Proposed Fatigue Index for the Objective Detection of Muscle Fatigue Using Surface Electromyography and a Double-Step Binary Classifier
Abstract
1. Introduction
Literature Review and Motivation
2. Materials and Method
2.1. Sample Size
2.2. Choosing the Appropriate Muscle and Fatigue Classification
- I.
- Slight muscle cramp or tightening.
- II.
- Sustained muscle cramp with a sort of painful feeling.
- III.
- A continuous feeling of burning pain.
- IV.
- Further painful feeling and lack of ability to maintain the activity.
2.3. Preparation of Subjects and Protocol Performance
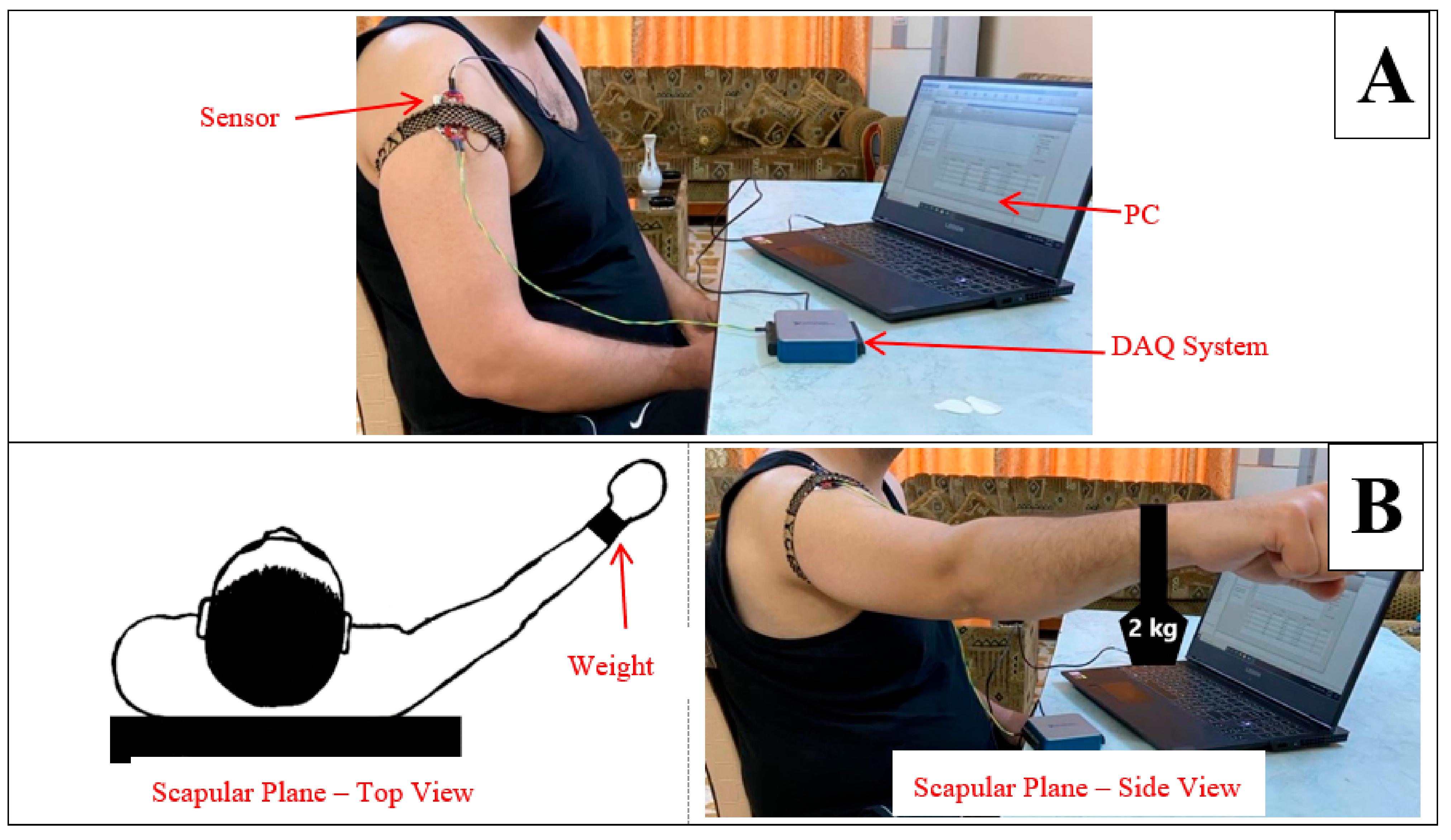
- I.
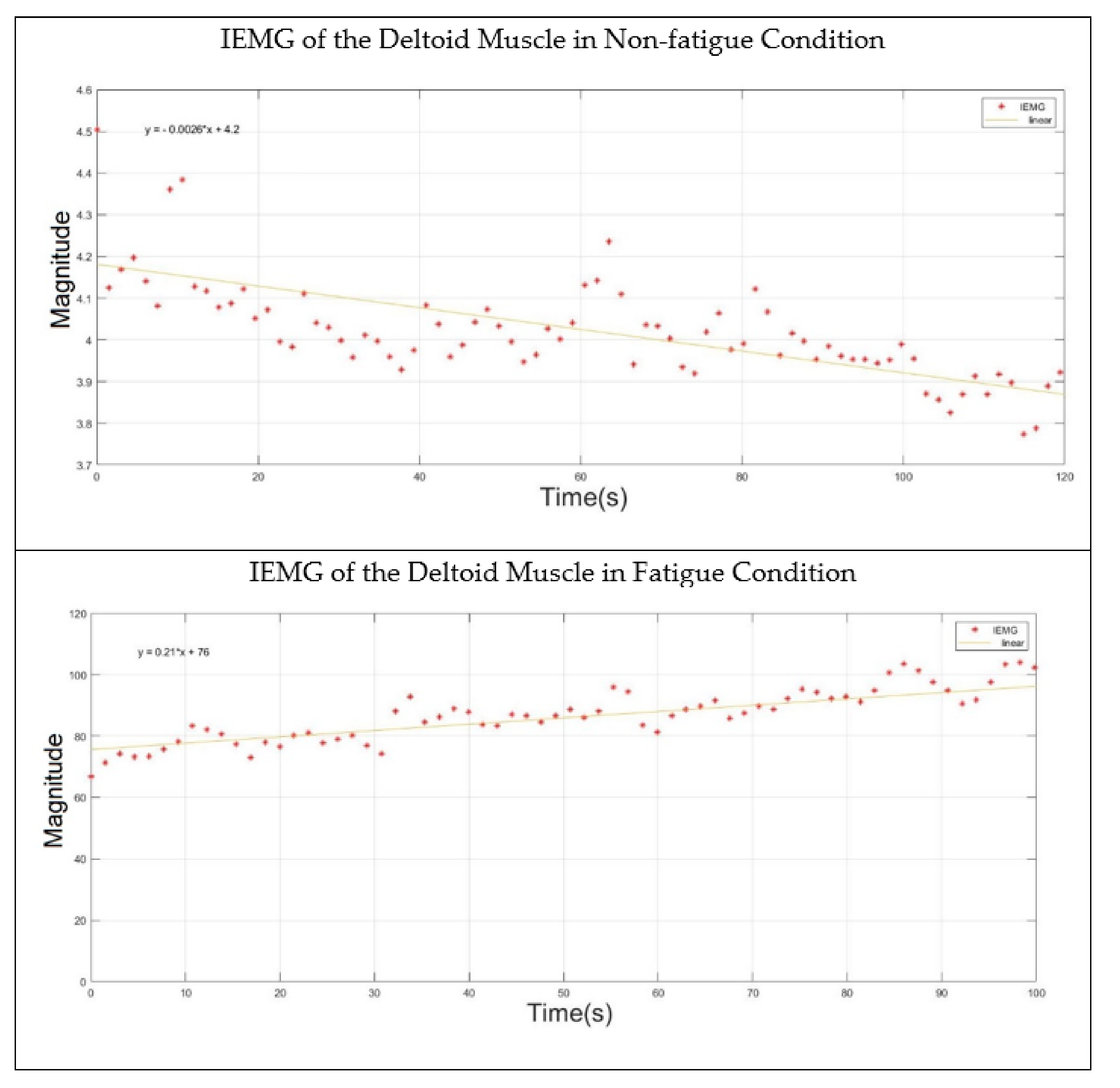
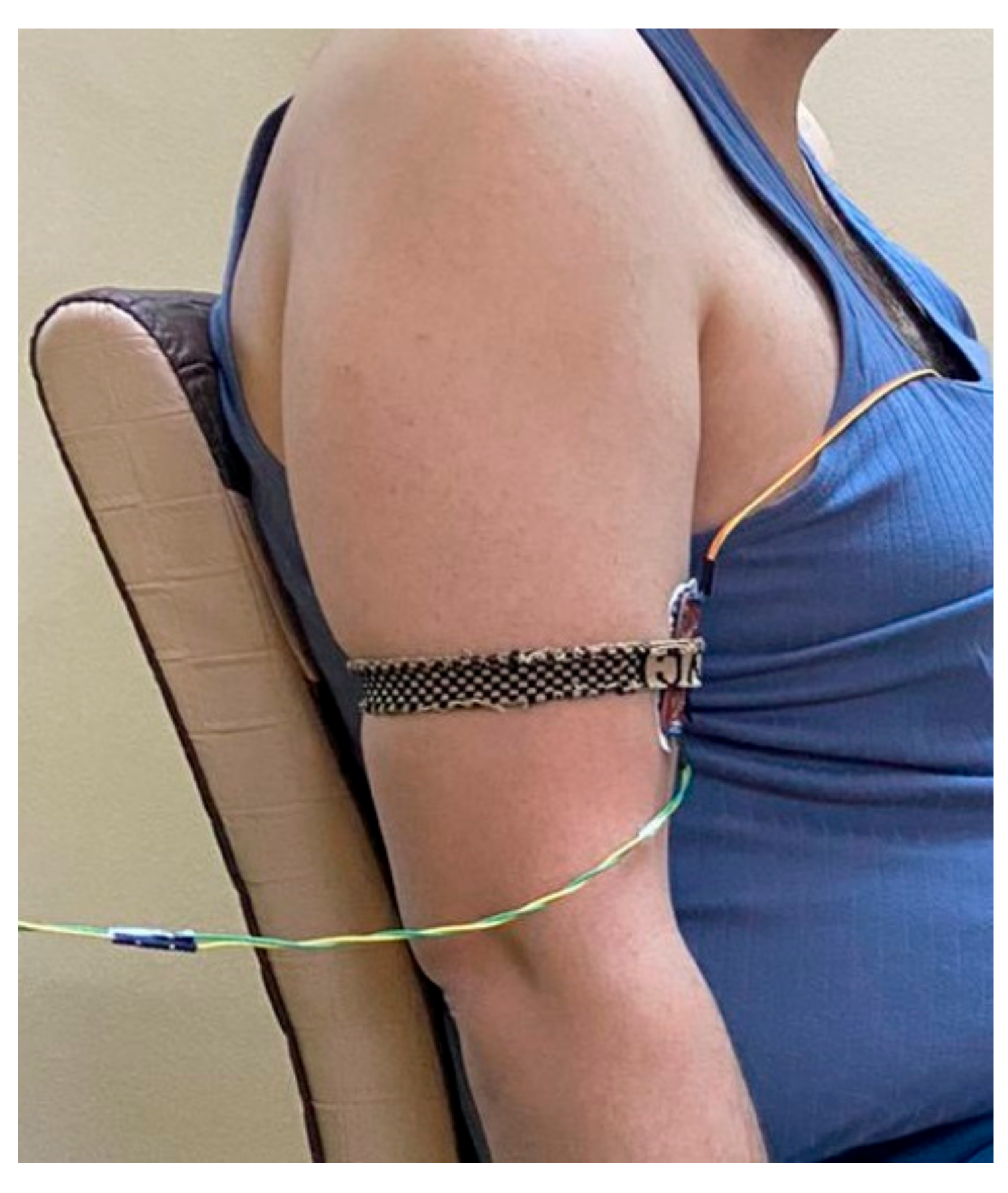
- Muscle in Non-fatigue Condition: The subjects were asked to relax their middle deltoid muscles by performing no action (Figure 1A) and, simultaneously, the sEMG signal was recorded. This procedure was aimed at evaluating the performance of the proposed algorithm in detecting the non-fatigued