Hardware Selection and Performance of Low-Cost Fluorometers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fluorometer Design and Components Tested
2.2. Experiment 1: Benchmarking Low-Cost Fluorometer to Commercial Unit Performance
2.3. Experiment 2: Assessment of the Functional Resolution and Sensitivity of Low-Cost Sensors
2.4. Experiment 3: Robustness of Performance with Temperature and Turbidity
3. Results
3.1. Low-Cost Fluorometer Performance
3.1.1. Comparison of Components for Low-Cost Fluorometers (Bit Rate and Sampling Frequency)
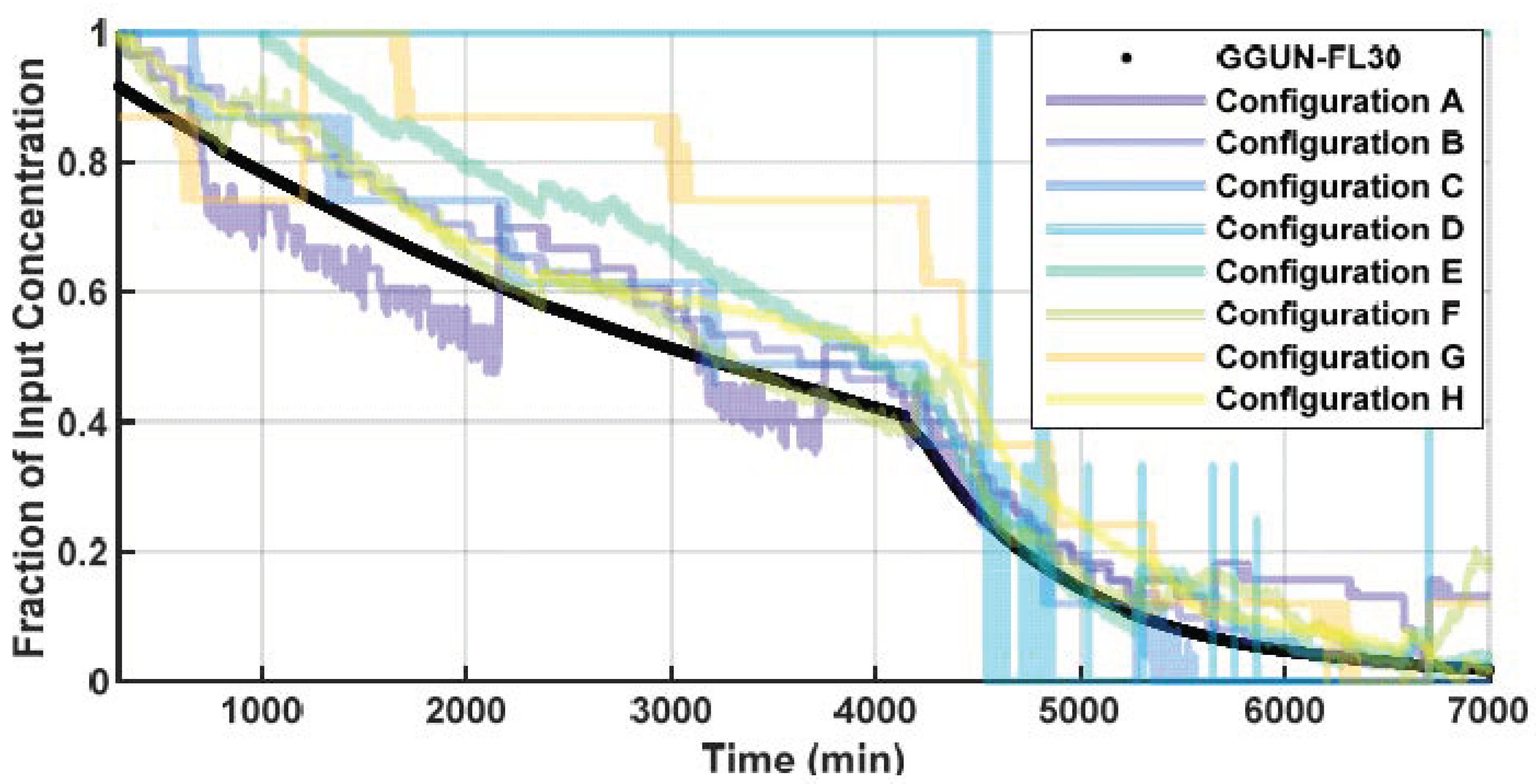
3.1.2. Performance Comparison of Low-Cost vs. Commercial (Comparison to GGUN)
3.2. Sensitivity of Low-Cost Fluorometers to Temperature and Turbidity
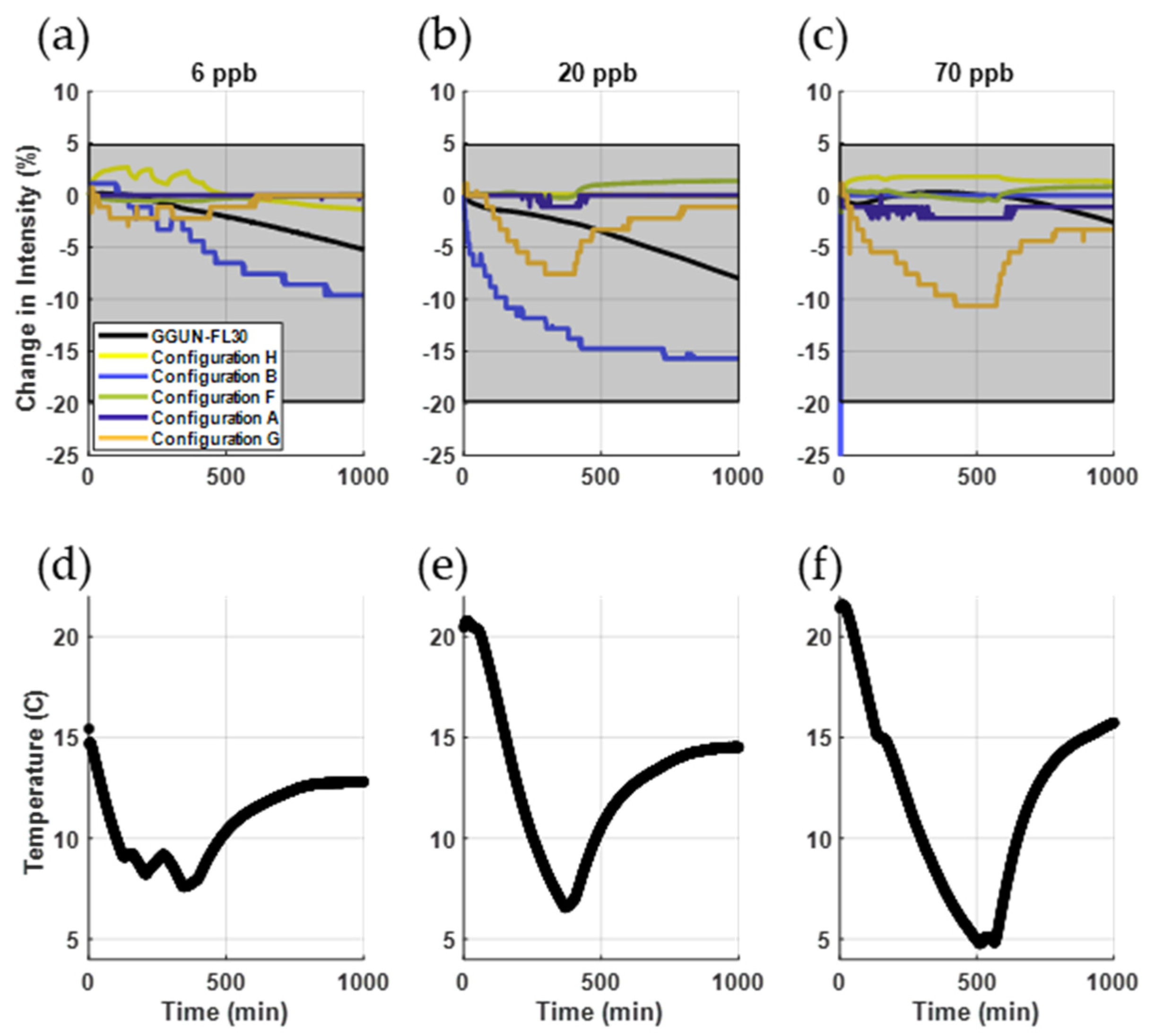
3.2.1. Thermal Sensitivity
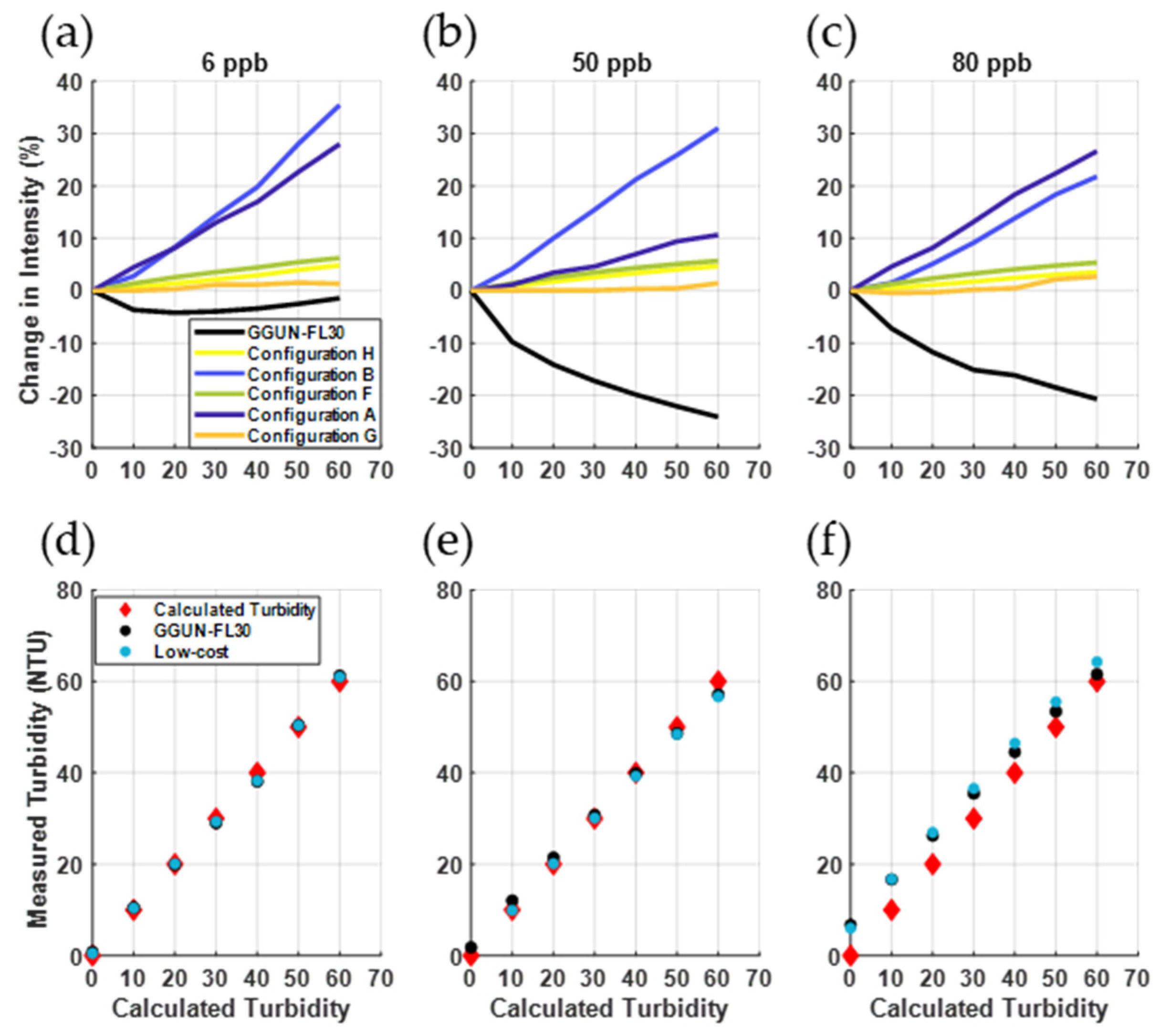
3.2.2. Turbidity Measurements and Interference with Fluorometry
4. Discussion & Conclusions
4.1. Low-Cost Fluorometers Can Achieve Fluorescent Tracer Sensitivity, Performing Comparably to Commercial Units across Operational Ranges for Solute Tracer Studies
4.2. Low-Cost Fluorometers Are Readily Customized for Different Tracers and Could Be Adapted for Multi-Tracer Applications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Höhne, A.; Mellerowicz, K.; Lischtschenko, O.; Lewandowski, J. A novel device for in situ point measurements of fluorescent tracers in sediment pore water. Adv. Water Resour. 2021, 148, 103827. [Google Scholar] [CrossRef]
- Runkel, R.L. On the use of rhodamine WT for the chatrcterization of stream hydrodynamics and transient storage. Water Resour. Res. 2015, 51, 6125–6142. [Google Scholar] [CrossRef]
