Metal Oxide Chemiresistors: A Structural and Functional Comparison between Nanowires and Nanoparticles
Abstract
:1. Introduction
2. Nanowires: Structure, Synthesis and Gas-Sensor Configurations
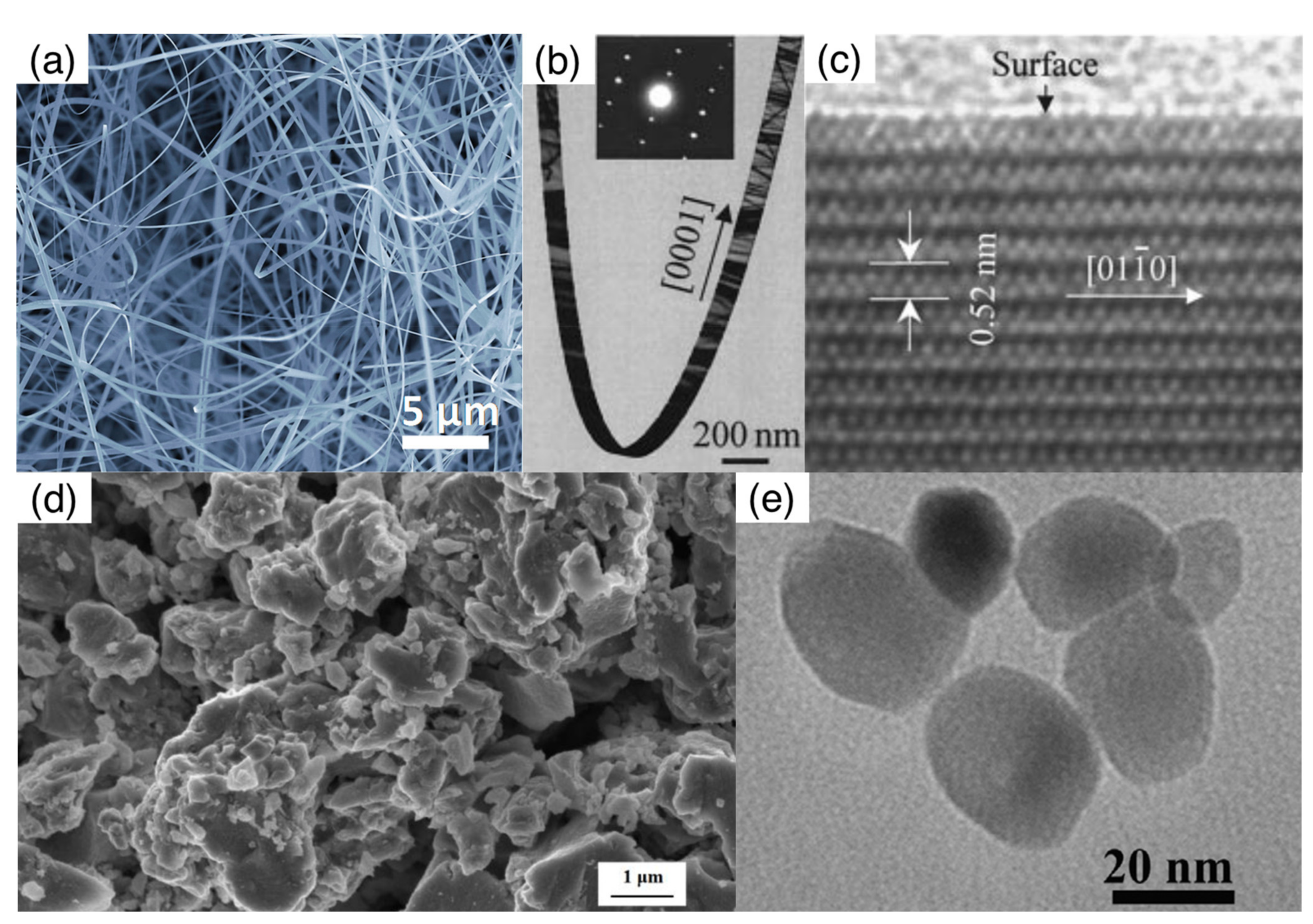
2.1. Structural and Morphological Features of Nanowires
2.2. Synthesis of Metal Oxide Nanowires

2.3. Nanowire-Based Chemiresistors: Device Configurations
3. Gas-Sensing Mechanism
3.1. Receptor Function
3.2. Transducer Function
3.3. Utility Factor
3.4. Size Effects
3.5. Shape Effects
4. Approaches Adopted to Control the Sensing Properties of Gas Sensors
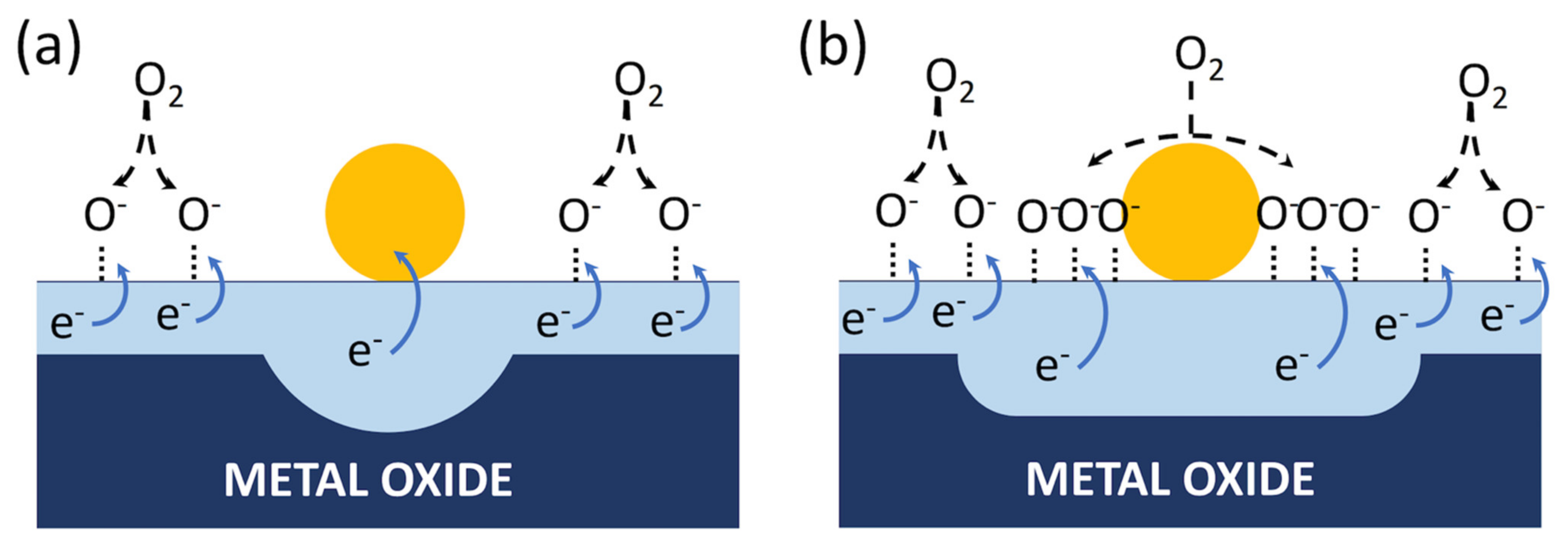
4.1. Porosity (Utility Factor) and Network Effects
| MOX, Morphology | T (°C) | Gas, Concentration | S | Ref. |
|---|---|---|---|---|
| SnO2, nanowire network | 250 | Ethanol, 100 ppm | 105 | [69] |
| SnO2, nanowire network | 300 | H2, 200 ppm | 200 | [69] |
| SnO2, nanocubes network | 250 | Ethanol, 100 ppm | 6000 | [69] |
| SnO2, nanocubes network | 300 | H2, 200 ppm | 270 | [69] |
| SnO2, nanowire network | 300 | Ethanol, 300 ppm | 1600 | [73] |
| SnO2, hierarchical flower-like assemblies of nanowires | 275 | Ethanol, 300 ppm | 4000 | [73] |
| SnO2, thick films | 300 | Ethanol, 100 ppm | 1520 | [71] |
| SnO2, thick films | 300 | H2, 200 ppm | 87 | [71] |
| SnO2, thick films | 300 | Ethanol, 100 ppm | 2400 | [74] |
| SnO2, hierarchical nanospheres of nanoparticles | 400 | Ethanol, 5 ppm | 316 | [75] |
| SnO2, hierarchical fibers of nanoparticles | 250 | H2, 100 ppm | 25 | [72] |
| SnO2, hierarchical fibers of nanoparticles | 150 | NO2, 125 ppb | 90 | [78] |
| WO3, 3D hierarchical assembly of nanowires | 300 | NO2, 50 ppb | 6 | [80] |
| WO3, thick film | 300 | NO2, 50 ppb | 1.5 | [77] |
| WO3, nanolamellae | 200 | NO2, 200 ppb | 70 | [79] |
| In2O3, nanowire network | RT | NO2, 50 ppb | 2 | [76] |
4.2. Surface Termination
4.3. Doped Nanostructures
4.4. Inorganic Heterostructures
4.4.1. Functionalization with Metallic Nanoparticles
| Supporting MOX, Morphology | Functionalization | T (°C) | Gas, Concentration | S | Ref. |
|---|---|---|---|---|---|
| SnO2, nanowire network | -- | 250 | Ethanol, 100 ppm | 105 | [69] |
| SnO2, nanowire network | Pd | 250 | Ethanol, 100 ppm | 1.1 × 105 | [69] |
| SnO2, nanowire network | -- | 300 | H2, 200 ppm | 200 | [69] |
| SnO2, nanowire network | Pd | 250 | H2, 200 ppm | 800 | [69] |
| SnO2, nanocubes network | -- | 250 | Ethanol, 100 ppm | 6000 | [69] |
| SnO2, nanocubes network | Pd | 250 | Ethanol, 100 ppm | 6000 | [69] |
| SnO2, nanocubes network | -- | 300 | H2, 200 ppm | 270 | [69] |
| SnO2, nanocubes network | Pd | 250 | H2, 200 ppm | 300 | [69] |
| SnO2, nanowire network | -- | 150 | H2, 40 ppm | no resp. | [18] |
| SnO2, nanowire network | Pd | 150 | H2, 40 ppm | 3 | [18] |
| SnO2, single nanowire | -- | 100 | H2, 1 ppm | no resp. | [129] |
| SnO2, single nanowire | Pd | 100 | H2, 1 ppm | 5 | [129] |
| SnO2, thick film | -- | 270 | Ethanol, 200 ppm | 28 | [130] |
| SnO2, thick film | Au | 220 | Ethanol, 200 ppm | 128 | [130] |
| SnO2, hollow spheres | -- | 325 | Ethanol, 5 ppm | 95 | [132] |
