Copper-Electroplating-Modified Liquid Metal Microfluidic Electrodes
Abstract
:1. Introduction
2. Experimental Details
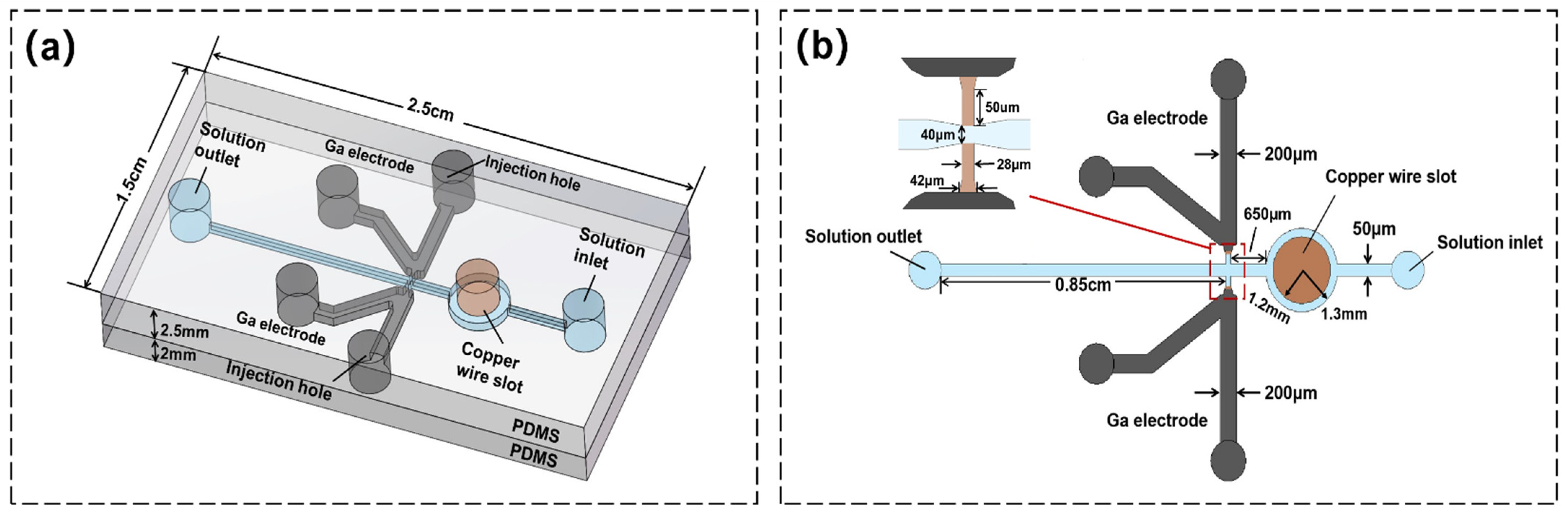
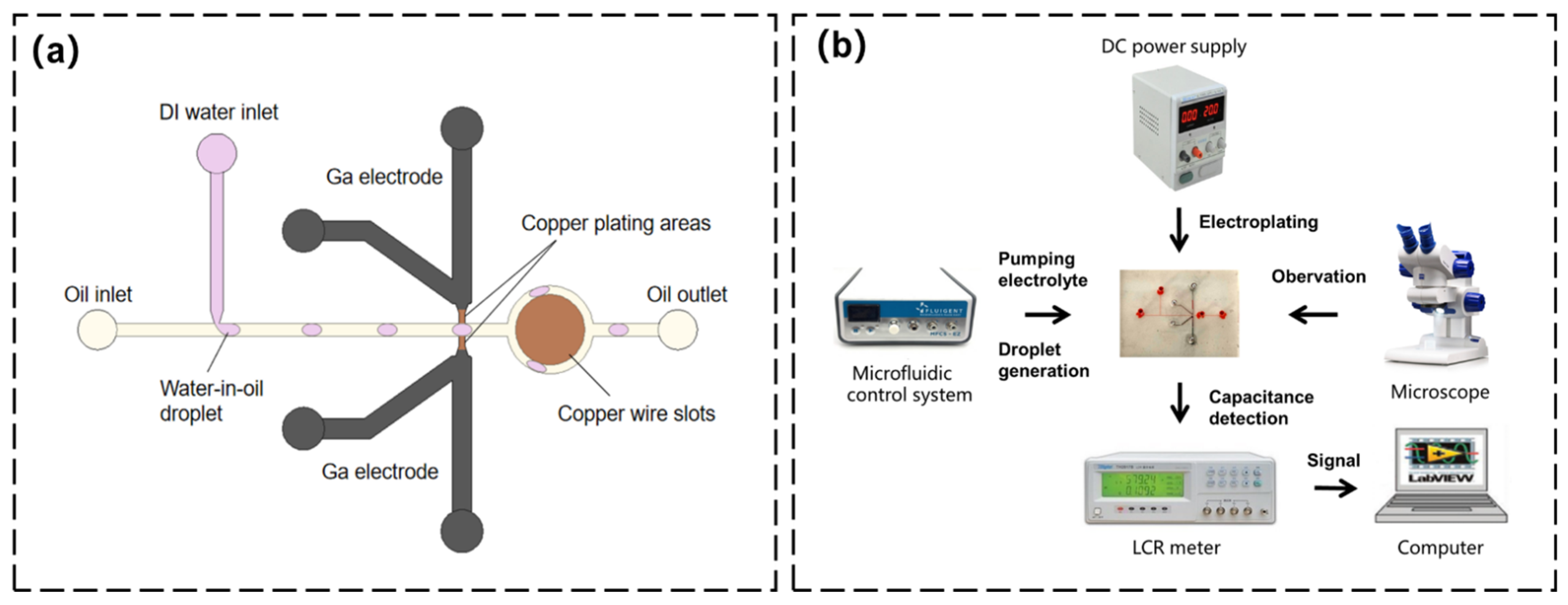
2.1. Design of the Chip
2.2. Fabrication of the Chip
2.3. Method of Microelectroplating
3. Results and Discussion
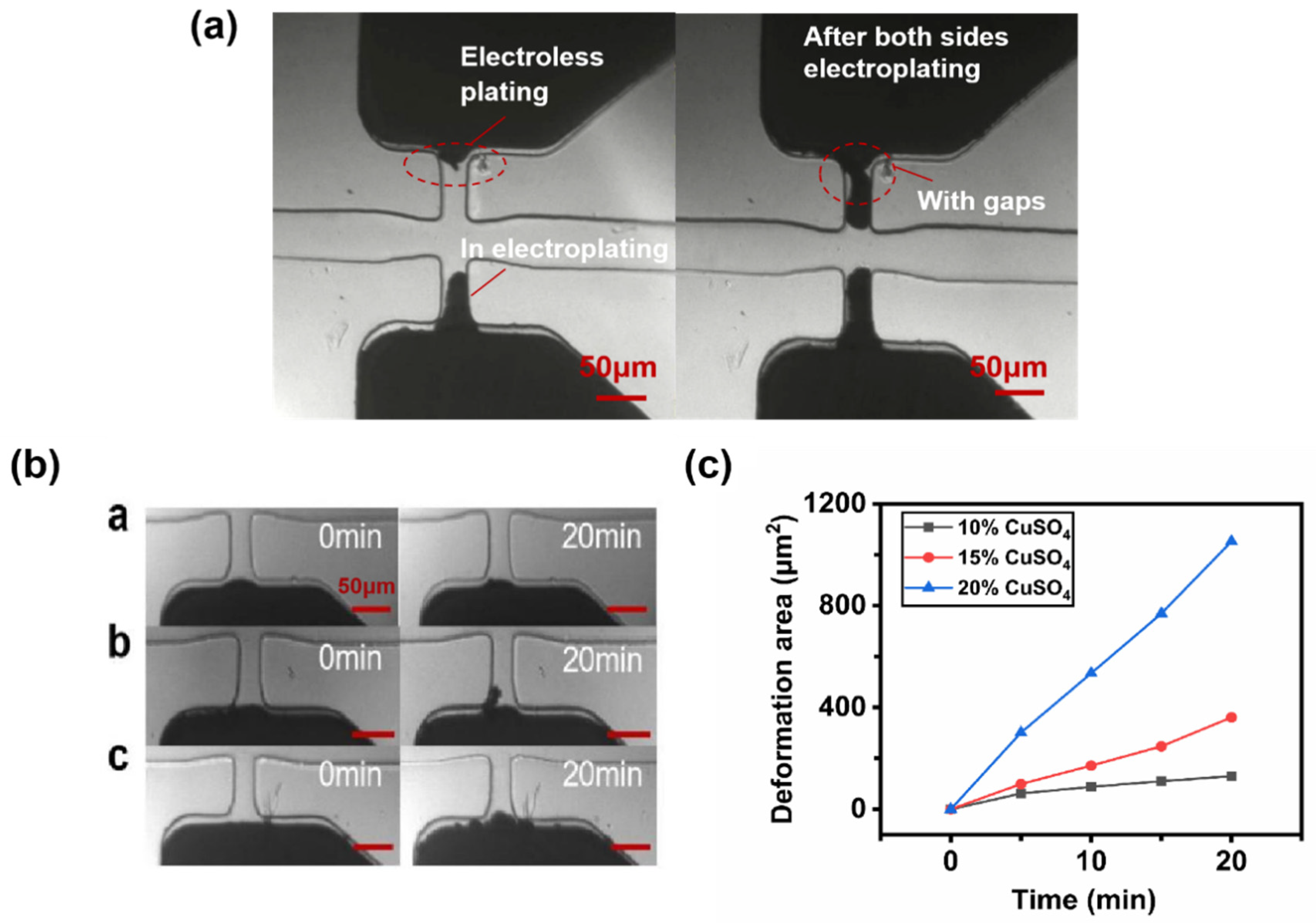
3.1. Effects of Electroless Plating
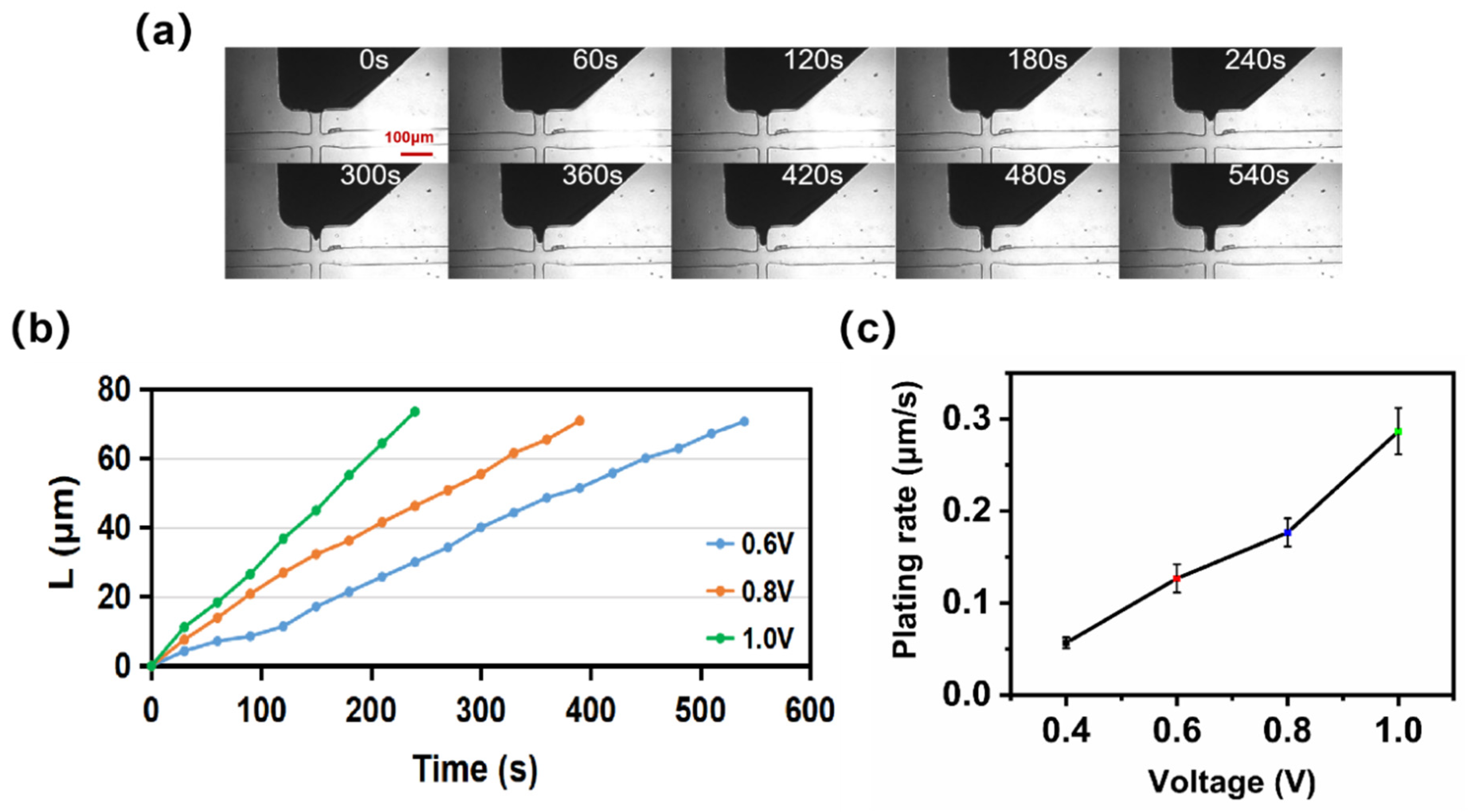
3.2. Voltage Effects on Microelectroplating
3.3. Microelectrode Stability
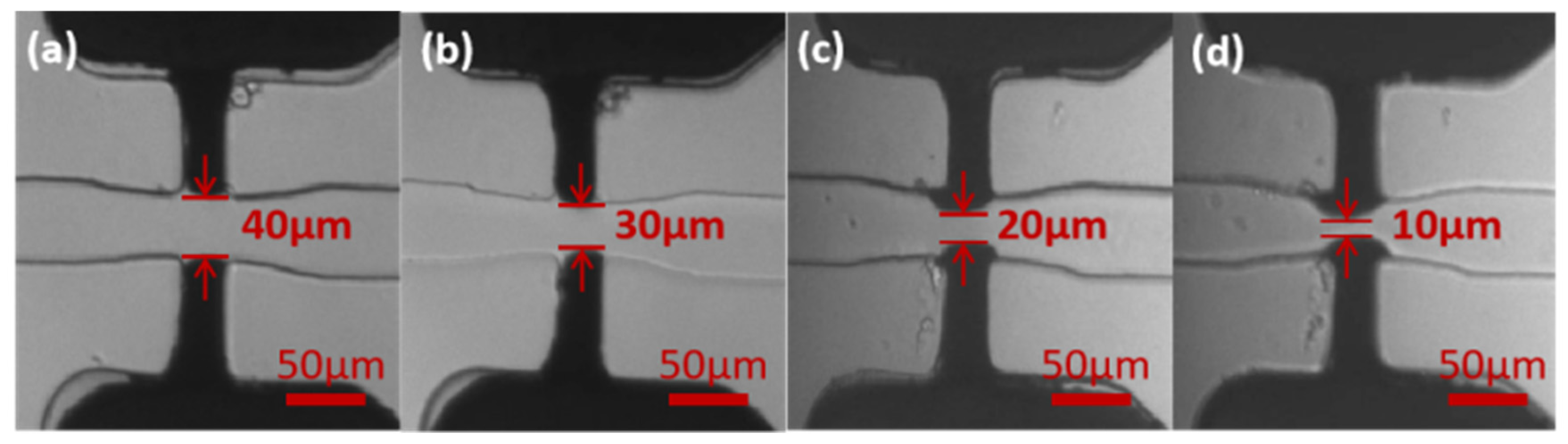
3.4. Fabrication of Microelectrodes with Different Spacings
4. Applications
4.1. Droplet Detection
4.2. Metal Particle Counting in Oil
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Meagher, R.J.; Hatch, A.V.; Renzi, R.F.; Singh, A.K. An integrated microfluidic platform for sensitive and rapid detection of biological toxins. Lab Chip 2008, 8, 2046–2053. [Google Scholar] [CrossRef]
- Sun, Y.; Quyen, T.L.; Hung, T.Q.; Chin, W.H.; Wolff, A.; Bang, D.D. A lab-on-a-chip system with integrated sample preparation and loop-mediated isothermal amplification for rapid and quantitative detection of Salmonella spp. in food samples. Lab Chip 2015, 15, 1898–1904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, L.H.; Keller, S.S.; Boisen, A. Microfabricated devices for oral drug delivery. Lab Chip 2018, 18, 2348–2358. [Google Scholar] [CrossRef] [Green Version]
