 96 | 13.5% |
| Smear location shift | 89 | 12.6% |
| Smear too thick | 49 | 6.9% |
| Atypical TB shape | 40 | 5.6% |
| Total | 709 | 100% |
| Automated System | Manual Microscopy | |
|---|---|---|
| True positive | 460 | 502 |
| True negative | 3197 | 3308 |
| False positive | 111 | 0 |
| False negative | 134 | 92 |
| Total cases | 3902 | 3902 |
| Sensitivity | 77.4% | 84.5% |
| Specificity | 96.6% | 100% |
| Accuracy | 93.7% | 97.6% |
| Automated System | Manual Microscopy | |
|---|---|---|
| True positive | 406 | 389 |
| True negative | 2634 | 2719 |
| False positive | 85 | 0 |
| False negative | 68 | 85 |
| Total cases | 3193 | 3193 |
| Sensitivity | 85.7% | 82.1% |
| Specificity | 96.9% | 100% |
| Accuracy | 95.2% | 97.3% |
| FinTotal Case No. | 3902 | Manual Microscopy | |
|---|---|---|---|
| True | False | ||
| Automated system | True | 3567 91.41% | 90 2.31% |
| False | 243 6.32% | 2 0.06% | |
| Total Case | 3193 | Manual Microscopy | |
|---|---|---|---|
| True | False | ||
| Automated system | True | 2957 92.61% | 83 2.60% |
| False | 151 4.73% | 2 0.06% | |
| Manual Microscopy | Automated System | Consistency (%) | |
|---|---|---|---|
| Trace | 192 | 75 | 39.1 |
| AFB 1+ | 137 | 122 | 89.1 |
| AFB 2+ | 89 | 89 | 100.0 |
| AFB 3+ | 47 | 47 | 100.0 |
| AFB 4+ | 37 | 37 | 100.0 |
| Not Found | 3308 | 3308 | 100.0 |
| Missed by System | Missed by Manual Examination | |
|---|---|---|
| Trace | 61 | 65 |
| 1+ | 5 | 19 |
| 2+ | 0 | 1 |
| Total | 66 | 85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, H.-T.; Tu, H.-Z.; Lee, H.-S.; Lin, Y.E.; Lin, C.-W. Evaluation of an AI-Based TB AFB Smear Screening System for Laboratory Diagnosis on Routine Practice. Sensors 2022, 22, 8497. https://doi.org/10.3390/s22218497
Fu H-T, Tu H-Z, Lee H-S, Lin YE, Lin C-W. Evaluation of an AI-Based TB AFB Smear Screening System for Laboratory Diagnosis on Routine Practice. Sensors. 2022; 22(21):8497. https://doi.org/10.3390/s22218497
Chicago/Turabian StyleFu, Hsiao-Ting, Hui-Zin Tu, Herng-Sheng Lee, Yusen Eason Lin, and Che-Wei Lin. 2022. "Evaluation of an AI-Based TB AFB Smear Screening System for Laboratory Diagnosis on Routine Practice" Sensors 22, no. 21: 8497. https://doi.org/10.3390/s22218497
APA StyleFu, H.-T., Tu, H.-Z., Lee, H.-S., Lin, Y. E., & Lin, C.-W. (2022). Evaluation of an AI-Based TB AFB Smear Screening System for Laboratory Diagnosis on Routine Practice. Sensors, 22(21), 8497. https://doi.org/10.3390/s22218497







