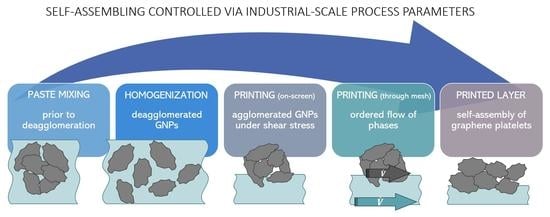
Self-Assembling Graphene Layers for Electrochemical Sensors Printed in a Single Screen-Printing Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Printing Pastes
2.3. Fabrication of the Electrodes
2.4. Physical Measurements
2.5. Electrochemical Measurements
2.6. Statistical Analysis
3. Results and Discussion
3.1. General Rheological Properties of Printing Pastes
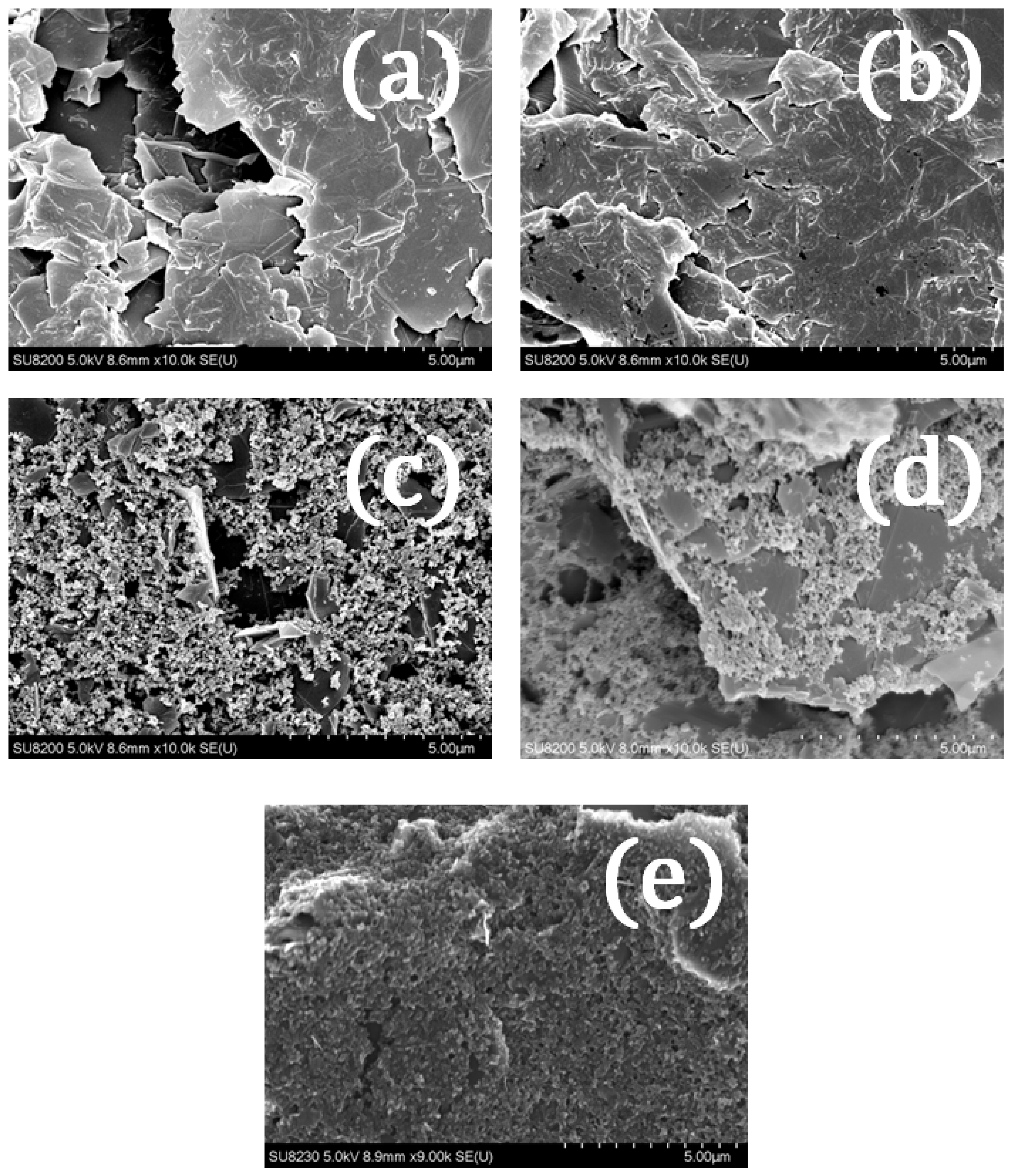
3.2. Influence of Shear-Thinning on the Filler Particles and Resulting Surface Morphology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Azeredo, N.F.B.; Santos, M.S.F.; Sempionatto, J.R.; Wang, J.; Angnes, L. Screen-Printed Technologies Combined with Flow Analysis Techniques: Moving from Benchtop to Everywhere. Anal. Chem. 2021, 94, 250–268. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.G.-M.; Rowley-Neale, S.J.; Banks, C.E. Screen-printed electrodes: Transitioning the laboratory in-to-the field. Talanta Open 2021, 3, 100032. [Google Scholar] [CrossRef]
- Mohamed, H.M. Screen-printed disposable electrodes: Pharmaceutical applications and recent developments. TrAC Trends Anal. Chem. 2016, 82, 1–11. [Google Scholar] [CrossRef]
- Serrano, N.; Castilla, Ò.; Ariño, C.; Diaz-Cruz, M.S.; Díaz-Cruz, J.M. Commercial Screen-Printed Electrodes Based on Carbon Nanomaterials for a Fast and Cost-Effective Voltammetric Determination of Paracetamol, Ibuprofen and Caffeine in Water Samples. Sensors 2019, 19, 4039. [Google Scholar] [CrossRef]
- Smart, A.; Crew, A.; Pemberton, R.; Hughes, G.; Doran, O.; Hart, J. Screen-printed carbon based biosensors and their applications in agri-food safety. TrAC Trends Anal. Chem. 2020, 127, 115898. [Google Scholar] [CrossRef]
- Hughes, G.; Westmacott, K.; Honeychurch, K.C.; Crew, A.; Pemberton, R.M.; Hart, J.P. Recent Advances in the Fabrication and Application of Screen-Printed Electrochemical (Bio)Sensors Based on Carbon Materials for Biomedical, Agri-Food and Environmental Analyses. Biosensors 2016, 6, 50. [Google Scholar] [CrossRef]
- Goldberg, H.D.; Brown, R.B.; Liu, D.P.; Meyerhoff, M.E. Screen printing: A technology for the batch fabrication of integrated chemical-sensor arrays. Sensors Actuators B Chem. 1994, 21, 171–183. [Google Scholar] [CrossRef]
