Recent Trends in Biosensors for Environmental Quality Monitoring
Abstract
:1. Introduction
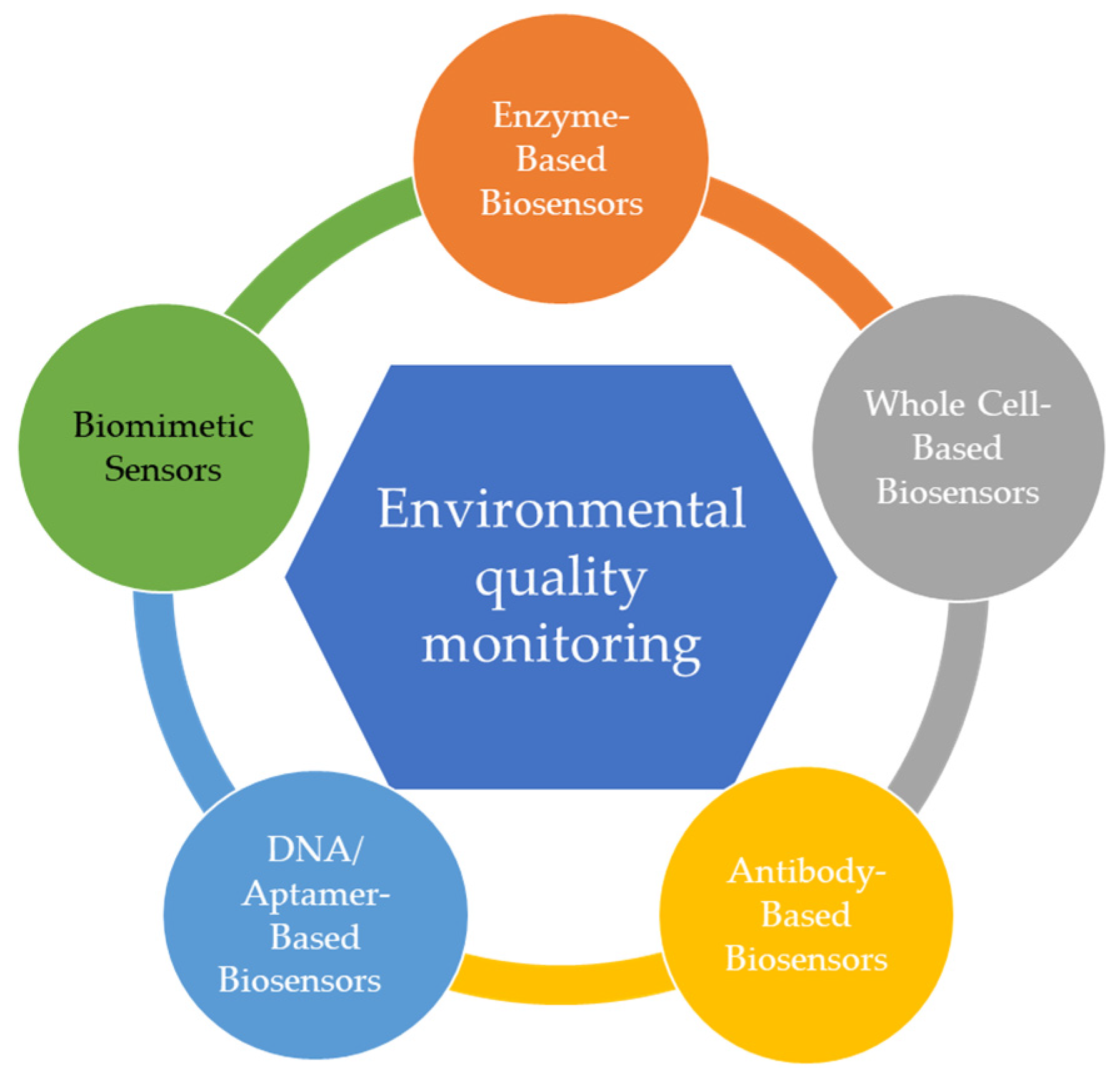
2. Sensors Used for Environmental Monitoring Overview
2.1. Enzyme-Based Biosensors
2.2. Whole Cell-Based Biosensors (Microbial)
2.3. Antibody-Based Biosensors
2.4. DNA/Aptamer-Based Biosensors
2.4.1. Aptamer-Based Biosensors
2.4.2. DNA-Based Biosensors
3. Biomimetic Sensors
4. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiong, J.; Sun, Z.; Yu, J.H.; Liu, H.; Wang, X.D. Thermal self-regulatory smart biosensor based on horseradish peroxidase-immobilized phase-change microcapsules for enhancing detection of hazardous substances. Chem. Eng. J. 2022, 430, 132982. [Google Scholar] [CrossRef]
- Justino, C.I.L.; Duarte, A.C.; Rocha-Santos, T.A.P. Recent Progress in Biosensors for Environmental Monitoring: A Review. Sensors 2017, 17, 2918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, F.; Zhang, D.; Yang, L.; Li, L.; Lu, Y.; Wang, J.; Fan, Y.; Zhu, Y.; Li, X.; Zhang, Y. Effects of antibiotics and heavy metals on denitrification in shallow eutrophic lakes. Chemosphere 2021, 291, 132948. [Google Scholar] [CrossRef]
- Li, L.; He, J.; Gan, Z.; Yang, P. Occurrence and fate of antibiotics and heavy metals in sewage treatment plants and risk assessment of reclaimed water in Chengdu, China. Chemosphere 2021, 272, 129730. [Google Scholar] [CrossRef]
- Wu, W.; Qu, S.; Nel, W.; Ji, J. Tracing and quantifying the sources of heavy metals in the upper and middle reaches of the Pearl River Basin: New insights from Sr-Nd-Pb multi-isotopic systems. Chemosphere 2022, 288, 132630. [Google Scholar] [CrossRef]
- Brunnbauer, L.; Gonzalez, J.; Lohninger, H.; Bode, J.; Vogt, C.; Nelhiebel, M.; Larisegger, S.; Limbeck, A. Strategies for trace metal quantification in polymer samples with an unknown matrix using Laser-Induced Breakdown Spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2021, 183, 106272. [Google Scholar] [CrossRef]
- Trapananti, A.; Eisenmann, T.; Giuli, G.; Mueller, F.; Moretti, A.; Passerini, S.; Bresser, D. Isovalent vs. aliovalent transition metal doping of zinc oxide lithium-ion battery anodes—In-depth investigation by ex situ and operando X-ray absorption spectroscopy. Mater. Today Chem. 2021, 20, 100478. [Google Scholar] [CrossRef]
- Dhote, S.S.; Deshmukh, L.; Paliwal, L. Miceller chromatographic method for the separation of heavy metal ions and spectrophotometric estimation of UO22+ on bismuth silicate layer. Int. J. Chem. Anal. Sci. 2013, 4, 85–90. [Google Scholar] [CrossRef]
- Murzyn, C.M.; Allen, D.J.; Baca, A.N.; Ching, M.L.; Marinis, R.T. Tunable Infrared Laser Absorption Spectroscopy of Aluminum Monoxide A2Πi−X2Σ+. J. Quant. Spectrosc. Radiat. Transf. 2021, 279, 108029. [Google Scholar] [CrossRef]
- Nigam, V.K.; Shukla, P. Enzyme Based Biosensors for Detection of Environmental Pollutants—A Review. J. Microbiol. Biotechnol. 2015, 25, 1773–1781. [Google Scholar] [CrossRef] [PubMed]
- Khanam, Z.; Gupta, S.; Verma, A. Endophytic fungi-based biosensors for environmental contaminants—A perspective. S. Afr. J. Bot. 2020, 134, 401–406. [Google Scholar] [CrossRef]
- Kumar, T.; Naik, S.; Jujjavarappu, S.E. A critical review on early-warning electrochemical system on microbial fuel cell-based biosensor for on-site water quality monitoring. Chemosphere 2021, 291, 133098. [Google Scholar] [CrossRef]
- Ivask, A.; Green, T.; Polyak, B.; Mor, A.; Kahru, A.; Virta, M.; Marks, R. Fibre-optic bacterial biosensors and their application for the analysis of bioavailable Hg and As in soils and sediments from Aznalcollar mining area in Spain. Biosens. Bioelectron. 2007, 22, 1396–1402. [Google Scholar] [CrossRef]
- Rathnayake, I.V.N.; Megharaj, M.; Naidu, R. Green fluorescent protein based whole cell bacterial biosensor for the detection of bioavailable heavy metals in soil environment. Environ. Technol. Innov. 2021, 23, 101785. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.N. Microbial-derived biosensors for monitoring environmental contaminants: Recent advances and future outlook. Process Saf. Environ. Prot. 2019, 124, 8–17. [Google Scholar] [CrossRef]
- Kumar, H.; Kumari, N.; Sharma, R. Nanocomposites (conducting polymer and nanoparticles) based electrochemical biosensor for the detection of environment pollutant: Its issues and challenges. Environ. Impact Assess. Rev. 2020, 85. [Google Scholar] [CrossRef]
- Tschmelak, J.; Proll, G.; Riedt, J.; Kaiser, J.; Kraemmer, P.; Barzaga, L.; Wilkinson, J.S.; Hua, P.; Hole, J.P.; Nudd, R.; et al. Biosensors for unattended, cost-effective and continuous monitoring of environmental pollution: Automated Water Analyser Computer Supported System (AWACSS) and River Analyser (RIANA). J. Environ. Anal. Chem. 2005, 85, 837–852. [Google Scholar] [CrossRef]
- Hashem, A.; Hossain, M.A.M.; Marlinda, A.R.; Mamun, M.A.; Simarani, K.; Johan, M.R. Nanomaterials based electrochemical nucleic acid biosensors for environmental monitoring: A review. Appl. Surf. Sci. Adv. 2021, 4, 100064. [Google Scholar] [CrossRef]
- Chung, T.H.; Meshref, M.N.A.; Dhar, B.R. A review and roadmap for developing microbial electrochemical cell-based biosensors for recalcitrant environmental contaminants, emphasis on aromatic compounds. Chem. Eng. J. 2021, 424, 130245. [Google Scholar] [CrossRef]
- Turner, A.P.F. Biosensors: Sense and sensibility. Chem. Soc. Rev. 2013, 42, 3184–3196. [Google Scholar] [CrossRef] [Green Version]
- Jain, U.; Saxena, K.; Hooda, V.; Balayan, S.; Singh, A.P.; Tikadar, M.; Chauhan, N. Emerging vistas on pesticides detection based on electrochemical biosensors—An update. Food Chem. 2022, 371. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, J.S. Optical biosensors: An exhaustive and comprehensive review. Analyst 2020, 145, 1605–1628. [Google Scholar] [CrossRef]
- Sethi, R.S. Transducer aspects of biosensors. GEC J. Res. 1991, 9, 81–96. [Google Scholar] [CrossRef]
- Mehrotra, P. Biosensors and their applications—A review. J. Oral Biol. Craniofac Res. 2016, 6, 153–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbasian, F.; Ghafar-Zadeh, E.; Magierowski, S. Microbiological sensing technologies: A review. Bioengineering 2018, 5, 20. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.Y.; Liu, J.C.; Yang, Z.C.; Wilkinson, J.S.; Zhou, X.H. Optical biosensors based on refractometric sensing schemes: A review. Biosens. Bioelectron. 2019, 144, 111693. [Google Scholar] [CrossRef] [PubMed]
