Detection of β-Lactoglobulin by a Porous Silicon Microcavity Biosensor Based on the Angle Spectrum
Abstract
:1. Introduction
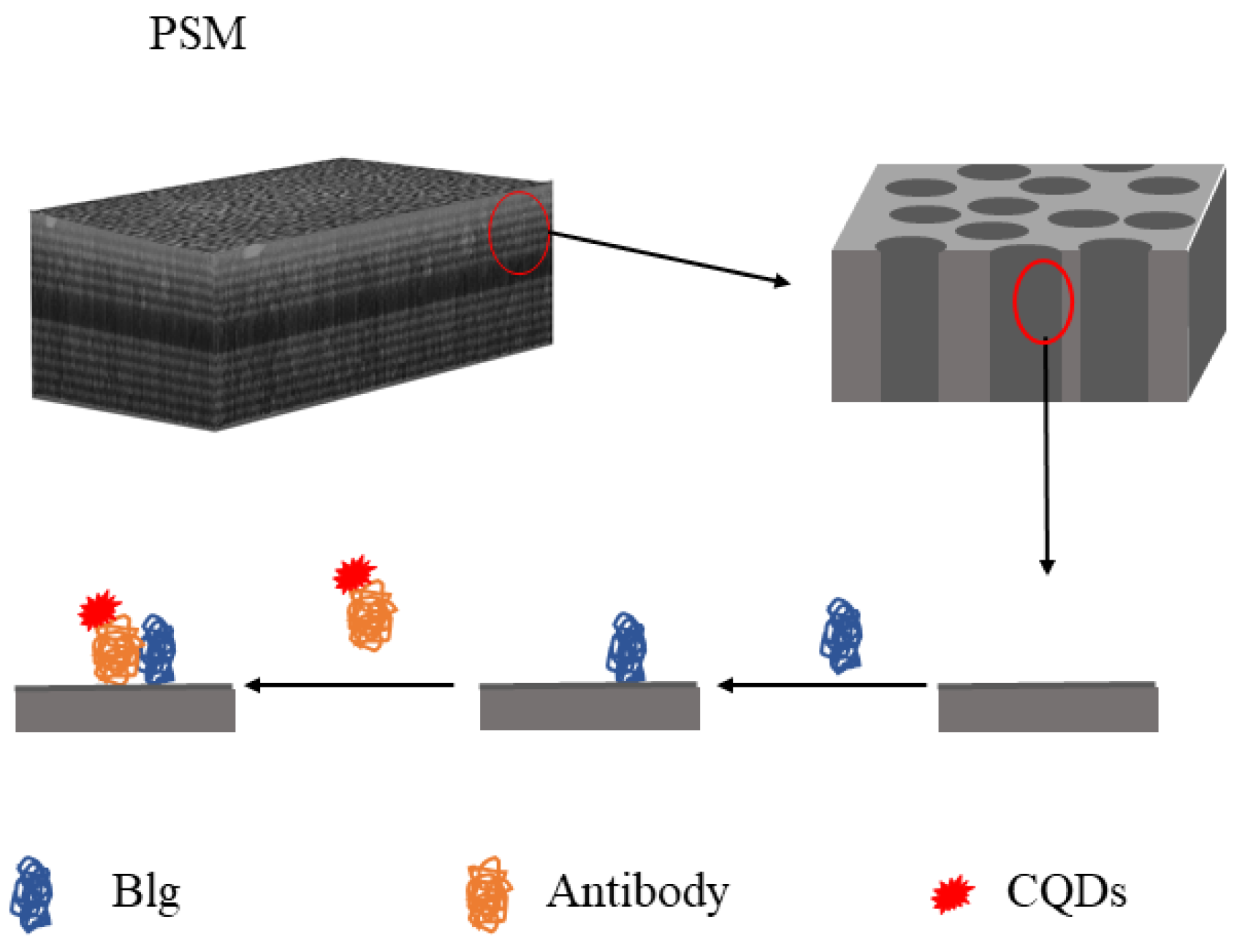
2. Experimental Principle
3. Materials and Methods
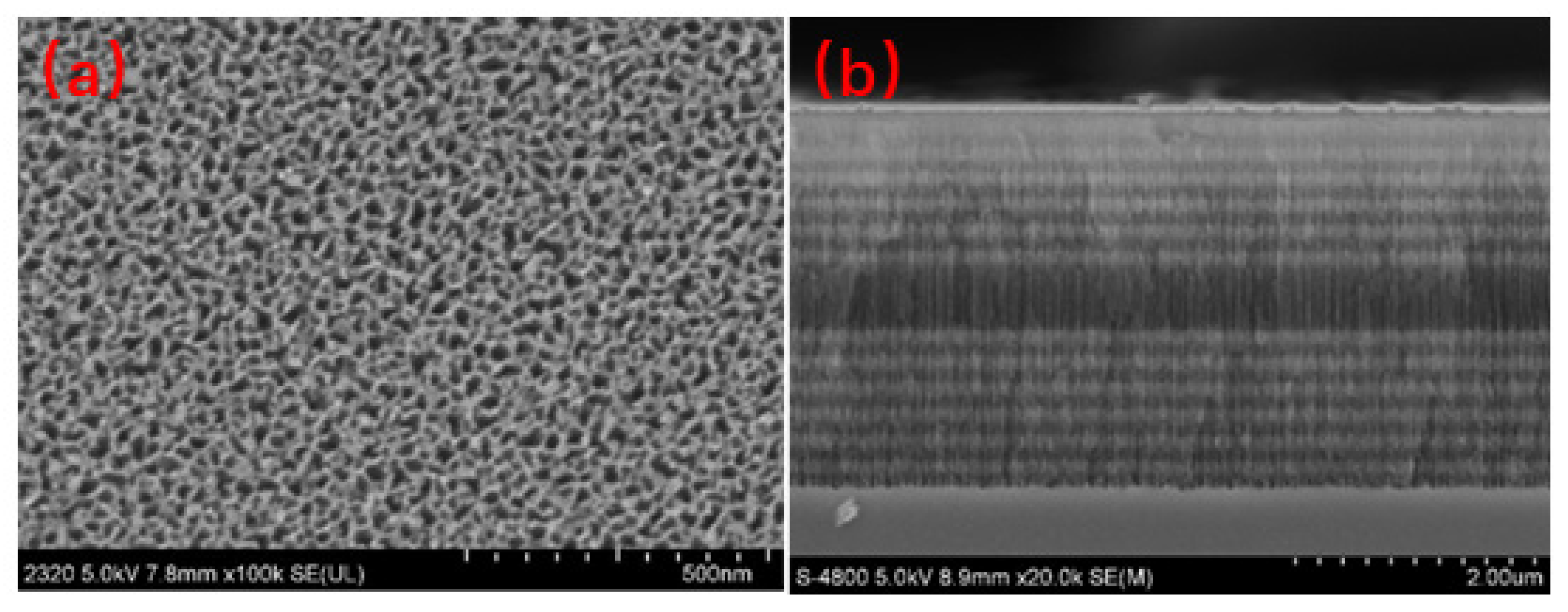
3.1. Preparation of the PSM
3.2. Functionalization of the PSM
3.3. Preparation of the Antibody Serum
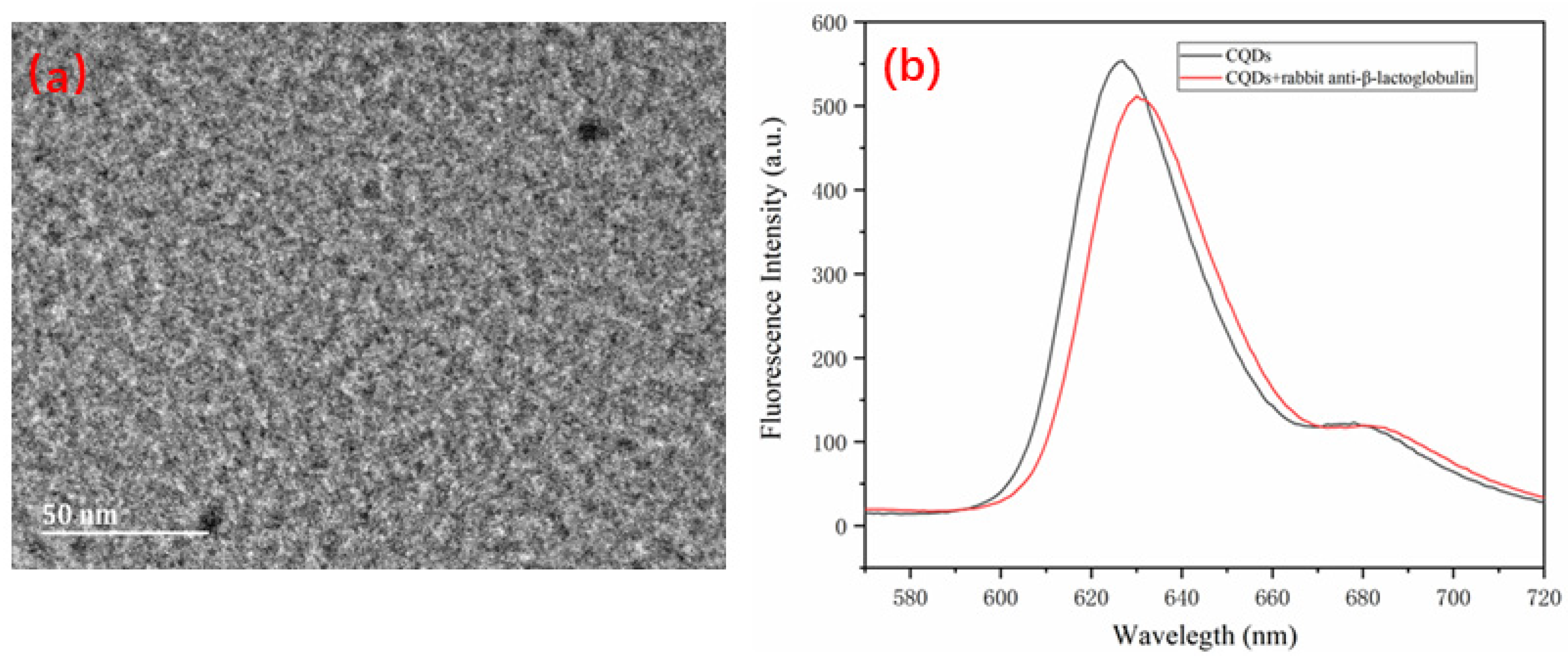
3.4. Preparation of the β-LG Antigen and Quantum-Dot-Modified β-LG Antibody
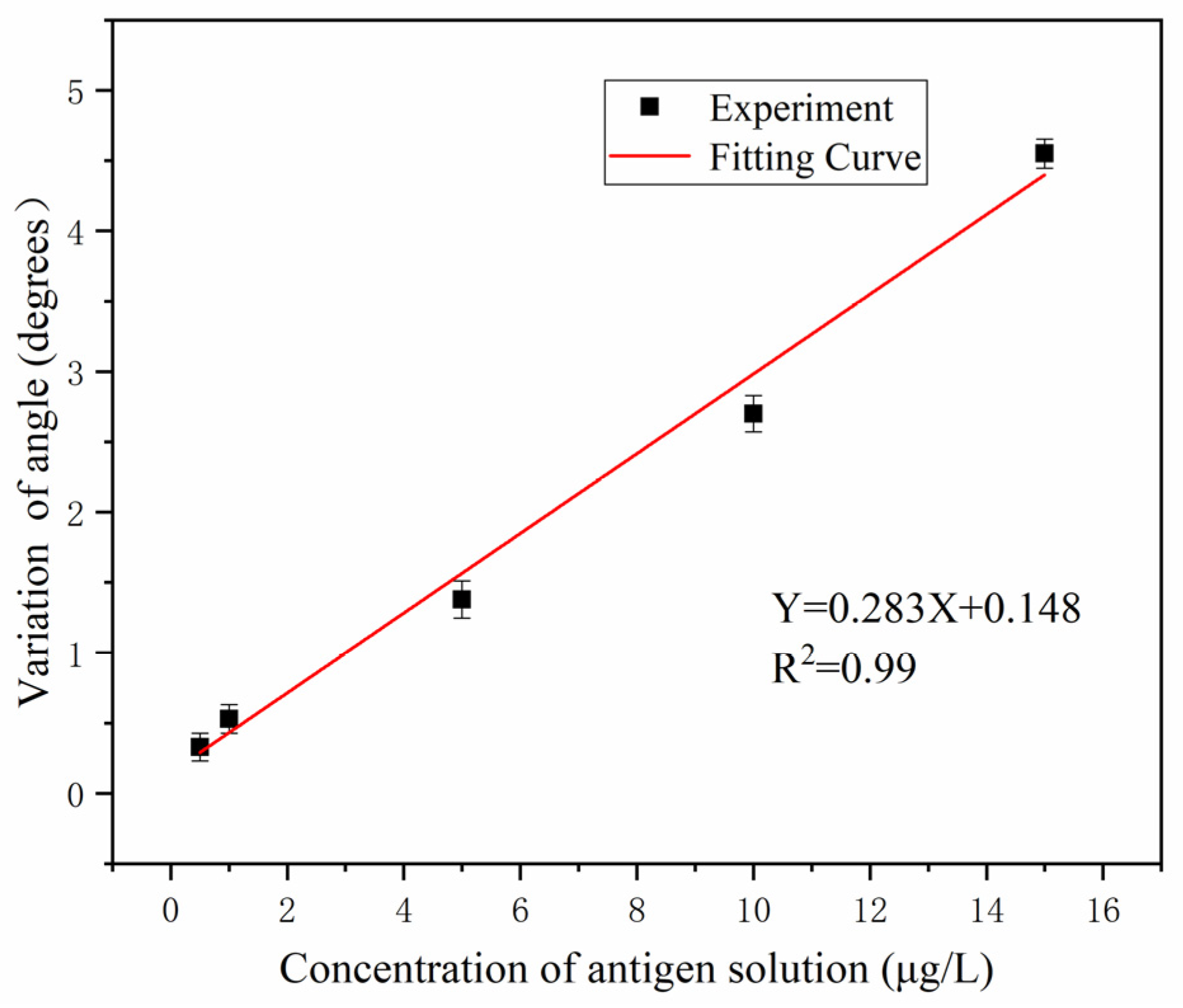
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Host, A.; Halken, S. A prospective study of cow milk allergy in Danish infants during the first 3 years of life. Clinical course in relation to clinical and immunological type of hypersensitivity reaction. Allergy 1990, 45, 587–596. [Google Scholar] [CrossRef]
- Exl, B.M. A review of recent developments in the use of moderately hydrolyzed whey formulae in infant nutrition. Nutr. Res. 2001, 21, 355–379. [Google Scholar] [CrossRef]
- Chaudhari, P.S.; Gokarna, A.; Kulkarni, M.; Karve, M.S.; Bhoraskar, S.V. Porous silicon as an entrapping matrix for the immobilization of urease. Sens. Actuators B Chem. 2005, 107, 258–263. [Google Scholar] [CrossRef]
- Meskini, O.; Abdelghani, A.; Tlili, A.; Mgaieth, R.; Jaffrezic-Renault, N.; Martelet, C. Porous silicon as functionalized material for immunosensor application. Talanta 2007, 71, 1430–1433. [Google Scholar] [CrossRef]
- Wei, X.; Weiss, S.M. Guided mode biosensor based on grating coupled porous silicon waveguide. Opt. Express 2011, 19, 11330. [Google Scholar] [CrossRef]