muscle. Despite that the subjects performed no action in this posture, the middle deltoid muscle still played an important role in stabilizing the shoulder joint [40,43]. Eventually, the middle deltoid was not fully relaxed and exhibited a sort of sEMG signal.
- II.
- Muscle in Fatigue Condition: The subjects were asked to elevate their upper limbs and keep them in the scapula plane with a 2 kg weight clutched on their forearms (Figure 1B). The subjects were encouraged to perform this activity until they could no longer maintain this posture, whereby the muscle was considered to have reached fatigue level. It should be noted that the weight was added to accelerate the generation of fatigue. This procedure was aimed at evaluating the performance of the proposed algorithm with regard to the objective detection of muscle fatigue.
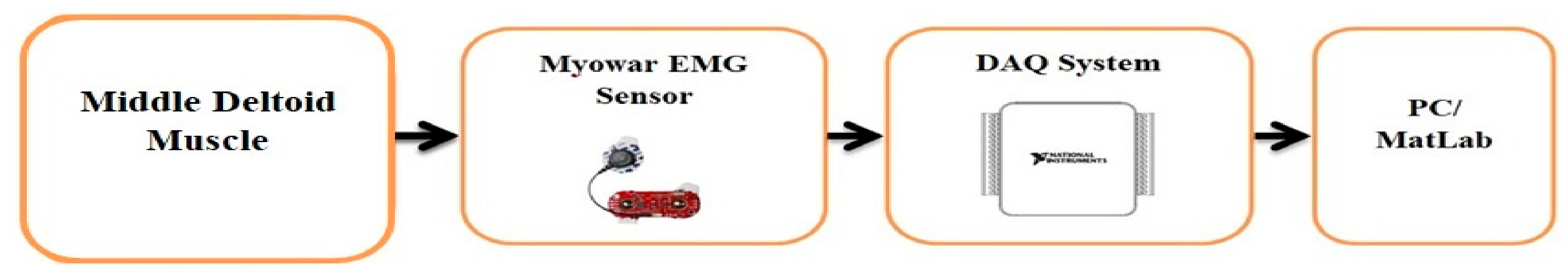
2.4. Acquisition of the sEMG and Hardware Setup
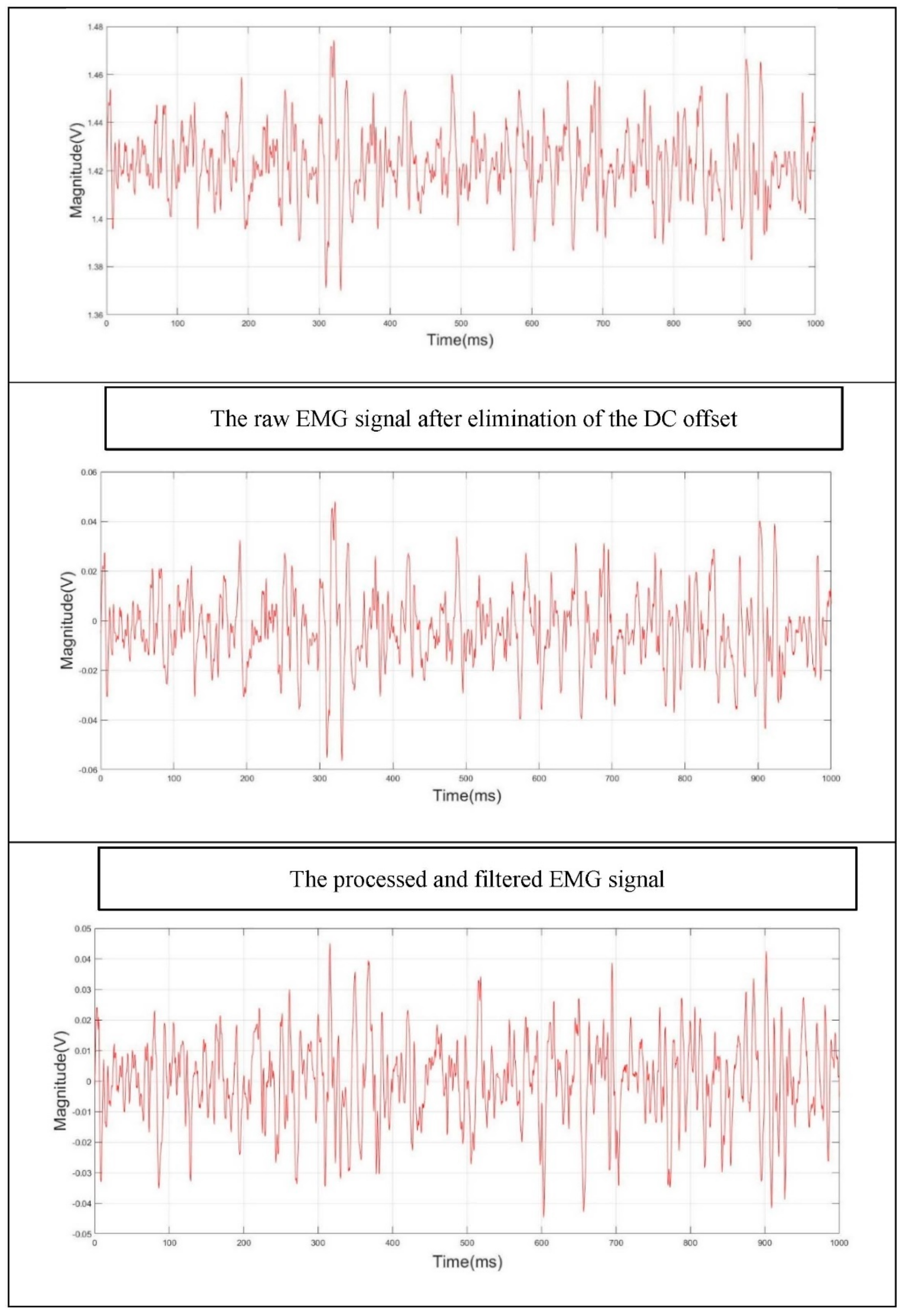
2.5. Pre-Processing of sEMG Signal
- I.
