An Electronic Nose as a Non-Destructive Analytical Tool to Identify the Geographical Origin of Portuguese Olive Oils from Two Adjacent Regions
Abstract
1. Introduction
2. Materials and Methods
2.1. Olive Oil Samples
2.2. Quality Physicochemical Parameters, Oxidative Stability, Total Phenols Content, and Sensory Analysis
2.3. Chromatographic Profile of the Volatile Fraction of the Studied Olive Oils
2.4. E-Nose Analysis
2.4.1. Lab-Made Device
2.4.2. Olive Oil Analysis and Signal Processing
2.5. Statistical Analysis
3. Results and Discussion
3.1. Quality Parametes, Oxidative Stability, Antioxidant Capacity, Sensory, and Volatiles Profiles of Olive Oils from Côa and Douro Adjacent Geographical Regions
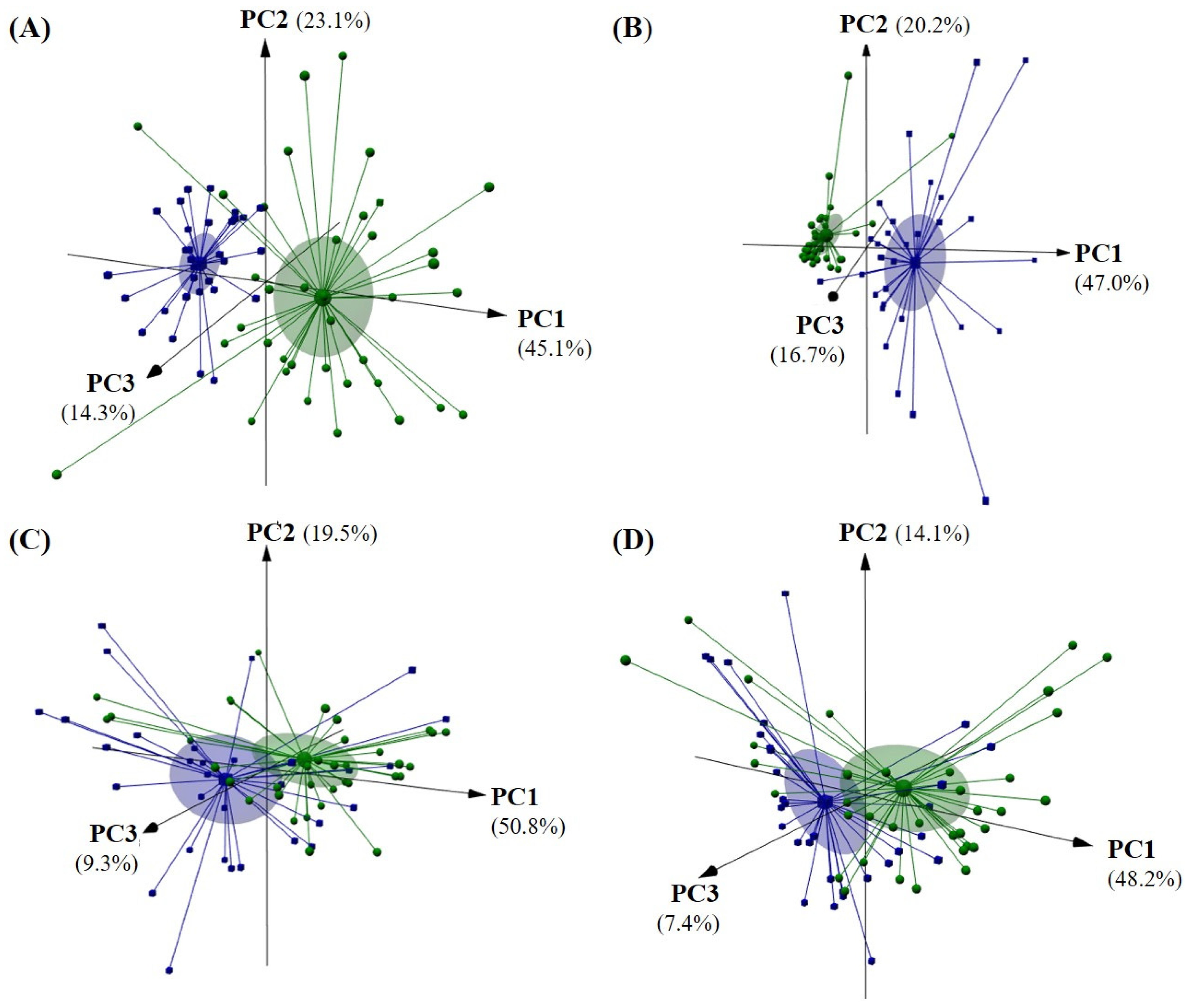
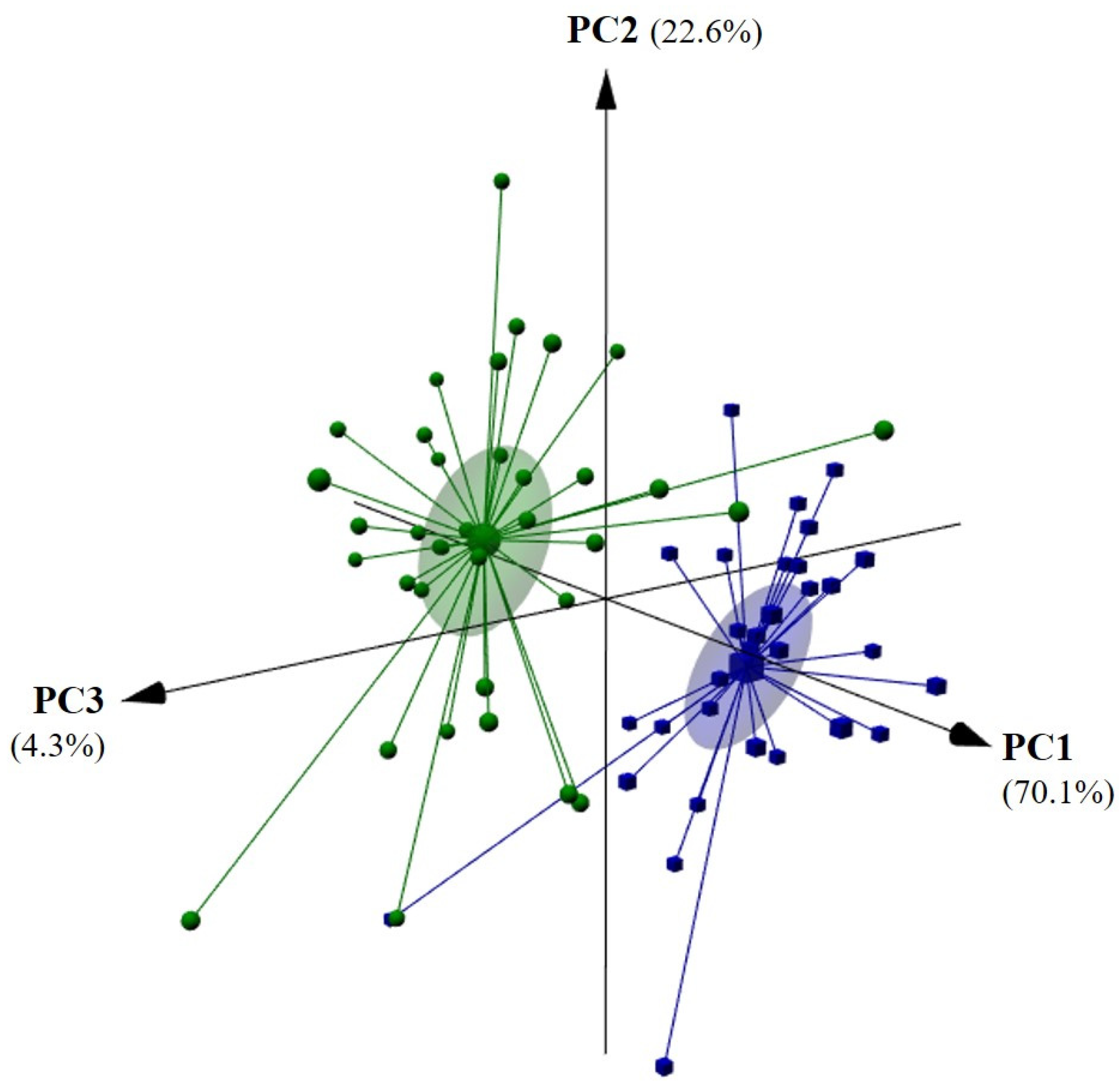
3.2. Identification of Olive Oil Geographical Origin and Assessment of the Alcohls, Aldehydes, and Total Volatiles Content of Oils from Côa and Douro Adjacent Regions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Zhang, Y.; Liu, Z.; Wang, W.; Sun, S.; Wang, J.; Zhu, Z.; Liu, J.; Yang, H.; Zhu, S.; et al. Quality assessment and geographical origin classification of extra-virgin olive oils imported into China. J. Food Compost. Anal. 2022, 113, 104713. [Google Scholar] [CrossRef]
- Aparicio-Ruiz, R.; Tena, N.; García-González, D.L. An International Survey on Olive Oils Quality and Traceability: Opinions from the Involved Actors. Foods 2022, 11, 1045. [Google Scholar] [CrossRef] [PubMed]