- Abid, Z.; Strindberg, S.; Javed, M.M.; Mazzoni, C.; Vaut, L.; Nielsen, L.H.; Gundlach, C.; Petersen, R.S.; Mullertz, A.; Boisen, A.; et al. Biodegradable microcontainers—Towards real life applications of microfabricated systems for oral drug delivery. Lab Chip 2019, 19, 2905–2914. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Hu, L.; Xu, C. Recent advances in the design of polymeric microneedles for transdermal drug delivery and biosensing. Lab Chip 2017, 17, 1373–1387. [Google Scholar] [CrossRef] [PubMed]
- Ueda, E.; Geyer, F.L.; Nedashkivska, V.; Levkin, P.A. Droplet Microarray: Facile formation of arrays of microdroplets and hydrogel micropads for cell screening applications. Lab Chip 2012, 12, 5218–5224. [Google Scholar] [CrossRef]
- Segaliny, A.I.; Li, G.; Kong, L.; Ren, C.; Chen, X.; Wang, J.K.; Baltimore, D.; Wu, G.; Zhao, W. Functional TCR T cell screening using single-cell droplet microfluidics. Lab Chip 2018, 18, 3733–3749. [Google Scholar] [CrossRef] [Green Version]
- Ye, N.; Qin, J.; Shi, W.; Liu, X.; Lin, B. Cell-based high content screening using an integrated microfluidic device. Lab Chip 2007, 7, 1696–1704. [Google Scholar] [CrossRef]
- Zhou, H.; Yao, S. Electrostatic charging and control of droplets in microfluidic devices. Lab Chip 2013, 13, 962–969. [Google Scholar] [CrossRef]
- Park, S.-Y.; Kalim, S.; Callahan, C.; Teitell, M.A.; Chiou, E.P.Y. A light-induced dielectrophoretic droplet manipulation platform. Lab Chip 2009, 9, 3228–3235. [Google Scholar] [CrossRef]
- Esquivel, J.P.; Del Campo, F.J.; Gómez de la Fuente, J.L.; Rojas, S.; Sabaté, N. Microfluidic fuel cells on paper: Meeting the power needs of next generation lateral flow devices. Energy Environ. Sci. 2014, 7, 1744–1749. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wang, H.; Meng, F.; Luo, Y.; Ling, F. Development status of microfluidic fuel cell. Chem. Ind. Eng. Prog. 2016, 35, 65–73. [Google Scholar]