- The DC offset was first eliminated. Matlab provides an efficient function for removing the DC level that the raw EMG data is mounted on [52].
- II.
- III.
- A nonzero-lag second order Butterworth IIR band-stop filter, with a cut-off frequency of 47–53 Hz, was also applied to eliminate the 50 Hz power line frequency.
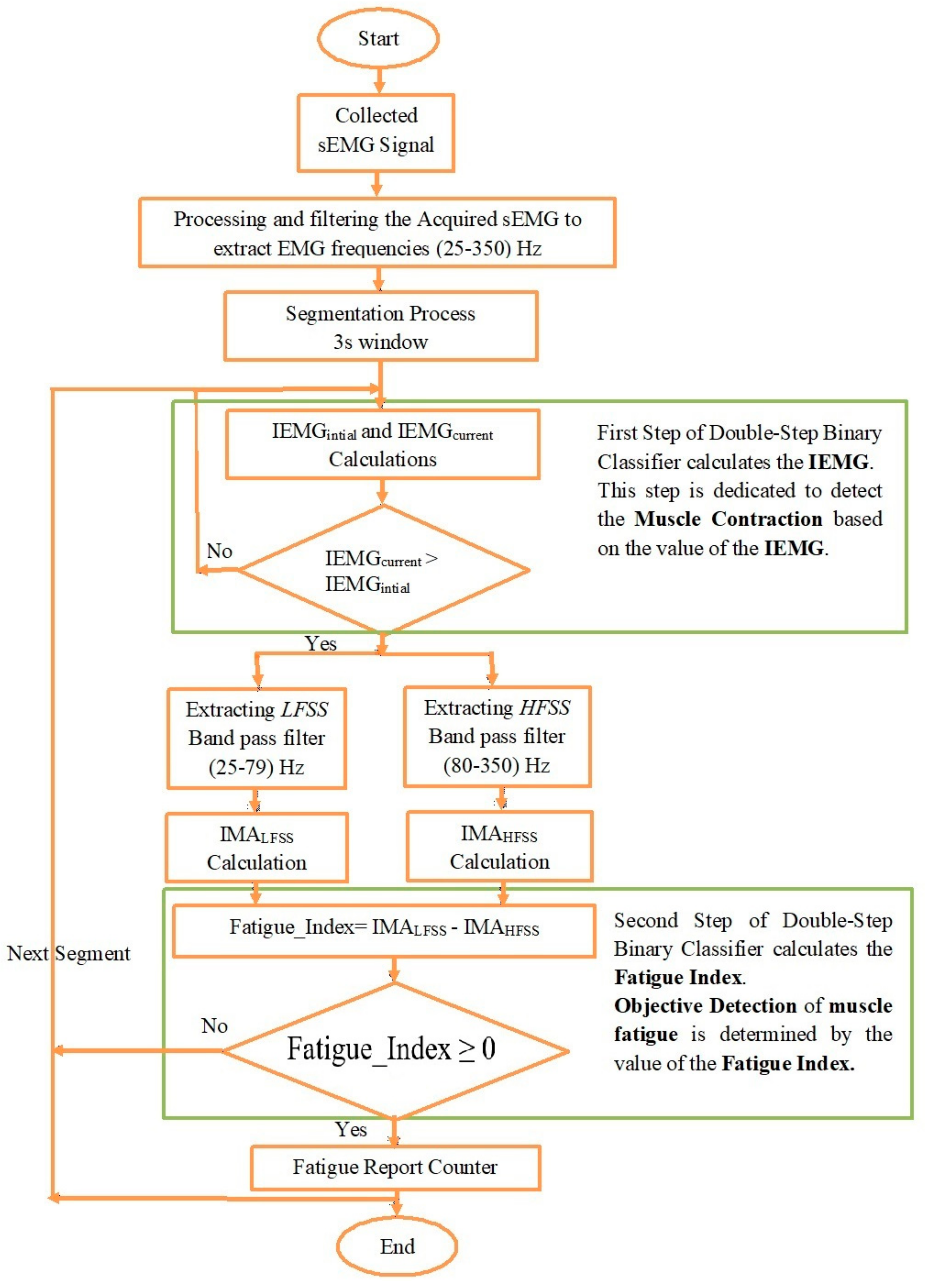
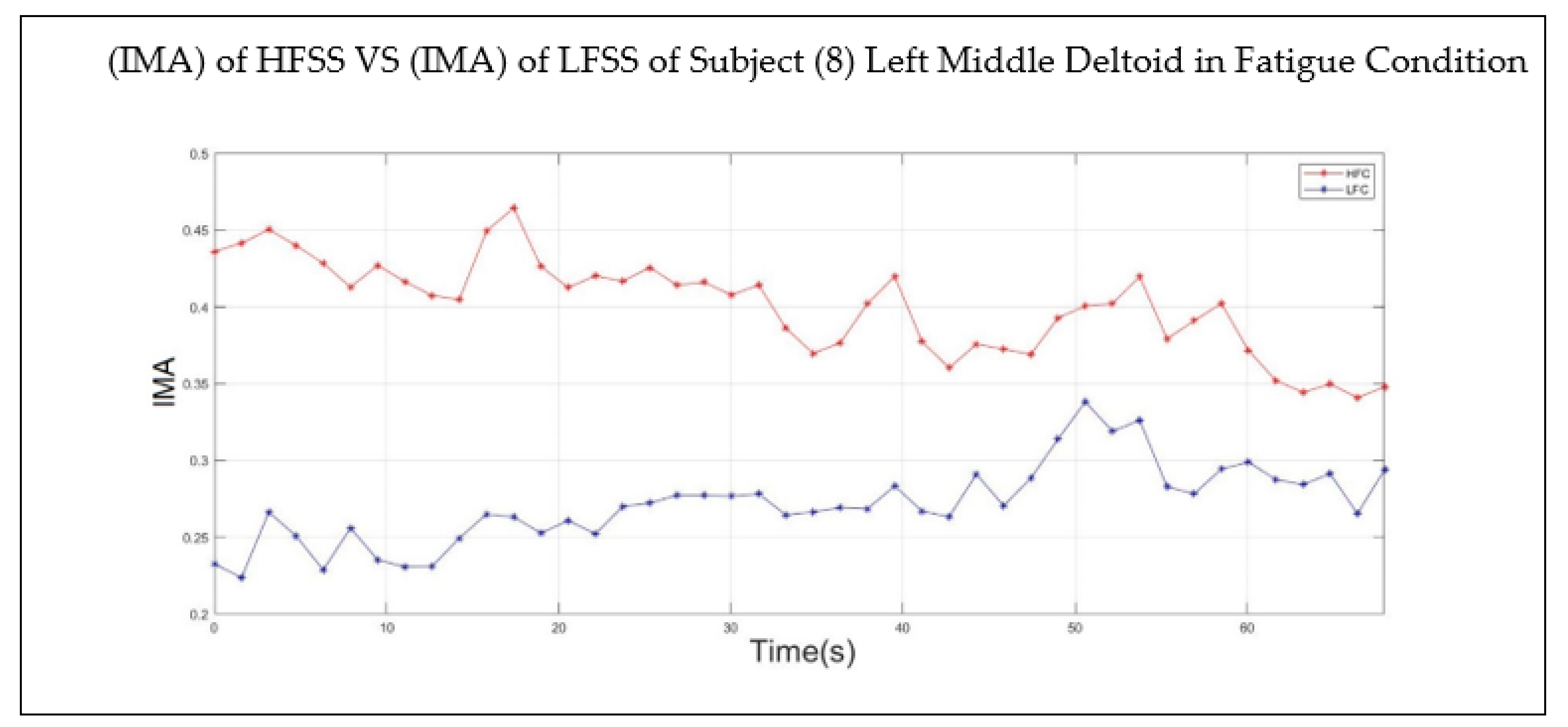
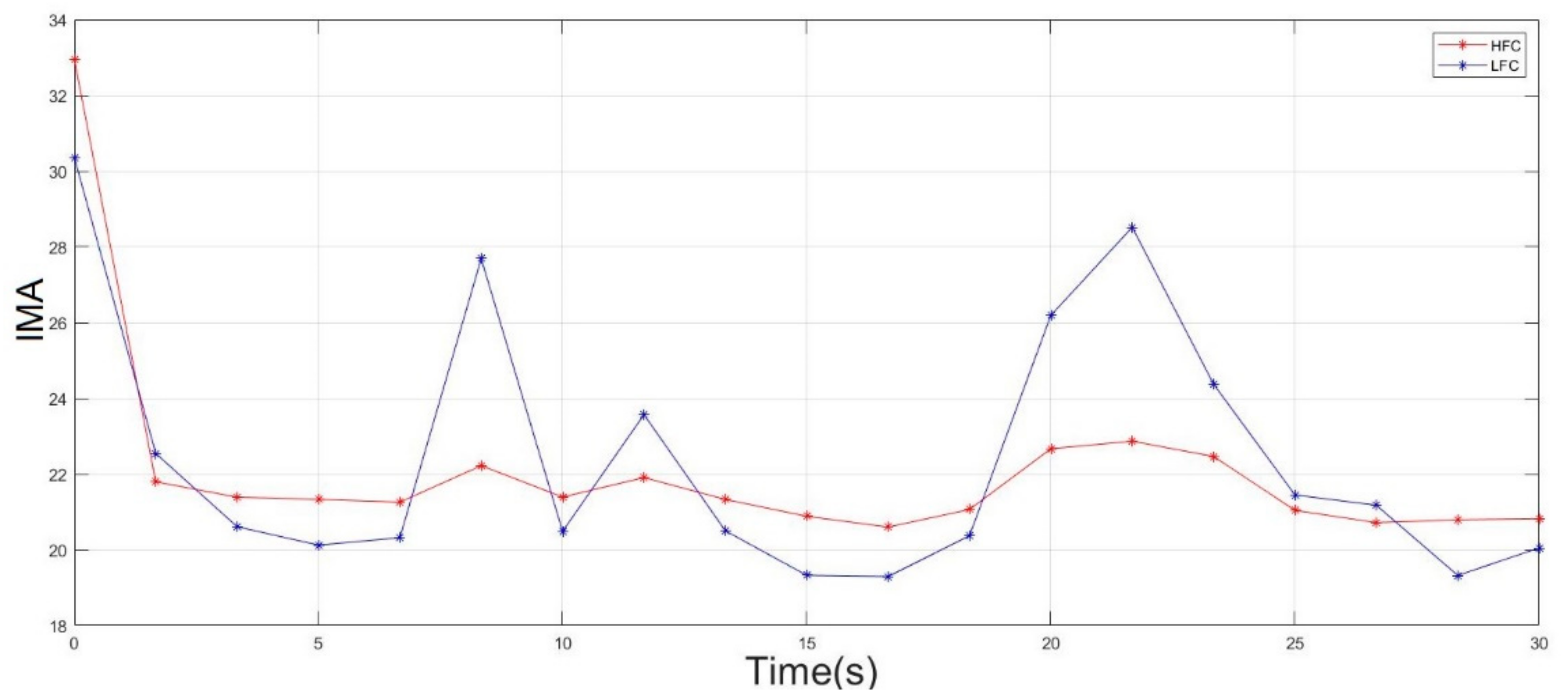
2.6. Double-Step Binary Classifier and Fatigue Index
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rampichini, S.; Vieira, T.M.; Castiglioni, P.; Merati, G. Complexity analysis of surface electromyography for assessing the myoelectric manifestation of muscle fatigue: A review. Entropy 2020, 22, 529. [Google Scholar] [CrossRef] [PubMed]
- Basmajian, J.V.; De Luca, C.J. Chapter 8. Muscle Fatigue and Time-Dependent Parameters of the Surface EMG Signal. In Muscles Alive: Their Functions Revealed by Electromyography; Williams & Wilkins: Baltimore, MD, USA, 1985; pp. 201–222. ISBN 0471675806. [Google Scholar]
- Mastaglia, F.L. The relationship between muscle pain and fatigue. Neuromuscul. Disord. 2012, 22, S178–S180. [Google Scholar] [CrossRef] [PubMed]
- Shair, E.F.; Ahmad, S.A.; Marhaban, M.H.; Tamrin, S.B.M.; Abdullah, A.R. EMG processing based measures of fatigue assessment during manual lifting. BioMed Res. Int. 2017, 2017, 3937254. [Google Scholar] [CrossRef] [PubMed]
- Hussain, J.; Sundaraj, K.; Low, Y.F.; Lam, C.K.; Sundaraj, S.; Ali, M.A. A systematic review on fatigue analysis in triceps brachii using surface electromyography. Biomed. Signal Process. Control 2018, 40, 396–414. [Google Scholar] [CrossRef]
- Mátrai, K.N.Z. Subjective and objective indicators in the research on health status. J. Hum. Sport Exerc. 2016, 11, S207–S217. [Google Scholar] [CrossRef]
- Al-Mulla, M.R.; Sepulveda, F.; Colley, M. sEMG Techniques to Detect and Predict Localised Muscle Fatigue. In EMG Methods for Evaluating Muscle and Nerve Function; InTech: Rijeka, Croatia, 2012. [Google Scholar] [CrossRef][Green Version]
- Sakurai, T.; Toda, M.; Sakurazawa, S.; Akita, J.; Kondo, K.; Nakamura, Y. Detection of muscle fatigue by the surface electromyogram and its application. In Proceedings of the 2010 IEEE/ACIS 9th International Conference on Computer and Information Science, Yamagata, Japan, 18–20 August 2010; pp. 43–47. [Google Scholar] [CrossRef]
- Elshafei, M.; Shihab, E. Towards detecting biceps muscle fatigue in gym activity using wearables. Sensors 2021, 21, 759. [Google Scholar] [CrossRef]
- Shindo, N. Electrical Stimulation. Physiotherapy 1988, 74, 74. [Google Scholar] [CrossRef]
- Nazmi, N.; Rahman, M.A.A.; Yamamoto, S.I.; Ahmad, S.A.; Zamzuri, H.; Mazlan, S.A. A review of classification techniques of EMG signals during isotonic and isometric contractions. Sensors 2016, 16, 1304. [Google Scholar] [CrossRef]
- Candotti, C.T.; Loss, J.F.; La Torre, M.; Melo, M.O.; Araújo, L.D.; Marcks, V.V. Use of electromyography to assess pain in the upper trapezius and lower back muscles within a fatigue protocol. Rev. Bras. Fisioter. 2009, 13, 144–151. [Google Scholar] [CrossRef][Green Version]
- Viitasalo, J.H.T.; Komi, P.V. Signal characteristics of EMG during fatigue. Eur. J. Appl. Physiol. Occup. Physiol. 1977, 37, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Cifrek, M.; Medved, V.; Tonković, S.; Ostojić, S. Surface EMG based muscle fatigue evaluation in biomechanics. Clin. Biomech. 2009, 24, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Too, J.; Abdullah, A.R.; Saad, N.M. Classification of Hand movements based on discrete wavelet transform and enhanced feature extraction. Int. J. Adv. Comput. Sci. Appl. 2019, 10, 83–89. [Google Scholar] [CrossRef]