- Carzedda, M.; Gallenti, G.; Troiano, S.; Cosmina, M.; Marangon, F.; de Luca, P.; Pegan, G.; Nassivera, F. Consumer preferences for origin and organic attributes of extra virgin olive oil: A choice experiment in the Italian market. Foods 2021, 10, 994. [Google Scholar] [CrossRef] [PubMed]
- Ben Hmida, R.; Gargouri, B.; Chtourou, F.; Sevim, D.; Bouaziz, M. Fatty acid and triacyglycerid as markers of virgin olive oil from Mediterranean region: Traceability and chemometric authentication. Eur. Food Res. Technol. 2022, 248, 1749–1764. [Google Scholar] [CrossRef]
- Baccouri, B.; Rajhi, I.; Mohamed, S.N.; Flamini, G. Monitoring the fatty acid and volatile compositions of Tunisian virgin olive oils using HS-SPME–GC–MS with regard to growing area. Eur. Food Res. Technol. 2022, 248, 2877–2885. [Google Scholar] [CrossRef]
- Eriotou, E.; Karabagias, I.K.; Maina, S.; Koulougliotis, D.; Kopsahelis, N. Geographical origin discrimination of “Ntopia” olive oil cultivar from Ionian islands using volatile compounds analysis and computational statistics. Eur. Food Res. Technol. 2021, 247, 3083–3098. [Google Scholar] [CrossRef]
- Quintanilla-Casas, B.; Torres-Cobos, B.; Guardiola, F.; Servili, M.; Alonso-Salces, R.M.; Valli, E.; Bendini, A.; Toschi, T.G.; Vichi, S.; Tres, A. Geographical authentication of virgin olive oil by GC–MS sesquiterpene hydrocarbon fingerprint: Verifying EU and single country label-declaration. Food Chem. 2022, 378, 132104. [Google Scholar] [CrossRef]