- Stream Solute Workshop. Concepts and Methods for Assessing Solute Dynamics in Stream Ecosystems. J. N. Am. Benthol. Soc. 1990, 9, 95–119. [Google Scholar] [CrossRef]
- Kvittingen, E.V.; Kvittingen, L.; Melø, T.B.; Sjursnes, B.J.; Verley, R. Demonstrating basic properties of spectroscopy using a self-constructed combined fluorimeter and UV-Photometer. J. Chem. Educ. 2017, 94, 1486–1491. [Google Scholar] [CrossRef]
- Bencala, K.E.; Rathbun, R.E.; Jackman, A.P.; Kennedy, V.C.; Zellweger, G.W.; Avanzino, R.J. Rhodamine Wt Dye Losses in a Mountain Stream Environment. JAWRA J. Am. Water Resour. Assoc. 1983, 19, 943–950. [Google Scholar] [CrossRef]
- Jankowski, K.; Schindler, D.E.; Lisi, P.J. Temperature sensitivity of community respiration rates in streams is associated with watershed geomorphic features. Ecology 2014, 95, 2707–2714. [Google Scholar] [CrossRef] [Green Version]
- Salafi, T.; Zeming, K.K.; Lim, J.W.; Raman, R.; Seah, A.W.R.; Tan, M.P.; Zhang, Y. Portable Smartphone-Based Platform for Real-Time Particle Detection in Microfluidics. Adv. Mater. Technol. 2019, 4, 1800359. [Google Scholar] [CrossRef]
- Fischer, H.B.; List, E.J.; Koh, R.C.Y.; Imberger, J.; Brooks, N.H. Mixing in Inland and Coastal Waters; Academic Press: Cambridge, MA, USA, 1979. [Google Scholar]
- Chung, S.W.; Gu, R.R. Estimating time-variable transformation rate of atrazine in a reservoir. Adv. Environ. Res. 2003, 7, 933–947. [Google Scholar] [CrossRef]
- Blaen, P.J.; Brekenfeld, N.; Comer-Warner, S.; Krause, S. Multitracer Field Fluorometry: Accounting for Temperature and Turbidity Variability during Stream Tracer Tests. Water Resour. Res. 2017, 53, 9118–9126. [Google Scholar] [CrossRef] [Green Version]
- Zepp, R.G.; Cline, D.M. Rates of Direct Photolysis in Aquatic Environment. Environ. Sci. Technol. 1977, 11, 359–366. [Google Scholar] [CrossRef]
