Pre-Anodized Graphite Pencil Electrode Coated with a Poly(Thionine) Film for Simultaneous Sensing of 3-Nitrophenol and 4-Nitrophenol in Environmental Water Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Instruments
2.3. Fabrication of APGE and APGE/PTH
3. Results and Discussion
3.1. Surface Morphology and FT-IR Measurements of APGE and APGE/PTH
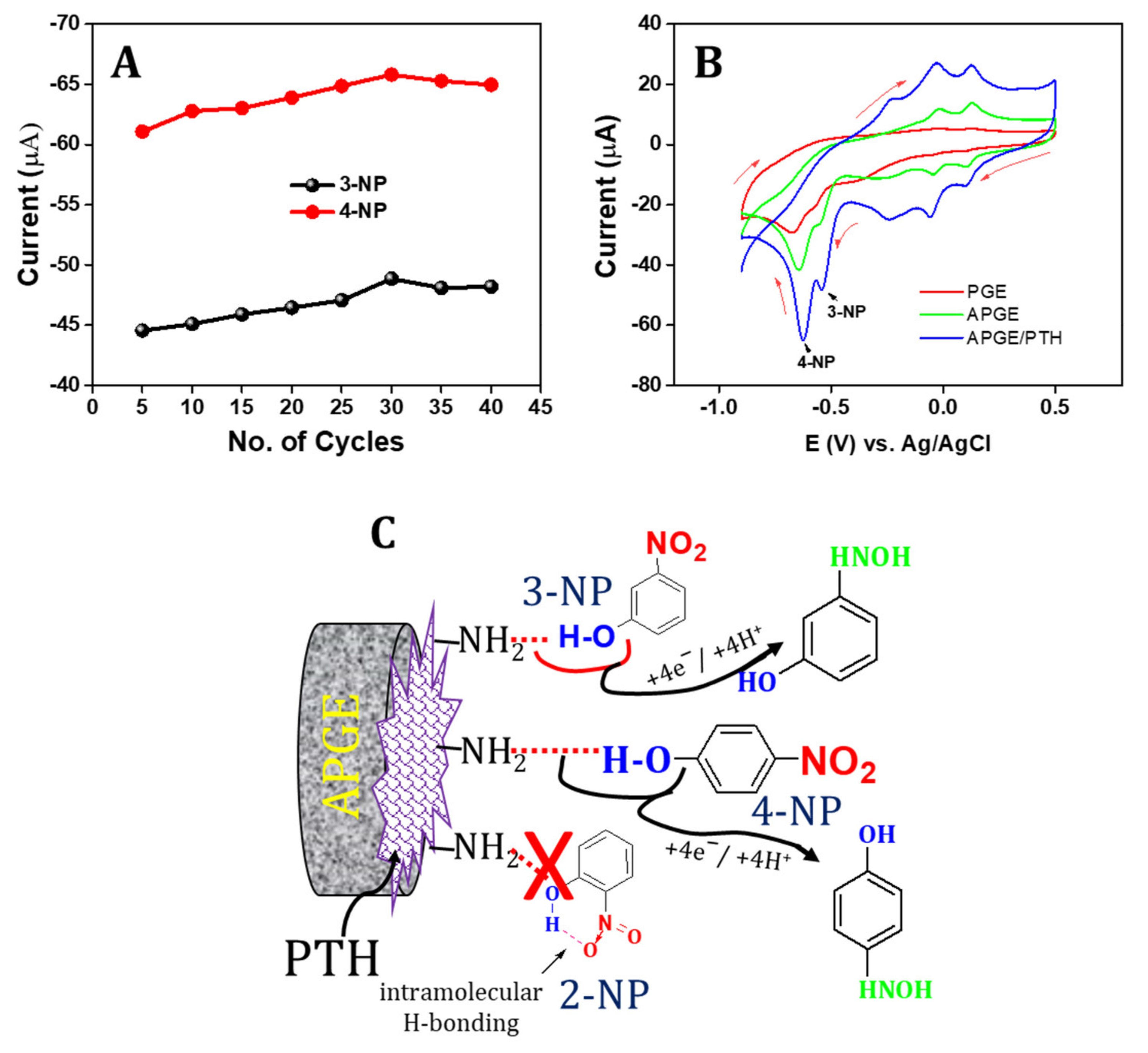
3.2. Electrochemical Characterization of APGE/PTH
3.3. Optimization of PTH on the APGE towards Sensing of 3-NP and 4-NP
3.4. Electrocatalytic Behavior of APGE/PTH towards Sensing of 3-NP and 4-NP
3.5. Electrocatalytic Mechanism towards 3-NP and 4-NP
3.6. pH Influence
3.7. Scan Rate Influence
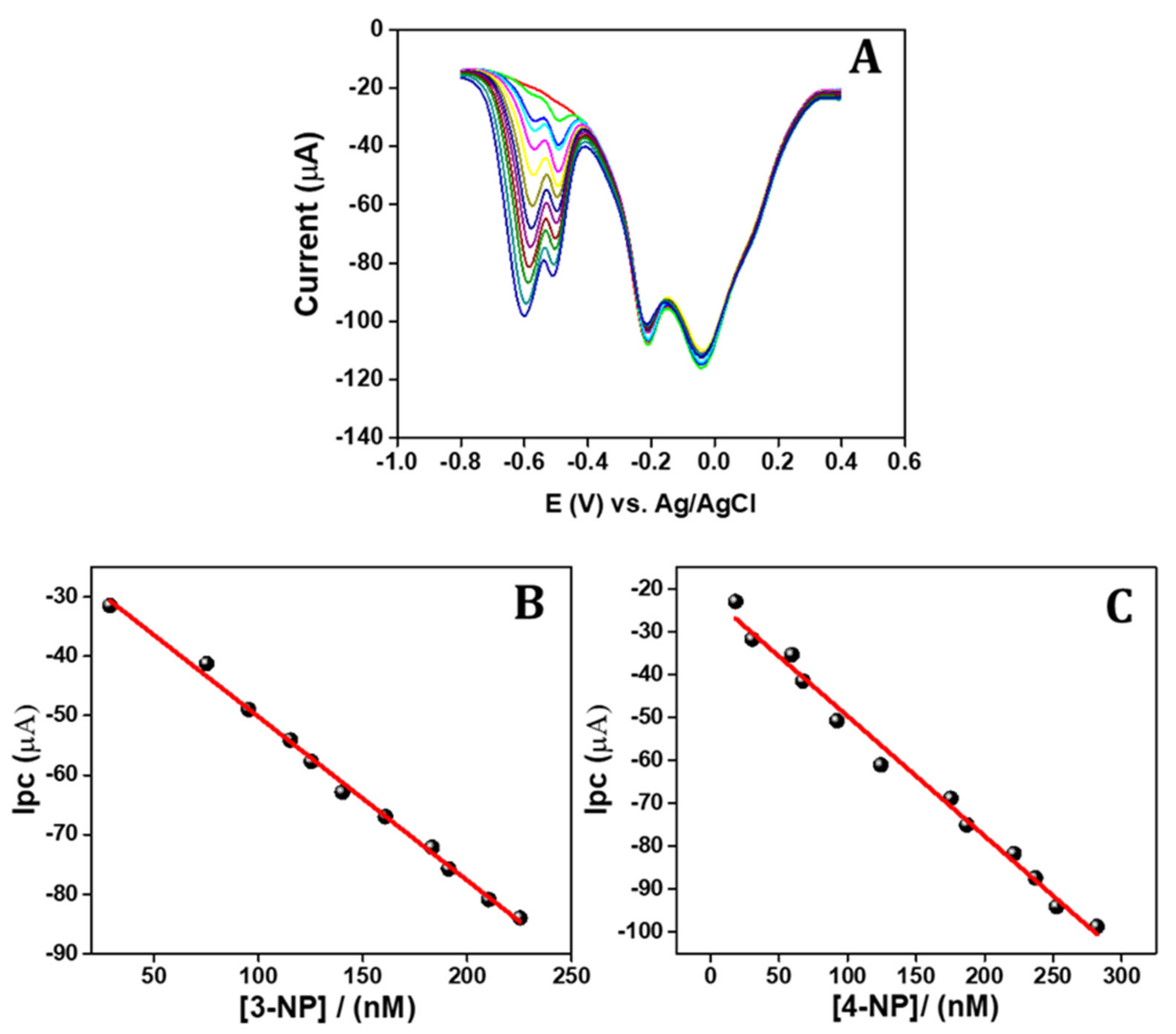
3.8. Simultaneous DPV Method Sensing of 3-NP and 4-NP
3.9. Selectivity of the APGE/PTH
3.10. Stability and Reproducibility of the APGE/PTH
3.11. Real Sample Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, T.; Huang, X.; Zeng, Q.; Wang, L. Simultaneous electrochemical determination of nitrophenol isomers with the polyfurfural film modified glassy carbon electrode. J. Electroanal. Chem. 2015, 743, 105–111. [Google Scholar] [CrossRef]
- Zhang, X.-D.; Zhao, Y.; Chen, K.; Guo, J.; Wang, P.; Wu, H.; Sun, W.-Y. Cucurbit[6]uril-based supramolecular assemblies incorporating metal complexes with multiaromatic ligands as structure-directing agent for detection of aromatic amines and nitroaromatic compounds. Sens. Actuators B Chem. 2018, 282, 844–853. [Google Scholar] [CrossRef]
- Shi, L.; Yin, Y.; Zhang, L.-C.; Wang, S.; Sillanpää, M.; Sun, H. Design and engineering heterojunctions for the photoelectrochemical monitoring of environmental pollutants: A review. Appl. Catal. B Environ. 2019, 248, 405–422. [Google Scholar] [CrossRef]
- Ramalingam, M.; Ponnusamy, V.K.; Sangilimuthu, S.N. Electrochemical determination of 4-nitrophenol in environmental water samples using porous graphitic carbon nitride-coated screen-printed electrode. Environ. Sci. Pollut. Res. 2019, 27, 17481–17491. [Google Scholar] [CrossRef] [PubMed]
- El Mhammedi, M.; Achak, M.; Bakasse, M.; Chtaini, A. Electrochemical determination of para-nitrophenol at apatite-modified carbon paste electrode: Application in river water samples. J. Hazard. Mater. 2009, 163, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kuang, D.; Feng, Y.; Zhang, F.; Xu, Z.; Liu, M. A graphene oxide-based electrochemical sensor for sensitive determination of 4-nitrophenol. J. Hazard. Mater. 2012, 201–202, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Yang, L.; Zhong, M.; Li, C.; Lu, X.; Kan, X. Selective recognition and electrochemical detection of p-nitrophenol based on a macroporous imprinted polymer containing gold nanoparticles. Microchim. Acta 2013, 180, 1461–1469. [Google Scholar] [CrossRef]