- Badihi-Mossberg, M.; Buchner, V.; Rishpon, J. Electrochemical Biosensors for pollutants in the environment. Electroanalysis 2007, 19, 2015–2028. [Google Scholar] [CrossRef]
- Asif, S.; Chaudhari, A.; Gireesh-Babu, P.; Chaudhuri, P.R.; Sen, R. Immobilization of fluorescent whole cell biosensors for the improved detection of heavy metal pollutants present in aquatic environment. In Proceedings of the International Conference on Advances in Bioprocess Engineering and Technology (ICABET), Kolkata, India, 20–22 January 2016; pp. 3492–3497. [Google Scholar]
- Huang, H.P.; Chen, Y.A.; Chen, Z.Z.; Chen, J.L.; Hu, Y.M.; Zhu, J.J. Electrochemical sensor based on Ce-MOF/carbon nanotube composite for the simultaneous discrimination of hydroquinone and catechol. J. Hazard. Mater. 2021, 416, 125895. [Google Scholar] [CrossRef]
- Lubineau, G.; Rahaman, A. A review of strategies for improving the degradation properties of laminated continuous-fiber/epoxy composites with carbon-based nanoreinforcements. Carbon 2012, 50, 2377–2395. [Google Scholar] [CrossRef]
- Martins, P.; Lanceros-Mendez, S. Polymer-Based Magnetoelectric Materials. Adv. Funct. Mater. 2013, 23, 3371–3385. [Google Scholar] [CrossRef]
- Nikbakt, S.; Kamarian, S.; Shakeri, M. A review on optimization of composite structures Part I: Laminated composites. Compos. Struct. 2018, 195, 158–185. [Google Scholar] [CrossRef]
- Porras, A.; Maranon, A. Development and characterization of a laminate composite material from polylactic acid (PLA) and woven bamboo fabric. Compos. Part B-Eng. 2012, 43, 2782–2788. [Google Scholar] [CrossRef]
- Sahay, R.; Kumar, P.S.; Sridhar, R.; Sundaramurthy, J.; Venugopal, J.; Mhaisalkar, S.G.; Ramakrishna, S. Electrospun composite nanofibers and their multifaceted applications. J. Mater. Chem. 2012, 22, 12953–12971. [Google Scholar] [CrossRef]
- Tornabene, F.; Viola, E.; Fantuzzi, N. General higher-order equivalent single layer theory for free vibrations of doubly-curved laminated composite shells and panels. Compos. Struct. 2013, 104, 94–117. [Google Scholar] [CrossRef]
- Treviso, A.; Van Genechten, B.; Mundo, D.; Tournour, M. Damping in composite materials: Properties and models. Compos. Ptart B-Eng. 2015, 78, 144–152. [Google Scholar] [CrossRef]
- Tang, L.; Dang, J.; He, M.K.; Li, J.Y.; Kong, J.; Tang, Y.S.; Gu, J.W. Preparation and properties of cyanate-based wave-transparent laminated composites reinforced by dopamine/POSS functionalized Kevlar cloth. Compos. Sci. Technol. 2019, 169, 120–126. [Google Scholar] [CrossRef]
- Tang, L.; He, M.K.; Na, X.Y.; Guan, X.F.; Zhang, R.H.; Zhang, J.L.; Gu, J.W. Functionalized glass fibers cloth/spherical BN fillers/epoxy laminated composites with excellent thermal conductivities and electrical insulation properties. Compos. Commun. 2019, 16, 5–10. [Google Scholar] [CrossRef]
- Tang, Y.S.; Dong, W.C.; Tang, L.; Zhang, Y.K.; Kong, J.; Gu, J.W. Fabrication and investigations on the polydopamine/KH-560 functionalized PBO fibers/cyanate ester wave-transparent composites. Compos. Commun. 2018, 8, 36–41. [Google Scholar] [CrossRef]
- Wang, G.L.; Yu, D.M.; Kelkar, A.D.; Zhang, L.F. Electrospun nanofiber: Emerging reinforcing filler in polymer matrix composite materials. Prog. Polym. Sci. 2017, 75, 73–107. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Q.; Shao, S.; Misra, A. Strength and plasticity of nanolaminated materials. Mater. Res. Lett. 2017, 5, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Yahaya, R.; Sapuan, S.M.; Jawaid, M.; Leman, Z.; Zainudin, E.S. Effect of layering sequence and chemical treatment on the mechanical properties of woven kenaf-aramid hybrid laminated composites. Mater. Des. 2015, 67, 173–179. [Google Scholar] [CrossRef]
- Yoon, J.; Shin, M.; Lee, T.; Choi, J.W. Highly sensitive biosensors based on biomolecules and functional nanomaterials depending on the types of nanomaterials: A perspective review. Materials 2020, 13, 299. [Google Scholar] [CrossRef] [Green Version]
- Kurbanoglu, S.; Ozkan, S.A.; Merkoçi, A. Nanomaterials-based enzyme electrochemical biosensors operating through inhibition for biosensing applications. Biosens. Bioelectron. 2017, 89, 886–898. [Google Scholar] [CrossRef]
- Cavalcante, F.T.T.; de Falcão, I.R.A.; da Souza, J.E.S.; Rocha, T.G.; de Sousa, I.G.; Cavalcante, A.L.G.; de Oliveira, A.L.B.; de Sousa, M.C.M.; dos Santos, J.C.S. Designing of Nanomaterials-Based Enzymatic Biosensors: Synthesis, Properties, and Applications. Electrochem 2021, 2, 149–184. [Google Scholar] [CrossRef]
- Gaviria-Arroyave, M.I.; Cano, J.B.; Peñuela, G.A. Nanomaterial-based fluorescent biosensors for monitoring environmental pollutants: A critical review. Talanta Open 2020, 2, 100006. [Google Scholar] [CrossRef]
- Yang, N.; Chen, X.; Ren, T.; Zhang, P.; Yang, D. Carbon nanotube based biosensors. Sens. Actuators B Chem. 2015, 207, 690–715. [Google Scholar] [CrossRef]
- Rahimi, P.; Joseph, Y. Enzyme-based biosensors for choline analysis: A review. TrAC Trends Anal. Chem. 2019, 110, 367–374. [Google Scholar] [CrossRef]
- Asal, M.; Ozen, O.; Sahinler, M.; Polatoglu, I. Recent Developments in Enzyme, DNA and Immuno-Based Biosensors. Sensors 2018, 18, 1924. [Google Scholar] [CrossRef] [Green Version]
- Economou, A.; Karapetis, S.K.; Nikoleli, G.-P.; Nikolelis, D.P.; Bratakou, S.; Varzakas, T.H. Enzyme-Based Sensors. In Advances in Food Diagnostics, 2nd ed.; Toldrá, F., Nollet, L.M.L., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2017; pp. 231–250. [Google Scholar]
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Lee, S.H.; Lee, U.J.; Fermin, C.D.; Kim, M. Immobilized Enzymes in Biosensor Applications. Materials 2019, 12, 121. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, H.H.; Kim, M. An Overview of Techniques in Enzyme Immobilization. Appl. Sci. Converg. Technol. 2017, 26, 157–163. [Google Scholar] [CrossRef]
- Amine, A.; Mohammadi, H.; Bourais, I.; Palleschi, G. Enzyme inhibition-based biosensors for food safety and environmental monitoring. Biosens. Bioelectron. 2006, 21, 1405–1423. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.M.; Tang, L.; Shen, G.L.; Huang, G.H.; Niu, C.G. Determination of trace chromium(VI) by an inhibition-based enzyme biosensor incorporating an electropolymerized aniline membrane and ferrocene as electron transfer mediator. J. Environ. Anal. Chem. 2004, 84, 761–774. [Google Scholar] [CrossRef]