| SnO2, hollow spheres | Pt | 325 | Ethanol, 5 ppm | 1400 | [132] |
| SnO2, nanorod network | Pt | 300 | Ethanol, 200 ppm | 40 | [133] |
| WO3, nanorod network | -- | 350 | H2S, 1 ppm | 4 | [134] |
| WO3, nanorod network | Au | 350 | H2S, 1 ppm | 100 | [134] |
| WO3, thick film | -- | 300 | H2S, 1 ppm | 3 | [135] |
| WO3, thick film | Au | 300 | H2S, 1 ppm | 7 | [135] |
| SnO2, thick film | -- | 300 | CO, 50 ppm | 10 | [128] |
| SnO2, thick film | Au | 300 | CO, 50 ppm | 100 | [128] |
| SnO2, thick film | Pd | 300 | CO, 50 ppm | 100 | [128] |
| SnO2, thick film | AuPd | 300 | CO, 50 ppm | 2.5 | [128] |
| SnO2, thick film | -- | 300 | Ethanol, 10 ppm | 50 | [128] |
| SnO2, thick film | Au | 300 | Ethanol, 10 ppm | 500 | [128] |
| SnO2, thick film | Pd | 300 | Ethanol, 10 ppm | 150 | [128] |
| SnO2, thick film | AuPd | 300 | Ethanol, 10 ppm | 40 | [128] |
| SnO2, thick film | -- | 300 | CH4, 1000 ppm | 12 | [128] |
| SnO2, thick film | Au | 300 | CH4, 1000 ppm | 30 | [128] |
| SnO2, thick film | Pd | 300 | CH4, 1000 ppm | 90 | [128] |
| SnO2, thick film | AuPd | 300 | CH4, 1000 ppm | 12 | [128] |
| SnO2, thick film | -- | 350 | CO, 20 ppm | 3 | [136] |
| SnO2, thick film | Au | 225 | CO, 20 ppm | 5 | [136] |
| SnO2, thick film | Pd | 100 | CO, 20 ppm | 3 | [136] |
| SnO2, thick film | AuPd | 350 | CO, 20 ppm | 9 | [136] |
| SnO2, thick film | -- | 500 | CH4, 50 ppm | 3 | [136] |
| SnO2, thick film | Au | 450 | CH4, 50 ppm | 4.5 | [136] |
| SnO2, thick film | Pd | 450 | CH4, 50 ppm | 3 | [136] |
| SnO2, thick film | AuPd | 400 | CH4, 50 ppm | 6.5 | [136] |
| SnO2, thick film | -- | 375 | NH3, 10 ppm | 2 | [136] |
| SnO2, thick film | Au | 350 | NH3, 10 ppm | 4 | [136] |
| SnO2, thick film | Pd | 350 | NH3, 10 ppm | 2 | [136] |
| SnO2, thick film | AuPd | 350 | NH3, 10 ppm | 6 | [136] |
| SnO2, nanosheet network | -- | 300 | Acetone, 50 ppm | 20 | [137] |
| SnO2, nanosheet network | Au | 275 | Acetone, 50 ppm | 80 | [137] |
| SnO2, nanosheet network | Pd | 250 | Acetone, 50 ppm | 40 | [137] |
| SnO2, nanosheet network | AuPd | 250 | Acetone, 50 ppm | 110 | [137] |
| WO3, nanowire network | -- | 300 | n-butanol, 100 ppm | 26 | [138] |
| WO3, nanowire network | Pd | 200 | n-butanol, 100 ppm | 69 | [138] |
| WO3, nanowire network | AuPd | 200 | n-butanol, 100 ppm | 93 | [138] |
4.4.2. Functionalization with Metal Oxide Nanoparticles
| Supporting MOX, Morphology | Functionalization | T (°C) | Gas, Concentration | S | Ref. |
|---|---|---|---|---|---|
| SnO2, hollow spheres | -- | 300 | Ethanol, 300 ppm | 11 | [61] |
| SnO2, hollow spheres | CuO | 300 | Ethanol, 300 ppm | 35 | [61] |
| SnO2, thick film | -- | 350 | H2S, 2 ppm | 100 | [46] |
| SnO2, thick film | CuO | 200 | H2S, 2 ppm | 600 | [46] |
| SnO2, thick film | -- | 350 | CO, 40 ppm | 3 | [46] |
| SnO2, thick film | CuO | 350 | CO, 40 ppm | 4 | [46] |
| SnO2, thick film | -- | 300 | NH3, 20 ppm | 2 | [46] |
| SnO2, thick film | CuO | 250 | NH3, 20 ppm | 2 | [46] |
| SnO2, nanowire network | -- | 400 | H2S, 2 ppm | 8 | [141] |
| SnO2, nanowire network | CuO | 200 | H2S, 2 ppm | 3261 | [141] |
| SnO2, nanowire network | -- | na | CO, 50 ppm | 7 | [141] |