- Ji, X.; Pan, J.; Zhou, J. Dielectrophoretic microfluidic chip with 3D vertical side-wall electrodes. In Proceedings of the 13th IEEE International Conference on Solid-State and Integrated-Circuit Technology (ICSICT), Hangzhou, China, 25–28 October 2016; pp. 269–271. [Google Scholar]
- Kadilak, A.L.; Liu, Y.; Shrestha, S.; Bernard, J.R.; Mustain, W.E.; Shor, L.M. Selective deposition of chemically-bonded gold electrodes onto PDMS microchannel side walls. J. Electroanal. Chem. 2014, 727, 141–147. [Google Scholar] [CrossRef]
- Kang, G.H.; No, K.Y.; Kim, G.M. Fabrication of microchannel with electrodes on side wall. Int. J. Mod. Phys. B 2006, 20, 4493–4498. [Google Scholar] [CrossRef]
- Fava, E.L.; Silva, T.A.; Prado, T.M.D.; Moraes, F.C.; Faria, R.C.; Fatibello-Filho, O. Electrochemical paper-based microfluidic device for high throughput multiplexed analysis. Talanta 2019, 203, 280–286. [Google Scholar] [CrossRef]
- Cao, J.T.; Zhu, Y.D.; Rana, R.K.; Zhu, J.J. Microfluidic chip integrated with flexible PDMS-based electrochemical cytosensor for dynamic analysis of drug-induced apoptosis on HeLa cells. Biosens. Bioelectron. 2014, 51, 97–102. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, S.; Zhang, S.; Li, Y.; Qu, Z.; Chen, Y.; Lu, B.; Wang, X.; Feng, X. Skin-like biosensor system via electrochemical channels for noninvasive blood glucose monitoring. Sci. Adv. 2017, 