- Phinyomark, A.; Thongpanja, S.; Hu, H.; Phukpattaranont, P.; Limsakul, C. The Usefulness of Mean and Median Frequencies in Electromyography Analysis. Comput. Intell. Electromyogr. Anal. A Perspect. Curr. Appl. Future Chall. 2012, 195–220. [Google Scholar] [CrossRef]
- Hameed, H.K.; Hasan, W.Z.W.; Shafie, S.; Ahmad, S.A.; Jaafar, H. An amplitude independent muscle activity detection algorithm based on adaptive zero crossing technique and mean instantaneous frequency of the sEMG signal. In Proceedings of the 2017 IEEE Regional Symposium on Micro and Nanoelectronics (RSM), Penang, Malaysia, 23–25 August 2017; pp. 183–186. [Google Scholar] [CrossRef]
- Tsai, A.C.; Hsieh, T.H.; Luh, J.J.; Lin, T. Te A comparison of upper-limb motion pattern recognition using EMG signals during dynamic and isometric muscle contractions. Biomed. Signal Process. Control 2014, 11, 17–26. [Google Scholar] [CrossRef]
- Qassim, H.M.; Wan Hassan, W.Z. A Review on Upper Limb Rehabilitation Robots Hassan. Appl. Sci. 2020, 10, 6976. [Google Scholar] [CrossRef]
- Han, J.; Song, W.; Kim, J. New EMG Pattern Recognition based on Soft Computing Techniques and Its Application to Control of a Rehabilitation Robotic Arm. In Proceedings of the 6th International Conference on Soft Computing (IIZUKA2000), Fukuoka, Japan, 1–4 October 2000. [Google Scholar]
- Asghari Oskoei, M.; Hu, H. Myoelectric control systems—A survey. Biomed. Signal Process. Control 2007, 2, 275–294. [Google Scholar] [CrossRef]
- Phinyomark, A.; Phukpattaranont, P.; Limsakul, C. Feature reduction and selection for EMG signal classification. Expert Syst. Appl. 2012, 39, 7420–7431. [Google Scholar] [CrossRef]
- Fernando, J.B.; Yoshioka, M.; Ozawa, J. Estimation of muscle fatigue by ratio of mean frequency to average rectified value from surface electromyography. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 5303–5306. [Google Scholar] [CrossRef]
- Wang, J.; Pang, M.; Yu, P.; Tang, B.; Xiang, K.; Ju, Z. Effect of Muscle Fatigue on Surface Electromyography-Based Hand Grasp Force Estimation. Appl. Bionics Biomech. 2021, 2021, 8817480. [Google Scholar] [CrossRef]
- Minning, S.; Eliot, C.A.; Uhl, T.L.; Malone, T.R. EMG analysis of shoulder muscle fatigue during resisted isometric shoulder elevation. J. Electromyogr. Kinesiol. 2007, 17, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Karthick, P.A.; Ghosh, D.M.; Ramakrishnan, S. Surface electromyography based muscle fatigue detection using high-resolution time-frequency methods and machine learning algorithms. Comput. Methods Programs Biomed. 2018, 154, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Kiryu, T.; Sasaki, I.; Shibai, K.; Tanaka, K. Providing appropriate exercise levels for the elderly. IEEE Eng. Med. Biol. Mag. 2001, 20, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.M.T.; Iwashita, A.; Sato, T.; Yoshida, M. Estimation of muscle activity by EMG frequency analysis during exercise on a cycle ergometer. IEICE Tech. Rep. ME Bio Cybern. 2010, 110, 7–11. [Google Scholar]
- Kushida, A.K.D.; Aoki, T. Estimation of muscle fatigue based on the frequency analysis of EMG. In Proceedings of the Life Engineering Symposium, Yokohama, Japan, 12 September 2013; pp. 281–284. [Google Scholar]
- Karthick, P.A.; Ramakrishnan, S. Muscle fatigue analysis using surface EMG signals and time–frequency based medium-to-low band power ratio. Electron. Lett. 2016, 52, 185–186. [Google Scholar] [CrossRef]
- Gross, D.; Grassino, A.; Ross, W.R.D. Macklem Electromyogram pattern of diaphragmatic fatigue. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1979, 46, 1–7. [Google Scholar] [CrossRef] [PubMed]