- Nasr, E.G.; Epova, E.N.; Sebilo, M.; Larivière, D.; Hammami, M.; Souissi, R.; Abderrazak, H.; Donard, O.F.X. Olive Oil Traceability Studies Using Inorganic and Isotopic Signatures: A Review. Molecules 2022, 27, 2014. [Google Scholar] [CrossRef]
- Maestrello, V.; Solovyev, P.; Bontempo, L.; Mannina, L.; Camin, F. Nuclear magnetic resonance spectroscopy in extra virgin olive oil authentication. Compr. Rev. Food Sci. Food Saf. 2022, in press. [Google Scholar] [CrossRef]
- Rodrigues, N.; Peres, F.; Casal, S.; Santamaria-Echart, A.; Barreiro, F.; Peres, A.M.; Alberto Pereira, J. Geographical discrimination of olive oils from Cv. ‘Galega Vulgar’. Food Chem. 2023, 398, 133945. [Google Scholar] [CrossRef]
- Scatigno, C.; Festa, G. FTIR coupled with machine learning to unveil spectroscopic benchmarks in the Italian EVOO. Int. J. Food Sci. Technol. 2022, 57, 4156–4162. [Google Scholar] [CrossRef]
- Scatigno, C.; Festa, G. A first elemental pattern and geo-discrimination of Italian EVOO by energy dispersive X-ray fluorescence and chemometrics. Microchem. J. 2021, 171, 106863. [Google Scholar] [CrossRef]
- Fort, A.; Ruisánchez, I.; Callao, M.P. Chemometric strategies for authenticating extra virgin olive oils from two geographically adjacent Catalan protected designations of origin. Microchem. J. 2021, 169, 106611. [Google Scholar] [CrossRef]
- Teixeira, G.G.; Dias, L.G.; Rodrigues, N.; Marx, Í.M.G.; Veloso, A.C.A.; Pereira, J.A.; Peres, A.M. Application of a lab-made electronic nose for extra virgin olive oils commercial classification according to the perceived fruitiness intensity. Talanta 2021, 226, 122122. [Google Scholar] [CrossRef]