- Arduini, F.; Micheli, L.; Moscone, D.; Palleschi, G.; Piermarini, S.; Ricci, F.; Volpe, G. Electrochemical biosensors based on nanomodified screen-printed electrodes: Recent applications in clinical analysis. TrAC Trends Anal. Chem. 2016, 79, 114–126. [Google Scholar] [CrossRef]
- Cinti, S.; Arduini, F. Graphene-based screen-printed electrochemical (bio)sensors and their applications: Efforts and criticisms. Biosens. Bioelectron. 2017, 89, 107–122. [Google Scholar] [CrossRef]
- Torres-Rivero, K.; Florido, A.; Bastos-Arrieta, J. Recent Trends in the Improvement of the Electrochemical Response of Screen-Printed Electrodes by Their Modification with Shaped Metal Nanoparticles. Sensors 2021, 21, 2596. [Google Scholar] [CrossRef]
- Squissato, A.L.; Munoz, R.A.A.; Banks, C.E.; Richter, E.M. An Overview of Recent Electroanalytical Applications Utilizing Screen-Printed Electrodes Within Flow Systems. ChemElectroChem 2020, 7, 2211–2221. [Google Scholar] [CrossRef]
- Gao, Y.; Nguyen, D.T.; Yeo, T.; Bin Lim, S.; Tan, W.X.; Madden, L.E.; Jin, L.; Long, J.Y.K.; Aloweni, F.A.B.; Liew, Y.J.A.; et al. A flexible multiplexed immunosensor for point-of-care in situ wound monitoring. Sci. Adv. 2021, 7, eabg9614. [Google Scholar] [CrossRef] [PubMed]
- Białobrzeska, W.; Dziąbowska, K.; Lisowska, M.; Mohtar, M.; Muller, P.; Vojtesek, B.; Krejcir, R.; O’Neill, R.; Hupp, T.; Malinowska, N.; et al. An Ultrasensitive Biosensor for Detection of Femtogram Levels of the Cancer Antigen AGR2 Using Monoclonal Antibody Modified Screen-Printed Gold Electrodes. Biosensors 2021, 11, 184. [Google Scholar] [CrossRef] [PubMed]
- Janczak, D.; Zych, M.; Raczyński, T.; Dybowska-Sarapuk, Ł.; Pepłowski, A.; Krzemiński, J.; Sosna-Głębska, A.; Znajdek, K.; Sibiński, M.; Jakubowska, M. Stretchable and Washable Electroluminescent Display Screen-Printed on Textile. Nanomaterials 2019, 9, 1276. [Google Scholar] [CrossRef]
- Ogończyk, D.; Tymecki, Ł.; Wyżkiewicz, I.; Koncki, R.; Głąb, S. Screen-printed disposable urease-based biosensors for inhibitive detection of heavy metal ions. Sens. Actuators B Chem. 2005, 106, 450–454. [Google Scholar] [CrossRef]
- Cagnani, G.R.; Ibáñez-Redín, G.; Tirich, B.; Gonçalves, D.; Balogh, D.T.; Oliveira, O.N. Fully-printed electrochemical sensors made with flexible screen-printed electrodes modified by roll-to-roll slot-die coating. Biosens. Bioelectron. 2020, 165, 112428. [Google Scholar] [CrossRef]
- Jewell, E.; Philip, B.; Greenwood, P. Improved Manufacturing Performance of Screen Printed Carbon Electrodes through Material Formulation. Biosensors 2016, 6, 30. [Google Scholar] [CrossRef]