- Joshi, K.A.; Tang, J.; Haddon, R.; Wang, J.; Chen, W.; Mulchandani, A. A disposable biosensor for organophosphorus nerve agents based on carbon nanotubes modified thick film strip electrode. Electroanalysis 2005, 17, 54–58. [Google Scholar] [CrossRef]
- Dzyadevych, S.V.; Soldatkin, A.P.; Arkhypova, V.N.; El’skaya, A.V.; Chovelon, J.M.; Georgiou, C.A.; Martelet, C.; Jaffrezic-Renault, N. Early-warning electrochemical biosensor system for environmental monitoring based on enzyme inhibition. Sens. Actuators B Chem. 2005, 105, 81–87. [Google Scholar] [CrossRef]
- Tortolini, C.; Bollella, P.; Antiochia, R.; Favero, G.; Mazzei, F. Inhibition-based biosensor for atrazine detection. Sens. Actuators B Chem. 2016, 224, 552–558. [Google Scholar] [CrossRef]
- Guan, Y.; Liu, L.; Chen, C.; Kang, X.; Xie, Q. Effective immobilization of tyrosinase via enzyme catalytic polymerization of l-DOPA for highly sensitive phenol and atrazine sensing. Talanta 2016, 160, 125–132. [Google Scholar] [CrossRef]
- Tang, S.; Ma, W.Y.; Xie, G.Z.; Su, Y.J.; Jiang, Y.D. Acetylcholinesterase-reduced graphene oxide hybrid films for organophosphorus neurotoxin sensing via quartz crystal microbalance. Chem. Phys. Lett. 2016, 660, 199–204. [Google Scholar] [CrossRef]
- Devic, E.; Li, D.H.; Dauta, A.; Henriksen, P.; Codd, G.A.; Marty, J.L.; Fournier, D. Detection of anatoxin-a(s) in environmental samples of cyanobacteria by using a biosensor with engineered acetylcholinesterases. Appl. Environ. Microbiol. 2002, 68, 4102–4106. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.-W.; Kim, Y.-K.; Song, S.-Y.; Lee, I.-h.; Lee, W.H. Optical biosensor consisting of glutathione-S-transferase for detection of captan. Biosens. Bioelectron. 2003, 18, 1461–1466. [Google Scholar] [CrossRef]
- Polatoglu, İ.; Kızılkaya, M.; Eren, Ü. Development of a gold nanoparticle based electrochemical biosensor for detection of phenolic compounds. J. Turk. Chem. Soc. Sect. B Chem. Eng. 2016, 1, 115–126. [Google Scholar]
- Fang, Y.; Umasankar, Y.; Ramasamy, R.P. A novel bi-enzyme electrochemical biosensor for selective and sensitive determination of methyl salicylate. Biosens. Bioelectron. 2016, 81, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.J.; Voyvodic, P.L.; Zuniga, A.; Bonnet, J. Microbially derived biosensors for diagnosis, monitoring and epidemiology. Microb. Biotechnol. 2017, 10, 1031–1035. [Google Scholar] [CrossRef] [Green Version]
- Lei, Y.; Chen, W.; Mulchandani, A. Microbial biosensors. Anal. Chim. Acta 2006, 568, 200–210. [Google Scholar] [CrossRef]
- D’Souza, S.F. Microbial biosensors. Biosens. Bioelectron. 2001, 16, 337–353. [Google Scholar] [CrossRef]
- Chung, T.H.; Dhar, B.R. Paper-based platforms for microbial electrochemical cell-based biosensors: A review. Biosens. Bioelectron. 2021, 192, 113485. [Google Scholar] [CrossRef]
- Moraskie, M.; Roshid, M.H.O.; O’Connor, G.; Dikici, E.; Zingg, J.-M.; Deo, S.; Daunert, S. Microbial whole-cell biosensors: Current applications, challenges, and future perspectives. Biosens. Bioelectron. 2021, 191, 113359. [Google Scholar] [CrossRef]
- Lim, J.W.; Ha, D.; Lee, J.; Lee, S.K.; Kim, T. Review of Micro/Nanotechnologies for Microbial Biosensors. Front. Bioeng. Biotechnol. 2015, 3, 61. [Google Scholar] [CrossRef] [Green Version]
- Gupta, N.; Renugopalakrishnan, V.; Liepmann, D.; Paulmurugan, R.; Malhotra, B.D. Cell-based biosensors: Recent trends, challenges and future perspectives. Biosens. Bioelectron. 2019, 141, 111435. [Google Scholar] [CrossRef]
- Do, M.H.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Pandey, A.; Sharma, P.; Varjani, S.; Nguyen, T.A.H.; Hoang, N.B. A dual chamber microbial fuel cell based biosensor for monitoring copper and arsenic in municipal wastewater. Sci. Total Environ. 2021, 811, 152261. [Google Scholar] [CrossRef]
- Webster, D.P.; TerAvest, M.A.; Doud, D.F.R.; Chakravorty, A.; Holmes, E.C.; Radens, C.M.; Sureka, S.; Gralnick, J.A.; Angenent, L.T. An arsenic-specific biosensor with genetically engineered Shewanella oneidensis in a bioelectrochemical system. Biosens. Bioelectron. 2014, 62, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.Y.; Fang, D.Y.; Yu, Y.; Wu, L.Z.; Wang, Y.; Zhi, J.F. A double-mediator based whole cell electrochemical biosensor for acute biotoxicity assessment of wastewater. Talanta 2017, 167, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lim, J.W.; Jeong, H.; Lee, S.-J.; Lee, D.-W.; Kim, T.; Lee, S.J. Development of a highly specific and sensitive cadmium and lead microbial biosensor using synthetic CadC-T7 genetic circuitry. Biosens. Bioelectron. 2016, 79, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Lim, J.-W.; Kim, T. Reusable and storable whole-cell microbial biosensors with a microchemostat platform for in situ on-demand heavy metal detection. Sens. Actuators B Chem. 2018, 264, 372–381. [Google Scholar] [CrossRef]
- Vopálenská, I.; Váchová, L.; Palková, Z. New biosensor for detection of copper ions in water based on immobilized genetically modified yeast cells. Biosens. Bioelectron. 2015, 72, 160–167. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, T.; Liang, B.; Han, D.; Zeng, L.; Zheng, C.; Li, T.; Wei, M.; Liu, A. Sensitive electrochemical microbial biosensor for p-nitrophenylorganophosphates based on electrode modified with cell surface-displayed organophosphorus hydrolase and ordered mesopore carbons. Biosens. Bioelectron. 2014, 60, 137–142. [Google Scholar] [CrossRef]
- Tucci, M.; Grattieri, M.; Schievano, A.; Cristiani, P.; Minteer, S.D. Microbial amperometric biosensor for online herbicide detection: Photocurrent inhibition of Anabaena variabilis. Electrochim. Acta 2019, 302, 102–108. [Google Scholar] [CrossRef]
- Tsopela, A.; Laborde, A.; Salvagnac, L.; Ventalon, V.; Bedel-Pereira, E.; Seguy, I.; Temple-Boyer, P.; Juneau, P.; Izquierdo, R.; Launay, J. Development of a lab-on-chip electrochemical biosensor for water quality analysis based on microalgal photosynthesis. Biosens. Bioelectron. 2016, 79, 568–573. [Google Scholar] [CrossRef] [Green Version]
- Haigh-Flórez, D.; de la Hera, C.; Costas, E.; Orellana, G. Microalgae dual-head biosensors for selective detection of herbicides with fiber-optic luminescent O2 transduction. Biosens. Bioelectron. 2014, 54, 484–491. [Google Scholar] [CrossRef]