| SnO2, nanowire network | CuO | na | CO, 50 ppm | 7 | [141] |
| SnO2, nanowire network | -- | na | NH3, 17 ppm | 4 | [141] |
| SnO2, nanowire network | CuO | na | NH3, 17 ppm | 4 | [141] |
| SnO2, single nanowire | -- | 250 | H2S, 10 ppm | 1.5 | [141] |
| SnO2, single nanowire | CuO | 250 | H2S, 10 ppm | 26 | [141] |
| SnO2, porous fiber | -- | 260 | Ethanol, 100 ppm | 120 | [64] |
| SnO2, porous fiber | ZnO | 260 | Ethanol, 100 ppm | 360 | [64] |
| SnO2, porous fiber | -- | 260 | Acetone, 100 ppm | 10 | [64] |
| SnO2, porous fiber | ZnO | 260 | Acetone, 100 ppm | 30 | [64] |
| SnO2, porous opal | -- | 250 | Acetone, 100 ppm | 13 | [142] |
| SnO2/ZnO, porous opal | -- | 275 | Acetone, 100 ppm | 45 | [142] |
| ZnO, porous opal | -- | 350 | Acetone, 100 ppm | 17 | [142] |
| SnO2, porous opal | -- | 250 | Ethanol, 100 ppm | 22 | [142] |
| SnO2/ZnO, porous opal | -- | 250 | Ethanol, 100 ppm | 23 | [142] |
| ZnO, porous opal | -- | 325 | Ethanol, 100 ppm | 12 | [142] |
| SnO2, nanowire network | -- | 300 | NO2, 5 ppm | 2 | [144] |
| SnO2, nanowire network | Pd | 300 | NO2, 5 ppm | 4 | [144] |
| SnO2, nanowire network | ZnO | 300 | NO2, 5 ppm | 4 | [144] |
| SnO2, nanowire network | ZnO + Pd | 300 | NO2, 5 ppm | 6 | [144] |
4.4.3. Core–Shell Nanostructures
4.4.4. Hierarchical, Branched Nanostructures

4.5. Inorganic–Organic Heterostructures
4.5.1. Graphene and Related Materials

4.5.2. Organic Receptors
4.6. Self-Heating Effect
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pan, Z.W.; Dai, Z.R.; Wang, Z.L. Nanobelts of Semiconducting Oxides. Science 2001, 291, 1947–1949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comini, E.; Faglia, G.; Sberveglieri, G.; Pan, Z.; Wang, Z.L. Stable and Highly Sensitive Gas Sensors Based on Semiconducting Oxide Nanobelts. Appl. Phys. Lett. 2002, 81, 1869–1871. [Google Scholar] [CrossRef]
- Banerjee, S.; Dan, A.; Chakravorty, D. Review Synthesis of Conducting Nanowires. J. Mater. Sci. 2002, 37, 4261–4271. [Google Scholar] [CrossRef]
- Cheng, C.; Fan, H.J. Branched Nanowires: Synthesis and Energy Applications. Nano Today 2012, 7, 327–343. [Google Scholar] [CrossRef]
- Manzano, C.V.; Philippe, L.; Serrà, A. Recent Progress in the Electrochemical Deposition of ZnO Nanowires: Synthesis Approaches and Applications. Crit. Rev. Solid State Mater. Sci. 2021, in press. [Google Scholar] [CrossRef]
- Wang, M.C.P.; Gates, B.D. Directed Assembly of Nanowires. Mater. Today 2009, 12, 34–43. [Google Scholar] [CrossRef]
- Fan, Z.; Ho, J.C.; Takahashi, T.; Yerushalmi, R.; Takei, K.; Ford, A.C.; Chueh, Y.-L.; Javey, A. Toward the Development of Printable Nanowire Electronics and Sensors. Adv. Mater. 2009, 21, 3730–3743. [Google Scholar] [CrossRef]
- Adam, T.; Dhahi, T.S.; Gopinath, S.C.B.; Hashim, U. Novel Approaches in Fabrication and Integration of Nanowire for Micro/Nano Systems. Crit. Rev. Anal. Chem. 2021, in press. [Google Scholar] [CrossRef]
- Milano, G.; Porro, S.; Valov, I.; Ricciardi, C. Recent Developments and Perspectives for Memristive Devices Based on Metal Oxide Nanowires. Adv. Electron. Mater. 2019, 5, 1800909. [Google Scholar] [CrossRef]
