Effect of Surface Morphology Changes on Optical Properties of Silicon Nanowire Arrays
Abstract
:1. Introduction
2. Experiment
2.1. The Preparatory Work
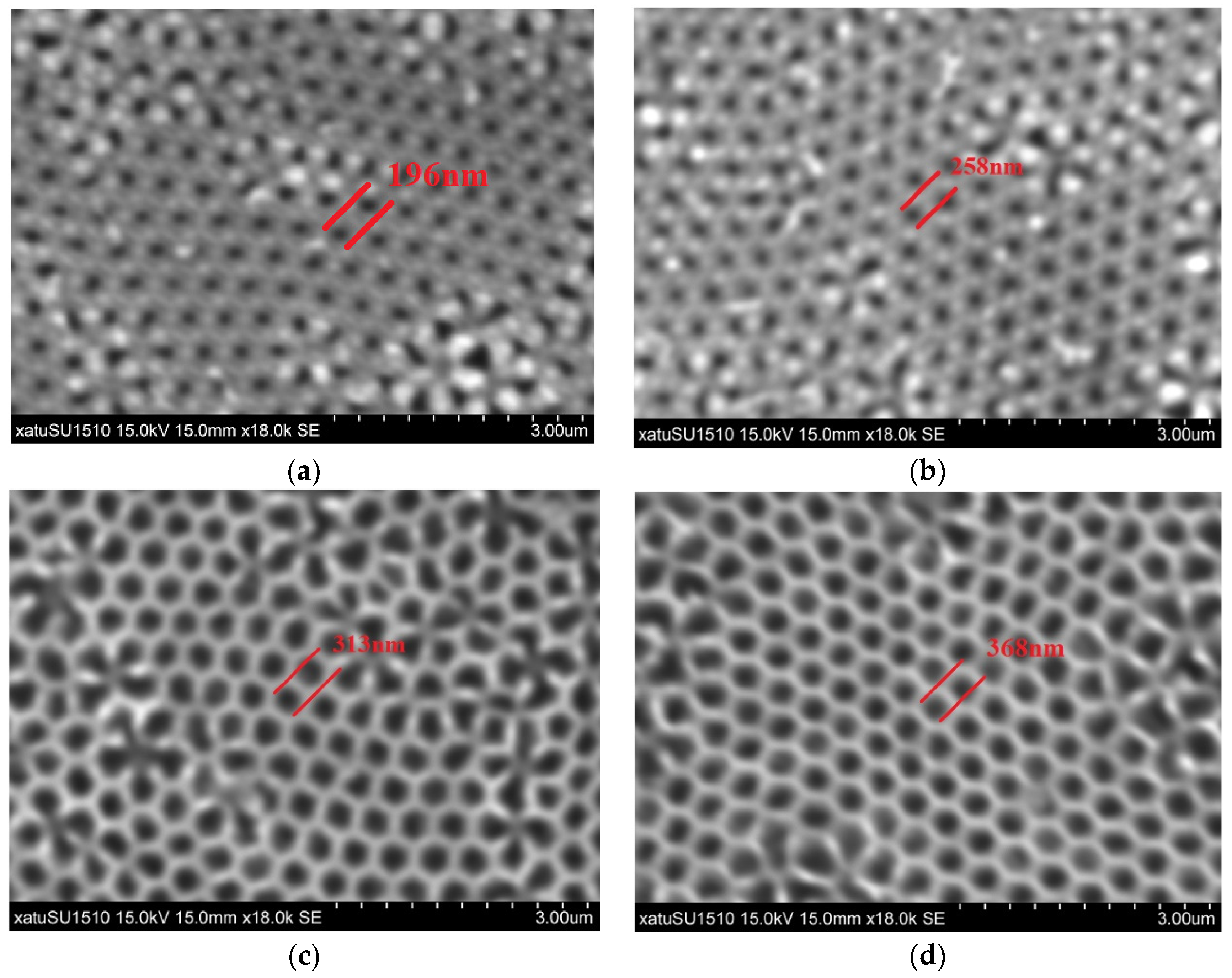
2.2. Formation of Au Films
2.3. Transferring of Au Films
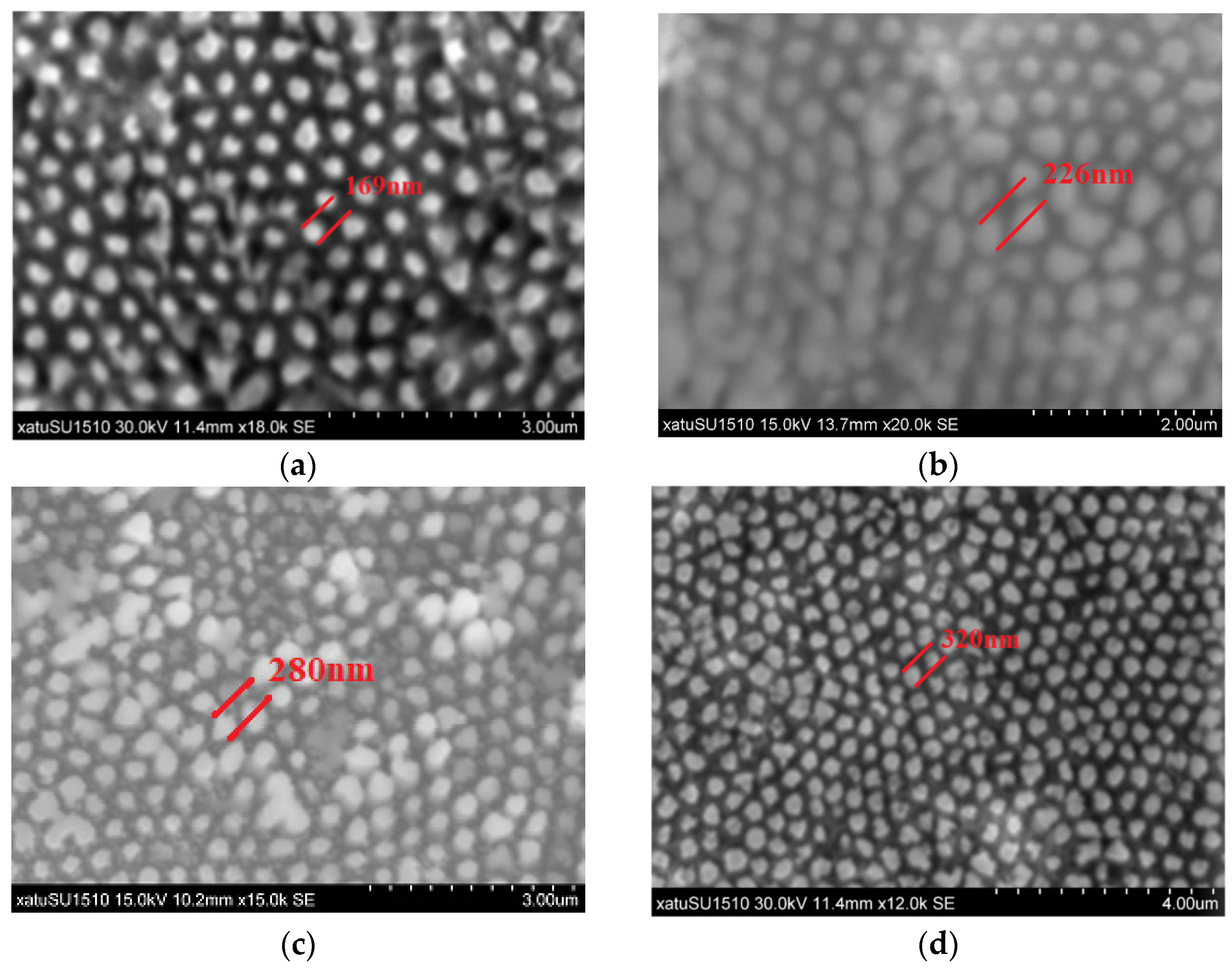
2.4. The Etching Process
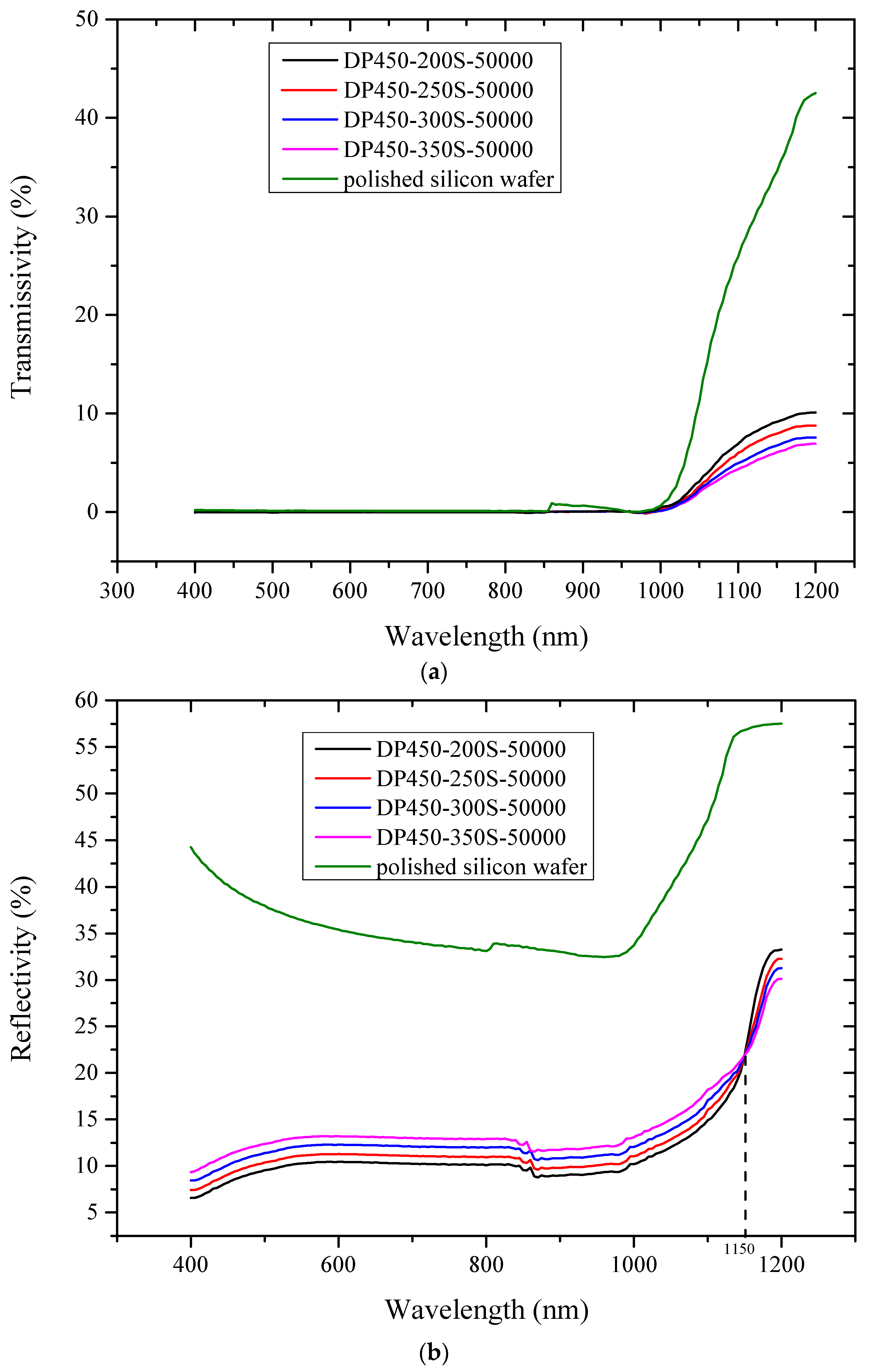
3. Optical Characteristics Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qin, Y.X.; Wang, X.Y.; Zang, J.S. Room-temperature ethanol sensor based on ZIF-67 modified silicon nanowires with expanded detection range and enhanced moisture resistance. Chem. Phys. Lett. 2021, 765, 138302. [Google Scholar] [CrossRef]
- Kapic, A.; Tsirou, A.; Verdini, P.G.; Carrara, S. Humidity Sensors for High Energy Physics Applications: A Review. IEEE Sens. J. 2020, 20, 10335–10344. [Google Scholar] [CrossRef]
- Cui, J.G.; Yu, Y.X.; Chu, X.X.; Zhao, R.Y.; Zhu, M.; Zhang, W.D.; Zhang, G.J. Research on Characteristics of Broadband Acoustic Sensor Based on Silicon-Based Grooved Microring Resonator. Micromachines 2021, 12, 1338. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.M.; Sun, Y.M.; Zhao, H. Quantum confinement effect induced topological phase transitions in anisotropic Weyl semimetal. Superlattices Microstruct. 2020, 138, 106386. [Google Scholar] [CrossRef]
- Agafonov, O.B.; Dais, C.; Grutzmacher, D.; Haug, R.J. Quantum confinement effects in Si/Ge heterostructures with spatially ordered arrays of self-asssembled quantum dots. Appl. Phys. Lett. 2010, 96, 222107. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Tang, Y.; Lam, C.; Wang, N.; Lee, C.S.; Bello, I.; Lee, S.T. Bulk-quantity Si nanowires synthesized by SiO sublimation. J. Cryst. Growth 2000, 212, 115–118. [Google Scholar] [CrossRef]