- Sharma, S.; Byrne, H.; O’Kennedy, R.J. Antibodies and Antibody-Derived Analytical Biosensors. In Biosensor Technologies for Detection of Biomolecules; Estrela, P., Ed.; Essays in Biochemistry; Portland Press: South Portland, ME, USA, 2016; Volume 60, pp. 9–18. [Google Scholar]
- Felix, F.S.; Angnes, L. Electrochemical immunosensors—A powerful tool for analytical applications. Biosens. Bioelectron. 2018, 102, 470–478. [Google Scholar] [CrossRef]
- Omidfar, K.; Khorsand, F.; Azizi, M.D. New analytical applications of gold nanoparticles as label in antibody based sensors. Biosens. Bioelectron. 2013, 43, 336–347. [Google Scholar] [CrossRef]
- Cristea, C.; Florea, A.; Tertiș, M.; Săndulescu, R. Immunosensors. In Biosensors-Micro and Nanoscale Applications; IntechOpen: London, UK, 2015; pp. 165–202. [Google Scholar]
- Fang, L.; Liao, X.; Jia, B.; Shi, L.; Kang, L.; Zhou, L.; Kong, W. Recent progress in immunosensors for pesticides. Biosens. Bioelectron. 2020, 164, 112255. [Google Scholar] [CrossRef]
- Farka, Z.; Juriik, T.; Kovaar, D.; Trnkova, L.; Sklaadal, P. Nanoparticle-Based Immunochemical Biosensors and Assays: Recent Advances and Challenges. Chem. Rev. 2017, 117, 9973–10042. [Google Scholar] [CrossRef]
- Shao, Y.N.; Zhou, H.; Wu, Q.P.; Xiong, Y.H.; Wang, J.; Ding, Y. Recent advances in enzyme-enhanced immunosensors. Biotechnol. Adv. 2021, 53, 107867. [Google Scholar] [CrossRef]
- Rhouati, A.; Catanante, G.; Nunes, G.; Hayat, A.; Marty, J.-L. Label-Free Aptasensors for the Detection of Mycotoxins. Sensors 2016, 16, 2178. [Google Scholar] [CrossRef]
- Jia, M.X.; Liao, X.F.; Fang, L.; Jia, B.Y.; Liu, M.; Li, D.H.; Zhou, L.D.; Kong, W.J. Recent advances on immunosensors for mycotoxins in foods and other commodities. TrAC—Trends Anal. Chem. 2021, 136, 116193. [Google Scholar] [CrossRef]
- Rogers, K.R. Recent advances in biosensor techniques for environmental monitoring. Anal. Chim. Acta 2006, 568, 222–231. [Google Scholar] [CrossRef]
- Jia, H.Y.; Guo, Y.M.; Sun, X.; Wang, X.Y. An Electrochemical Immunosensor Based on Microfluidic Chip for Detection of Chlorpyrifos. Int. J. Electrochem. Sci. 2015, 10, 8750–8758. [Google Scholar]
- Zhang, Z.; Dong, S.; Ge, D.; Zhu, N.; Wang, K.; Zhu, G.; Xu, W.; Xu, H. An ultrasensitive competitive immunosensor using silica nanoparticles as an enzyme carrier for simultaneous impedimetric detection of tetrabromobisphenol A bis(2-hydroxyethyl) ether and tetrabromobisphenol A mono(hydroxyethyl) ether. Biosens. Bioelectron. 2018, 105, 77–80. [Google Scholar] [CrossRef]
- Belkhamssa, N.; Justino, C.I.L.; Santos, P.S.M.; Cardoso, S.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T.; Ksibi, M. Label-free disposable immunosensor for detection of atrazine. Talanta 2016, 146, 430–434. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Chen, M.; Li, H.; Yan, F.; Pang, P.; Wang, H.; Wu, Z.; Yang, W. A molybdenum disulfide/gold nanorod composite-based electrochemical immunosensor for sensitive and quantitative detection of microcystin-LR in environmental samples. Sens. Actuators B Chem. 2017, 244, 606–615. [Google Scholar] [CrossRef]
- McNamee, S.E.; Elliott, C.T.; Delahaut, P.; Campbell, K. Multiplex biotoxin surface plasmon resonance method for marine biotoxins in algal and seawater samples. Environ. Sci. Pollut. Res. 2013, 20, 6794–6807. [Google Scholar] [CrossRef] [PubMed]
- Antunes, J.; Justino, C.; da Costa, J.P.; Cardoso, S.; Duarte, A.C.; Rocha-Santos, T. Graphene immunosensors for okadaic acid detection in seawater. Microchem. J. 2018, 138, 465–471. [Google Scholar] [CrossRef]
- Enrico, D.L.; Manera, M.G.; Montagna, G.; Cimaglia, F.; Chiesa, M.; Poltronieri, P.; Santino, A.; Rella, R. SPR based immunosensor for detection of Legionella pneumophila in water samples. Opt. Commun. 2013, 294, 420–426. [Google Scholar] [CrossRef]
- Huang, L.; Wang, X.; Liu, S.; Gao, Z.-X. Application of Aptamer-based Biosensor in Bisphenol A Detection. Chin. J. Anal. Chem. 2021, 49, 172–183. [Google Scholar] [CrossRef]
- Wang, T.; Chen, C.Y.; Larcher, L.M.; Barrero, R.A.; Veedu, R.N. Three decades of nucleic acid aptamer technologies: Lessons learned, progress and opportunities on aptamer development. Biotechnol. Adv. 2019, 37, 28–50. [Google Scholar] [CrossRef] [PubMed]
- Monosik, R.; Stredanský, M.; Sturdik, E. Biosensors-classification, characterization and new trends. Acta Chim. Slovaca 2012, 5, 109. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Tao, Z.-H.; Xu, L.; Liu, Y.-Q. Research and Development of Functionalized Aptamer based Biosensor. Chin. J. Anal. Chem. 2014, 42, 298–304. [Google Scholar] [CrossRef]
- Muhammad, M.; Huang, Q. A review of aptamer-based SERS biosensors: Design strategies and applications. Talanta 2021, 227, 122188. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Kwon, Y.S.; Gu, M.B. Aptamer-based environmental biosensors for small molecule contaminants. Curr. Opin. Biotechnol. 2017, 45, 15–23. [Google Scholar] [CrossRef]
- McConnell, E.M.; Nguyen, J.; Li, Y.F. Aptamer-Based Biosensors for Environmental Monitoring. Front. Chem. 2020, 8, 8. [Google Scholar] [CrossRef]
- Xie, M.J.; Zhao, F.G.; Zhang, Y.P.; Xiong, Y.; Han, S.Y. Recent advances in aptamer-based optical and electrochemical biosensors for detection of pesticides and veterinary drugs. Food Control 2022, 131, 108399. [Google Scholar] [CrossRef]
- Mishra, G.K.; Sharma, V.; Mishra, R.K. Electrochemical Aptasensors for Food and Environmental Safeguarding: A Review. Biosensors 2018, 8, 28. [Google Scholar] [CrossRef] [Green Version]
- Komarova, N.; Kuznetsov, A. Inside the Black Box: What Makes SELEX Better? Molecules 2019, 24, 3598. [Google Scholar] [CrossRef] [Green Version]
- Lyu, C.; Khan, I.M.; Wang, Z.P. Capture-SELEX for aptamer selection: A short review. Talanta 2021, 229, 122274. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, T.T.; Wu, Z.Y.; Yu, R.Q. Internal standard-based SERS aptasensor for ultrasensitive quantitative detection of Ag+ ion. Talanta 2018, 185, 30–36. [Google Scholar] [CrossRef]
- Wu, Y.G.; Liu, L.; Zhan, S.S.; Wang, F.Z.; Zhou, P. Ultrasensitive aptamer biosensor for arsenic(III) detection in aqueous solution based on surfactant-induced aggregation of gold nanoparticles. Analyst 2012, 137, 4171–4178. [Google Scholar] [CrossRef]