- Rodrigues, N.; Silva, K.; Veloso, A.C.A.; Pereira, J.A.; Peres, A.M. The use of electronic nose as alternative non-destructive technique to discriminate flavored and unflavored olive oils. Foods 2021, 10, 2886. [Google Scholar] [CrossRef] [PubMed]
- Marchal, P.C.; Sanmartin, C.; Martínez, S.S.; Ortega, J.G.; Mencarelli, F.; García, J.G. Prediction of fruity aroma intensity and defect presence in virgin olive oil using an electronic nose. Sensors 2021, 21, 2298. [Google Scholar] [CrossRef] [PubMed]
- Tata, A.; Massaro, A.; Damiani, T.; Piro, R.; Dall’Asta, C.; Suman, M. Detection of soft-refined oils in extra virgin olive oil using data fusion approaches for LC-MS, GC-IMS and FGC-Enose techniques: The winning synergy of GC-IMS and FGC-Enose. Food Cont. 2022, 133, 108645. [Google Scholar] [CrossRef]
- Zarezadeh, M.R.; Aboonajmi, M.; Varnamkhasti, M.G.; Azarikia, F. Olive Oil Classification and Fraud Detection Using E-Nose and Ultrasonic System. Food Anal. Methods 2021, 14, 2199–2210. [Google Scholar] [CrossRef]
- Abu-Khalaf, N. Identification and quantification of olive oil quality parameters using an electronic nose. Agriculture 2021, 11, 674. [Google Scholar] [CrossRef]
- Commission Delegated Regulation (EU) 2015/1830 of 8th July 2015: Amending Regulation (EEC) No 2568/91 on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis. Off. J. Eur. Union 2015, L266, 9–13.