- Gong, S.; Xiao, X.; Sam, D.K.; Liu, B.; Wei, W.; Yu, W.; Lv, X. Dispersed copper nanoparticles promote the electron mobility of nitrogen-rich graphitized carbon aerogel for electrochemical determination of 4-nitrophenol. Microchim. Acta 2019, 186, 853. [Google Scholar] [CrossRef]
- Luo, S.; Miao, Y.; Guo, J.; Sun, X.; Yan, G. Phosphorimetric determination of 4-nitrophenol using mesoporous molecular imprinting polymers containing manganese(II)-doped ZnS quantum dots. Microchim. Acta 2019, 186, 249. [Google Scholar] [CrossRef]
- Xu, G.; Li, B.; Wang, X.; Luo, X. Electrochemical sensor for nitrobenzene based on carbon paste electrode modified with a poly(3,4-ethylenedioxythiophene) and carbon nanotube nanocomposite. Microchim. Acta 2014, 181, 463–469. [Google Scholar] [CrossRef]
- Wijker, R.S.; Kurt, Z.; Spain, J.C.; Bolotin, J.; Zeyer, J.; Hofstetter, T.B. Isotope Fractionation Associated with the Biodegradation of 2- and 4-Nitrophenols via Monooxygenation Pathways. Environ. Sci. Technol. 2013, 47, 14185–14193. [Google Scholar] [CrossRef]
- Murphy, M.; Manoj, D.; Saravanakumar, D.; Thenmozhi, K.; Senthilkumar, S. Water insoluble, self-binding viologen functionalized ionic liquid for simultaneous electrochemical detection of nitrophenol isomers. Anal. Chim. Acta 2020, 1138, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Kubendhiran, S.; Sakthivel, R.; Chen, S.-M.; Mutharani, B.; Chen, T.-W. Innovative Strategy Based on a Novel Carbon-Black−β-Cyclodextrin Nanocomposite for the Simultaneous Determination of the Anticancer Drug Flutamide and the Environmental Pollutant 4-Nitrophenol. Anal. Chem. 2018, 90, 6283–6291. [Google Scholar] [CrossRef] [PubMed]
- Umar, A.; Kim, S.; Kumar, R.; Algarni, H.; Al-Assiri, M.S. Platinum nanoparticles decorated carbon nanotubes for highly sensitive 2-nitrophenol chemical sensor. Ceram. Int. 2016, 42, 9257–9263. [Google Scholar] [CrossRef]
- Gerent, G.G.; Spinelli, A. Magnetite-platinum nanoparticles-modified glassy carbon electrode as electrochemical detector for nitrophenol isomers. J. Hazard. Mater. 2017, 330, 105–115. [Google Scholar] [CrossRef]