- Siddiqui, M.F.; Khan, Z.A.; Jeon, H.; Park, S. SPE based soil processing and aptasensor integrated detection system for rapid on site screening of arsenic contamination in soil. Ecotoxicol. Environ. Saf. 2020, 196, 110559. [Google Scholar] [CrossRef]
- Zhou, B.; Yang, X.Y.; Wang, Y.S.; Yi, J.C.; Zeng, Z.; Zhang, H.; Chen, Y.T.; Hu, X.J.; Suo, Q.L. Label-free fluorescent aptasensor of Cd2+ detection based on the conformational switching of aptamer probe and SYBR green I. Microchem. J. 2019, 144, 377–382. [Google Scholar] [CrossRef]
- Tian, C.; Zhao, L.; Zhu, J.; Zhang, S.S. Ultrasensitive detection of trace Hg2+ by SERS aptasensor based on dual recycling amplification in water environment. J. Hazard. Mater. 2021, 416, 126251. [Google Scholar] [CrossRef]
- Lu, Y.L.; Zhong, J.; Yao, G.H.; Huang, Q. A label-free SERS approach to quantitative and selective detection of mercury (II) based on DNA aptamer-modified SiO2@Au core/shell nanoparticles. Sens. Actuators B Chem. 2018, 258, 365–372. [Google Scholar] [CrossRef]
- Zhu, Y.; Zeng, G.M.; Zhang, Y.; Tang, L.; Chen, J.; Cheng, M.; Zhang, L.H.; He, L.; Guo, Y.; He, X.X.; et al. Highly sensitive electrochemical sensor using a MWCNTs/GNPs-modified electrode for lead (II) detection based on Pb2+-induced G-rich DNA conformation. Analyst 2014, 139, 5014–5020. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; He, B.; Jin, H.; Suo, Z. A fluorescent aptasensor for Pb2+ detection based on gold nanoflowers and RecJf exonuclease-induced signal amplification. Anal. Chim. Acta 2021, 1192, 339329. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.H.; Wang, H.B.; Fang, B.Y.; Tan, F.; Cao, Y.C.; Zhao, Y.D. Visual detection of trace lead ion based on aptamer and silver staining nano-metal composite. Colloids Surf. B 2018, 162, 415–419. [Google Scholar] [CrossRef]
- Qi, Y.Y.; Xiu, F.R.; Zheng, M.F.; Li, B.X. A simple and rapid chemiluminescence aptasensor for acetamiprid in contaminated samples: Sensitivity, selectivity and mechanism. Biosens. Bioelectron. 2016, 83, 243–249. [Google Scholar] [CrossRef]
- Bala, R.; Kumar, M.; Bansal, K.; Sharma, R.K.; Wangoo, N. Ultrasensitive aptamer biosensor for malathion detection based on cationic polymer and gold nanoparticles. Biosens. Bioelectron. 2016, 85, 445–449. [Google Scholar] [CrossRef]
- Nair, R.V.; Chandran, P.R.; Mohamed, A.P.; Pillai, S. Sulphur-doped graphene quantum dot based fluorescent turn-on aptasensor for selective and ultrasensitive detection of omethoate. Anal. Chim. Acta 2021, 1181, 338893. [Google Scholar] [CrossRef]
- Zhao, Y.W.; Wang, Y.; Yang, R.M.; Zhang, H.; Zhao, Y.F.; Miao, X.M.; Lu, L.H. A zero-background fluorescent aptasensor for ultrasensitive detection of pesticides based on magnetic three-dimensional DNA walker and poly(T)-templated copper nanoparticles. Sens. Actuators B Chem. 2021, 343, 130172. [Google Scholar] [CrossRef]
- Guo, Z.J.; Jiang, K.T.; Jiang, H.H.; Zhang, H.; Liu, Q.; You, T.Y. Photoelectrochemical aptasensor for sensitive detection of tetracycline in soil based on CdTe-BiOBr heterojunction: Improved photoactivity enabled by Z-scheme electron transfer pathway. J. Hazard. Mater. 2022, 424, 127498. [Google Scholar] [CrossRef]
- Kokkinos, C. Electrochemical DNA Biosensors Based on Labeling with Nanoparticles. Nanomaterials 2019, 9, 1361. [Google Scholar] [CrossRef] [Green Version]
- Saidur, M.R.; Aziz, A.R.A.; Basirun, W.J. Recent advances in DNA-based electrochemical biosensors for heavy metal ion detection: A review. Biosens. Bioelectron. 2017, 90, 125–139. [Google Scholar] [CrossRef]
- Bacchu, M.S.; Ali, M.R.; Das, S.; Akter, S.; Sakamoto, H.; Suye, S.I.; Rahman, M.M.; Campbell, K.; Khan, M.Z.H. A DNA functionalized advanced electrochemical biosensor for identification of the foodborne pathogen Salmonella enterica serovar Typhi in real samples. Anal. Chim. Acta 2021, 1192, 339332. [Google Scholar] [CrossRef]
- Hamed, K.-K.; Vahideh, R.; Ali, E.; Fatemeh, S. DNA Biosensors Techniques and Their Applications in Food Safety, Environmental Protection and Biomedical Research: A mini-review. J. Cell Dev. Biol. 2020, 3, 28–35. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, J.; Huang, Y.; Du, Y.C.; Zhang, Y.; Cui, Y.X.; Kong, D.M. Development of the DNA-based biosensors for high performance in detection of molecular biomarkers: More rapid, sensitive, and universal. Biosens. Bioelectron. 2022, 197, 113739. [Google Scholar] [CrossRef]
- Sun, C.T.; Ou, X.W.; Cheng, Y.; Zhai, T.Y.; Liu, B.F.; Lou, X.D.; Xia, F. Coordination-induced structural changes of DNA-based optical and electrochemical sensors for metal ions detection. Dalton Trans. 2019, 48, 5879–5891. [Google Scholar] [CrossRef]
- He, Z.Y.; Yin, H.L.; Chang, C.C.; Wang, G.Q.; Liang, X.G. Interfacing DNA with Gold Nanoparticles for Heavy Metal Detection. Biosensors 2020, 10, 167. [Google Scholar] [CrossRef]
- Ono, A.; Togashi, H. Highly selective oligonucleotide-based sensor for mercury(II) in aqueous solutions. Angew. Chem. Int. Ed. 2004, 43, 4300–4302. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Zhang, C.; Ma, R.; Du, X.; Dong, W.H.; Chen, Y.; Chen, Q. An ultra-sensitive Au nanoparticles functionalized DNA biosensor for electrochemical sensing of mercury ions. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 75, 175–181. [Google Scholar] [CrossRef]
- Ravikumar, A.; Panneerselvam, P.; Radhakrishnan, K.; Morad, N.; Anuradha, C.D.; Sivanesan, S. DNAzyme Based Amplified Biosensor on Ultrasensitive Fluorescence Detection of Pb (II) Ions from Aqueous System. J. Fluoresc. 2017, 27, 2101–2109. [Google Scholar] [CrossRef]
- Radhakrishnan, K.; Kumar, P.S. Target-receptive structural switching of ssDNA as selective and sensitive biosensor for subsequent detection of toxic Pb2+ and organophosphorus pesticide. Chemosphere 2022, 287, 132163. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Karimi, F.; Fu, L.; Sanati, A.L.; Alizadeh, M.; Karaman, C.; Orooji, Y. Cyanazine herbicide monitoring as a hazardous substance by a DNA nanostructure biosensor. J. Hazard. Mater. 2022, 423, 127058. [Google Scholar] [CrossRef] [PubMed]
- Peyman, H.; Roshanfekr, H.; Ansari, S. DNA-based electrochemical biosensor using chitosan-carbon nanotubes composite film for biodetection of Pirazon. Eurasian Chem. Commun. 2020, 2, 213–225. [Google Scholar] [CrossRef] [Green Version]