- Rodrigues, N.; Casal, S.; Peres, A.M.; Baptista, P.; Pereira, J.A. Seeking for sensory differentiated olive oils? The urge to preserve old autochthonous olive cultivars. Food Res. Int. 2020, 128, 108759. [Google Scholar] [CrossRef]
- Pizarro, M.L.; Becerra, M.; Sayago, A.; Beltrán, M.; Beltrán, R. Comparison of Different Extraction Methods to Determine Phenolic Compounds in Virgin Olive Oil. Food Anal. Methods 2013, 6, 123–132. [Google Scholar] [CrossRef]
- Cherif, M.; Rodrigues, N.; Veloso, A.C.A.; Pereira, J.A.; Peres, A.M. Kinetic study of the microwave-induced thermal degradation of cv. Arbequina olive oils flavored with lemon verbena essential oil. J. Am. Oil Chem. Soc. 2021, 98, 1021–1032. [Google Scholar] [CrossRef]
- IOC, International Olive Council. Sensory Analysis of Olive Oil—Method for the Organoleptic Assessment of Virgin Olive Oil Applying to Use a Designation of Origin. COI/T.20/Doc. no. 22 November 2005. 29p. Available online: https://www.internationaloliveoil.org/what-we-do/chemistry-standardisation-unit/standards-and-methods/ (accessed on 1 September 2022).
- Rodrigues, N.; Peres, A.M.; Baptista, P.; Pereira, J.A. Olive Oil Sensory Analysis as a Tool to Preserve and Valorize the Heritage of Centenarian Olive Trees. Plants 2022, 11, 257. [Google Scholar] [CrossRef]
- Silva, K.; Rodrigues, N.; Pereira, J.A.; Ramalhosa, E. Characterisation of olive oils from the Douro valley, Portugal: Study of the volatile fraction and its relationship with sensory characteristics. Appl. Sci. 2022, 12, 9246. [Google Scholar] [CrossRef]
- Teixeira, G.G.; Peres, A.M.; Estevinho, L.; Geraldes, P.; Garcia-Cabezon, C.; Martin-Pedrosa, F.; Rodriguez-Mendez, M.L.; Dias, L.G. Enose lab made with vacuum sampling: Quantitative applications. Chemosensors 2022, 10, 261. [Google Scholar] [CrossRef]
- Gila, D.M.M.; García, J.G.; Bellincontro, A.; Mencarelli, F.; Ortega, J.G. Fast tool based on electronic nose to predict olive fruit quality after harvest. Postharvest Biol. Technol. 2020, 160, 111058. [Google Scholar] [CrossRef]