- Ni, Y.; Wang, L.; Kokot, S. Simultaneous determination of nitrobenzene and nitro-substituted phenols by differential pulse voltammetry and chemometrics. Anal. Chim. Acta 2001, 431, 101–113. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, D.; Lv, X.; Xu, X.; Zhou, H.; Liu, P.; Cui, B.; Wang, L. Simultaneous electrochemical determination of nitrophenol isomers Based on spirofluorene-based microporous polymer film modified electrodes through one-step electropolymerization strategy. Sens. Actuators B Chem. 2021, 333, 129568. [Google Scholar] [CrossRef]
- Doan, V.-D.; Phan, T.L.; Le, V.T.; Vasseghian, Y.; Evgenievna, L.O.; Tran, D.L.; Le, V.T. Efficient and fast degradation of 4-nitrophenol and detection of Fe(III) ions by Poria cocos extract stabilized silver nanoparticles. Chemosphere 2022, 286, 131894. [Google Scholar] [CrossRef]
- Kumar, S.; Sekar, S.; Kaliamurthy, A.K.; Lee, S. Bifunctional rGO-NiCo2S4 MOF hybrid with high electrochemical and catalytic activity for supercapacitor and nitroarene reduction. J. Mater. Res. Technol. 2021, 12, 2489–2501. [Google Scholar] [CrossRef]
- Karaová, J.; Barek, J.; Schwarzová-Pecková, K. Oxidative and Reductive Detection Modes for Determination of Nitrophenols by High-Performance Liquid Chromatography with Amperometric Detection at a Boron Doped Diamond Electrode. Anal. Lett. 2016, 49, 66–79. [Google Scholar] [CrossRef]
- Belloli, R.; Barletta, B.; Bolzacchini, E.; Meinardi, S.; Orlandi, M.; Rindone, B. Determination of toxic nitrophenols in the atmosphere by high-performance liquid chromatography. J. Chromatogr. A 1999, 846, 277–281. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, M.; Zhao, J.; Shi, Z. Sandwich-type spontaneous injection of nitrophenols for capillary electrophoresis analysis. Anal. Methods 2012, 4, 2177–2182. [Google Scholar] [CrossRef]
- Miró, M.; Cladera, A.; Estela, J.M.; Cerdà, V. Dual wetting-film multi-syringe flow injection analysis extraction: Application to the simultaneous determination of nitrophenols. Anal. Chim. Acta 2001, 438, 103–116. [Google Scholar] [CrossRef]
- Ahmed, G.H.G.; Laíño, R.B.; Calzón, J.A.G.; García, M.E.D. Highly fluorescent carbon dots as nanoprobes for sensitive and selective determination of 4-nitrophenol in surface waters. Mikrochim. Acta 2015, 182, 51–59. [Google Scholar] [CrossRef]
- Wang, F.; Fu, X.; Chai, X.; Han, Q.; Wang, H.; Hao, Q. Highly selective fluorometric detection of para-nitrophenol from its isomers by nitrogen-doped graphene quantum dots. Microchem. J. 2021, 168, 106389. [Google Scholar] [CrossRef]
- Ashok Kumar, E.; Riswana Barveen, N.; Wang, T.-J.; Kokulnathan, T.; Chang, Y.-H. Development of SERS platform based on ZnO multipods decorated with Ag nanospheres for detection of 4-nitrophenol and rhodamine 6G in real samples. Microchem. J. 2021, 170, 106660. [Google Scholar] [CrossRef]