- Zhou, Z.; Lan, C.; Wei, R.; Ho, J.C. Transparent Metal-Oxide Nanowires and Their Applications in Harsh Electronics. J. Mater. Chem. C 2019, 7, 202–217. [Google Scholar] [CrossRef]
- Yang, B.; Myung, N.V.; Tran, T.-T. 1D Metal Oxide Semiconductor Materials for Chemiresistive Gas Sensors: A Review. Adv. Electron. Mater. 2021, 7, 2100271. [Google Scholar] [CrossRef]
- Cai, B.; Song, Z.; Tong, Y.; Tang, Q.; Shaymurat, T.; Liu, Y. A Single Nanobelt Transistor for Gas Identification: Using a Gas-Dielectric Strategy. Sensors 2016, 16, 917. [Google Scholar] [CrossRef] [Green Version]
- Xue, N.; Zhang, Q.; Zhang, S.; Zong, P.; Yang, F. Highly Sensitive and Selective Hydrogen Gas Sensor Using the Mesoporous SnO2 Modified Layers. Sensors 2017, 17, 2351. [Google Scholar] [CrossRef] [Green Version]
- Xiao, B.; Wang, F.; Zhai, C.; Wang, P.; Xiao, C.; Zhang, M. Facile Synthesis of In2O3 Nanoparticles for Sensing Properties at Low Detection Temperature. Sens. Actuators B Chem. 2016, 235, 251–257. [Google Scholar] [CrossRef]
- Zhu, Z.; Suzuki, M.; Nagashima, K.; Yoshida, H.; Kanai, M.; Meng, G.; Anzai, H.; Zhuge, F.; He, Y.; Boudot, M.; et al. Rational Concept for Reducing Growth Temperature in Vapor–Liquid–Solid Process of Metal Oxide Nanowires. Nano Lett. 2016, 16, 7495–7502. [Google Scholar] [CrossRef]
- Klamchuen, A.; Suzuki, M.; Nagashima, K.; Yoshida, H.; Kanai, M.; Zhuge, F.; He, Y.; Meng, G.; Kai, S.; Takeda, S.; et al. Rational Concept for Designing Vapor–Liquid–Solid Growth of Single Crystalline Metal Oxide Nanowires. Nano Lett. 2015, 15, 6406–6412. [Google Scholar] [CrossRef]
- Abokifa, A.A.; Haddad, K.; Fortner, J.; Lo, C.S.; Biswas, P. Sensing Mechanism of Ethanol and Acetone at Room Temperature by SnO2 Nano-Columns Synthesized by Aerosol Routes: Theoretical Calculations Compared to Experimental Results. J. Mater. Chem. A 2018, 6, 2053–2066. [Google Scholar] [CrossRef]
- Nguyen, K.; Hung, C.M.; Ngoc, T.M.; Thanh Le, D.T.; Nguyen, D.H.; Nguyen Van, D.; Nguyen Van, H. Low-Temperature Prototype Hydrogen Sensors Using Pd-Decorated SnO2 Nanowires for Exhaled Breath Applications. Sens. Actuators B Chem. 2017, 253, 156–163. [Google Scholar] [CrossRef]
- Hernandez-Ramirez, F.; Tarancon, A.; Casals, O.; Pellicer, E.; Rodriguez, J.; Romano-Rodriguez, A.; Morante, J.R.; Barth, S.; Mathur, S. Electrical Properties of Individual Tin Oxide Nanowires Contacted to Platinum Electrodes. Phys. Rev. B 2007, 76, 085429. [Google Scholar] [CrossRef] [Green Version]
- Vomiero, A.; Ponzoni, A.; Comini, E.; Ferroni, M.; Faglia, G.; Sberveglieri, G. Direct Integration of Metal Oxide Nanowires into an Effective Gas Sensing Device. Nanotechnology 2010, 21, 145502. [Google Scholar] [CrossRef]
- Klamchuen, A.; Yanagida, T.; Kanai, M.; Nagashima, K.; Oka, K.; Rahong, S.; Gang, M.; Horprathum, M.; Suzuki, M.; Hidaka, Y.; et al. Study on Transport Pathway in Oxide Nanowire Growth by Using Spacing-Controlled Regular Array. Appl. Phys. Lett. 2011, 99, 193105. [Google Scholar] [CrossRef] [Green Version]