- Association Française de Normalisation (AFNOR). Recueil des Normes Françaises, Qualité de l’Eau, 2nd ed.; Association Française de Normalisation (AFNOR): Paris, France, 1997; Volume 1, p. 293. [Google Scholar]
- Garcia, B.; Magalhães, J.; Fregapane, G.; Salvador, M.D.; Paiva-Martins, F. Potential of selected Portuguese cultivars for the production of high quality monovarietal virgin olive oil. Eur. J. Lipid Sci. Technol. 2012, 114, 1070–1082. [Google Scholar] [CrossRef]
- Mariotti, R.; Núñez-Carmona, E.; Genzardi, D.; Sberveglieri, S.P.V.; Mousavi, S. Volatile Olfactory Profiles of Umbrian Extra Virgin Olive Oils and Their Discrimination through MOX Chemical Sensors. Sensors 2022, 22, 7164. [Google Scholar] [CrossRef]

| Physicochemical, Stability and Antioxidant Data | Olive Oil’s Geographical Origin | p-Value * | |
|---|---|---|---|
| Côa Valley | Douro Velley | ||
| FA (goleic acid/100 g) | 0.22 ± 0.07a | 0.23 ± 0.04a | 0.3085 # |
| PV (mEq O2/kg) | 5.63 ± 2.09a | 4.82 ± 1.41b | 0.0046 # |
| K232 | 1.97 ± 0.29a | 1.82 ± 0.27b | 0.0024 $ |
| K268 | 0.15 ± 0.03a | 0.13 ± 0.02b | <0.0001 # |
| OS (h) | 17.5 ± 7.4a | 13.9 ± 6.4b | 0.0017 $ |
| TPC (mg GAE/kg) | 505 ± 188a | 274 ± 77b | <0.0001 # |
| DPPH (%) | 53.2 ± 17.2a | 13.9 ± 6.4b | <0.0001 # |
| Sensory Attributes | Olive Oil Geographical Origin | p-Value * | ||||
|---|---|---|---|---|---|---|
| Côa Valley | Oils with Perceived Sensation | Douro Valley | Oils with Perceived Sensation | |||
| Olfactory sensations | ||||||
| Fruity | Greenly | 3.4 ± 1.9a | 81% | 1.1 ± 1.8b | 33% | <0.0001 $ |
| Ripely | 0.9 ± 2.0b | 19% | 2.9 ± 2.4a | 67% | <0.0001 $ | |
| Fruit sensations | Apple | 4.5 ± 0.6a | 100% | 3.9 ± 1.7b | 93% | 0.0034 $ |
| Banana | 2.0 ± 2.4a | 44% | 1.9 ± 2.7a | 38% | 0.7603 $ | |
| Cabbage | 2.5 ± 2.4a | 57% | 1.2 ± 2.2b | 27% | 0.0007 $ | |
| Dry fruits | 3.4 ± 0.7a | 100% | 2.9 ± 1.2b | 95% | 0.0032 $ | |
| Tomato | 4.5 ± 2.0a | 89% | 2.5 ± 2.4b | 63% | <0.0001 $ | |