- Liu, J.; Chen, H.; Lin, Z.; Lin, J.-M. Preparation of Surface Imprinting Polymer Capped Mn-Doped ZnS Quantum Dots and Their Application for Chemiluminescence Detection of 4-Nitrophenol in Tap Water. Anal. Chem. 2010, 82, 7380–7386. [Google Scholar] [CrossRef]
- Delnavaz, E.; Amjadi, M. An ultrasensitive chemiluminescence assay for 4-nitrophenol by using luminol–NaIO4 reaction catalyzed by copper, nitrogen co-doped carbon dots. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 241, 118608. [Google Scholar] [CrossRef]
- Ramalingam, M.; Kokulnathan, T.; Tsai, P.-C.; Valan Arasu, M.; Al-Dhabi, N.A.; Prakasham, K.; Ponnusamy, V.K. Ultrasonication-assisted synthesis of gold nanoparticles decorated ultrathin graphitic carbon nitride nanosheets as a highly efficient electrocatalyst for sensitive analysis of caffeic acid in food samples. Appl. Nanosci. 2021. [Google Scholar] [CrossRef]
- Kaya, H.O.; Cetin, A.E.; Azimzadeh, M.; Topkaya, S.N. Pathogen detection with electrochemical biosensors: Advantages, challenges and future perspectives. J. Electroanal. Chem. 2021, 882, 114989. [Google Scholar] [CrossRef]
- Liu, G.; Xiong, Z.; Yang, L.; Shi, H.; Fang, D.; Wang, M.; Shao, P.; Luo, X. Electrochemical approach toward reduced graphene oxide-based electrodes for environmental applications: A review. Sci. Total Environ. 2021, 778, 146301. [Google Scholar] [CrossRef] [PubMed]
- Hashim, H.S.; Fen, Y.W.; Omar, N.A.S.; Fauzi, N.I.M. Sensing Methods for Hazardous Phenolic Compounds Based on Graphene and Conducting Polymers-Based Materials. Chemosensors 2021, 9, 291. [Google Scholar] [CrossRef]
- Hui, Y.; Bian, C.; Xia, S.; Tong, J.; Wang, J. Synthesis and electrochemical sensing application of poly (3, 4-ethylenedioxythiophene)-based materials: A review. Anal. Chim. Acta 2018, 1022, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Zhao, J.; Hao, Z.; Guo, M.; Li, L.; Li, N.; Sun, X.; Zhang, P.; Cui, J. Simultaneous electrochemical determination of nitrophenol isomers based on macroporous carbon functionalized with amino-bridged covalent organic polycalix[4]arenes. J. Hazard. Mater. 2022, 423, 127034. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Huang, F.; Li, W.; Yu, S.; Zhang, S.; Kong, J. Sensitive detection of trifluoperazine using a poly-ABSA/SWNTs film-modified glassy carbon electrode. Talanta 2008, 74, 815–820. [Google Scholar] [CrossRef]
- Jeevagan, A.J.; John, S.A. Electrochemical determination of L-methionine using the electropolymerized film of non-peripheral amine substituted Cu(II) phthalocyanine on glassy carbon electrode. Bioelectrochemistry 2012, 85, 50–55. [Google Scholar] [CrossRef]