- De Luca, C.J. Use of the surface EMG signal for performance evaluation of back muscles. Muscle Nerve 1993, 16, 210–216. [Google Scholar] [CrossRef]
- Eng, J. Sample size estimation: How many individuals should be studied? Radiology 2003, 227, 309–313. [Google Scholar] [CrossRef]
- Ahsan, M.R.; Ibrahimy, M.I.; Khalifa, O.O. The use of artificial neural network in the classification of EMG signals. In Proceedings of the 2012 Third FTRA International Conference on Mobile, Ubiquitous, and Intelligent Computing, Washington, DC, USA, 26–28 June 2012; pp. 225–229. [Google Scholar] [CrossRef]
- Arjunan, S.P.; Kumar, D.K. Decoding subtle forearm flexions using fractal features of surface electromyogram from single and multiple sensors. J. Neuroeng. Rehabil. 2010, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, M.; Benussi, C.; Lourenco, J.L. Single channel surface EMG control of advanced prosthetic hands: A simple, low cost and efficient approach. Expert Syst. Appl. 2017, 79, 322–332. [Google Scholar] [CrossRef]
- Mane, S.M.; Kambli, R.A.; Kazi, F.S.; Singh, N.M. Hand motion recognition from single channel surface EMG using wavelet & artificial neural network. Procedia Comput. Sci. 2015, 49, 58–65. [Google Scholar] [CrossRef]
- Kim, J.; Mastnik, S.; André, E. EMG-based hand gesture recognition for realtime biosignal interfacing. In Proceedings of the 13th International Conference on Intelligent User Interfaces, Canaria, Spain, 13–16 January 2008; pp. 30–39. [Google Scholar] [CrossRef]
- Wu, Y.; Liang, S.; Zhang, L.; Chai, Z.; Cao, C.; Wang, S. Gesture recognition method based on a single-channel semg envelope signal. EURASIP J. Wirel. Commun. Netw. 2018, 2018, 35. [Google Scholar] [CrossRef]
- Miniato, M.A.; Caire, M.J. Anatomy, Shoulder and Upper Limb, Shoulder; StatPearls: Treasure Island, FL, USA, 2018. [Google Scholar]
- Gopura, R.A.R.C.; Bandara, D.S.V.; Kiguchi, K.; Mann, G.K.I. Understanding the response of the shoulder complex to the demands of repetitive work. Rob. Auton. Syst. 2016, 75, 203–220. [Google Scholar] [CrossRef]
- Öberg, T.; Sandsjö, L.; Kadefors, R. Subjective and objective evaluation of shoulder muscle fatigue. Ergonomics 1994, 37, 1323–1333. [Google Scholar] [CrossRef] [PubMed]
- Dohle, C.I.; Rykman, A.; Chang, J.; Volpe, B.T. Pilot study of a robotic protocol to treat shoulder subluxation in patients with chronic stroke. J. Neuroeng. Rehabil. 2013, 10, 88. [Google Scholar] [CrossRef]
- Troiano, A.; Naddeo, F.; Sosso, E.; Camarota, G.; Merletti, R.; Mesin, L. Assessment of force and fatigue in isometric contractions of the upper trapezius muscle by surface EMG signal and perceived exertion scale. Gait Posture 2008, 28, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Del Toro, S.F.; Santos-Cuadros, S.; Olmeda, E.; Álvarez-Caldas, C.; Díaz, V.; San Román, J.L. Is the Use of a Low-Cost sEMG Sensor Valid to Measure Muscle Fatigue? Sensors 2019, 19, 3204. [Google Scholar] [CrossRef]
- Omama, Y.; Haddad, C.; MacHaalany, M.; Hamoudi, A.; Hajj-Hassan, M.; Ali, M.A.; Hamawy, L. Surface EMG Classification of Basic Hand Movement. In Proceedings of the 2019 Fifth International Conference on Advances in Biomedical Engineering (ICABME), Tripoli, Lebanon, 17–19 October 2019; pp. 1–4. [Google Scholar] [CrossRef]
- Ahamed, N.U.; Sundaraj, K.; Ahmad, R.B.; Rahman, M.; Islam, M.A. Analysis of right arm biceps brachii muscle activity with varying the electrode placement on three male age groups during isometric contractions using a wireless EMG sensor. Procedia Eng. 2012, 41, 61–67. [Google Scholar] [CrossRef]
- Myoware. 3-Lead Muscle/Electromyography Sensor for Microcontroller Applications. Available online: https://cdn.sparkfun.com/assets/a/3/a/f/a/AT-04-001.pdf (accessed on 24 January 2022).