- Kokai, F.; Inoue, S.; Hidaka, H.; Uchiyama, K.; Takahashi, Y.; Koshio, A. Catalyst-free growth of amorphous silicon nanowires by laser ablation. Appl. Phys. A 2013, 112, 1–7. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, K.; Miao, B.; Wang, B.; Hou, J. Temperature dependence of morphology and diameter of silicon nanowires synthesized by laser ablation. Chem. Phys. Lett. 2002, 358, 396–400. [Google Scholar] [CrossRef]
- Huang, J.; He, Y.R.; Chen, M.J.; Wang, X.Z. Separating photo-thermal conversion and steam generation process for evaporation enhancement using a solar absorber. Appl. Energy 2019, 236, 244–252. [Google Scholar] [CrossRef]
- Kolb, F.M.; Hofmeister, H.; Scholz, R.; Zacharias, M.; Gösele, U.; Ma, D.D.; Lee, S.T. Analysis of silicon nanowires grown by combining SiO evaporation with the VLS mechanism. J. Electrochem. Soc. 2004, 151, G472–G475. [Google Scholar] [CrossRef]
- Albuschies, J.; Baus, M.; Winkler, O.; Hadam, B.; Spangenberg, B.; Kurz, H. High-density silicon nanowire growth from self-assembled Au nanoparticles. Microelectron. Eng. 2006, 83, 1530–1533. [Google Scholar] [CrossRef]
- Takeda, S.; Ueda, K.; Ozaki, N.; Ohno, Y. Formation mechanism of nanocatalysts for the growth of silicon nanowires on a hydrogen-terminated Si{111} surface template. Appl. Phys. Lett. 2003, 82, 979–981. [Google Scholar] [CrossRef]
- Muñoz, D.; Voz, C.; Fonrodona, M.; Garin, M.; Orpella, A.; Vetter, M.; Puigdollers, J.; Alcubilla, R.; Villar, F.; Bertomeu, J.; et al. Characterization of bifacial heterojunction silicon solar cells obtained by hot wire CVD. J. Non-Cryst. Solids 2006, 352, 1953–1957. [Google Scholar] [CrossRef]