3, e1701629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Susloparova, A.; Koppenhoefer, D.; Law, J.K.Y.; Vu, X.T.; Ingebrandt, S. Electrical cell-substrate impedance sensing with field-effect transistors is able to unravel cellular adhesion and detachment processes on a single cell level. Lab Chip 2015, 15, 668–679. [Google Scholar] [CrossRef]
- Scherer, B.; Surrette, C.; Li, H.; Torab, P.; Kvam, E.; Galligan, C.; Go, S.; Grossmann, G.; Hammond, T.; Johnson, T.; et al. Digital electrical impedance analysis for single bacterium sensing and antimicrobial susceptibility testing. Lab Chip 2021, 21, 1073–1083. [Google Scholar] [CrossRef]
- Zhang, R.; Ye, Z.; Gao, M.; Gao, C.; Zhang, X.; Li, L.; Gui, L. Liquid metal electrode-enabled flexible microdroplet sensor. Lab Chip 2020, 20, 496–504. [Google Scholar] [CrossRef]
- Zuo, Z.; Wang, K.; Gao, L.; Ho, V.; Mao, H.; Qian, D. A Novel Mass-Producible Capacitive Sensor with Fully Symmetric 3D Structure and Microfluidics for Cells Detection. Sensors 2019, 19, 325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, M.M.; Narakathu, B.B.; Emamian, S.; Chlaihawi, A.A.; Aljanabi, F.; Maddipatla, D.; Bazuin, B.J.; Atashbar, M.Z. Eutectic Ga-In Liquid Metal Based Flexible Capacitive Pressure Sensor. In Proceedings of the 15th IEEE Sensors Conference, Orlando, FL, USA, 30 October–3 November 2016. [Google Scholar]
- Shi, H.; Zhang, H.; Ma, L.; Rogers, F.; Zhao, X.; Zeng, L. An Impedance Debris Sensor Based on a High-Gradient Magnetic Field for High Sensitivity and High Throughput. IEEE Trans. Ind. Electron. 2021, 68, 5376–5384. [Google Scholar] [CrossRef]