| Herbaceous sensations | Dry herbs | 0.9 ± 1.9b | 19% | 1.5 ± 2.0a | 42% | 0.0440 $ |
| Fresh herbs | 3.3 ± 1.9a | 79% | 1.2 ± 1.8b | 33% | <0.0001 $ | |
| Tomato leaves | 1.7 ± 2.2a | 42% | 1.2 ± 1.9a | 30% | 0.1540 $ | |
| Harmony | 8.0 ± 0.5a | ---- | 7.8 ± 0.9b | ---- | 0.0079 $ | |
| Gustatory sensations | ||||||
| Fruity | Greenly | 1.0 ± 2.2b | 19% | 2.8 ± 2.6a | 58% | <0.0001 # |
| Ripely | 4.0 ± 2.3a | 81% | 1.6 ± 2.1b | 42% | <0.0001 $ | |
| Basic taste | Bitter | 3.7 ± 1.3a | 100% | 2.3 ± 1.0b | 100% | <0.0001 # |
| Sweet | 3.4 ± 3.2b | 100% | 4.6 ± 2.0a | 100% | 0.0060 # | |
| Kinesthetic sensation | Pungent | 4.4 ± 1.2a | 100% | 3.2 ± 1.0b | 100% | <0.0001 $ |
| Fruit sensations | Apple | 4.6 ± 0.8a | 100% | 4.2 ± 1.7b | 93% | 0.0462 # |
| Banana | 2.8 ± 2.7a | 56% | 2.9 ± 2.8a | 60% | 0.7369 $ | |
| Cabbage | 3.5 ± 2.7a | 68% | 1.1 ± 2.2b | 24% | 0.0429 # | |
| Dry fruits | 4.3 ± 3.6a | 99% | 3.2 ± 1.1 | 97% | 0.0097 # | |
| Plum | 0.7 ± 1.6a | 18% | 0.3 ± 1.1b | 7% | 0.0370 # | |
| Tomato | 4.5 ± 1.9a | 92% | 2.5 ± 2.4b | 56% | <0.0001 # | |
| Herbaceous sensations | Dry herbs | 0.8 ± 1.9a | 18% | 1.4 ± 2.1a | 34% | 0.1291 $ |
| Fresh herbs | 3.1 ± 2.1a | 75% | 1.4 ± 2.0b | 36% | <0.0001 $ | |
| Olive leaves | 1.1 ± 2.0a | 24% | 0.1 ± 0.7b | 3% | 0.0001 # | |
| Tomato leaves | 2.4 ± 2.3a | 56% | 1.0 ± 1.7b | 25% | <0.0001 # | |
| Harmony | 7.6 ± 0.5a | ---- | 7.7 ± 0.9a | ---- | 0.9235 $ | |
| Global sensations | ||||||
| Complexity | 6.6 ± 0.8a | ---- | 6.5 ± 1.0a | ---- | 0.7432 # | |
| Persistence | 7.5 ± 0.8a | ---- | 7.2 ± 1.1 | ---- | 0.0108 # | |
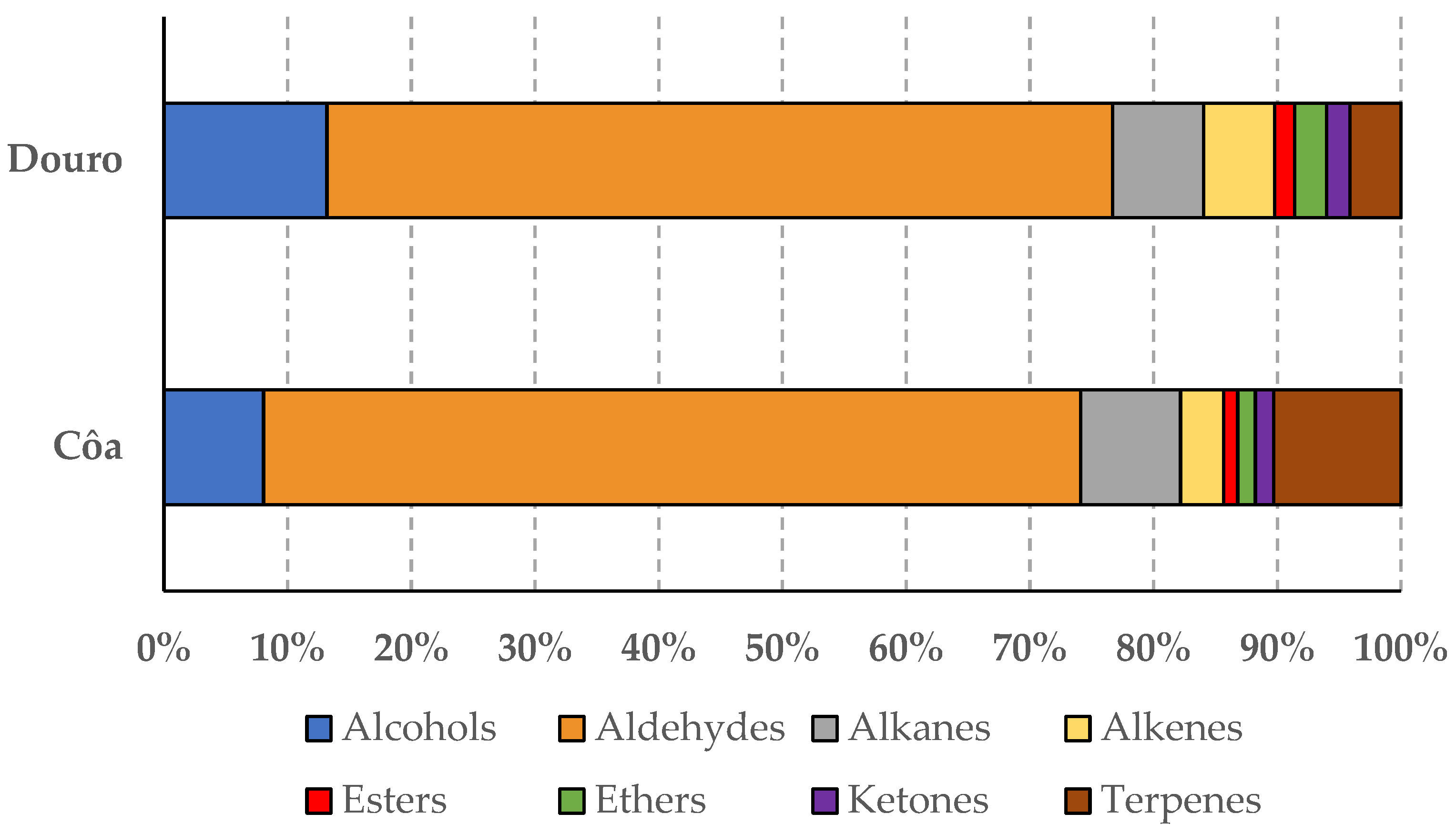
| Chemical Family of Volatile Compounds § | Olive Oil’s Geographical Origin (mg/kg) | p-Value * | |
|---|---|---|---|
| Côa Valley | Douro Valley | ||
| Alcohols | 1.2 ± 3.4b | 5.1 ± 4.9a | 0.0003 # |
| Aldehydes | 9.8 ± 9.9b | 27.7 ± 18.0a | <0.0001 # |
| Alkanes | 1.1 ± 0.7b | 2.9 ± 1.7a | <0.0001 # |
| Alkenes | 0.4 ± 0.4b | 2.2 ± 0.9a | <0.0001 # |