- Antonio, A.L.; Faro, M.J.L.; Irrera, A. Silicon Nanowires Synthesis by Metal-Assisted Chemical Etching: A Review. Nanomaterials 2021, 11, 383. [Google Scholar]
- Schmidt, V.; Wittemann, J.V.; Senz, S.; Gösele, U. Silicon nanowires: A review on aspects of their growth and their electrical properties. Adv. Mater. 2009, 21, 2681–2702. [Google Scholar] [CrossRef]
- Huang, Z.; Geyer, N.; Werner, P.; de Boor, J.; Gösele, U. Metal-assisted chemical etching of silicon: A Review. Adv. Mater. 2011, 23, 285–308. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, R.; Ding, J.; Li, M.; Humphrey, M.G.; Zhang, C. Low-cost, large-scale, and facile production of Si nanowires exhibiting enhanced third-order optical nonlinearity. ACS Appl. Mater. Interfaces 2012, 4, 1553–1559. [Google Scholar] [CrossRef]
- Huang, Z.; Fang, H.; Zhu, J. Fabrication of silicon nanowire arrays with controlled diameter, length, and density. Adv. Mater. 2007, 19, 744–748. [Google Scholar] [CrossRef]
- Leonardi, A.A.; Faro, M.J.L.; Irrera, A. CMOS-Compatible and Low-Cost Thin Film MACE Approach for Light-Emitting Si NWs Fabrication. Nanomater. 2020, 10, 966. [Google Scholar] [CrossRef]
- Jungkil, K.; Hee, H.; Young, H.K.; Suk-Ho, C.; Jae-Cheon, K.; Woo, L. Au/Ag Bilayered Metal Mesh as a Si Etching Catalyst for Controlled Fabrication of Si Nanowires. ACS Nano 2011, 5, 3222–3229. [Google Scholar]
- Tsujino, K.; Imai, S.; Lee, C.L.; Matsumura, M.; Mizushima, S. Local wet etching of glasses by acidification utilizing electrochemistry. J. Micromech. Micro Eng. 2008, 18, 115023. [Google Scholar] [CrossRef]
