A Comparison between High and Low Cuff Pressures on Muscle Oxygen Saturation and Recovery Responses Following Blood-Flow Restriction Resistance Exercise
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Experimental Protocol
2.3. Isometric Force Testing
2.4. Ultrasound Measurements
2.5. Blood Flow Restriction Protocols
2.6. Muscle Oxygen Saturation
2.7. Muscle Soreness and RPE
2.8. Statistics
3. Results
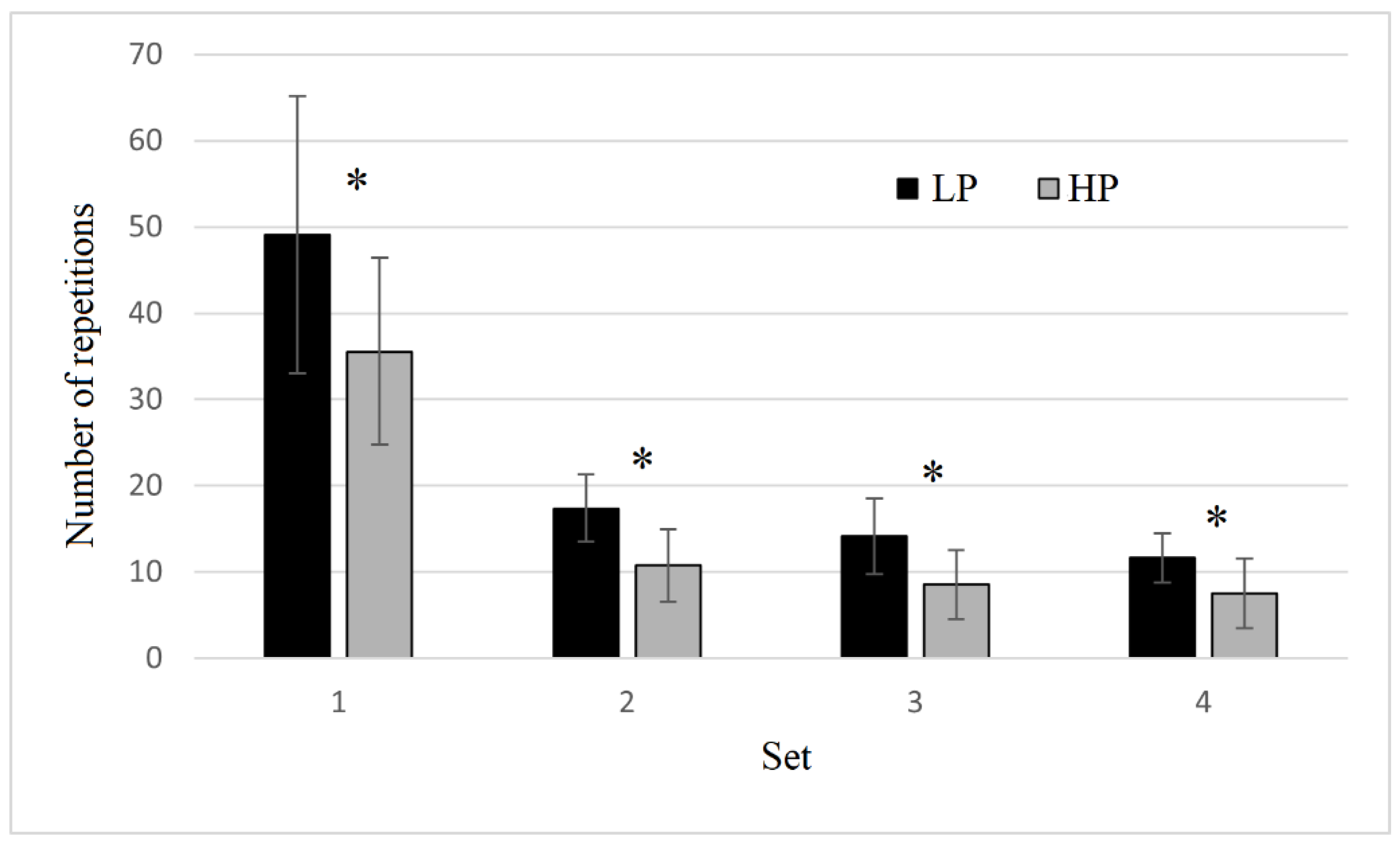
3.1. Number of Repetitions and Muscle Oxygen Saturation
3.2. Muscle Morphology, Muscle Soreness and RPE Assessments
3.3. Isometric Strength Assessments
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Minniti, M.C.; Statkevich, A.P.; Kelly, R.L.; Rigsby, V.P.; Exline, M.M.; Rhon, D.I.; Clewley, D. The safety of blood flow restriction training as a therapeutic intervention for patients with musculoskeletal disorders: A systematic review. Am. J. Sports Med. 2020, 48, 1773–1785. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Kawamoto, K.; Yasuda, T.; Kearns, C.F.; Midorikawa, T.; Sato, Y. Eight days KAATSU-resistance training improved sprint but not jump performance in collegiate male track and field athletes. Int. J. KAATSU Train. Res. 2005, 1, 19–23. [Google Scholar] [CrossRef]
- Patterson, S.D.; Ferguson, R.A. Enhancing strength and postocclusive calf blood flow in older people with training with blood-flow restriction. J. Aging Phys. Act. 2011, 19, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Pearson, S.J.; Hussain, S.R. A review on the mechanisms of blood-flow restriction resistance training-induced muscle hypertrophy. Sports Med. 2015, 45, 187–200. [Google Scholar] [CrossRef]
- Takarada, Y.; Takazawa, H.; Sato, Y.; Tanaka, Y.; Ishii, N. Effects of resistance exercise combined with moderate vascular occlusion on muscular function in humans. J. Appl. Physiol. 2000, 88, 2097–2106. [Google Scholar] [CrossRef]
- Loenneke, J.P.; Thiebaud, R.S.; Abe, T. Does blood flow restriction result in skeletal muscle damage? A critical review of available evidence. Scand. J. Med. Sci. Sports 2014, 24, e415–e422. [Google Scholar] [CrossRef]
- Wernbom, M.; Paulsen, G.; Nilsen, T.S.; Hisdal, J.; Raastad, T. Contractile function and sarcolemmal permeability after acute low-load resistance exercise with blood flow restriction. Eur. J. Appl. Physiol. 2012, 112, 2051–2063. [Google Scholar] [CrossRef] [PubMed]
- Umbel, J.D.; Hoffman, R.L.; Dearth, D.J.; Chleboun, G.S.; Manini, T.M.; Clark, B.C. Delayed-onset muscle soreness induced by low-load blood flow-restricted exercise. Eur. J. Appl. Physiol. 2009, 107, 687–695. [Google Scholar] [CrossRef]