- Ahammad, A.J.S.; Rahman, M.; Xu, G.-R.; Kim, S.; Lee, J.-J. Highly sensitive and simultaneous determination of hydroquinone and catechol at poly(thionine) modified glassy carbon electrode. Electrochimica Acta 2011, 56, 5266–5271. [Google Scholar] [CrossRef]
- Ge, C.-Y.; Rahman, M.; Li, X.-B.; Lee, J.-J. Simultaneous and Interference-Free Detection of Hydroquinone and Catechol on Poly (Evans Blue)-Modified Glassy Carbon Electrode. J. Electrochem. Soc. 2016, 163, B556. [Google Scholar] [CrossRef]
- Rahman, M.; Lee, J.-J. Sensitivity control of dopamine detection by conducting poly(thionine). Electrochem. Commun. 2021, 125, 107005. [Google Scholar] [CrossRef]
- Rahman, M.; Lopa, N.S.; Kim, Y.J.; Choi, D.-K.; Lee, J.-J. Label-Free DNA Hybridization Detection by Poly(Thionine)-Gold Nanocomposite on Indium Tin Oxide Electrode. J. Electrochem. Soc. 2016, 163, B153. [Google Scholar] [CrossRef]
- Dalkiran, B.; Brett, C.M.A. A novel nanostructured poly(thionine)-deep eutectic solvent/CuO nanoparticle film-modified disposable pencil graphite electrode for determination of acetaminophen in the presence of ascorbic acid. Anal. Bioanal. Chem. 2021, 413, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Cui, X.; Yang, F.; Ma, Y.; Yang, X. Preparation of poly(thionine) modified screen-printed carbon electrode and its application to determine NADH in flow injection analysis system. Biosens. Bioelectron. 2003, 19, 277–282. [Google Scholar] [CrossRef]
- Ghica, M.E.; Ferreira, G.M.; Brett, C.M.A. Poly(thionine)-carbon nanotube modified carbon film electrodes and application to the simultaneous determination of acetaminophen and dipyrone. J. Solid State Electrochem. 2015, 19, 2869–2881. [Google Scholar] [CrossRef]
- Dalkıran, B.; Fernandes, I.P.G.; David, M.; Brett, C.M.A. Electrochemical synthesis and characterization of poly(thionine)-deep eutectic solvent/carbon nanotube–modified electrodes and application to electrochemical sensing. Mikrochim. Acta 2020, 187, 609. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kan, X. A boronic acid carbon nanodots/poly(thionine) sensing platform for the accurate and reliable detection of NADH. Bioelectrochemistry 2019, 130, 107344. [Google Scholar] [CrossRef]
- Shamspur, T.; Biniaz, Z.; Mostafavi, A.; Torkzadeh-Mahani, M.; Mohamadi, M. An Electrochemical Immunosensor Based on Poly(Thionine)-Modified Carbon Paste Electrode for the Determination of Prostate Specific Antigen. IEEE Sens. J. 2018, 18, 4861–4868. [Google Scholar] [CrossRef]