- Somalu, M.; Yufit, V.; Shapiro, I.; Xiao, P.; Brandon, N. The impact of ink rheology on the properties of screen-printed solid oxide fuel cell anodes. Int. J. Hydrogen Energy 2013, 38, 6789–6801. [Google Scholar] [CrossRef]
- Chiticaru, E.A.; Pilan, L.; Damian, C.-M.; Vasile, E.; Burns, J.S.; Ioniţă, M. Influence of Graphene Oxide Concentration when Fabricating an Electrochemical Biosensor for DNA Detection. Biosensors 2019, 9, 113. [Google Scholar] [CrossRef]
- Randviir, E.P.; Brownson, D.A.C.; Metters, J.P.; Kadara, R.O.; Banks, C.E. The fabrication, characterisation and electrochemical investigation of screen-printed graphene electrodes. Phys. Chem. Chem. Phys. 2014, 16, 4598–4611. [Google Scholar] [CrossRef]
- Beitollahi, H.; Mohammadi, S.Z.; Safaei, M.; Tajik, S. Applications of electrochemical sensors and biosensors based on modified screen-printed electrodes: A review. Anal. Methods 2020, 12, 1547–1560. [Google Scholar] [CrossRef]
- Hassani, S.; Akmal, M.R.; Salek-Maghsoudi, A.; Rahmani, S.; Ganjali, M.R.; Norouzi, P.; Abdollahi, M. Novel label-free electrochemical aptasensor for determination of Diazinon using gold nanoparticles-modified screen-printed gold electrode. Biosens. Bioelectron. 2018, 120, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Abellán-Llobregat, A.; Jeerapan, I.; Bandodkar, A.; Vidal, L.; Canals, A.; Wang, J.; Morallón, E. A stretchable and screen-printed electrochemical sensor for glucose determination in human perspiration. Biosens. Bioelectron. 2017, 91, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Glennon, T.; O’Quigley, C.; McCaul, M.; Matzeu, G.; Beirne, S.; Wallace, G.G.; Stroiescu, F.; O’Mahoney, N.; White, P.; Diamond, D. ‘SWEATCH’: A Wearable Platform for Harvesting and Analysing Sweat Sodium Content. Electroanalysis 2016, 28, 1283–1289. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Molinnus, D.; Mirza, O.; Guinovart, T.; Windmiller, J.R.; Valdés-Ramírez, G.; Andrade, F.J.; Schoening, M.J.; Wang, J. Epidermal tattoo potentiometric sodium sensors with wireless signal transduction for continuous non-invasive sweat monitoring. Biosens. Bioelectron. 2013, 54, 603–609. [Google Scholar] [CrossRef]
- Lin, H.-W.; Chang, C.-P.; Hwu, W.-H.; Ger, M.-D. The rheological behaviors of screen-printing pastes. J. Mater. Process. Technol. 2008, 197, 284–291. [Google Scholar] [CrossRef]