- Foudeh, A.M.; Trigui, H.; Mendis, N.; Faucher, S.P.; Veres, T.; Tabrizian, M. Rapid and specific SPRi detection of L-pneumophila in complex environmental water samples. Anal. Bioanal. Chem. 2015, 407, 5541–5545. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.R.; Bacchu, M.S.; Setu, M.A.A.; Akter, S.; Hasan, M.N.; Chowdhury, F.T.; Rahman, M.M.; Ahommed, M.S.; Khan, M.Z.H. Development of an advanced DNA biosensor for pathogenic Vibrio cholerae detection in real sample. Biosens. Bioelectron. 2021, 188, 113338. [Google Scholar] [CrossRef]
- Rochelet, M.; Vienney, F.; Solanas, S.; Membrilla, A.; Hartmann, A. An electrochemical DNA biosensor for the detection of CTX-M extended-spectrum β-lactamase-producing Escherichia coli in soil samples. J. Microbiol. Methods 2013, 92, 153–156. [Google Scholar] [CrossRef]
- Raju, V.M.; Bhavana, V.; Gayathri, G.K.; Suryan, S.; Reddy, R.; Reddy, N.; Ravikumar, C.R.; Santosh, M.S. A novel disposable electrochemical DNA biosensor for the rapid detection of Bacillus thuringiensis. Microchem. J. 2020, 159, 105434. [Google Scholar] [CrossRef]
- Toldra, A.; Alcaraz, C.; Diogene, J.; O’Sullivan, C.K.; Campas, M. Detection of Ostreopsis cf. ovata in environmental samples using an electrochemical DNA-based biosensor. Sci. Total Environ. 2019, 689, 655–661. [Google Scholar] [CrossRef]
- Arriaza-Echanes, C.; Campo-Giraldo, J.L.; Quezada, C.P.; Espinoza-González, R.; Rivas-Álvarez, P.; Pacheco, M.; Bravo, D.; Pérez-Donoso, J.M. Biomimetic synthesis of CuInS2 nanoparticles: Characterization, cytotoxicity, and application in quantum dots sensitized solar cells. Arab. J. Chem. 2021, 14, 103176. [Google Scholar] [CrossRef]
- Romanholo, P.V.V.; Razzino, C.A.; Raymundo-Pereira, P.A.; Prado, T.M.; Machado, S.A.S.; Sgobbi, L.F. Biomimetic electrochemical sensors: New horizons and challenges in biosensing applications. Biosens. Bioelectron. 2021, 185, 113242. [Google Scholar] [CrossRef]
- Lowe, C.R. Chemoselective biosensors. Curr. Opin. Chem. Biol. 1999, 3, 106–111. [Google Scholar] [CrossRef]
- Khan, S.; Wong, A.; Zanoni, M.V.B.; Sotomayor, M.D.P.T. Electrochemical sensors based on biomimetic magnetic molecularly imprinted polymer for selective quantification of methyl green in environmental samples. Mater. Sci. Eng. C 2019, 103, 109825. [Google Scholar] [CrossRef]
- Yang, S.; Liu, M.; Deng, F.; Mao, L.; Yuan, Y.; Huang, H.; Chen, J.; Liu, L.; Zhang, X.; Wei, Y. Biomimetic modification of silica nanoparticles for highly sensitive and ultrafast detection of DNA and Ag+ ions. Appl. Surf. Sci. 2020, 510, 145421. [Google Scholar] [CrossRef]
- Nasir, M.; Rauf, S.; Muhammad, N.; Nawaz, M.H.; Chaudhry, A.A.; Malik, M.H.; Shahid, S.A.; Hayat, A. Biomimetic nitrogen doped titania nanoparticles as a colorimetric platform for hydrogen peroxide detection. J. Colloid Interface Sci. 2017, 505, 1147–1157. [Google Scholar] [CrossRef]
- Cao, W.; Ju, P.; Wang, Z.; Zhang, Y.; Zhai, X.; Jiang, F.; Sun, C. Colorimetric detection of H2O2 based on the enhanced peroxidase mimetic activity of nanoparticles decorated Ce2(WO4)3 nanosheets. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 239, 118499. [Google Scholar] [CrossRef]
- Vena, M.P.; Jobbagy, M.; Bilmes, S.A. Microorganism mediated biosynthesis of metal chalcogenides; a powerful tool to transform toxic effluents into functional nanomaterials. Sci. Total Environ. 2016, 565, 804–810. [Google Scholar] [CrossRef]
- Queirós, R.B.; Guedes, A.; Marques, P.; Noronha, J.; Sales, M.G.F.; Chemical, A.B. Recycling old screen-printed electrodes with newly designed plastic antibodies on the wall of carbon nanotubes as sensory element for in situ detection of bacterial toxins in water. Sensors 2013, 189, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.Q.; Lin, F.F.; Yuan, Q.P.; Liu, J.W.; Li, Y.; Liang, H. Robust magnetic laccase-mimicking nanozyme for oxidizing o-phenylenediamine and removing phenolic pollutants. J. Environ. Sci. 2020, 88, 103–111. [Google Scholar] [CrossRef]
- Zhai, C.; Miao, L.; Zhang, Y.; Zhang, L.; Li, H.; Zhang, S. An enzyme response-regulated colorimetric assay for pattern recognition sensing application using biomimetic inorganic-protein hybrid nanoflowers. Chem. Eng. J. 2022, 431, 134107. [Google Scholar] [CrossRef]
- Chu, W.; Zhang, Y.; Li, D.; Barrow, C.J.; Wang, H.; Yang, W. A biomimetic sensor for the detection of lead in water. Biosens. Bioelectron. 2015, 67, 621–624. [Google Scholar] [CrossRef]
- Niu, X.H.; He, Y.F.; Li, X.; Zhao, H.L.; Pan, J.M.; Qiu, F.X.; Lan, M.B. A peroxidase-mimicking nanosensor with Hg2+-triggered enzymatic activity of cysteine-decorated ferromagnetic particles for ultrasensitive Hg2+ detection in environmental and biological fluids. Sens. Actuators B Chem. 2019, 281, 445–452. [Google Scholar] [CrossRef]
- Wujcik, E.K.; Londoño, N.J.; Duirk, S.E.; Monty, C.N.; Masel, R.I. An acetylcholinesterase-inspired biomimetic toxicity sensor. Chemosphere 2013, 91, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Fan, Y.; Li, G.; Du, W.; Li, R.; Liu, Y.; Cheng, Z.; Xu, J. Biomimetic synthesis of zeolitic imidazolate frameworks and their application in high performance acetone gas sensors. Sens. Actuators B Chem. 2020, 302, 127187. [Google Scholar] [CrossRef]
- Machini, W.B.S.; Teixeira, M.F.S. Application of oxo-manganese complex immobilized on ion-exchange polymeric film as biomimetic sensor for nitrite ions. Sens. Actuators B Chem. 2015, 217, 58–64. [Google Scholar] [CrossRef] [Green Version]
- Cao, Q.; Xiao, Y.; Liu, N.; Huang, R.; Ye, C.; Huang, C.; Liu, H.; Han, G.; Wu, L. Synthesis of Yolk/Shell heterostructures MOF@MOF as biomimetic sensing platform for catechol detection. Sens. Actuators B Chem. 2021, 329, 129133. [Google Scholar] [CrossRef]
- Cheng, Y.; Chen, T.; Fu, D.; Liu, J. A molecularly imprinted nanoreactor based on biomimetic mineralization of bi-enzymes for specific detection of urea and its analogues. Sens. Actuators B Chem. 2022, 350, 130909. [Google Scholar] [CrossRef]
- Wong, A.; de Vasconcelos Lanza, M.R.; Sotomayor, M.D.P.T. Sensor for diuron quantitation based on the P450 biomimetic catalyst nickel(II) 1,4,8,11,15,18,22,25-octabutoxy-29H,31H-phthalocyanine. J. Electroanal. Chem. 2013, 690, 83–88. [Google Scholar] [CrossRef] [Green Version]
- Sgobbi, L.F.; Machado, S.A.S. Functionalized polyacrylamide as an acetylcholinesterase-inspired biomimetic device for electrochemical sensing of organophosphorus pesticides. Biosens. Bioelectron. 2018, 100, 290–297. [Google Scholar] [CrossRef]