- Xiao, Y.; Ju, H.-X.; Chen, H.-Y. A reagentless hydrogen peroxide sensor based on incorporation of horseradish peroxidase in poly(thionine) film on a monolayer modified electrode. Anal. Chim. Acta 1999, 391, 299–306. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, H.; Wu, Y.; Yu, A. Amperometric hydrogen peroxide biosensor based on a glassy carbon electrode modified with polythionine and gold nanoparticles. Microchim. Acta 2012, 176, 279–285. [Google Scholar] [CrossRef]
- Borisenko, K.B.; Bock, C.W.; Hargittai, I. Intramolecular Hydrogen Bonding and Molecular Geometry of 2-Nitrophenol from a Joint Gas-Phase Electron Diffraction and ab Initio Molecular Orbital Investigation. J. Phys. Chem. 1994, 98, 1442–1448. [Google Scholar] [CrossRef]
- Kovács, A.; Keresztury, G.; Izvekov, V. Intramolecular hydrogen-bonding in 2-nitroresorcinol. A combined FT-IR, FT-Raman and computational study. Chem. Phys. 2000, 253, 193–204. [Google Scholar] [CrossRef]
- Manikandan, R.; Deepa, P.; Narayanan, S.S. Fabrication and characterization of poly 2-napthol orange film modified electrode and its application to selective detection of dopamine. J. Solid State Electrochem. 2017, 21, 3567–3578. [Google Scholar] [CrossRef]
- Vishnu, N.; Kumar, A.S. A preanodized 6B-pencil graphite as an efficient electrochemical sensor for mono-phenolic preservatives (phenol and meta-cresol) in insulin formulations. Anal. Methods 2015, 7, 1943–1950. [Google Scholar] [CrossRef]
- Nath, N.C.D.; Sarker, S.; Rahman, M.; Lee, H.J.; Kim, Y.J.; Lee, J.-J. A facile template-free chemical synthesis of poly(thionine) nanowires. Chem. Phys. Lett. 2013, 559, 56–60. [Google Scholar] [CrossRef]
- Thangaraj, R.; Kumar, A.S. Graphitized mesoporous carbon modified glassy carbon electrode for selective sensing of xanthine, hypoxanthine and uric acid. Anal. Methods 2012, 4, 2162–2171. [Google Scholar] [CrossRef]
- Vishnu, N.; Kumar, A.S. Development of Prussian Blue and Fe(bpy)32+ hybrid modified pencil graphite electrodes utilizing its intrinsic iron for electroanalytical applications. J. Electroanal. Chem. 2017, 786, 145–153. [Google Scholar] [CrossRef]
- Kumar, A.S.; Shanmugam, R.; Vishnu, N.; Pillai, K.C.; Kamaraj, S. Electrochemical immobilization of ellagic acid phytochemical on MWCNT modified glassy carbon electrode surface and its efficient hydrazine electrocatalytic activity in neutral pH. J. Electroanal. Chem. 2016, 782, 215–224. [Google Scholar] [CrossRef]
- Zen, J.-M.; Lai, Y.-Y.; Yang, H.-H.; Kumar, A.S. Multianalyte sensor for the simultaneous determination of hypoxanthine, xanthine and uric acid based on a preanodized nontronite-coated screen-printed electrode. Sens. Actuators B Chem. 2002, 84, 237–244. [Google Scholar] [CrossRef]
