Quantification of Head Tremors in Medical Conditions: A Comparison of Analyses Using a 2D Video Camera and a 3D Wireless Inertial Motion Unit
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Clinical Assessment of Head Tremor Severity
2.3. Materials
2.4. Procedure
- In a sitting position for 60 s (sitting condition);
- Counting backward, aloud, for 30 s while sitting (counting condition);
- With arms outstretched, against gravity, for 30 s while in a sitting position (arms extended condition).
2.5. Data Processing
2.5.1. Data Preprocessing
2.5.2. Data Transformation
- Amplitude of head tremor (HTA): For each linear component of head tremor obtained from the video camera (i.e., x, y) and each angular component of head motion obtained from the IMMU (i.e., roll, pitch, and yaw), the amplitude of head tremor was quantified using time domain analysis by the peak-to-peak value of the corresponding filtered displacement signal.
- Head tremor frequency: For each linear component of head tremor obtained from the video camera (i.e., x, y) and each angular component of head motion obtained from the IMMU (i.e., roll, pitch, and yaw), the frequency of head tremor was quantified using both frequency domain and time-frequency domain analyses. In comparison to frequency domain analysis, time-frequency domain analysis allows simultaneously extracting the temporal and spectral information contained in the signals analyzed. It makes it possible to determine which frequencies are present in the signal at a particular time, and thus takes into account non-stationary signals whose frequency behavior changes with time.
- Frequency domain analysis: the head tremor frequency calculated in the frequency domain (HTF-F) was defined as the mean frequency of the power spectrum of the filtered displacement signal obtained by using a fast Fourier transform (FFT).
- Time-frequency domain analysis: the head tremor frequency calculated in the time-frequency domain (HTF-TF) was defined as the average over time of the mean frequency obtained at each time instant from the time-frequency power spectrum of the filtered displacement signal. The time-frequency transform was performed using Morlet wavelet with the WavCrossSpec software [22] adapted from the MATLAB package developed by [23]. In WavCrossSpec, the scale resolution of the wavelet (parameter ‘nvoice’), the number of scales used in the wavelet analysis (parameter ‘J1′), and the Morlet mother wavelet parameter (parameter ‘wavenumber’) were set to 50, 3.92, and 16, respectively, to provide a satisfactory compromise between time and frequency resolution for the analysis of the spectral content of head tremor.
2.6. Statistics
3. Results
3.1. Clinical Severity of Head Tremor (TOTHEADTREM)
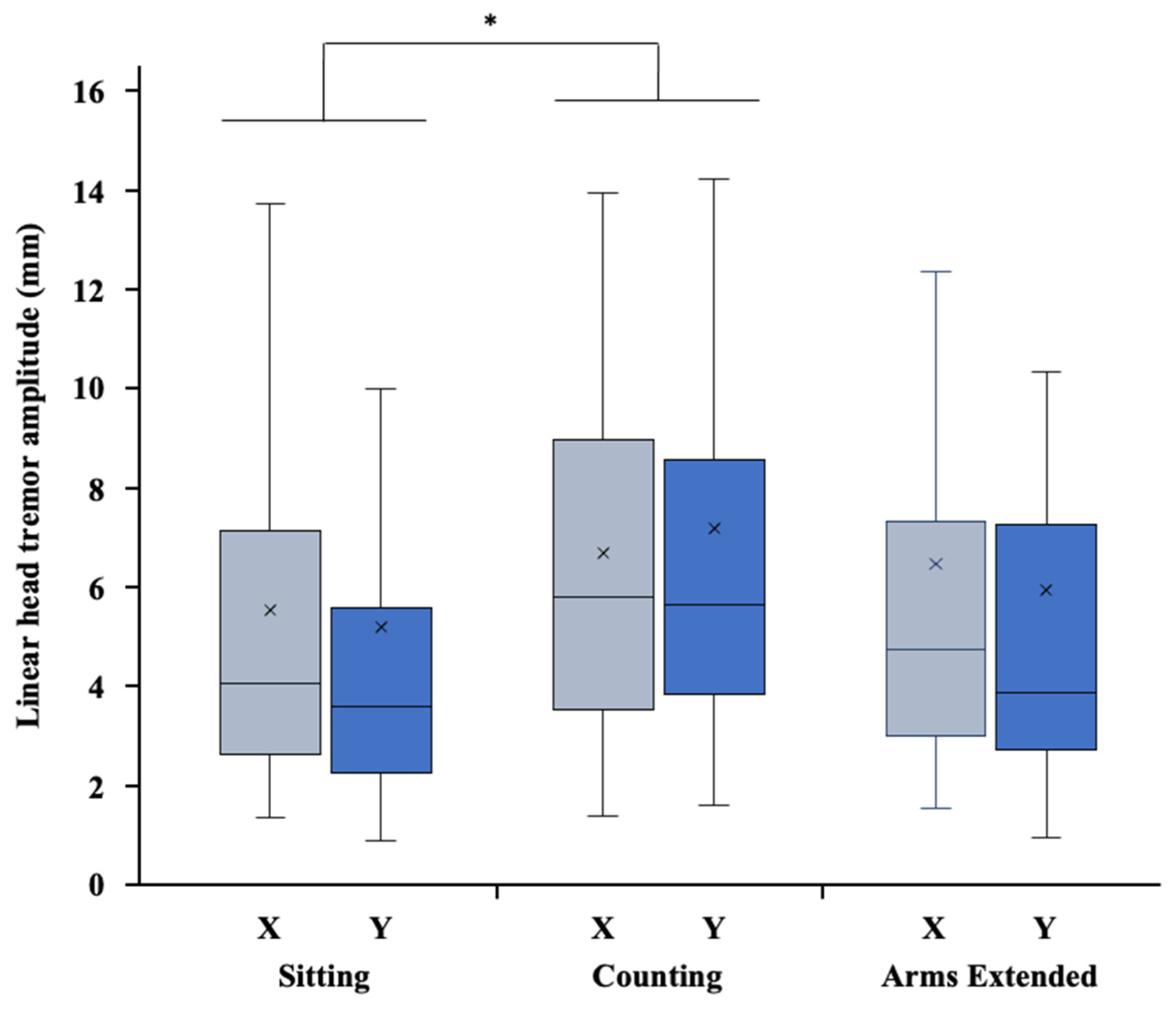
3.2. Amplitude of Head Tremor (HTA)
3.3. Frequency of Head Tremor (HTF)
3.4. Correlations between the Severity and the Amplitude of Head Tremor