- Wang, J.; Jia, Z. Metal Nanoparticles/Porous Silicon Microcavity Enhanced Surface Plasmon Resonance Fluorescence for the Detection of DNA. Sensors 2018, 18, 661. [Google Scholar] [CrossRef] [Green Version]
- Maniya, N.H.; Srivastava, D.N. Fabrication of porous silicon-based label-free optical biosensor for heat shock protein 70 detection. Mater. Sci. Semicond. Process. 2020, 115, 105126. [Google Scholar] [CrossRef]
- Myndrul, V.; Viter, R.; Savchuk, M.; Koval, M.; Starodub, N.; Silamiķelis, V.; Smyntyna, V.; Ramanavicius, A.; Iatsunskyi, I. Gold coated porous silicon nanocomposite as a substrate for photoluminescence-based immunosensor suitable for the determination of aflatoxin b1. Talanta 2017, 175, 297–304. [Google Scholar] [CrossRef]
- Zhou, Z.; Sohn, H. A Novel Chemical Gas Vapor Sensor Based on Photoluminescence Enhancement of Rugate Porous Silicon Filters. Sensors 2020, 20, 2722. [Google Scholar] [CrossRef]
- Dardano, P.; Caliò, A.; Politi, J.; Rea, I.; Rendina, I.; De Stefano, L.E. Optically monitored drug delivery patch based on porous silicon and polymer microneedles. Biomed. Opt. Express 2016, 7, 1645–1655. [Google Scholar] [CrossRef] [Green Version]
- Bahadori, H.; Majidi, M.R.; Alipour, E.J. An electrochemical sensor for simultaneous determination of some pharmaceutical compounds using ionic liquid and Pd nanoparticles supported on porous silicon doped carbon-ceramic electrode as a renewable surface composite electrode. Microchem. J. 2021, 161, 105724. [Google Scholar] [CrossRef]