- Debell, T.; Goertzen, L.; Larson, L.; Selbie, W.; Selker, J.; Udell, C. OPEnS Hub: Real-Time Data Logging, Connecting Field Sensors to Google Sheets. Front. Earth Sci. 2019, 7, 137. [Google Scholar] [CrossRef] [Green Version]
- Gillett, D.; Marchiori, A. A low-cost continuous turbidity monitor. Sensors 2019, 19, 3039. [Google Scholar] [CrossRef] [Green Version]
- Hossain, A.; Canning, J.; Ast, S.; Rutledge, P.J.; Li Yen, T.; Jamalipour, A. Lab-in-a-Phone: Smartphone-Based Portable Fluorometer for pH Measurements of Environmental Water. IEEE Sens. J. 2015, 15, 5095–5102. [Google Scholar] [CrossRef] [Green Version]
- Kelley, C.D.; Krolick, A.; Brunner, L.; Burklund, A.; Kahn, D.; Ball, W.P.; Weber-Shirk, M. An affordable open-source turbidimeter. Sensors 2014, 14, 7142–7155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leeuw, T.; Boss, E.S.; Wright, D.L. In situ measurements of phytoplankton fluorescence using low cost electronics. Sensors 2013, 13, 7872–7883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Wang, W.; Ding, H.; Yi, D. Smartphone-Based Hand-Held Optical Fiber Fluorescence Sensor for On-Site pH Detection. IEEE Sens. J. 2019, 19, 9441–9446. [Google Scholar] [CrossRef]
- Porter, L.A.; Chapman, C.A.; Alaniz, J.A. Simple and inexpensive 3D printed filter fluorometer designs: User-friendly instrument models for laboratory learning and outreach activities. J. Chem. Educ. 2017, 94, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Wigton, B.T.; Chohan, B.S.; McDonald, C.; Johnson, M.; Schunk, D.; Kreuter, R.; Sykes, D. A portable, low-cost, LED fluorimeter for middle school, high school, and undergraduate chemistry labs. J. Chem. Educ. 2011, 88, 1182–1187. [Google Scholar] [CrossRef]