- Ho, S.-T.; Wang, C.-Y.; Liu, H.-L.; Lin, H.-N. Catalyst-Free Selective-Area Growth of Vertically Aligned Zinc Oxide Nanowires. Chem. Phys. Lett. 2008, 463, 141–144. [Google Scholar] [CrossRef]
- Qin, Y.; Xie, W.; Liu, Y.; Ye, Z. Thermal-Oxidative Growth of Aligned W18O49 Nanowire Arrays for High Performance Gas Sensor. Sens. Actuators B Chem. 2016, 223, 487–495. [Google Scholar] [CrossRef]
- Steinhauer, S.; Brunet, E.; Maier, T.; Mutinati, G.C.; Köck, A.; Freudenberg, O.; Gspan, C.; Grogger, W.; Neuhold, A.; Resel, R. Gas Sensing Properties of Novel CuO Nanowire Devices. Sens. Actuators B Chem. 2013, 187, 50–57. [Google Scholar] [CrossRef]
- Ponzoni, A.; Russo, V.; Bailini, A.; Casari, C.S.; Ferroni, M.; Li Bassi, A.; Migliori, A.; Morandi, V.; Ortolani, L.; Sberveglieri, G.; et al. Structural and Gas-Sensing Characterization of Tungsten Oxide Nanorods and Nanoparticles. Sens. Actuators B Chem. 2011, 153, 340–346. [Google Scholar] [CrossRef]
- Zeng, H.; Zhang, G.; Nagashima, K.; Takahashi, T.; Hosomi, T.; Yanagida, T. Metal–Oxide Nanowire Molecular Sensors and Their Promises. Chemosensors 2021, 9, 41. [Google Scholar] [CrossRef]
- Zhang, B.; Fu, W.; Meng, X.; Ruan, A.; Su, P.; Yang, H. Enhanced Ethanol Sensing Properties Based on Spherical-Coral-like SnO2 Nanorods Decorated with α-Fe2O3 Nanocrystallites. Sens. Actuators B Chem. 2018, 261, 505–514. [Google Scholar] [CrossRef]
- Kuchibhatla, S.V.N.T.; Karakoti, A.S.; Bera, D.; Seal, S. One Dimensional Nanostructured Materials. Prog. Mater. Sci. 2007, 52, 699–913. [Google Scholar] [CrossRef]
- Jeon, J.-Y.; Park, S.-J.; Ha, T.-J. Functionalization of Zinc Oxide Nanoflowers with Palladium Nanoparticles via Microwave Absorption for Room Temperature-Operating Hydrogen Gas Sensors in the Ppb Level. ACS Appl. Mater. Interfaces 2021, 13, 25082–25091. [Google Scholar] [CrossRef]
- Lv, Y.; Huang, K.; Zhang, W.; Ran, S.; Chi, F.; Yang, B.; Liu, X. High-Performance Gas-Sensing Properties of Octahedral NiO Crystals Prepared via One-Step Controllable Synthesis Route. Cryst. Res. Technol. 2014, 49, 109–115. [Google Scholar] [CrossRef]
- Kooti, M.; Keshtkar, S.; Askarieh, M.; Rashidi, A. Progress toward a Novel Methane Gas Sensor Based on SnO2 Nanorods-Nanoporous Graphene Hybrid. Sens. Actuators B Chem. 2019, 281, 96–106. [Google Scholar] [CrossRef]
- Chang, C.-M.; Hon, M.-H.; Leu, I.-C. Outstanding H2 Sensing Performance of Pd Nanoparticle-Decorated ZnO Nanorod Arrays and the Temperature-Dependent Sensing Mechanisms. ACS Appl. Mater. Interfaces 2013, 5, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Lee, S.; Cho, W.; Kwon, Y.M.; Baik, J.M.; Shin, H. Development of a Novel Gas-Sensing Platform Based on a Network of Metal Oxide Nanowire Junctions Formed on a Suspended Carbon Nanomesh Backbone. Sensors 2021, 21, 4525. [Google Scholar] [CrossRef] [PubMed]
- Fort, A.; Mugnaini, M.; Rocchi, S.; Vignoli, V.; Comini, E.; Faglia, G.; Ponzoni, A. Metal-Oxide Nanowire Sensors for CO Detection: Characterization and Modeling. Sens. Actuators B Chem. 2010, 148, 283–291. [Google Scholar] [CrossRef]