- Park, S.J.; Han, H.; Rhu, H.; Baik, S.; Lee, W. A versatile ultra-thin Au nanomesh from a reusable anodic aluminium oxide (AAO) membrane. J. Mater. Chem. C 2013, 1, 5330–5335. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Liu, H.; Han, J. Comprehensive Study of Au Nano-Mesh as a Catalyst in the Fabrication of Silicon Nanowires Arrays by Metal-Assisted Chemical Etching. Coatings 2019, 9, 149. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.J.; Song, J.H. Finite-element analysis of fatigue crack closure under plane strain conditions: Stabilization behaviour and mesh size effect. Fatigue Fract. Eng. Mater. Struct. 2005, 28, 333–342. [Google Scholar] [CrossRef]
- Wang, S.; Han, J.; Song, D.; Wang, X. Effect of NaOH solution concentration on the quality of controllable silicon nanowires array fabrication. Mater. Res. Express 2019, 6, 1250e9. [Google Scholar] [CrossRef]
- Kelzenberg, M.D.; Boettcher, S.W.; Petykiewicz, J.A.; Turner-Evans, D.B.; Putnam, M.C.; Warren, E.L.; Spurgeon, J.M.; Briggs, R.M.; Lewis, N.S.; Atwater, H.A. Enhanced absorption and carrier collection in Si wire arrays for photovoltaic applications. Nat. Mater. 2010, 9, 239–244. [Google Scholar] [CrossRef]
- Garnett, E.; Yang, P. Light Trapping in Silicon Nanowire Solar Cells. Nano Lett. 2010, 10, 1082–1087. [Google Scholar] [CrossRef]
- Huang, Z.; Geyer, N.; Liu, L.; Li, M.; Zhong, P. Metal-assisted electrochemical etching of silicon. Nanotechnology 2010, 21, 465301. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, X.; Reiche, M.; Liu, L.; Lee, W.; Shimizu, T.; Senz, S.; Gösele, U. Extended arrays of vertically aligned sub-10 nm diameter [100] Si nanowires by metal-assisted chemical etching. Nano Lett. 2008, 8, 3046–3051. [Google Scholar] [CrossRef]