- Choi, J.-W.; Rosset, S.; Niklaus, M.; Adleman, J.R.; Shea, H.; Psaltis, D. 3-dimensional electrode patterning within a microfluidic channel using metal ion implantation. Lab Chip 2010, 10, 783–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Flanagan, L.; Lee, A.P. Side-Wall Vertical Electrodes for Lateral Field Microfluidic Applications. J. Microelectromech. Syst. 2007, 16, 454–461. [Google Scholar] [CrossRef]
- Harnett, C.K.; Templeton, J.; Dunphy-Guzman, K.A.; Senousy, Y.M.; Kanouff, M.P. Model based design of a microfluidic mixer driven by induced charge electroosmosis. Lab Chip 2008, 8, 565–572. [Google Scholar] [CrossRef]
- Richards, A.L.; Dickey, M.D.; Kennedy, A.S.; Buckner, G.D. Design and demonstration of a novel micro-Coulter counter utilizing liquid metal electrodes. J. Micromech. Microeng. 2012, 22, 115012. [Google Scholar] [CrossRef]
- Gao, M.; Gui, L. Development of a Multi-Stage Electroosmotic Flow Pump Using Liquid Metal Electrodes. Micromachines 2016, 7, 165. [Google Scholar] [CrossRef] [Green Version]
- So, J.H.; Dickey, M.D. Inherently aligned microfluidic electrodes composed of liquid metal. Lab Chip 2011, 11, 905–911. [Google Scholar] [CrossRef]
- Li, S.; Li, M.; Hui, Y.S.; Cao, W.; Li, W.; Wen, W. A novel method to construct 3D electrodes at the sidewall of microfluidic channel. Microfluid. Nanofluid. 2013, 14, 499–508. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, M.; Zhang, L.; Deng, Z.; Gui, L. A Handy Flexible Micro-Thermocouple Using Low-Melting-Point Metal Alloys. Sensors 2019, 19, 314. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Simon, M.G.; Jiang, A.Y.L.; Nelson, E.L.; Lee, A.P.; Li, G.-P.; Bachman, M. 3-D In-Bi-Sn Electrodes for Lab-on-PCB Cell Sorting. IEEE Trans. Compon. Packag. Manuf. Technol. 2016, 6, 1295–1300. [Google Scholar] [CrossRef]