- Barsan, N.; Weimar, U. Conduction Model of Metal Oxide Gas Sensors. J. Electroceramics 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Ponce, M.A.; Bueno, P.R.; Varela, J.; Castro, M.S.; Aldao, C.M. Impedance Spectroscopy Analysis of SnO2 Thick-Films Gas Sensors. J. Mater. Sci. Mater. Electron. 2008, 19, 1169–1175. [Google Scholar] [CrossRef]
- Vodolazskaya, I.V.; Eserkepov, A.V.; Akhunzhanov, R.K.; Tarasevich, Y.Y. Effect of Tunneling on the Electrical Conductivity of Nanowire-Based Films: Computer Simulation within a Core–Shell Model. J. Appl. Phys. 2019, 126, 244903. [Google Scholar] [CrossRef] [Green Version]
- Ponzoni, A. The Contributions of Junctions and Nanowires/Nanotubes in Conductive Networks. Appl. Phys. Lett. 2019, 114, 153105. [Google Scholar] [CrossRef] [Green Version]
- Gomes da Rocha, C.; Manning, H.G.; O’Callaghan, C.; Ritter, C.; Bellew, A.T.; Boland, J.J.; Ferreira, M.S. Ultimate Conductivity Performance in Metallic Nanowire Networks. Nanoscale 2015, 7, 13011–13016. [Google Scholar] [CrossRef]
- Kim, D.; Nam, J. Analyzing Conducting Rod Networks Using Centrality. Electrochim. Acta 2021, 370, 137725. [Google Scholar] [CrossRef]
- Lee, S.P. Electrodes for Semiconductor Gas Sensors. Sensors 2017, 17, 683. [Google Scholar] [CrossRef]
- Zhang, Z.; Yao, K.; Liu, Y.; Jin, C.; Liang, X.; Chen, Q.; Peng, L.-M. Quantitative Analysis of Current–Voltage Characteristics of Semiconducting Nanowires: Decoupling of Contact Effects. Adv. Funct. Mater. 2007, 17, 2478–2489. [Google Scholar] [CrossRef]
- Hernández-Ramírez, F.; Tarancón, A.; Casals, O.; Rodríguez, J.; Romano-Rodríguez, A.; Morante, J.R.; Barth, S.; Mathur, S.; Choi, T.Y.; Poulikakos, D.; et al. Fabrication and Electrical Characterization of Circuits Based on Individual Tin Oxide Nanowires. Nanotechnology 2006, 17, 5577–5583. [Google Scholar] [CrossRef]
- Yamazoe, N. New Approaches for Improving Semiconductor Gas Sensors. Sens. Actuators B Chem. 1991, 5, 7–19. [Google Scholar] [CrossRef]
- Yamazoe, N.; Shimanoe, K. Theory of Power Laws for Semiconductor Gas Sensors. Sens. Actuators B Chem. 2008, 128, 566–573. [Google Scholar] [CrossRef]
- Krivetskiy, V.; Ponzoni, A.; Comini, E.; Badalyan, S.; Rumyantseva, M.; Gaskov, A. Selectivity Modification of SnO2-Based Materials for Gas Sensor Arrays. Electroanalysis 2010, 22, 2809–2816. [Google Scholar] [CrossRef]
- Gurlo, A.; Bârsan, N.; Ivanovskaya, M.; Weimar, U.; Göpel, W. In2O3 and MoO3–In2O3 Thin Film Semiconductor Sensors: Interaction with NO2 and O3. Sens. Actuators B Chem. 1998, 47, 92–99. [Google Scholar] [CrossRef]
- Lantto, V.; Rompplainen, P.; Leppävuori, S. A Study of the Temperature Dependence of the Barrier Energy in Porous Tin Dioxide. Sens. Actuators 1988, 14, 149–163. [Google Scholar] [CrossRef]
- Sinkkonen, J. DC Conductivity of a Random Barrier Network. Phys. Status Solidi 1980, 102, 621–627. [Google Scholar] [CrossRef]
- Ponzoni, A.; Comini, E.; Concina, I.; Ferroni, M.; Falasconi, M.; Gobbi, E.; Sberveglieri, V.; Sberveglieri, G. Nanostructured Metal Oxide Gas Sensors, a Survey of Applications Carried out at SENSOR Lab, Brescia (Italy) in the Security and Food Quality Fields. Sensors 2012, 12, 17023–17045. [Google Scholar] [CrossRef]