- Xu, C.; Willenbacher, N. How rheological properties affect fine-line screen printing of pastes: A combined rheological and high-speed video imaging study. J. Coatings Technol. Res. 2018, 15, 1401–1412. [Google Scholar] [CrossRef]
- Qi, X.; Ha, H.; Hwang, B.; Lim, S. Printability of the Screen-Printed Strain Sensor with Carbon Black/Silver Paste for Sensitive Wearable Electronics. Appl. Sci. 2020, 10, 6983. [Google Scholar] [CrossRef]
- Phillips, C.; Al-Ahmadi, A.; Potts, S.-J.; Claypole, T.; Deganello, D. The effect of graphite and carbon black ratios on conductive ink performance. J. Mater. Sci. 2017, 52, 9520–9530. [Google Scholar] [CrossRef]
- Suresh, R.R.; Lakshmanakumar, M.; Jayalatha, J.B.B.A.; Rajan, K.S.; Sethuraman, S.; Krishnan, U.M.; Rayappan, J.B.B. Fabrication of screen-printed electrodes: Opportunities and challenges. J. Mater. Sci. 2021, 56, 8951–9006. [Google Scholar] [CrossRef]
- Janczak, D.; Peplowski, A.; Wroblewski, G.; Gorski, L.; Zwierkowska, E.; Jakubowska, M. Investigations of Printed Flexible pH Sensing Materials Based on Graphene Platelets and Submicron RuO2Powders. J. Sensors 2017, 2017, 2190429. [Google Scholar] [CrossRef]
- Ram, R.; Rahaman, M.; Aldalbahi, A.; Khastgir, D. Determination of percolation threshold and electrical conductivity of polyvinylidene fluoride (PVDF)/short carbon fiber (SCF) composites: Effect of SCF aspect ratio. Polym. Int. 2017, 66, 573–582. [Google Scholar] [CrossRef]
- Stauffer, D.; Aharony, A. Introduction to Percolation Theory, 2nd ed.; Taylor & Francis: London, UK, 2018. [Google Scholar] [CrossRef]
- Charlaix, E. Percolation threshold of a random array of discs: A numerical simulation. J. Phys. A Math. Gen. 1986, 19, L533–L536. [Google Scholar] [CrossRef]
- Lu, W.; Lin, H.; Wu, D.; Chen, G. Unsaturated polyester resin/graphite nanosheet conducting composites with a low percolation threshold. Polymer 2006, 47, 4440–4444. [Google Scholar] [CrossRef]
- Pepłowski, A.; Walter, P.A.; Janczak, D.; Górecka, Ż.; Święszkowski, W.; Jakubowska, M. Solventless Conducting Paste Based on Graphene Nanoplatelets for Printing of Flexible, Standalone Routes in Room Temperature. Nanomaterials 2018, 8, 829. [Google Scholar] [CrossRef] [PubMed]