- Lumibao, C.Y.; Tillekeratne, L.M.V.; Kirchhoff, J.R.; Fouchard, D.M.D.; Hudson, R.A. Electrochemical and Electrocatalytic Properties of Imidazole Analogues of the Redox Cofactor Pyrroloquinoline Quinone. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2008, 20, 2177–2184. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.-L.; Lai, G.-S.; Yu, A.-M.; Huang, Y.-M.; Han, D.-Y. Amperometric NADH Biosensor Based on Magnetic Chitosan Microspheres/Poly(thionine) Modified Glassy Carbon Electrode. Electroanalysis 2010, 22, 1725–1732. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, Y.; Yang, L.; He, P.-G.; Fang, Y.-Z. Direct Electrochemical Detection of Oligonucleotide Hybridization on Poly(thionine) Film. Chin. J. Chem. 2005, 23, 1665–1670. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, X.; Zhou, L.; You, T. Simultaneous electrochemical determination of dihydroxybenzene isomers using electrospun nitrogen-doped carbon nanofiber film electrode. Sens. Actuators B Chem. 2016, 224, 568–576. [Google Scholar] [CrossRef]
- Zhang, T.; Lang, Q.; Yang, D.; Li, L.; Zeng, L.; Zheng, C.; Li, T.; Wei, M.; Liu, A. Simultaneous voltammetric determination of nitrophenol isomers at ordered mesoporous carbon modified electrode. Electrochimica Acta 2013, 106, 127–134. [Google Scholar] [CrossRef]
- Huang, Y.; Bai, S.; Huang, J.; Ma, Y.; Zeng, Q.; Wang, M.; Wang, L. Simultaneous detection of nitrophenol isomers using an easy-to-fabricate thiophene-based microporous polymer film modified electrode. Microchem. J. 2019, 153, 104465. [Google Scholar] [CrossRef]
- Yao, C.; Sun, H.; Fu, H.-F.; Tan, Z.-C. Sensitive simultaneous determination of nitrophenol isomers at poly(p-aminobenzene sulfonic acid) film modified graphite electrode. Electrochimica Acta 2015, 156, 163–170. [Google Scholar] [CrossRef]




| Modified Electrode | Technique | Analytical Parameter | 3-NP | 4-NP | Ref |
|---|---|---|---|---|---|
| APGE/PTH | DPV | Linear range (nmol/L) | 20–230 | 15–280 | Proposed work |
| LOD (nmol/L) | 4.5 | 4 | |||
| Polyfurfural/GCE | DPV | Linear range (mol/L) | 750–100,000 | 750–100,000 | [1] |
| LOD (nmol/L) | 50 | 40 | |||
| PSF/GCE | DPV | Linear range (nmol/L) | 200–100,000 | 100–120,000 | [17] |
| LOD (nmol/L) | |||||
| (Fe3O4-Pt NPs)/GCE | DPV | Linear range (nmol/L) | 100–1500 | 100–1500 | [15] |
| LOD (nmol/L) | 45.3 | 48.2 | |||
| CalCOP-MPC/GCE | DPV | Linear range (nmol/L) | 1000–400,000 | 1000–400,000 | [34] |
| LOD (nmol/L) | 122 | 212 | |||