- D’Amico, A.; Di Natale, C. A Contribution on Some Basic Definitions of Sensors Properties. IEEE Sens. J. 2001, 1, 183–190. [Google Scholar] [CrossRef]
- Prades, J.D.; Jimenez-Diaz, R.; Manzanares, M.; Hernandez-Ramirez, F.; Cirera, A.; Romano-Rodriguez, A.; Mathur, S.; Morante, J.R. A Model for the Response towards Oxidizing Gases of Photoactivated Sensors Based on Individual SnO2 nanowires. Phys. Chem. Chem. Phys. 2009, 11, 10881–10889. [Google Scholar] [CrossRef] [PubMed]
- Sakai, G.; Matsunaga, N.; Shimanoe, K.; Yamazoe, N. Theory of Gas-Diffusion Controlled Sensitivity for Thin Film Semiconductor Gas Sensor. Sens. Actuators B Chem. 2001, 80, 125–131. [Google Scholar] [CrossRef]
- Panchapakesan, B.; DeVoe, D.L.; Widmaier, M.R.; Cavicchi, R.; Semancik, S. Nanoparticle Engineering and Control of Tin Oxide Microstructures for Chemical Microsensor Applications. Nanotechnology 2001, 12, 336–349. [Google Scholar] [CrossRef]
- Yamazoe, N.; Shimanoe, K. Roles of Shape and Size of Component Crystals in Semiconductor Gas Sensors: I. Response to oxygen. J. Electrochem. Soc. 2008, 155, J85. [Google Scholar] [CrossRef]
- Yamazoe, N.; Shimanoe, K. Roles of Shape and Size of Component Crystals in Semiconductor Gas Sensors: II. Response to NO2 and H2. J. Electrochem. Soc. 2008, 155, J93. [Google Scholar] [CrossRef]
- Hua, Z.; Qiu, Z.; Li, Y.; Zeng, Y.; Wu, Y.; Tian, X.; Wang, M.; Li, E. A Theoretical Investigation of the Power-Law Response of Metal Oxide Semiconductor Gas Sensors ΙI: Size and Shape Effects. Sens. Actuators B Chem. 2018, 255, 3541–3549. [Google Scholar] [CrossRef]
- Rebholz, J.; Bonanati, P.; Weimar, U.; Barsan, N. Grain Shape Influence on Semiconducting Metal Oxide Based Gas Sensor Performance: Modeling versus Experiment. Anal. Bioanal. Chem. 2014, 406, 3977–3983. [Google Scholar] [CrossRef]
- Ottaviano, L.; Maccallini, E.; Santucci, S. Visualisation of the Preferential Adsorption Sites of Oxygen onto WO3 Nano-Particles. Surf. Sci. 2001, 492, L700–L704. [Google Scholar] [CrossRef]
- Lee, J.-H. Gas Sensors Using Hierarchical and Hollow Oxide Nanostructures: Overview. Sens. Actuators B Chem. 2009, 140, 319–336. [Google Scholar] [CrossRef]
- Li, M.; Zhu, H.; Cheng, J.; Zhao, M.; Yan, W. Synthesis and Improved Ethanol Sensing Performance of CuO/SnO2 Based Hollow Microspheres. J. Porous Mater. 2017, 24, 507–518. [Google Scholar] [CrossRef]
- Liu, J.; Dai, M.; Wang, T.; Sun, P.; Liang, X.; Lu, G.; Shimanoe, K.; Yamazoe, N. Enhanced Gas Sensing Properties of SnO2 Hollow Spheres Decorated with CeO2 Nanoparticles Heterostructure Composite Materials. ACS Appl. Mater. Interfaces 2016, 8, 6669–6677. [Google Scholar] [CrossRef]
- Motsoeneng, R.G.; Kortidis, I.; Rikhotso, R.; Swart, H.C.; Ray, S.S.; Motaung, D.E. Temperature-Dependent Response to C3H7OH and C2H5OH Vapors Induced by Deposition of Au Nanoparticles on SnO2/NiO Hollow Sphere-Based Conductometric Sensors. Sens. Actuators B Chem. 2020, 316, 128041. [Google Scholar] [CrossRef]
- Li, H.; Chu, S.; Ma, Q.; Li, H.; Che, Q.; Wang, J.; Wang, G.; Yang, P. Multilevel Effective Heterojunctions Based on SnO2/ZnO 1D Fibrous Hierarchical Structure with Unique Interface Electronic Effects. ACS Appl. Mater. Interfaces 2019, 11, 31551–31561. [Google Scholar] [CrossRef]