- Yan, X.; Kong, D.; Jin, R.; Zhao, X.; Li, H.; Liu, F.; Lin, Y.; Lu, G.J.S.; Chemical, A.B. Fluorometric and colorimetric analysis of carbamate pesticide via enzyme-triggered decomposition of Gold nanoclusters-anchored MnO2 nanocomposite. Sensors Actuators B Chem. 2019, 290, 640–647. [Google Scholar] [CrossRef]
- Jin, R.; Kong, D.S.; Zhao, X.; Li, H.X.; Yan, X.; Liu, F.M.; Sun, P.; Du, D.; Lin, Y.H.; Lu, G.Y. Tandem catalysis driven by enzymes directed hybrid nanoflowers for on-site ultrasensitive detection of organophosphorus pesticide. Biosens. Bioelectron. 2019, 141, 111473. [Google Scholar] [CrossRef]
- Raymundo-Pereira, P.A.; Gomes, N.O.; Machado, S.A.S.; Oliveira, O.N. Simultaneous, ultrasensitive detection of hydroquinone, paracetamol and estradiol for quality control of tap water with a simple electrochemical method. J. Electroanal. Chem. 2019, 848. [Google Scholar] [CrossRef]
- Li, L.; Zou, J.-Y.; Zhang, L.; You, S.-Y.; Xie, X.; Chen, G.-H. Sensitive detection of the antibiotic pollutants by a solvent-stable luminescent sensor based on a europium(III) metal-organic framework. J. Solid State Chem. 2022, 305, 122668. [Google Scholar] [CrossRef]
- Martins, T.S.; Bott-Neto, J.L.; Oliveira, O.N., Jr.; Machado, S.A.S. Paper-based electrochemical sensors with reduced graphene nanoribbons for simultaneous detection of sulfamethoxazole and trimethoprim in water samples. J. Electroanal. Chem. 2021, 882, 114985. [Google Scholar] [CrossRef]
- Sekli Belaïdi, F.; Farouil, L.; Salvagnac, L.; Temple-Boyer, P.; Séguy, I.; Heully, J.L.; Alary, F.; Bedel-Pereira, E.; Launay, J. Towards integrated multi-sensor platform using dual electrochemical and optical detection for on-site pollutant detection in water. Biosens. Bioelectron. 2019, 132, 90–96. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Macias, D.; Dean, Z.S.; Kreger, N.R.; Wong, P.K. A UAV-mounted whole cell biosensor system for environmental monitoring applications. IEEE Trans. Nanobiosci. 2015, 14, 811–817. [Google Scholar] [CrossRef] [Green Version]



| Immobilization of Enzymes | Method’s Characteristics |
|---|---|
| Adsorption | Simple, inexpensive, less destructive to enzymatic activity, no additional reagent necessary |
| Microencapsulation | Preservation of structural and acting integrities of enzymes, due to their protection against environmental conditions |
| Entrapment | High stability conferred to the enzymes |
| Cross-linking | Improved efficiency and stability of enzymes by strong and stable bindings |
| Covalent bondings | More stability for enzymes and enzymes-support complexes, meanwhile stronger bindings than in adsorption case |
| Analyte | Enzyme(s) | Immobilization Method | Transducer | Target | LOD | Linearity | Reference |
|---|---|---|---|---|---|---|---|
| Hg2+, Cu2+, Cd2+ | Urease | Entrapment in sol-gel matrix | Optical | River water | 10 nM, 50 μM, 500 μM | - | [54] |
| Chromium | GOx | Cross-linking with GA and covering with aniline membrane | Amperometric | Soil | 0.49 µg L−1 | 0.49–95.73 mgL−1 95.73–8.05 mgL−1 | [55] |
| Paraoxon | AChE | Dropping on the multiwall carbon nanotubes | Amperometric | Water | 0.5 nmol L−1 | 6.9 nM | [56] |
| Paraoxon-ethyl, diisopropyl fluorophosphates | AChE | Cross-linking with BSA in a saturated glutaraldehyde vapor | Conductometric | Soil | 1 × 10−8, 5 × 10−11 | - | [57] |
| Atrazine | Tyrosinase | Cross-linking with PVA-SbQ | Amperometric | Spiked drinking water s | 0.3 ppm | 0.5–20 ppm | [58] |
| Atrazine | Tyrosinase | Entrapping in poly(L-DOPA) | Amperometric | Water | 10 ppb | 50 ppb–3.0 ppm | [59] |
| Organophosphorus neurotoxin | AChE | Cross-linking with GA | Piezoelectric | Water | 50 mg/m3 | 0–50 mg/m3 | [60] |
| Captan | Glutathione-S-transferase | Entrapment in gel sodium alginate | Optical | Water | 2 ppm | - | [61] |
| Anatoxin-a | AChE | Entrapment in PVA-SbQ | Amperometric | Water | 1 µg L−1 | 0–2.0 ppm | [62] |
| Catechol | Tyrosinase | Chitosan-gold nanoparticles | Amperometric | Environmental monitoring | 27 × 10−6 mM | 0.046–50 μM | [63] |
| Methyl salicylate | Alcohol oxidase and peroxidase | Molecular tetherings in carbon nanotube matrix | Amperometric | Environmental monitoring | 0.00098 mM | - | [64] |
| Analyte | Microorganism | Immobilization Method | Transducer | Target | LOD | Reference |
|---|---|---|---|---|---|---|
| As3+ | Genetically engineered S. oneidensis | Biofilm formation | Electrochemical | Environmental monitoring | 40 μM | [73] |
| Cu2+, Cd2+, Ni2+, Pb2+ | Saccharomyces cerevisiae S288C | Physical adsorption on BND-chitosan hydrogell polymer on GCE | Amperometric | Wastewater | - | [74] |
| As3+, Cd2+, Pb2+, Zn2+ | E. coli | Microbial culture in microfluidic device | Fluorescent | Water | - | [75] |
| Pb2+ | E. Coli DH5α | Microbial culture in a microfluidic device | Fluorescent | Environmental monitoring | [76] | |
| Cd2+, Cu2+, Zn2+ | Bacillus megaterium VR1 | Entrapment in sol-gel matrix | Fluorescent | Soil | 1.42 × 10−4, 3.16 × 10−4, 2.42 × 10−4 | [14] |
| Cu2+ | S. Cerevisiae | Entrapment in alginate beads | Colorimetric | Water | 1 µM | [77] |
| Paraoxon, parathion, methylparathion | Genetically engineered Escherihia coli | Biofilm on GCE modified with OMCs | Amperometric | Environmmental monitoring | 9 nM, 10 nM, 15 nMz | [78] |
| Atrazine (herbicide) | Anabaena variabilis | Entrapment in alginate | Amperometric | Environmmental monitoring | 0.07 µM | [79] |
| Diuron (herbicide) | Chlamydomonas reinhardtii | Ti/TiO2 ultramicroe-lectrodes in algal suspension | Chronoamperometric | Water | 0.2 µM | [80] |
| Simazine (herbicide) | Dictyosphaerium chlorelloides Dc1M | Adsorption on porous silicone disks | Luminescent | Drinking water | 40.8 µg L−1 | [81] |
| Analyte | Transducer | Electrode/Sensing Material | Target | LOD | Linearity | Reference |
|---|---|---|---|---|---|---|
| Chlorpyrifos | Impedimetric | Chip modified with gold nanoparticles | - | 0.5 ng mL−1 | 0.5–500 ng/ml | [92] |
| TBBPA-DHEE and TBBPA-MHEE | Impedimetric | Silica nanoparticles | Aquatic environments | 0.08 ng mL−1 | 0.21–111.31 ng/mL | [93] |
| Atrazine | Electrochemical | SWCNT | Seawater, riverine water | 0.01 ng mL−1 | - | [94] |
| Microcystin-LR | Impedimetric | Gold electrodes with MoS2 andgold nanorods | Water | 5 ng L−1 | 0.01–20 gL−1 | [95] |