- Clarkson, M.J.; May, A.K.; Warmington, S.A. Is there rationale for the cuff pressures prescribed for blood flow restriction exercise? A systematic review. Scand. J. Med. Sci. Sports 2022, 30, 1318–1336. [Google Scholar] [CrossRef]
- Abe, T.; Kearns, C.F.; Sato, Y. Muscle size and strength are increased following walk training with restricted venous blood flow from the leg muscle, Kaatsu-walk training. J. Appl. Physiol. 2006, 100, 1460–1466. [Google Scholar] [CrossRef]
- Mattocks, K.T.; Jessee, M.B.; Counts, B.R.; Buckner, S.L.; Mouser, J.G.; Dankel, S.J.; Loenneke, J.P. The effects of upper body exercise across different levels of blood flow restriction on arterial occlusion pressure and perceptual responses. Physiol. Behav. 2017, 171, 181–186. [Google Scholar] [CrossRef]
- Bell, Z.W.; Buckner, S.L.; Jessee, M.B.; Mouser, J.G.; Mattocks, K.T.; Dankel, S.J.; Abe, T.; Loenneke, J.P. Moderately heavy exercise produces lower cardiovascular, RPE, and discomfort compared to lower load exercise with and without blood flow restriction. Eur. J. Appl. Physiol. 2018, 118, 1473–1480. [Google Scholar] [CrossRef]
- Dankel, S.J.; Jessee, M.B.; Buckner, S.L.; Mouser, J.G.; Mattocks, K.T.; Loenneke, J.P. Are higher blood flow restriction pressures more beneficial when lower loads are used? Physiol. Intern. 2017, 104, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Bartolomei, S.; Nigro, F.; Ruggeri, S.; Lanzoni Malagoli, I.; Ciacci, S.; Merni, F.; Sadres, E.; Hoffman, J.R.; Semprini, G. Comparison between bench press throw and ballistic push-up tests to assess upper-body power in trained individuals. J. Strength Cond. Res. 2018, 32, 1503–1510. [Google Scholar] [CrossRef] [PubMed]
- Miyatani, M.; Kanehisa, H.; Ito, M.; Kawakami, Y.; Fukunaga, T. The accuracy of volume estimates using ultrasound muscle thickness measurements in different muscle groups. Eur. J. Appl. Physiol. 2004, 91, 264–272. [Google Scholar] [CrossRef]
- Gualano, B.; Ugrinowitsch, C.; Neves, M., Jr.; Lima, F.R.; Pinto, A.L.S.; Laurentino, G.; Tricoli, V.A.; Lancha, A.H., Jr.; Roschel, H. Vascular occlusion training for inclusion body myositis: A novel therapeutic approach. J. Visual Exp. 2010, 40. Available online: https://d1wqtxts1xzle7.cloudfront.net/44924606/Vascular_occlusion_training_for_inclusio20160420-4627-rioj2c-with-cover-page-v2.pdf?Expires=1669342046&Signature=JCz~sKR7lEPIQKScqghgQS1Wn6QXCXKTxW6QCIyuz3gGsctCPOzmhh6Ze~ChDcDoWD0iF7ksrBMbnopFlvkBGgwgwSx7MrypMg06nqTYF5gmxMiwmDmjmvRWMCgDwaIF7ak6bYJKYi5BfzunDtRmBAuqZ2xab0INzGnQf2ve~I5bxgdSjVlixsICA137Qvf~A1QqPUK0kMg40g5RvqZvnxpJFr8nRKReIR9cuJUJ1k7jj-~-4ytfFnmJyfBiA6F37DW9wfH6e6Rp8eHxf05EMo01cfJdnlCNPFEyAOF4j75JYuwlTbKHaIqBOYsYVgvjzd289S3hp~zu15VemMOYSQ__&Key-Pair-Id=APKAJLOHF5GGSLRBV4ZA (accessed on 20 October 2022).