| SBCD-rGO/GCE | DPV | Linear range (nmol/L) | 100–800,000 | 100–800,000 | [17] |
| LOD (nmol/L) | 30 | 50 | |||
| OMCs/GCE | DPV | Linear range (nmol/L) | 1000–100,000 | 2000–90,000 | [62] |
| LOD (nmol/L) | 60 | 100 | |||
| PTTB/GCE | DPV | Linear range (nmol/L) | 300–12,500 | 300–15,000 | [63] |
| LOD (nmol/L) | 50 | 50 | |||
| Poly(p-ABSA)/GCE | DPV | Linear range (nmol/L) | 3000–800,000 | 3000–800,000 | [64] |
| LOD (nmol/L) | 500 | 300 |
| Samples | Initial Conc. | Added (nmol/L) | Observed (nmol/L) | Recovery (%) | RSD (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| 3-NP | 4-NP | 3-NP | 4-NP | 3-NP | 4-NP | 3-NP | 4-NP | ||
| Tap water | NS * | 30.0 | 20.0 | 30.07 (±0.05) | 19.89 (±0.03) | 100.23 | 99.45 | 2.19 | 1.98 |
| 50.0 | 50.0 | 49.78 (±0.08) | 50.07 (±0.01) | 99.56 | 100.14 | 2.32 | 2.14 | ||
| 100.0 | 100.0 | 99.85 (±0.03) | 100.08 (±0.07) | 99.85 | 100.08 | 2.18 | 2.21 | ||
| Lake water | NS * | 30.0 | 20.0 | 30.09 (±0.08) | 20.07 (±0.01) | 100.30 | 100.35 | 1.87 | 1.56 |
| 50.0 | 50.0 | 51.01 (±0.05) | 49.08 (±0.08) | 102.02 | 98.16 | 1.98 | 1.23 | ||
| 100.0 | 100.0 | 99.03 (±0.01) | 100.10 (±0.03) | 99.03 | 100.10 | 3.23 | 3.18 | ||
| River water | NS * | 30.0 | 20.0 | 31.03 (±0.09) | 20.08 (±0.03) | 103.43 | 100.40 | 1.89 | 1.34 |
| 50.0 | 50.0 | 49.96 (±0.05) | 50.65 (±0.08) | 99.92 | 101.30 | 2.33 | 2.12 | ||
| 100.0 | 100.0 | 99.82 (±0.02) | 100.08 (±0.07) | 99.82 | 100.08 | 3.16 | 3.26 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sree, V.G.; Sohn, J.I.; Im, H. Pre-Anodized Graphite Pencil Electrode Coated with a Poly(Thionine) Film for Simultaneous Sensing of 3-Nitrophenol and 4-Nitrophenol in Environmental Water Samples. Sensors 2022, 22, 1151. https://doi.org/10.3390/s22031151
Sree VG, Sohn JI, Im H. Pre-Anodized Graphite Pencil Electrode Coated with a Poly(Thionine) Film for Simultaneous Sensing of 3-Nitrophenol and 4-Nitrophenol in Environmental Water Samples. Sensors. 2022; 22(3):1151. https://doi.org/10.3390/s22031151
Chicago/Turabian StyleSree, Vijaya Gopalan, Jung Inn Sohn, and Hyunsik Im. 2022. "Pre-Anodized Graphite Pencil Electrode Coated with a Poly(Thionine) Film for Simultaneous Sensing of 3-Nitrophenol and 4-Nitrophenol in Environmental Water Samples" Sensors 22, no. 3: 1151. https://doi.org/10.3390/s22031151
APA StyleSree, V. G., Sohn, J. I., & Im, H. (2022). Pre-Anodized Graphite Pencil Electrode Coated with a Poly(Thionine) Film for Simultaneous Sensing of 3-Nitrophenol and 4-Nitrophenol in Environmental Water Samples. Sensors, 22(3), 1151. https://doi.org/10.3390/s22031151