| Okadaic acid Domoic acid | Optical (SPR) | Gold electrode with carboxymethylated surface | Seawater | 0.36 ng mL−1 1.66 ng mL−1 | - | [96] |
| Okadaic acid | Impedimetric | Graphene | Seawater | 0.05 ng mL−1 | - | [97] |
| Legionella pneumophila | Optical (SPR) | Gold substrate | Water | 103 CFU mL−1 | - | [98] |
| Analyte | Detection Method | Target | LOD | Linearity | Reference |
|---|---|---|---|---|---|
| Ag+ | SERS based on Au@Ag core–shell nanoparticles | Tap water, river water | 50 × 10−12 mg L−1 | 0.1–100 nM | [110] |
| As3+ | Colorimetric with GNPs | Wastewater | 0.0006 mg L−1 | 1–400 range/ppm | [111] |
| As3+ | Colorimetric with AuNPs | Soil | 1.97 ppm | - | [112] |
| Cd2+ | Fluorescence with use of SYBR green I as signal reporter | Tap water, river water | 3 × 10−9 mg L−1 | 1.12–224.82 μg L−1 | [113] |
| Hg2+ | SERS based on dual recycling | Water environment | 0.11 fM | 0.2–125 fM | [114] |
| Hg2+ | SERS based on SiO2@Au core/shell nanoparticles | Lake water | 10 × 10−9 mg L−1 | - | [115] |
| Pb2+ | Electrochemical (Impedance), G-rich aptamer/MWCNTs/GNPs | Water | 4.3 × 10–15 M | 5.0 × 10−11–1.0 × 10−14 M | [116] |
| Pb2+ | Fluorescence based on gold nanoflowers | Tap water | 0.285 nM | 0.01–850 nM | [117] |
| Pb2+ | Colorimetric with use of silver staining | Soil | 5.0 × 10−7 mg L−1 | - | [118] |
| Acetampirid | Chemiluminescence with use of AuNPs | Wastewater Soil | 62 × 10−12 mg L−1 1.0 × 10−9 mg L−1 | - | [119] |
| Malathion | Colorimetric based on AuNPs and cationic polymer | Lake water | 6 × 10−14 mg L−1 | 0.5–1000 pM | [120] |
| Omethoate | Fluorescence based on S-GQD | - | 1 ppb | 0–200 ppm | [121] |
| Organophosphorus pesticides | Fluorescence with poly(T) CuNPs | Lake water | 0.22 nM | 0–200 nM | [122] |
| Tetracycline | Photoelectrochemical based on CdTe-BiOBr heterojunction | Soil | 9.25 pM | 10–1500 pM | [123] |
| Analyte | Transducer | Target | LOD | Linearity | Reference |
|---|---|---|---|---|---|
| Hg2+ | Electrochemical | Tap water, river water | 0.05 nM | 0.1–200 nM | [132] |
| Pb2+ | Fluorescent | Aqueous systems | 5 nM | 0–50 nM | [133] |
| Pb2+ | Fluorescent | Lake water | 0.6 nM | 2–10 nM | [134] |
| Organophosphorus pesticides | Fluorescent | Lake water | 0.018 µg L−1 | 2–10 μg/L | [134] |
| Cyanazine | Impedimetric | Water | 0.8 nM | 4.0 nM–70 μM | [135] |
| Pirazon | Impedimetric | Water | 1 × 10−10 M | 5 × 10−9–5 × 10−5 M | [136] |
| Legionella pneumophila | Optical (SPRi) | Water | 104 CFU mL−1 | - | [137] |
| Vibrio cholerae | Impedimetric | - | 7.41 × 10−30 mol L−1 | 10−8–10−14 mol L−1 | [138] |
| Escherichia coli | Amperometric | Soil | 100 cells/g soil | - | [139] |
| Bacillus thuringiensis | Impedimetric | - | 0.997 × 10−12 M | 1 pM–1 μM | [140] |
| Ostreopsis cf. ovata | Colorimetric | Plankton, bentonite | 9 pg/μL | - | [141] |
| Analyte | Mimetic Structure | Transducer | Target | Sensibility (LOD) | Linearity | Reference |
|---|---|---|---|---|---|---|
| Heavy metals | ||||||
| Cu2+, Cr3+, Fe3+, Pb2+, Fe2+, Cd2+, Cr6+, Co2+, Zn2+, Ag+, Al3+ | Enzyme immobilization Metal phosphates-acetylcholinesterase nanoflowers | Colorimetric | Water | Cu2+—0.81 μM, Cr3+—0.75 μM Al3+—1.06 μM | 2.5–500 μM. | [152] |
| Pb2+ | Gold nanoparticles with glutathione linker | UV–vis spectroscopic | Water | 47.6 nM (9.9 ppb) | 2–14 mM | [153] |
| Hg2+ | Cysteine-decorated ferromagnetic particle (Cys-Fe3O4) | Colorimetric | River water | 5.9 pM. | 0.02–90 nM | [154] |
| Chemicals | ||||||
| Methyl green | Magnetic molecularly imprinted polymer | Square-wave adsorptive anodic stripping voltammetry | River waterIndustrial wastewater | 1.0 × 10−8 mol L−1 | 9.9 × 10−8–1.8 × 10−6 mol L−1 | [145] |
| Acetylcholinesterase inhibitors | Microchannel 1-phenyl-1,2,3-butanetrione 2-oxime (PBO)-based microsensor | Potentiometric | Surface waters used for municipal drinking water supplies | LD50, LC50 | 2–1360 mg kg−1 | [155] |
| Acetone gas | Zeolitic imidazolate framework-90 polyhedron crystals | quartz crystal microbalance | Air | Lower than 20 ppb | - | [156] |
| Nitrite ions | Oxo-bridged dinuclear manganese-phenanthroline complex immobilized into an ion-exchange Polymeric film deposited on glassy carbon electrode | Cyclic voltammetry | Environmental samples | 6.50 × 10−6 mol L−1 | 2.49 × 10−6–9.90 × 10−6 mol L−1 | [157] |
| Catechol | Metal-organic frameworks | Water | 33 nmol L−1 | - | [158] | |
| Urea | Embedding urease and bovine hemoglobin in metal-organic frameworks through biomimetic mineralization | Colorimetric | Sewage | 0.02 mM | 0.08–20.00 mM | [159] |
| Pesticides | ||||||
| Diurone | Carbon paste electrode modified with the nickel(II) 1,4,8,11,15,18,22,25-octabutoxy-29H,31H-phthalocyanine complex | Cyclic voltammetry and amperometry | River water, soil | 6.14 × 10−6 mol L−1, | 9.9 ×10−6 and 1.5 × 10−4 mol L−1 | [160] |
| Organophosphorus pesticides | Employing a functionalized polyacrylamide, polyhydroxamicalkanoate | Amperometric | Water supply | 0.26 μmol L−1 | - | [161] |
| Carbamate | Gold nanoclusters-anchored MnO2 (AuNCs-MnO2) nanocomposite | Fluorimetric/Colorimetric | Soil, water | 0.125 µg L−1. | - | [162] |
| Paraoxon | Cu3(PO4)2·3H2O, AChE and ChO -based lab-on paper platform | Cyclic voltammetry and Colorimetric | Tap and river water | 6 fg mL−1 | - | [163] |
| Toxins | ||||||
| Bacterial toxins | Microcystins inserted into a polymeric matrix | Potentiometric | Water | below the guideline value establishedby WHO | 7.24 × 10−10–1.28 × 10−9 M | [150] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gavrilaș, S.; Ursachi, C.Ș.; Perța-Crișan, S.; Munteanu, F.-D. Recent Trends in Biosensors for Environmental Quality Monitoring. Sensors 2022, 22, 1513. https://doi.org/10.3390/s22041513
Gavrilaș S, Ursachi CȘ, Perța-Crișan S, Munteanu F-D. Recent Trends in Biosensors for Environmental Quality Monitoring. Sensors. 2022; 22(4):1513. https://doi.org/10.3390/s22041513
Chicago/Turabian StyleGavrilaș, Simona, Claudiu Ștefan Ursachi, Simona Perța-Crișan, and Florentina-Daniela Munteanu. 2022. "Recent Trends in Biosensors for Environmental Quality Monitoring" Sensors 22, no. 4: 1513. https://doi.org/10.3390/s22041513
APA StyleGavrilaș, S., Ursachi, C. Ș., Perța-Crișan, S., & Munteanu, F.-D. (2022). Recent Trends in Biosensors for Environmental Quality Monitoring. Sensors, 22(4), 1513. https://doi.org/10.3390/s22041513