- Guardado, I.; Guerra, A.M.; Pino, B.S.; Camacho, G.; Andrada, R. Acute responses of muscle oxygen saturation during different cluster training configurations in resistance-trained individuals. Biol. Sport 2021, 38, 367–376. [Google Scholar] [CrossRef]
- Gómez-Carmona, C.D.; Bastida-Castillo, A.; Rojas-Valverde, D.; de la Cruz Sánchez, E.; García-Rubio, J.; Ibáñez, S.J.; Pino-Ortega, J. Lower-limb dynamics of muscle oxygen saturation during the back-squat exercise: Effects of training load and effort level. J. Strength Cond. Res. 2020, 34, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Foster, C.; Florhaug, J.A.; Franklin, J.; Gottschall, L.; Hrovatin, L.A.; Parker, S.; Doleshal, P.; Dodge, C. A new approach to monitoring exercise training. J. Strength Cond. Res. 2001, 15, 109–115. [Google Scholar] [PubMed]
- Lee, K.A.; Hicks, G.; Nino-Murcia, G. Validity and reliability of a scale to assess fatigue. Psychol. Res. 1991, 36, 291–298. [Google Scholar] [CrossRef]
- Stevens, J.P. Applied Multivariate Statistics for the Social Science, 5th ed.; Taylor and Francis: New York, NY, USA, 2009. [Google Scholar]
- Cook, S.B.; Clark, B.C.; Ploutz-Snyder, L.L. Effects of exercise load and blood-flow restriction on skeletal muscle function. Med. Sci. Sports Exerc. 2007, 39, 1708–1713. [Google Scholar] [CrossRef]
- Karabulut, M.; Cramer, J.T.; Abe, T.; Sato, Y.; Bemben, M.G. Neuromuscular fatigue following low-intensity dynamic exercise with externally applied vascular restriction. J. Electromyogr. Kinesiol. 2010, 20, 440–447. [Google Scholar] [CrossRef]
- Husmann, F.; Mittlmeier, T.; Bruhn, S.; Zschorlich, V.; Behrens, M. Impact of blood flow restriction exercise on muscle fatigue development and recovery. Med. Sci. Sports Exerc. 2018, 50, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.G.; Lamb, G.D.; Westerblad, H. Skeletal muscle fatigue: Cellular mechanisms. Physiol. Rev. 2008, 88, 287–332. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, G.; Cotter, J.A.; Reuland, W.; Cerussi, A.E.; Tromberg, B.J.; Galassetti, P. Effect of blood flow restriction on tissue oxygenation during knee extension. Med. Sci. Sports Exerc. 2015, 47, 185. [Google Scholar] [CrossRef]
- Gonzalez, A.M.; Pinzone, A.G.; Lipes, S.E.; Mangine, G.T.; Townsend, J.R.; Allerton, T.D.; Sell, K.M.; Ghigiarelli, J.J. Effect of watermelon supplementation on exercise performance, muscle oxygenation, and vessel diameter in resistance-trained men. Eur. J. Appl. Physiol. 2022, 122, 1627–1638. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.F.; Koga, S.; Barstow, T.J. Dynamics of noninvasively estimated microvascular O2 extraction during ramp exercise. J. Appl. Physiol. 2007, 103, 1999–2004. [Google Scholar] [CrossRef] [PubMed]