- Wigton, B.T.; Chohan, B.S.; Kreuter, R.; Sykes, D. The characterization of an easy-to-operate inexpensive student-built fluorimeter. J. Chem. Educ. 2011, 88, 1188–1193. [Google Scholar] [CrossRef]
- Ali, A.S.; Zanzinger, Z.; Debose, D.; Stephens, B. Open Source Building Science Sensors (OSBSS): A low-cost Arduino-based platform for long-term indoor environmental data collection. Build. Environ. 2016, 100, 114–126. [Google Scholar] [CrossRef] [Green Version]
- Laidlaw, I.M.S.; Smart, P.L. An Evaluation of Some Fluorescent Dyes for Water \t\t\t Tracing. Water Resour. Res. 1977, 13, 15–33. [Google Scholar] [CrossRef]
- Steppuhn, H.; Meiman, J.R.; Goodell, B.C. Automatic detection of water-borne fluorescent tracers. Int. Assoc. Sci. Hydrol. Bull. 1971, 16, 83–89. [Google Scholar] [CrossRef]
- Smart, P.L.; Finlayson, B.L.; Rylands, W.D.; Ball, C.M. The relation of fluorescence to dissolved organic carbon in surface waters. Water Res. 1976, 10, 805–811. [Google Scholar] [CrossRef]
- Lorenzen, C.J. A method for the continuous measurement of in vivo chlorophyll concentration. Deep Res. 1966, 13, 223–227. [Google Scholar] [CrossRef]
- Schreiber, U.; Schliwa, U.; Bilger, W. Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth. Res. 1986, 10, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Schnegg, P.; Kennedy, K. A new borehole fluorometer for double tracer tests. In Proceedings of the Mass Transport in Fractured Aquifers and Aquitards, Copenhagen, Denmark, 14–16 May 1998; pp. 14–17. [Google Scholar]
- Jeong, H.; Shin, S.; Hwang, J.; Kim, Y.J.; Choi, S. Open-Source Fluorescence Spectrometer for Noncontact Scientific Research and Education. J. Chem. Educ. 2021, 98, 3493–3501. [Google Scholar] [CrossRef]