| Esters | 0.2 ± 0.2b | 0.6 ± 0.7a | 0.0002 # |
| Ethers | 0.2 ± 0.3b | 1.1 ± 0.6a | <0.0001 # |
| Ketones | 0.2 ± 0.2b | 0.7 ± 0.7a | 0.0004 # |
| Terpenes | 1.4 ± 0.8a | 1.4 ± 0.9a | 0.9730 $ |
| Total | 14.5 ± 11.6b | 41.6 ± 19.8a | <0.0001 # |
| Geographical Origin | Volatiles Chemical Class | Concentration Range (mg/kg oil) a | Nº of Feature Extracted Variables b | E-Nose-MOS-SA Models (LOO-CV c) | |
| Determination Coefficient (R2) | Root Mean Square Errors (RMSE, mg/kg Oil) | ||||
| Côa Valley | Alcohols | [0.17, 19.1] | 20 d | 0.986 | 0.40 |
| Aldehydes | [0.41, 64.4] | 25 e | 0.982 | 1.34 | |
| Total | [6.24, 78.8] | 25 f | 0.990 | 1.19 | |
| Douro Valley | Alcohols | [1.11, 22.1] | 21 g | 0.998 | 0.23 |
| Aldehydes | [10.9, 83.9] | 20 h | 0.984 | 2.29 | |
| Total | [20.2, 104.9] | 19 i | 0.981 | 2.79 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, N.; Ferreiro, N.; Veloso, A.C.A.; Pereira, J.A.; Peres, A.M. An Electronic Nose as a Non-Destructive Analytical Tool to Identify the Geographical Origin of Portuguese Olive Oils from Two Adjacent Regions. Sensors 2022, 22, 9651. https://doi.org/10.3390/s22249651
Rodrigues N, Ferreiro N, Veloso ACA, Pereira JA, Peres AM. An Electronic Nose as a Non-Destructive Analytical Tool to Identify the Geographical Origin of Portuguese Olive Oils from Two Adjacent Regions. Sensors. 2022; 22(24):9651. https://doi.org/10.3390/s22249651
Chicago/Turabian StyleRodrigues, Nuno, Nuno Ferreiro, Ana C. A. Veloso, José A. Pereira, and António M. Peres. 2022. "An Electronic Nose as a Non-Destructive Analytical Tool to Identify the Geographical Origin of Portuguese Olive Oils from Two Adjacent Regions" Sensors 22, no. 24: 9651. https://doi.org/10.3390/s22249651
APA StyleRodrigues, N., Ferreiro, N., Veloso, A. C. A., Pereira, J. A., & Peres, A. M. (2022). An Electronic Nose as a Non-Destructive Analytical Tool to Identify the Geographical Origin of Portuguese Olive Oils from Two Adjacent Regions. Sensors, 22(24), 9651. https://doi.org/10.3390/s22249651