- Dybowska-Sarapuk, Ł.; Janczak, D.; Wróblewski, G.; Sloma, M.; Jakubowska, M. The influence of graphene screen printing paste’s composition on its viscosity. In Proceedings of the Photonics Applications in Astronomy, Communications, Industry, and High-Energy Physics Experiments; SPIE: Washington, DC, USA, 2015; Volume 966242. [Google Scholar] [CrossRef]
- Dybowska-Sarapuk, Ł.; Szałapak, J.; Wróblewski, G.; Wyżkiewicz, I.; Słoma, M.; Jakubowska, M. Rheology of inks for various techniques of printed electronics. In Advances in Intelligent Systems and Computing; Springer: Cham, Switzerland, 2015; Volume 393, pp. 447–451. [Google Scholar] [CrossRef]
- Potts, S.-J.; Phillips, C.; Claypole, T.; Jewell, E. The Effect of Carbon Ink Rheology on Ink Separation Mechanisms in Screen-Printing. Coatings 2020, 10, 1008. [Google Scholar] [CrossRef]
- Tran, T.S.; Dutta, N.K.; Choudhury, N.R. Graphene inks for printed flexible electronics: Graphene dispersions, ink formulations, printing techniques and applications. Adv. Colloid Interface Sci. 2018, 261, 41–61. [Google Scholar] [CrossRef]
- Reinhardt, K.; Hofmann, N.; Eberstein, M. The importance of shear thinning, thixotropic and viscoelastic properties of thick film pastes to predict effects on printing performance. In Proceedings of the EMPC 2017-21st European Microelectronics and Packaging Conference and Exhibition, Warsaw, Poland, 10–13 September 2017; pp. 1–7. [Google Scholar] [CrossRef]
- Sajjad, M.; Otsuki, A. Coupling Flotation Rate Constant and Viscosity Models. Metals 2022, 12, 409. [Google Scholar] [CrossRef]
- Vermant, J.; Solomon, M. Flow-induced structure in colloidal suspensions. J. Physics: Condens. Matter 2005, 17, R187. [Google Scholar] [CrossRef]
- Zaccone, A.; Wu, H.; Gentili, D.; Morbidelli, M. Theory of activated-rate processes under shear with application to shear-induced aggregation of colloids. Phys. Rev. E 2009, 80, 051404. [Google Scholar] [CrossRef]
- Shvets, A.A. Theory of colloidal stabilization by unattached polymers. arXiv 2020, arXiv:2010.08110. [Google Scholar] [CrossRef]



| Paste Symbol | GNP Content (wt%) | CB Content (wt%) | DBP Content (μL/g) |
|---|---|---|---|
| GNP-1 | 15 | 0 | 0 |
| GNP-2 | 12 | 0 | 0 |
| GNP-3 | 10 | 3 | 0 |
| GNP-4 | 10 | 3 | 100 |
| Material | GNP-1 | GNP-2 | GNP-3 | GNP-4 | PF-407 A w/Mixing | PF-407 A w/o Mixing | |
|---|---|---|---|---|---|---|---|
| Parameter | |||||||
| σ electrical conductivity | S | 513.6 | 612.7 | 1248.56 | 1027.99 | 1684.24 | 530.33 |