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhatia, K.P.; Bain, P.; Bajaj, N.; Elble, R.J.; Hallett, M.; Louis, E.D.; Raethjen, J.; Stamelou, M.; Testa, C.M.; Deuschl, G.; et al. Consensus Statement on the classification of tremors. from the task force on tremor of the International Parkinson and Movement Disorder Society. Mov. Disord. 2018, 33, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Bain, P.G.; Findley, L.J.; Atchison, P.; Behari, M.; Vidailhet, M.; Gresty, M.; Rothwell, J.C.; Thompson, P.D.; Marsden, C.D. Assessing tremor severity. J. Neurol. Neurosurg. Psychiatry 1993, 56, 868–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elble, R.J. The Essential Tremor Rating Assessment Scale. J. Neurol. Neuromed. 2016, 1, 34–38. [Google Scholar]
- Fahn, S.; Tolosa, E. Clinical rating scale for tremor. In Parkinson’s Disease and Movement Disorders; Jankovic, J., Tolosa, E., Eds.; Williams and Wilkins: Baltimore, MD, USA, 1993; pp. 271–280. [Google Scholar]
- Ondo, W.; Hashem, V.; LeWitt, P.A.; Pahwa, R.; Shih, L.; Tarsy, D.; Zesiewicz, T.; Elble, R. Comparison of the Fahn-Tolosa-Marin Clinical Rating Scale and the Essential Tremor Rating Assessment Scale. Mov. Disord. Clin. Pr. 2017, 5, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Elble, R.; Bain, P.; Forjaz, M.J.; Haubenberger, D.; Testa, C.; Goetz, C.G.; Leentjens, A.F.G.; Martinez-Martin, P.; Traon, A.P.; Post, B.; et al. Task force report: Scales for screening and evaluating tremor: Critique and recommendations. Mov. Disord. 2013, 28, 1793–1800. [Google Scholar] [CrossRef] [PubMed]
- Mostile, G.; Terranova, R.; Rascunà, C.; Terravecchia, C.; Cicero, C.E.; Giuliano, L.; Davì, M.; Chisari, C.; Luca, A.; Preux, P.-M.; et al. Clinical-Instrumental patterns of neurodegeneration in Essential Tremor: A data-driven approach. Park. Relat. Disord. 2021, 87, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Deuschl, G.; Raethjen, J.; Hellriegel, H.; Elble, R. Treatment of patients with essential tremor. Lancet Neurol. 2011, 10, 148–161. [Google Scholar] [CrossRef]
- Giuffrida, J.P.; Riley, D.E.; Maddux, B.N.; Heldman, D.A. Clinically deployable Kinesia technology for automated tremor assessment. Mov. Disord. 2009, 24, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Hess, C.W.; Pullman, S.L. Tremor: Clinical Phenomenology and Assessment Techniques. Tremor Other Hyperkinetic Mov. 2012, 2. [Google Scholar] [CrossRef]
- Kostikis, N.; Hristu-Varsakelis, D.; Arnaoutoglou, M.; Kotsavasiloglou, C. Smartphone-based evaluation of parkinsonian hand tremor: Quantitative measurements vs. clinical assessment scores. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2014, 2014, 906–909. [Google Scholar] [PubMed]