- Choi, M.S.; Na, H.G.; Mirzaei, A.; Bang, J.H.; Oum, W.; Han, S.; Choi, S.-W.; Kim, M.; Jin, C.; Kim, S.S.; et al. Room-temperature NO2 sensor based on electrochemically etched porous silicon. J. Alloys Compd. 2019, 811, 151975. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, J.; Jia, Z. Detection of Ammonia-Oxidizing Bacteria (AOB) Using a Porous Silicon Optical Biosensor Based on a Multilayered Double Bragg Mirror Structure. Sensors 2018, 18, 105. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Lawrie, J.L.; Beavers, K.R.; Laibinis, P.E.; Weiss, S.M. Effect of DNA-Induced Corrosion on Passivated Porous Silicon Biosensors. ACS Appl. Mater. Interfaces 2014, 6, 13510–13519. [Google Scholar] [CrossRef]
- Li, P.; Jia, Z.; Lu, X.; Liu, Y.; Ning, X.; Mo, J.; Wang, J. Spectrometer-free biological detection method using porous silicon microcavity devices. Opt. Express 2015, 23, 24626–24633. [Google Scholar] [CrossRef]
- Vilensky, R.; Bercovici, M.; Segal, E. Oxidized Porous Silicon Nanostructures Enabling Electrokinetic Transport for Enhanced DNA Detection. Adv. Funct. Mater. 2015, 25, 6725–6732. [Google Scholar] [CrossRef]
- Li, Y.; Jia, Z.; Lv, G.; Wen, H.; Li, P.; Zhang, H.; Wang, J. Detection of Echinococcus granulosus antigen by a quantum dot/porous silicon optical biosensor. Biomed. Opt. Express 2017, 8, 3458–3469. [Google Scholar] [CrossRef] [PubMed]
- Kou, D.; Zhang, S.; Lutkenhaus, J.; Wang, L.; Tang, B.; Ma, W.J. Porous organic/inorganic hybrid one-dimensional photonic crystals for rapid visual detection of organic solvents. J. Mater. Chem. C 2018, 6, 2704–2711. [Google Scholar] [CrossRef]
- Massad-Ivanir, N.; Bhunia, S.K.; Jelinek, R.; Segal, E. Porous Silicon Bragg Reflector/Carbon Dot Hybrids: Synthesis, Nanostructure, and Optical Properties. Front. Chem. 2018, 6, 574. [Google Scholar] [CrossRef] [Green Version]