- Fuentes Del Toro, S.; Wei, Y.; Olmeda, E.; Ren, L.; Guowu, W.; Díaz, V. Validation of a Low-Cost Electromyography (EMG) System via a Commercial and Accurate EMG Device: Pilot Study. Sensors 2019, 19, 5214. [Google Scholar] [CrossRef] [PubMed]
- Me, R. Standards for Reporting EMG Data. J. Electromyogr. Kinesiol. 2018, 42, I–II. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Merletti, R.; Stegeman, D.; Blok, J.; Rau, G.; Disselhorst-Klug, C.; Hägg, G. European Recommendations for Surface ElectroMyoGraphy. Roessingh Res. Dev. 1999, 8, 13–54. [Google Scholar]
- Sarmiento, D.L.R. Digital Processing of Electromyographic Signals for Control. Available online: https://www.mathworks.com/matlabcentral/fileexchange/68245-digital-processing-of-electromyographic-signals-for-control (accessed on 24 January 2022).
- Liu, S.H.; Lin, C.B.; Chen, Y.; Chen, W.; Huang, T.S.; Hsu, C.Y. An EMG patch for the real-time monitoring of muscle-fatigue conditions during exercise. Sensors 2019, 19, 3108. [Google Scholar] [CrossRef]
- Venugopal, G.; Navaneethakrishna, M.; Ramakrishnan, S. Extraction and analysis of multiple time window features associated with muscle fatigue conditions using sEMG signals. Expert Syst. Appl. 2014, 41, 2652–2659. [Google Scholar] [CrossRef]
- Kumar, P.; Sebastian, A.; Potluri, C.; Yihun, Y.; Anugolu, M.; Creelman, J.; Urfer, A.; Naidu, D.S.; Schoen, M.P. Spectral analysis of sEMG signals to investigate skeletal muscle fatigue. In Proceedings of the 2011 50th IEEE Conference on Decision and Control and European Control Conference, Orlando, FL, USA, 12–15 December 2011; pp. 47–52. [Google Scholar] [CrossRef]
- Sadoyama, T.; Miyano, H. Frequency analysis of surface EMG to evaluation of muscle fatigue. Eur. J. Appl. Physiol. Occup. Physiol. 1981, 47, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Karthick, P.A.; Ramakrishnan, S. Surface electromyography based muscle fatigue progression analysis using modified B distribution time-frequency features. Biomed. Signal Process. Control 2016, 26, 42–51. [Google Scholar] [CrossRef]
- Naik, G.R. Applications, Challenges, and Advancements in Electromyography Signal Processing; IGI Global: Hershey, PA, USA, 2014. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Y.; Zhang, C.; Ruan, Z.; Meng, W.; Cai, Y.; Ai, Q. sEMG-Based Dynamic Muscle Fatigue Classification Using SVM with Improved Whale Optimization Algorithm. IEEE Internet Things J. 2021, 8, 16835–16844. [Google Scholar] [CrossRef]
- Edward Jeroa, S.; Divya Bharathia, K.; Karthickb, P.A.; Ramakrishnana, S. Muscle fatigue analysis in isometric contractions using geometric features of surface electromyography signals. Biomed. Signal Process. Control 2021, 68, 102603. [Google Scholar] [CrossRef]
- Al-Mulla, M.R.; Sepulveda, F.; Colley, M. Evolved pseudo-wavelet function to optimally decompose sEMG for automated classification of localized muscle fatigue. Med. Eng. Phys. 2011, 33, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Dotan, R.; Mitchell, C.; Cohen, R.; Klentrou, P.; Gabriel, D.; Falk, B. Child-adult differences in muscle activation—A review. Pediatr. Exerc. Sci. 2012, 24, 2–21. [Google Scholar] [CrossRef]
- Konrad, P. The ABC of EMG, A Practical Introduction to Kinesiological Electromyography; Noraxon Inc.: Scottsdale, AZ, USA, 2005; ISBN 0977162214. [Google Scholar]
- Chaytor, C.P.; Forman, D.; Byrne, J.; Loucks-Atkinson, A.; Power, K.E. Changes in muscle activity during the flexion and extension phases of arm cycling as an effect of power output are muscle-specific. PeerJ 2020, 8, e9759. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Y.; Zhang, Z.; Fang, Y.; Liu, H.; Yao, C. Exploring the relation between EMG sampling frequency and hand motion recognition accuracy. In Proceedings of the 2017 IEEE International Conference on Systems, Man, and Cybernetics (SMC), Banff, AB, Canada, 5–8 October 2017; pp. 1139–1144. [Google Scholar] [CrossRef]
- Cavallaro, E.E.; Rosen, J.; Perry, J.C.; Burns, S. Real-time myoprocessors for a neural controlled powered exoskeleton arm. IEEE Trans. Biomed. Eng. 2006, 53, 2387–2396. [Google Scholar] [CrossRef]


| Ref | Accuracy % |
|---|---|
| [34] | 88.4 |
| [35] | 90 |
| [36] | 90 |
| [37] | 93.5 |
| [38] | 94 |
| [39] | 79.4 |
| Mean (μ) = 89.21 |
| Parameter | Detail |
|---|---|
| Input Impedance | 110 GΩ |
| Supply Voltage | 3.3 V |
| Common Mode Rejection Ratio (CMRR) | 110 |
| Gain | Adjustable |
| Reference | Middle Deltoid Muscle | Mean Frequency (MNF) Hz | Median Frequency (MDF) Hz | Reference | Middle Deltoid Muscle | Mean Frequency (MNF) Hz | Median Frequency (MDF) Hz |
|---|---|---|---|---|---|---|---|
| Subject 1 | Left muscle | 85 | 73 | Subject 20 | Left muscle | 79 | 72 |
| Right muscle | 84 | 76 | Right muscle | 85 | 76 | ||
| Subject 2 | Left muscle | 83 | 77 | Subject 21 | Left muscle | 78 | 70 |
| Right muscle | 98 | 90 | Right muscle | 84 | 78 | ||
| Subject 3 | Left muscle | 88 | 80 | Subject 22 | Left muscle | 84 | 76 |
| Right muscle | Not-Examined | Not-Examined | Right muscle | 86 | 78 | ||
| Subject 4 | Left muscle | 85 | 70 | Subject 23 | Left muscle | 83 | 75 |
| Right muscle | 84 | 73 | Right muscle | 79 | 74 | ||
| Subject 5 | Left muscle | 88 | 79 | Subject 24 | Left muscle | 87 | 79 |
| Right muscle | 89 | 80 | Right muscle | 72 | 68 | ||
| Subject 6 | Left muscle | 77 | 72 | Subject 25 | Left muscle | 75 | 70 |
| Right muscle | 89 | 84 | Right muscle | 85 | 75 | ||
| Subject 7 | Left muscle | 69 | 67 | Subject 26 | Left muscle | 86 | 80 |
| Right muscle | 65 | 63 | Right muscle | 83 | 75 | ||
| Subject 8 | Left muscle | 89 | 80 | Subject 27 | Left muscle | 93 | 86 |
| Right muscle | 83 | 77 | Right muscle | 83 | 75 | ||
| Subject 9 | Left muscle | 86 | 73 | Subject 28 | Left muscle | 89 | 77 |
| Right muscle | 96 | 89 | Right muscle | 83 | 76 | ||
| Subject 10 | Left muscle | 89 | 81 | Subject 29 | Left muscle | 77 | 73 |
| Right muscle | 90 | 83 | Right muscle | 86 | 78 | ||
| Subject 11 | Left muscle | 86 | 75 | Subject 30 | Left muscle | 85 | 80 |
| Right muscle | 92 | 85 | Right muscle | 90 | 80 | ||
| Subject 12 | Left muscle | 87 | 80 | Subject 31 | Left muscle | 89 | 82 |
| Right muscle | 89 | 82 | Right muscle | 84 | 75 | ||
| Subject 13 | Left muscle | 88 | 78 | Subject 32 | Left muscle | 82 | 74 |
| Right muscle | 93 | 84 | Right muscle | 84 | 76 | ||
| Subject 14 | Left muscle | 83 | 74 | Subject 33 | Left muscle | 81 | 75 |
| Right muscle | 81 | 73 | Right muscle | 85 | 74 | ||
| Subject 15 | Left muscle | 82 | 74 | Subject 34 | Left muscle | 86 | 74 |
| Right muscle | 84 | 79 | Right muscle | 83 | 76 | ||
| Subject 16 | Left muscle | 86 | 75 | Subject 35 | Left muscle | 85 | 78 |
| Right muscle | 85 | 76 | Right muscle | 83 | 76 | ||
| Subject 17 | Left muscle | 82 | 74 | Subject 36 | Left muscle | 93 | 88 |
| Right muscle | 87 | 77 | Right muscle | 79 | 73 | ||
| Subject 18 | Left muscle | 81 | 74 | Subject 37 | Left muscle | 89 | 82 |
| Right muscle | 92 | 86 | Right muscle | 80 | 72 | ||
| Subject 19 | Left muscle | 80 | 72 | Subject 38 | Left muscle | 87 | 76 |
| Right muscle | 87 | 81 | Right muscle | 84 | 76 |
| Reference | Middle Deltoid Muscle | Slope’s Value Non-Fatigue | Slope’s Value Fatigue | Reference | Middle Deltoid Muscle | Slope’s Value Non-Fatigue | Slope’s Value Fatigue |