- Akita, T.; Tomie, M.; Ikuta, R.; Egoshi, H.; Hayase, M. Observation of the Behavior of Additives in Copper Electroplating Using a Microfluidic Device. J. Electrochem. Soc. 2018, 166, D3058–D3065. [Google Scholar] [CrossRef]
- Bao, B.; Ji, B.; Wang, M.; Gao, K.; Yang, B.; Chen, X.; Wang, X.; Liu, J. Development and characterisation of electroplating silver/silver chloride modified microelectrode arrays. Micro Nano Lett. 2019, 14, 299–303. [Google Scholar] [CrossRef]
- Polk, B.J.; Bernard, M.; Kasianowicz, J.J.; Misakian, M.; Gaitan, M. Microelectroplating Silver on Sharp Edges toward the Fabrication of Solid-State Nanopores. J. Electrochem. Soc. 2004, 151, C559. [Google Scholar] [CrossRef]
- Zheng, W.; Guo, Z.; Luo, H.; Jiang, S.; Mo, Y. Comparison Between Micro-chemical Etching and Microelectroplating Processes for Preparing Metal Mold of Microfluidic Chip. Corros. Sci. Prot. Technol. 2015, 27, 264–268. [Google Scholar]
- He, Q.; Chen, G.; Chen, X.; Yao, C. Application of Oil Analysis to the Condition Monitoring of Large Engineering Machinery. In Proceedings of the 8th International Conference on Reliability, Maintainability and Safety (ICRMS 2009), Chengdu, China, 20–24 July 2009. [Google Scholar]