- Surdo, S.; Barillaro, G. Impact of Fabrication and Bioassay Surface Roughness on the Performance of Label-Free Resonant Biosensors Based on One-Dimensional Photonic Crystal Microcavities. ACS Sens. 2020, 5, 2894–2902. [Google Scholar] [CrossRef]
- Mangaiyarkarasi, D.; Breese, M.; Ow, Y.S.; Vijila, C. Controlled blueshift of the resonant wavelength in porous silicon microcavities using ion irradiation. Appl. Phys. Lett. 2020, 89, 021910. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Jia, Z.; Lv, C. Enhanced Raman scattering in porous silicon grating. Opt. Express 2018, 26, 6507. [Google Scholar] [CrossRef]
- Li, S.; Huang, J.; Cai, L. A porous silicon optical microcavity for sensitive bacteria detection. Nanotechnology 2011, 22, 425502. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Chen, L.; Zhang, H.; Mo, J.; Zhong, F.; Lv, C.; Ma, J.; Jia, Z. Hybridization assay of insect antifreezing protein gene by novel multilayered porous silicon nucleic acid biosensor. Biosens. Bioelectron. 2013, 39, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Macleod, H.A. Thin-Film Optical Filters; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Lv, X.Y.; Mo, J.Q.; Tu, Y.X.; Zhong, F.R.; Jiang, T.; Jia, Z.H.; Zhang, L. Porous silicon optical cavity as an immunosensor platform. Opt. Lett. 2010, 6, 314–316. [Google Scholar] [CrossRef]
- Zhou, R.; Jia, Z.; Lv, X.; Huang, H. The Enhanced Sensitivity of a Porous Silicon Microcavity Biosensor Based on an Angular Spectrum Using CdSe/ZnS Quantum Dots. Sensors 2019, 19, 4872. [Google Scholar] [CrossRef] [Green Version]