- Senova, S.; Querlioz, D.; Thiriez, C.; Jedynak, P. Using the Accelerometers Integrated in Smartphones to Evaluate Essential Tremor. Ster. Funct. Neurosurg. 2015, 93, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Berbakov, L.; Čarna, J.; Svetel, M.; Vasiljević, J.; Dimić, G.; Radulović, N. Quantitative Assessment of Head Tremor in Patients with Essential Tremor and Cervical Dystonia by Using Inertial Sensors. Sensors 2019, 19, 4246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elble, R.J.; Hellriegel, H.; Raethjen, J.; Deuschl, G. Assessment of Head Tremor with Accelerometers Versus Gyroscopic Transducers. Mov. Disord. Clin. Pr. 2016, 4, 205–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koller, W.C.; Vetere-Overfield, B.; Barter, R. Tremors in Early Parkinson’s Disease. Clin. Neuropharmacol. 1989, 12, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Pahwa, R.; Busenbark, K.; Swanson-Hyland, E.F.; Dubinsky, R.M.; Hubble, J.P.; Gray, C.; Koller, W.C. Botulinum toxin treatment of essential head tremor. Neurology 1995, 45, 822–824. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, A.G.; Jinnah, H.A.; Tripp, R.M.; Optican, L.M.; Ramat, S.; Lenz, F.A.; Zee, D.S. Irregularity distinguishes limb tremor in cervical dystonia from essential tremor. J. Neurol. Neurosurg. Psychiatry 2008, 79, 187–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wissel, J.; Masuhr, F.; Schelosky, L.; Ebersbach, G.; Poewe, W. Quantitative assessment of botulinum toxin treatment in 43 patients with head tremor. Mov. Disord. 1997, 12, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Olsson, F.; Medvedev, A. Nonparametric Time-Domain Tremor Quantification With Smart Phone for Therapy Individualization. IEEE Trans. Control Syst. Technol. 2018, 28, 118–129. [Google Scholar] [CrossRef]
- Paulich, M.; Schepers, M.; Rudigkeit, N.; Bellusci, G. Xsens MTw Awinda: Miniature Wireless Inertial-Magnetic Motion Tracker for Highly Accurate 3D Kinematic Applications; Technical Report; Xsens Technologies BV: Enschede, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Riviere, C.N.; Reich, S.G.; Thakor, N.V. Adaptive Fourier modeling for quantification of tremor. J. Neurosci. Methods 1997, 74, 77–87. [Google Scholar] [CrossRef]
- Bigot, J.; Longcamp, M.; Maso, F.D.; Amarantini, D. A new statistical test based on the wavelet cross-spectrum to detect time–frequency dependence between non-stationary signals: Application to the analysis of cortico-muscular interactions. NeuroImage 2011, 55, 1504–1518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grinsted, A.; Moore, J.C.; Jevrejeva, S. Application of the cross wavelet transform and wavelet coherence to geophysical time series. Nonlinear Process. Geophys. 2004, 11, 561–566. [Google Scholar] [CrossRef]
- Elble, R.J.; Pullman, S.L.; Matsumoto, J.Y.; Raethjen, J.; Deuschl, G.; Tintner, R. Tremor amplitude is logarithmically related to 4- and 5-point tremor rating scales. Brain 2006, 129, 2660–2666. [Google Scholar] [CrossRef] [PubMed]
- Elble, R.J.; McNames, J. Using Portable Transducers to Measure Tremor Severity. Tremor Other Hyperkinetic Mov. 2016, 6, 375. [Google Scholar] [CrossRef]