- Cucci, C.; Mignani, A.G.; Dall’Asta, C.; Pela, R.; Dossena, A. A portable fluorometer for the rapid screening of M1 aflatoxin. Sens. Actuators B Chem. 2007, 126, 467–472. [Google Scholar] [CrossRef]
- Tseng, Y.C.; Chu, S.W. High spatio-temporal-resolution detection of chlorophyll fluorescence dynamics from a single chloroplast with confocal imaging fluorometer. Plant Methods 2017, 13, 43. [Google Scholar] [CrossRef] [Green Version]
- Bullis, R.; Coker, J.; Belding, J.; De Groodt, A.; Mitchell, D.W.; Velazquez, N.; Bell, A.; Hall, J.; Gunderson, W.A.; Gunderson, J.E.C. The Fluorino: A Low-Cost, Arduino-Controlled Fluorometer. J. Chem. Educ. 2021, 98, 3892–3897. [Google Scholar] [CrossRef]
- Bates, H.; Zavafer, A.; Szabó, M.; Ralph, P.J. A guide to Open-JIP, a low-cost open-source chlorophyll fluorometer. Photosynth. Res. 2019, 142, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Khosravani, M.R.; Reinicke, T. 3D-printed sensors: Current progress and future challenges. Sens. Actuators A Phys. 2020, 305, 111916. [Google Scholar] [CrossRef]
- Pascua, J.A.A.; Prado, A.J.A.; Solis, B.R.B.; Cid-andres, A.P.; Cambiador, C.J.B. Trends in fabrication, data gathering, validation, and application of molecular fluorometer and spectrofluoromete. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 220, 116837. [Google Scholar] [CrossRef] [PubMed]
- Schnegg, P.A. An inexpensive field fluorometer for hydrogeological tracer tests with three tracers and turbidity measurement. Groundw. Hum. Dev. 2002, 1484–1488. [Google Scholar]
- Hixson, J.; Ward, A.S. Supporting Data for Hixson and Ward, Hardware Selection and Performance of Low-Cost Fluorometers, HydroShare. 2021. Available online: https://www.hydroshare.org/resource/7e89f4916e84454e84d2000e61da6b47 (accessed on 10 November 2021).