| γ˙th thinning shear rate for η ≈ 0 | s−1 | 500.0 | 327.6 | 999.9 | 1000.0 | 500.0 | 500.0 |
| η0 static viscosity at γ˙ = 0 | Pa·s | 50.9 | 65.0 | 66.9 | 79.9 | 554.5 | 273.3 |
| ηp viscosity at γ˙ = 172 s−1 | Pa·s | 4.3 | 2.6 | 9 | 12.9 | 15.3 | 21.9 |
| η∞ asymptotic viscosity, γ˙→∞ | Pa·s | 1.0 | 0.1 | 1.3 | 3.1 | 0.6 | 0.3 |
| γ yield stress | Pa | 1200 | 1800 | 900 | 1600 | 15,000 | 8000 |
| Ra surface roughness | μm | 5.2 | 7.1 | 5.6 | 6.5 | 2.2 | 2.1 |
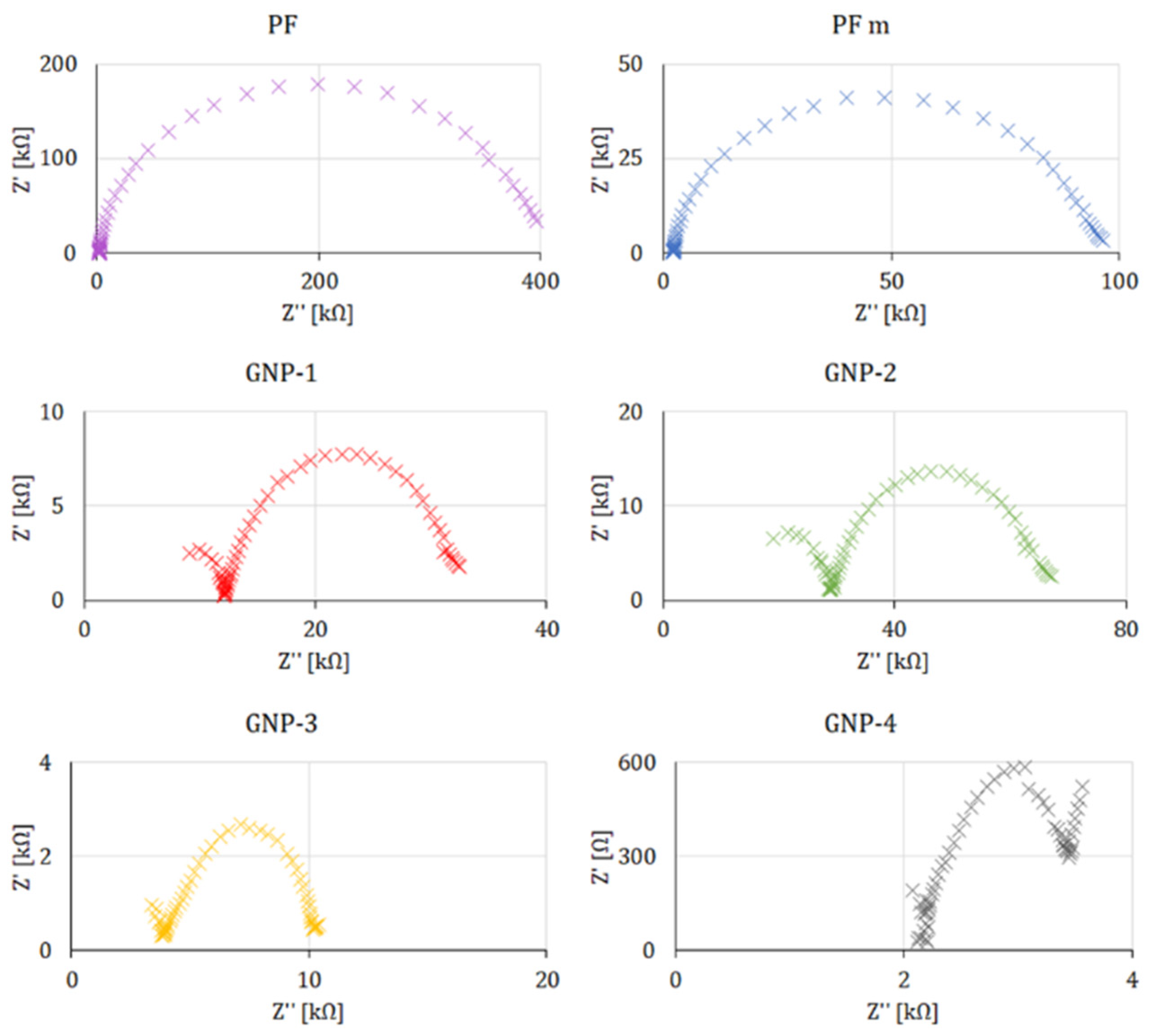
| Ret electron transfer | kΩ | 42.4 | 21.7 | 8.37 | 3.12 | 88.7 | 163.0 |
| ΔEredox peaks potential separation | V | 1.24 | 1.03 | 0.62 | 0.43 | 0.99 | 1.01 |
| ia anodic peak current | μA | 0.525 | 1.57 | 4.04 | 5.54 | 4.35 | 4.52 |
| ic cathodic peak current | μA | −0.409 | −1.08 | −6.18 | −10.3 | −2.22 | −2.12 |
| electrochem. surface | mm2 | 0.36 | 0.39 | 1.67 | 1.43 | 1.03 | 1.46 |
| Parameters Correlated | Pearson’s Correlation Coefficient ρ | p-Value |
|---|---|---|
| Ret ↔ η0 | 96.3% | 0.0020 |
| ΔEredox ↔ γ˙th | −89.2% | 0.0168 |
| ic ↔ γ˙th | −91.0% | 0.0118 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pepłowski, A.; Budny, F.; Jarczewska, M.; Lepak-Kuc, S.; Dybowska-Sarapuk, Ł.; Baraniecki, D.; Walter, P.; Malinowska, E.; Jakubowska, M. Self-Assembling Graphene Layers for Electrochemical Sensors Printed in a Single Screen-Printing Process. Sensors 2022, 22, 8836. https://doi.org/10.3390/s22228836
Pepłowski A, Budny F, Jarczewska M, Lepak-Kuc S, Dybowska-Sarapuk Ł, Baraniecki D, Walter P, Malinowska E, Jakubowska M. Self-Assembling Graphene Layers for Electrochemical Sensors Printed in a Single Screen-Printing Process. Sensors. 2022; 22(22):8836. https://doi.org/10.3390/s22228836
Chicago/Turabian StylePepłowski, Andrzej, Filip Budny, Marta Jarczewska, Sandra Lepak-Kuc, Łucja Dybowska-Sarapuk, Dominik Baraniecki, Piotr Walter, Elżbieta Malinowska, and Małgorzata Jakubowska. 2022. "Self-Assembling Graphene Layers for Electrochemical Sensors Printed in a Single Screen-Printing Process" Sensors 22, no. 22: 8836. https://doi.org/10.3390/s22228836
APA StylePepłowski, A., Budny, F., Jarczewska, M., Lepak-Kuc, S., Dybowska-Sarapuk, Ł., Baraniecki, D., Walter, P., Malinowska, E., & Jakubowska, M. (2022). Self-Assembling Graphene Layers for Electrochemical Sensors Printed in a Single Screen-Printing Process. Sensors, 22(22), 8836. https://doi.org/10.3390/s22228836