|---|---|---|---|---|---|---|---|
| Subject 1 | Left muscle | −0.0018 | 0.038 | Subject 20 | Left muscle | −0.00011 | 0.037 |
| Right muscle | −0.0038 | 0.4 | Right muscle | −0.00004 | 0.045 | ||
| Subject 2 | Left muscle | −0.00016 | 0.14 | Subject 21 | Left muscle | −0.0002 | 0.203 |
| Right muscle | −0.0002 | 1.4 | Right muscle | −0.00234 | 0.038 | ||
| Subject 3 | Left muscle | −0.00081 | 0.15 | Subject 22 | Left muscle | −0.0018 | 0.04 |
| Right muscle | Not-Examined | Not-Examined | Right muscle | −0.0007 | 0.8 | ||
| Subject 4 | Left muscle | −0.0021 | 0.057 | Subject 23 | Left muscle | −0.00016 | 0.16 |
| Right muscle | −0.0016 | 0.074 | Right muscle | −0.0002 | 1.8 | ||
| Subject 5 | Left muscle | −0.00021 | 0.0014 | Subject 24 | Left muscle | −0.00009 | 0.6 |
| Right muscle | −0.00001 | 0.014 | Right muscle | −0.0021 | 0.1 | ||
| Subject 6 | Left muscle | −0.00015 | 0.33 | Subject 25 | Left muscle | 0.0008 | 0.08 |
| Right muscle | −0.00008 | 0.28 | Right muscle | −0.00021 | 0.002 | ||
| Subject 7 | Left muscle | −0.0017 | 0.15 | Subject 26 | Left muscle | −0.0056 | 0.023 |
| Right muscle | −0.0014 | 0.098 | Right muscle | −0.00078 | 0.23 | ||
| Subject 8 | Left muscle | −0.0046 | 0.048 | Subject 27 | Left muscle | −0.0067 | 0.28 |
| Right muscle | −0.0043 | 0.053 | Right muscle | −0.0067 | 0.18 | ||
| Subject 9 | Left muscle | −0.0013 | 0.27 | Subject 28 | Left muscle | −0.009 | 0.1 |
| Right muscle | −0.00008 | 0.09 | Right muscle | −0.0067 | 0.05 | ||
| Subject 10 | Left muscle | −0.005 | 0.087 | Subject 29 | Left muscle | −0.0021 | 0.06 |
| Right muscle | −0.0073 | 0.09 | Right muscle | 0.0034 | 0.15 | ||
| Subject 11 | Left muscle | −0.0002 | 0.024 | Subject 30 | Left muscle | −0.00076 | 0.1 |
| Right muscle | −0.00023 | 0.059 | Right muscle | −0.0099 | 0.02 | ||
| Subject 12 | Left muscle | −0.0044 | 0.027 | Subject 31 | Left muscle | −0.00045 | 0.96 |
| Right muscle | −0.000013 | 0.0044 | Right muscle | −0.0002 | 0.024 | ||
| Subject 13 | Left muscle | −0.00067 | 0.036 | Subject 32 | Left muscle | −0.0045 | 0.033 |
| Right muscle | −0.0026 | 0.21 | Right muscle | −0.0045 | 0.03 | ||
| Subject 14 | Left muscle | −0.00001 | 0.039 | Subject 33 | Left muscle | −0.344 | 0.005 |
| Right muscle | −0.000005 | 0.022 | Right muscle | −0.00067 | 0.068 | ||
| Subject 15 | Left muscle | −0.000007 | 0.045 | Subject 34 | Left muscle | −0.00045 | 0.2 |
| Right muscle | −0.00057 | 0.024 | Right muscle | −0.00001 | 0.04 | ||
| Subject 16 | Left muscle | −0.00023 | 0.02 | Subject 35 | Left muscle | −0.004 | 0.05 |
| Right muscle | −0.00012 | 0.019 | Right muscle | −0.0067 | 0.05 | ||
| Subject 17 | Left muscle | −0.0009 | 0.047 | Subject 36 | Left muscle | −0.00057 | 0.033 |
| Right muscle | −0.0022 | 0.019 | Right muscle | −0.00023 | 0.02 | ||
| Subject 18 | Left muscle | −0.0033 | 0.085 | Subject 37 | Left muscle | −0.0067 | 0.03 |
| Right muscle | −0.00067 | 0.011 | Right muscle | −0.0006 | 0.06 | ||
| Subject 19 | Left muscle | −0.0006 | 0.05 | Subject 38 | Left muscle | −0.000046 | 0.035 |
| Right muscle | −0.0003 | 0.05 | Right muscle | −0.0000078 | 0.019 |
| Reference | Middle Deltoid Muscle | Fatigue Index Value | Time Consumed (s) | Reference | Middle Deltoid Muscle | Fatigue Index Value | Time Consumed (s) | ||
|---|---|---|---|---|---|---|---|---|---|
| First Segment (No Fatigue) | Last Segment (Fatigue Reached) | First Segment (No Fatigue) | Last Segment (Fatigue Reached) | ||||||
| Subject 1 | Left muscle | −0.682 | 0.063 | 25 | Subject 20 | Left muscle | −0.438 | 0.068 | 41 |
| Right muscle | −0.648 | 0.072 | 31 | Right muscle | −0.398 | 0.045 | 56 | ||
| Subject 2 | Left muscle | −0.468 | 0.006 | 33 | Subject 21 | Left muscle | −0.425 | 0.041 | 44 |
| Right muscle | −0.248 | 0.003 | 60 | Right muscle | −0.438 | 0.009 | 68 | ||
| Subject 3 | Left muscle | −0.314 | 0.010 | 70 | Subject 22 | Left muscle | −0.145 | 0.019 | 94 |
| Right muscle | Not-Examined | Not-Examined | Not-Examined | Right muscle | −0.245 | 0.067 | 100 | ||
| Subject 4 | Left muscle | −0.122 | 0.021 | 94 | Subject 23 | Left muscle | −0.488 | 0.017 | 67 |
| Right muscle | −0.142 | 0.096 | 100 | Right muscle | −0.134 | 0.003 | 83 | ||
| Subject 5 | Left muscle | −0.300 | 0.020 | 51 | Subject 24 | Left muscle | −0.257 | 0.001 | 85 |
| Right muscle | −0.266 | 0.004 | 113 | Right muscle | −0.490 | 0.011 | 95 | ||
| Subject 6 | Left muscle | −0.239 | 0.002 | 109 | Subject 25 | Left muscle | −0.130 | 0.023 | 53 |
| Right muscle | −0.558 | 0.001 | 115 | Right muscle | −0.290 | −0.023 | 34 | ||
| Subject 7 | Left muscle | −0.126 | 0.033 | 67 | Subject 26 | Left muscle | −0.290 | 0.027 | 42 |
| Right muscle | −0.253 | 0.050 | 75 | Right muscle | −0.309 | 0.012 | 54 | ||
| Subject 8 | Left muscle | −0.203 | −0.053 | 12 | Subject 27 | Left muscle | −0.463 | 0.018 | 80 |
| Right muscle | −0.326 | 0.022 | 20 | Right muscle | −0.523 | 0.017 | 85 | ||
| Subject 9 | Left muscle | −0.363 | 0.027 | 85 | Subject 28 | Left muscle | −0.285 | 0.008 | 49 |
| Right muscle | −0.450 | 0.012 | 92 | Right muscle | −0.137 | 0.062 | 76 | ||
| Subject 10 | Left muscle | −0.431 | 0.005 | 45 | Subject 29 | Left muscle | −0.389 | 0.010 | 70 |
| Right muscle | −0.428 | 0.071 | 83 | Right muscle | −0.652 | 0.023 | 76 | ||
| Subject 11 | Left muscle | −0.358 | 0.020 | 73 | Subject 30 | Left muscle | −0.398 | 0.023 | 81 |
| Right muscle | −0.454 | 0.003 | 75 | Right muscle | −0.378 | 0.034 | 89 | ||
| Subject 12 | Left muscle | −0.424 | −0.012 | 65 | Subject 31 | Left muscle | −0.289 | 0.028 | 59 |
| Right muscle | −0.473 | 0.023 | 66 | Right muscle | −0.478 | 0.035 | 69 | ||
| Subject 13 | Left muscle | −0.479 | 0.037 | 61 | Subject 32 | Left muscle | −0.145 | 0.012 | 65 |
| Right muscle | −0.518 | 0.012 | 74 | Right muscle | −0.537 | −0.059 | 59 | ||
| Subject 14 | Left muscle | −0.396 | 0.019 | 56 | Subject 33 | Left muscle | −0.405 | 0.047 | 60 |
| Right muscle | −0.439 | 0.033 | 67 | Right muscle | −0.407 | 0.063 | 72 | ||
| Subject 15 | Left muscle | −0.427 | 0.009 | 58 | Subject 34 | Left muscle | −0.537 | 0.059 | 59 |
| Right muscle | −0.363 | 0.045 | 64 | Right muscle | −0.469 | 0.033 | 85 | ||
| Subject 16 | Left muscle | −0.415 | −0.079 | 52 | Subject 35 | Left muscle | −0.425 | 0.006 | 63 |
| Right muscle | −0.340 | 0.023 | 92 | Right muscle | −0.526 | 0.032 | 78 | ||
| Subject 17 | Left muscle | −0.352 | 0.016 | 52 | Subject 36 | Left muscle | −0.397 | 0.048 | 49 |
| Right muscle | −0.523 | 0.002 | 78 | Right muscle | −0.465 | 0.063 | 65 | ||
| Subject 18 | Left muscle | −0.392 | 0.098 | 52 | Subject 37 | Left muscle | −0.324 | 0.055 | 59 |
| Right muscle | −0.560 | 0.003 | 69 | Right muscle | −0.372 | 0.034 | 73 | ||
| Subject 19 | Left muscle | −0.521 | 0.004 | 49 | Subject 38 | Left muscle | −0.426 | 0.056 | 64 |
| Right muscle | −0.482 | 0.022 | 65 | Right muscle | −0.293 | 0.036 | 70 | ||
| Reference | Middle Deltoid Muscle | Segments Fatigue Classification | |||||
|---|---|---|---|---|---|---|---|
| 1st Seg. | 2nd Seg. | 3rd Seg. | (N−2)th Seg. | (N−1)th Seg. | Nth Seg. | ||
| Subject 1 | Left muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue |
| Right muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue | |
| Subject 2 | Left muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue |
| Right muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue | Fatigue | |
| Subject 3 | Left muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue |
| Right muscle | Not-Examined | Not-Examined | Not-Examined | Not-Examined | Not-Examined | Not-Examined | |
| Subject 4 | Left muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue |
| Right muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue | |
| Subject 5 | Left muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue |
| Right muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue | |
| Subject 6 | Left muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue |
| Right muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue | |
| Subject 7 | Left muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue |
| Right muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue | |
| Subject 8 | Left muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue |
| Right muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue | |
| Subject 9 | Left muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue |
| Right muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue | |
| Subject 10 | Left muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue |
| Right muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue | |
| Subject 11 | Left muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue |
| Right muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue | |
| Subject 12 | Left muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue |
| Right muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue | |
| Subject 13 | Left muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue |
| Right muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue | |
| Subject 14 | Left muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue |
| Right muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue | |
| Subject 15 | Left muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue |
| Right muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue | |
| Subject 16 | Left muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue |
| Right muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue | |
| Subject 17 | Left muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue |
| Right muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue | |
| Subject 18 | Left muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue |
| Right muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue | |
| Subject 19 | Left muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue |
| Right muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue | |
| Subject 20 | Left muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue |
| Right muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue | |
| Subject 21 | Left muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue |
| Right muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue | |
| Subject 22 | Left muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue |
| Right muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue | |
| Subject 23 | Left muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue |
| Right muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue | |
| Subject 24 | Left muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue |
| Right muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue | |
| Subject 25 | Left muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue |
| Right muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | |
| Subject 26 | Left muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue |
| Right muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue | |
| Subject 27 | Left muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue |
| Right muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue | |
| Subject 28 | Left muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue |
| Right muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue | |
| Subject 29 | Left muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue | Fatigue |
| Right muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue | |
| Subject 30 | Left muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue |
| Right muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue | |
| Subject 31 | Left muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue |
| Right muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue | |
| Subject 32 | Left muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue |
| Right muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | |
| Subject 33 | Left muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue |
| Right muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue | |
| Subject 34 | Left muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue |
| Right muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue | |
| Subject 35 | Left muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue |
| Right muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue | |
| Subject 36 | Left muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue |
| Right muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue | |
| Subject 37 | Left muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue |
| Right muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue | Fatigue | |
| Subject 38 | Left muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue |
| Right muscle | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Non-fatigue | Fatigue | |
| Reference | Type of Employed Classifier | Obtained Accuracy |
|---|---|---|
| [59] | Support Vector Machine (SVM) | 85.5% |
| [60] | Multilayer Perceptron (MLP) | 86% |
| [26] | Support Vector Machine (SVM) | 91.39% |
| [61] | Linear Discriminant Analysis (LDA) | 88.41% |
| Our work | Double-Step Binary Classifier | 94.66% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qassim, H.M.; Hasan, W.Z.W.; Ramli, H.R.; Harith, H.H.; Mat, L.N.I.; Ismail, L.I. Proposed Fatigue Index for the Objective Detection of Muscle Fatigue Using Surface Electromyography and a Double-Step Binary Classifier. Sensors 2022, 22, 1900. https://doi.org/10.3390/s22051900
Qassim HM, Hasan WZW, Ramli HR, Harith HH, Mat LNI, Ismail LI. Proposed Fatigue Index for the Objective Detection of Muscle Fatigue Using Surface Electromyography and a Double-Step Binary Classifier. Sensors. 2022; 22(5):1900. https://doi.org/10.3390/s22051900
Chicago/Turabian StyleQassim, Hassan M., Wan Zuha Wan Hasan, Hafiz R. Ramli, Hazreen Haizi Harith, Liyana Najwa Inche Mat, and Luthffi Idzhar Ismail. 2022. "Proposed Fatigue Index for the Objective Detection of Muscle Fatigue Using Surface Electromyography and a Double-Step Binary Classifier" Sensors 22, no. 5: 1900. https://doi.org/10.3390/s22051900
APA StyleQassim, H. M., Hasan, W. Z. W., Ramli, H. R., Harith, H. H., Mat, L. N. I., & Ismail, L. I. (2022). Proposed Fatigue Index for the Objective Detection of Muscle Fatigue Using Surface Electromyography and a Double-Step Binary Classifier. Sensors, 22(5), 1900. https://doi.org/10.3390/s22051900