- Lemke, D.; Schnegg, P.A.; Schwientek, M.; Osenbrück, K.; Cirpka, O.A. On-line fluorometry of multiple reactive and conservative tracers in streams. Environ. Earth Sci. 2013, 69, 349–358. [Google Scholar] [CrossRef]
- Flett, V.; Maurice, L.; Finlayson, A.; Black, A.R.; MacDonald, A.M.; Everest, J.; Kirkbride, M.P. Meltwater flow through a rapidly deglaciating glacier and foreland catchment system: Virkisjökull, Se Iceland. Hydrol. Res. 2017, 48, 1666–1681. [Google Scholar] [CrossRef]
- Flury, M.; Wai, N.N. Dyes as tracers for vadose zone hydrology. Rev. Geophys. 2003, 41, 1002. [Google Scholar] [CrossRef]
- Khamis, K.; Sorensen, J.P.R.; Bradley, C.; Hannah, D.M.; Lapworth, D.J.; Stevens, R. In situ tryptophan-like fluorometers: Assessing turbidity and temperature effects for freshwater applications. Environ. Sci. Process. Impacts 2015, 17, 740–752. [Google Scholar] [CrossRef] [Green Version]
- Leibundgut, C.; Maloszewski, P.; Külls, C. Tracers in Hydrology; John Wiley: Chichester, UK, 2009; ISBN 9780470518854. [Google Scholar]
- Duff, J.H.; Murphy, F.; Fuller, C.C.; Triska, F.J. A mini drivepoint sampler for measuring pore water solute concentrations in the hyporheic zone of sand-bottom streams. Limnol. Oceanogr. 1998, 43, 1378–1383. [Google Scholar] [CrossRef]



| Commercial Unit | 2020 Price (USD) | Manufacturer’s Stated Sensitivity for Uranine | Intended Application |
|---|---|---|---|
| Hach DR3900 | 7779 | 2 ppb | Spectrofluorometer |
| Hach DR900 | 1491 | 10 ppb | Field colorimeter |
| AquaLog | 38,950 | <1 ppb | Spectrometer |
| GGUN FL 30 | 8000 | 0.02 ppb | Field fluorometer |
| Trilogy Laboratory Fluorometer with Uranine Module | 6865 | 0.01 ppb | Laboratory fluorometer |
| Cyclops-7F (Uranine Optics) | 1865 | 0.01 ppb | Field fluorometer |
| Cyclops-6K (Chlorophyll Optics) | 6375 | 0.25 ppb | Field fluorometer |
| Config. ID | Unique Hardware | Bit | r2 | Range of Measurement Uncertainty a | Unique Component Price (USD) | Total Price (USD) |
|---|---|---|---|---|---|---|
| A | Photocell w/1 kΩ resistor | 10 | 0.95 | 0.0–90% | $0.95 | $59.13 |
| B | Photocell w/10 kΩ resistor | 10 | >0.999 | 0.0–54% | $0.95 | $59.13 |
| C | Photocell w/100 kΩ resistor | 8 | 0.99 | 0.0–119% | $0.95 | $59.13 |
| D | Analog Light Sensor (ALS-PT19) | 8 | 0.35 | 0.0–127% | $2.50 | $60.68 |
| E | High Dynamic Range Digital Light Sensor (TSL2591) | 16 | 0.99 | 0.6–154% | $6.95 | $65.13 |
| F | Log-scale Analog Light Sensor (GA1A12S202) | 16 | 0.92 | −317–2860% | $3.95 | $62.13 |
| G | Log-scale Analog Light Sensor (GA1A12S202) w/optical filter b | 8 | 0.85 | −1897–1558% | $80.95 c | $139.13 |
| H | Photocell w/10 kΩ resistor | 16 | 0.99 | 0.3–53% | $0.95 | $59.13 |
| I | Log-scale Analog Light Sensor (GA1A12S202) | 8 | 0.37 | 0.0–157% | $3.95 | $62.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hixson, J.L.; Ward, A.S. Hardware Selection and Performance of Low-Cost Fluorometers. Sensors 2022, 22, 2319. https://doi.org/10.3390/s22062319
Hixson JL, Ward AS. Hardware Selection and Performance of Low-Cost Fluorometers. Sensors. 2022; 22(6):2319. https://doi.org/10.3390/s22062319
Chicago/Turabian StyleHixson, Jase L., and Adam S. Ward. 2022. "Hardware Selection and Performance of Low-Cost Fluorometers" Sensors 22, no. 6: 2319. https://doi.org/10.3390/s22062319