- Ma, S.; Yang, D.; Liang, B.; Jin, Y. Impact of metal particle size on partial discharge characteristics of moving metal particles in transformer oil. Chem. Phys. Lett. 2019, 731, 136577. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Liu, D. A Compact On-line Particle Counter Sensor for Hydraulic Oil Contamination Detection. In Proceedings of the 3rd International Conference on Mechanical and Electronics Engineering (ICMEE 2011), Hefei, China, 23–25 September 2012. [Google Scholar]
- Zhang, R.-c.; Yu, X.; Hu, Y.-l.; Zang, H.-j.; Shu, W. Active control of hydraulic oil contamination to extend the service life of aviation hydraulic system. Int. J. Adv. Manuf. Technol. 2018, 96, 1693–1704. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, H.; Ma, L.; Sun, Y.; Chen, H.; Zhao, X.; Bai, C.; Zhang, Y. Inductive-Capacitive Coulter Counting: Detection and Differentiation of Multi-Contaminants in Hydraulic Oil Using a Microfluidic Sensor. IEEE Sens. J. 2021, 21, 2067–2076. [Google Scholar] [CrossRef]
- Murali, S.; Jagtiani, A.V.; Xia, X.; Carletta, J.; Zhe, J. A microfluidic Coulter counting device for metal wear detection in lubrication oil. Rev. Sci. Instrum. 2009, 80, 016105. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, J.; Liu, B.; Zhang, P.; Zhang, H.; Gui, L. Copper-Electroplating-Modified Liquid Metal Microfluidic Electrodes. Sensors 2022, 22, 1820. https://doi.org/10.3390/s22051820
Gong J, Liu B, Zhang P, Zhang H, Gui L. Copper-Electroplating-Modified Liquid Metal Microfluidic Electrodes. Sensors. 2022; 22(5):1820. https://doi.org/10.3390/s22051820
Chicago/Turabian StyleGong, Jiahao, Bingxin Liu, Pan Zhang, Huimin Zhang, and Lin Gui. 2022. "Copper-Electroplating-Modified Liquid Metal Microfluidic Electrodes" Sensors 22, no. 5: 1820. https://doi.org/10.3390/s22051820