- Yu, X.H.; Rong, J.; Zhan, Z.L.; Liu, Z.; Liu, J.X. Effects of grain size and thermodynamic energy on the lattice parameters of metallic nanomaterials. Mater. Des. 2015, 85, 159–163. [Google Scholar] [CrossRef]
- Wu, Q.; Miao, W.S.; Zhang, Y.D.; Gao, H.J.; Hui, D. Mechanical properties of nanomaterials: A review. Nanotechnol. Rev. 2020, 9, 259–273. [Google Scholar] [CrossRef]
- Leonardi, A.A.; Faro, M.J.L.; Irrera, A. Biosensing platforms based on silicon nanostructures: A critical review. Anal. Chim. Acta 2021, 1160, 338393. [Google Scholar] [CrossRef] [PubMed]
- Kasai, H.; Kamatani, H.; Okada, S.; Oikawa, H.; Nakanishi, H. Size-dependent colors and luminescences of organic microcrystals. Jpn. J. Appl. Phys. 1996, 35, L221–L223. [Google Scholar] [CrossRef]
- Danos, L.; Halcovitch, N.R.; Wood, B. Silicon photosensitisation using molecular layers. Faraday Discuss. 2020, 222, 405–423. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Huang, S.; Zhao, J. Effect of Surface Morphology Changes on Optical Properties of Silicon Nanowire Arrays. Sensors 2022, 22, 2454. https://doi.org/10.3390/s22072454
Wang S, Huang S, Zhao J. Effect of Surface Morphology Changes on Optical Properties of Silicon Nanowire Arrays. Sensors. 2022; 22(7):2454. https://doi.org/10.3390/s22072454
Chicago/Turabian StyleWang, Shanshan, Shujia Huang, and Jijie Zhao. 2022. "Effect of Surface Morphology Changes on Optical Properties of Silicon Nanowire Arrays" Sensors 22, no. 7: 2454. https://doi.org/10.3390/s22072454