- Haubenberger, D.; Abbruzzese, G.; Bain, P.G.; Bajaj, N.; Benito-León, J.; Bhatia, K.P.; Deuschl, G.; Forjaz, M.J.; Hallett, M.; Louis, E.D.; et al. Transducer-based evaluation of tremor. Mov. Disord. 2016, 31, 1327–1336. [Google Scholar] [CrossRef] [PubMed]
- Roze, E.; Coêlho-Braga, M.C.; Gayraud, D.; Legrand, A.P.; Trocello, J.-M.; Fénelon, G.; Cochen, V.; Patte, N.; Viallet, F.; Vidailhet, M.; et al. Head tremor in Parkinson’s disease. Mov. Disord. 2006, 21, 1245–1248. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-Y.; Aziz, T.Z.; Stein, J.F.; Liu, X. Time–frequency analysis of transient neuromuscular events: Dynamic changes in activity of the subthalamic nucleus and forearm muscles related to the intermittent resting tremor. J. Neurosci. Methods 2005, 145, 151–158. [Google Scholar] [CrossRef] [PubMed]




| Method | Video | IMMU | |||
|---|---|---|---|---|---|
| Movement type | Translation | Rotation | |||
| Axis | x | y | x | y | z |
| Measured component of head motion | X (m) | Y (m) | Roll (°) | Pitch (°) | Yaw (°) |
| Dependent variables for head tremor | |||||
| Peak-to-peak head tremor amplitude (HTA) | HTAX (m) | HTAY (m) | HTARoll (°) | HTAPitch (°) | HTAYaw (°) |
| Head tremor frequency (HTF, in Hz) * | |||||
| Frequency domain | HTF-FVideo | – | – | HTF-FIMMU | |
| Time-frequency domain | HTF-TFVideo | – | – | HTF-TFIMMU | |
| Model | Condition | R | R2 | Estimate | SE | t | p |
|---|---|---|---|---|---|---|---|
| Resultant linear amplitude-TOTHEADTREM | Sitting | 0.277 | 0.075 | 0.072 | 0.037 | 1.95 | 0.058 |
| Arms extended | 0.293 | 0.048 | 0.057 | 0.037 | 1.54 | 0.130 | |
| Counting | 0.280 | 0.014 | 0.026 | 0.031 | 0.807 | 0.423 | |
| Angular distance-TOTHEADTREM | Sitting | 0.468 | 0.219 | 0.192 | 0.053 | 3.626 | <0.001 * |
| Arms extended | 0.521 | 0.272 | 0.148 | 0.035 | 4.19 | <0.001 * | |
| Counting | 0.207 | 0.043 | 0.057 | 0.039 | 1.45 | 0.154 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amarantini, D.; Rieu, I.; Castelnovo, G.; Fluchère, F.; Laurencin, C.; Degos, B.; Poujois, A.; Kreisler, A.; Sangla, S.; Tir, M.; et al. Quantification of Head Tremors in Medical Conditions: A Comparison of Analyses Using a 2D Video Camera and a 3D Wireless Inertial Motion Unit. Sensors 2022, 22, 2385. https://doi.org/10.3390/s22062385
Amarantini D, Rieu I, Castelnovo G, Fluchère F, Laurencin C, Degos B, Poujois A, Kreisler A, Sangla S, Tir M, et al. Quantification of Head Tremors in Medical Conditions: A Comparison of Analyses Using a 2D Video Camera and a 3D Wireless Inertial Motion Unit. Sensors. 2022; 22(6):2385. https://doi.org/10.3390/s22062385
Chicago/Turabian StyleAmarantini, David, Isabelle Rieu, Giovanni Castelnovo, Frédérique Fluchère, Chloé Laurencin, Bertrand Degos, Aurélia Poujois, Alexandre Kreisler, Sophie Sangla, Mélissa Tir, and et al. 2022. "Quantification of Head Tremors in Medical Conditions: A Comparison of Analyses Using a 2D Video Camera and a 3D Wireless Inertial Motion Unit" Sensors 22, no. 6: 2385. https://doi.org/10.3390/s22062385