- Yuan, F.; Li, S.; Fan, Z.; Meng, X.; Fan, L.; Yang, S. Shining carbon dots: Synthesis and biomedical and optoelectronic applications. Nano Today 2016, 11, 565–586. [Google Scholar] [CrossRef]
- Du, Y.; Guo, S. Chemically doped fluorescent carbon and graphene quantum dots for bioimaging, sensor, catalytic and photoelectronic applications. Nanoscale 2016, 8, 2532–2543. [Google Scholar] [CrossRef]
- Demir, B.; Lemberger, M.; Panagiotopoulou, M.; Medina-Rangel, P.; Timur, S.; Hirsch, T.; Bernadette, T.S.B.; Wegener, J.; Haupt, K. Tracking Hyaluronan: Molecularly Imprinted Polymer Coated Carbon Dots for Cancer Cell Targeting and Imaging. ACS Appl. Mater. Interfaces 2018, 10, 3305–3313. [Google Scholar] [CrossRef]
- Ray, S.C.; Saha, A.; Jana, N.R.; Sarkar, R. Fluorescent Carbon Nanoparticles: Synthesis, Characterization, and Bioimaging Application. J. Phys. Chem. C 2009, 113, 18546–18551. [Google Scholar] [CrossRef]
- Luo, P.G.; Sahu, S.; Yang, S.T.; Sonkar, S.K.; Sun, Y.-P. Carbon “quantum” dots for optical bioimaging. Theranostics 2013, 1, 2116–2127. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhang, Y.; Li, B. Ultracompact wavelength and polarization splitters in periodic dielectric waveguides. Opt. Express 2008, 16, 1600–1609. [Google Scholar] [CrossRef]
- Ma, N.; Jiang, W.; Li, T.; Zhang, Z.; Yang, M. Fluorescence aggregation assay for the protein biomarker mucin 1 using carbon dot-labeled antibodies and aptamers. Microchim. Acta 2015, 182, 443–447. [Google Scholar] [CrossRef]