- Bartolomei, S.; Totti, V.; Griggio, F.; Malerba, C.; Ciacci, S.; Semprini, G.; Di Michele, R. Upper-body resistance exercise reduces time to recover after a high-volume bench press protocol in resistance-trained men. J. Strength Cond. Res. 2021, 35, S180–S187. [Google Scholar] [CrossRef]
- Bartolomei, S.; Nigro, F.; D’Amico, A.; Cortesi, M.; Di Michele, R. Mud Pack with Menthol and Arnica Montana Accelerates Recovery Following a High-Volume Resistance Training Session for Lower Body in Trained Men. J. Strength Cond. Res. 2020, 36, 1909–1915. [Google Scholar] [CrossRef]
- Jessee, M.B.; Buckner, S.L.; Mouser, J.G.; Mattocks, K.T.; Dankel, S.J.; Abe, T.; Bell, Z.W.; Bentley, J.P.; Loenneke, J.P. Muscle adaptations to high-load training and very low-load training with and without blood flow restriction. Front. Physiol. 2018, 9, 1448. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.F.; Fatela, P.; Mendonca, G.V.; Vaz, J.R.; Valamatos, M.J.; Infante, J.; Mil-Homens, P.; Alves, F.B. Tissue oxygenation in response to different relative levels of blood-flow restricted exercise. Front. Physiol. 2019, 10, 407. [Google Scholar] [CrossRef]


| Timepoint | HP | LP |
|---|---|---|
| Baseline | 569.0 ± 69.4 | 569.0 ± 69.4 |
| 15P # | 528.1 ± 48.3 | 526.2 ± 39.3 |
| 60P # | 520.6 ± 82.1 | 498.6 ± 49.1 |
| 24hP | 558.0 ± 64.1 | 527.2 ± 66.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartolomei, S.; Montesanto, P.; Lanzoni, I.M.; Gatta, G.; Cortesi, M.; Fantozzi, S. A Comparison between High and Low Cuff Pressures on Muscle Oxygen Saturation and Recovery Responses Following Blood-Flow Restriction Resistance Exercise. Sensors 2022, 22, 9138. https://doi.org/10.3390/s22239138
Bartolomei S, Montesanto P, Lanzoni IM, Gatta G, Cortesi M, Fantozzi S. A Comparison between High and Low Cuff Pressures on Muscle Oxygen Saturation and Recovery Responses Following Blood-Flow Restriction Resistance Exercise. Sensors. 2022; 22(23):9138. https://doi.org/10.3390/s22239138
Chicago/Turabian StyleBartolomei, Sandro, Pasquale Montesanto, Ivan Malagoli Lanzoni, Giorgio Gatta, Matteo Cortesi, and Silvia Fantozzi. 2022. "A Comparison between High and Low Cuff Pressures on Muscle Oxygen Saturation and Recovery Responses Following Blood-Flow Restriction Resistance Exercise" Sensors 22, no. 23: 9138. https://doi.org/10.3390/s22239138
APA StyleBartolomei, S., Montesanto, P., Lanzoni, I. M., Gatta, G., Cortesi, M., & Fantozzi, S. (2022). A Comparison between High and Low Cuff Pressures on Muscle Oxygen Saturation and Recovery Responses Following Blood-Flow Restriction Resistance Exercise. Sensors, 22(23), 9138. https://doi.org/10.3390/s22239138