- He, S.; Li, X.; Gao, J.; Tong, P.; Chen, H. Development of a H2O2-sensitive quantum dots-based fluorescent sandwich ELISA for sensitive detection of bovine β-lactoglobulin by monoclonal antibody. J. Sci. Food Agric. 2018, 98, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; He, W.; Ji, K.; Wan, W.; Hu, D.; Hui, W.; Luo, X.; Liu, Z. A Simple and Fast Detection Method for Bovine Milk Residues in Foods: A2-Site Monoclonal Antibody Immunochromatography Assay. J. Food Sci. 2013, 78, M452–M457. [Google Scholar]
- Ruiz-Valdepeñas Montiel, V.; Campuzano, S.; Conzuelo, F.; Torrente-Rodríguez, R.M.; Gamella, M.; Reviejo, A.J.; Pingarrón, J.M. Electrochemical magnetoimmunosensing platform for determination of the milk allergen β-lactoglobulin. Talanta 2015, 131, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Ashley, J.; D’Aurelio, R.; Piekarska, M.; Temblay, J.; Pleasants, M.; Trinh, L.; Rodgers, T.L.; Tothill, I.E. Development of a β-Lactoglobulin Sensor Based on SPR for Milk Allergens Detection. Biosensors 2018, 8, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, L.; Gao, Y.; Wang, J.; Aili, T.; Jia, Z.; Lv, X.; Huang, X.; Yang, J. Detection of β-Lactoglobulin by a Porous Silicon Microcavity Biosensor Based on the Angle Spectrum. Sensors 2022, 22, 1912. https://doi.org/10.3390/s22051912
Bai L, Gao Y, Wang J, Aili T, Jia Z, Lv X, Huang X, Yang J. Detection of β-Lactoglobulin by a Porous Silicon Microcavity Biosensor Based on the Angle Spectrum. Sensors. 2022; 22(5):1912. https://doi.org/10.3390/s22051912
Chicago/Turabian StyleBai, Lanlan, Yun Gao, Jiajia Wang, Tuerxunnayi Aili, Zhenhong Jia, Xiaoyi Lv, Xiaohui Huang, and Jie Yang. 2022. "Detection of β-Lactoglobulin by a Porous Silicon Microcavity Biosensor Based on the Angle Spectrum" Sensors 22, no. 5: 1912. https://doi.org/10.3390/s22051912