- Li, R.; Chen, S.; Lou, Z.; Li, L.; Huang, T.; Song, Y.; Chen, D.; Shen, G. Fabrication of Porous SnO2 Nanowires Gas Sensors with Enhanced Sensitivity. Sens. Actuators B Chem. 2017, 252, 79–85. [Google Scholar] [CrossRef]
- Reddy, C.S.; Murali, G.; Reddy, A.S.; Park, S.; In, I. GO Incorporated SnO2 Nanotubes as Fast Response Sensors for Ethanol Vapor in Different Atmospheres. J. Alloy. Compd. 2020, 813, 152251. [Google Scholar] [CrossRef]
- Mohanapriya, P.; Segawa, H.; Watanabe, K.; Watanabe, K.; Samitsu, S.; Natarajan, T.S.; Jaya, N.V.; Ohashi, N. Enhanced Ethanol-Gas Sensing Performance of Ce-Doped SnO2 Hollow Nanofibers Prepared by Electrospinning. Sens. Actuators B Chem. 2013, 188, 872–878. [Google Scholar] [CrossRef]
- Wang, T.T.; Ma, S.Y.; Cheng, L.; Luo, J.; Jiang, X.H.; Jin, W.X. Preparation of Yb-Doped SnO2 Hollow Nanofibers with an Enhanced Ethanol–Gas Sensing Performance by Electrospinning. Sens. Actuators B Chem. 2015, 216, 212–220. [Google Scholar] [CrossRef]
- Kida, T.; Suematsu, K.; Hara, K.; Kanie, K.; Muramatsu, A. Ultrasensitive Detection of Volatile Organic Compounds by a Pore Tuning Approach Using Anisotropically Shaped SnO2 Nanocrystals. ACS Appl. Mater. Interfaces 2016, 8, 35485–35495. [Google Scholar] [CrossRef]
- Ponzoni, A. Morphological Effects in SnO2 Chemiresistors for Ethanol Detection: A Review in Terms of Central Performances and Outliers. Sensors 2021, 21, 29. [Google Scholar] [CrossRef]
- Lee, S.-H.; Galstyan, V.; Ponzoni, A.; Gonzalo-Juan, I.; Riedel, R.; Dourges, M.-A.; Nicolas, Y.; Toupance, T. Finely Tuned SnO2 Nanoparticles for Efficient Detection of Reducing and Oxidizing Gases: The Influence of Alkali Metal Cation on Gas-Sensing Properties. ACS Appl. Mater. Interfaces 2018, 10, 10173–10184. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zhang, Y.; Liu, J.; Li, H.-Y.; Hu, Z.; Luo, X.; Gao, N.; Zhang, B.; Jiang, J.; Zhong, A.; et al. Sensitive H2 Gas Sensors Based on SnO2 Nanowires. Sens. Actuators B Chem. 2021, 345, 130334. [Google Scholar] [CrossRef]
- Firooz, A.A.; Mahjoub, A.R.; Khodadadi, A.A. Highly Sensitive CO and Ethanol Nanoflower-like SnO2 Sensor among Various Morphologies Obtained by Using Single and Mixed Ionic Surfactant Templates. Sens. Actuators B Chem. 2009, 141, 89–96. [Google Scholar] [CrossRef]
- Ling-min, Y.; Sheng, L.; Bing, Y.; Miao-miao, H.; Meng-di, K.; Xinhui, F. A Highly Sensitive Ethanol Gas Sensor Based on Mesoporous SnO2 Fabricated from a Facile Double-Surfactant Template Method. Mater. Lett. 2015, 158, 409–412. [Google Scholar] [CrossRef]
- Yoon, J.-W.; Choi, S.H.; Kim, J.-S.; Jang, H.W.; Kang, Y.C.; Lee, J.-H. Trimodally Porous SnO2 Nanospheres with Three-Dimensional Interconnectivity and Size Tunability: A One-Pot Synthetic Route and Potential Application as an Extremely Sensitive Ethanol Detector. NPG Asia Mater. 2016, 8, e244. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Liu, Z.; Li, C.; Tang, T.; Liu, X.; Han, S.; Lei, B.; Zhou, C. Detection of NO2 down to Ppb Levels Using Individual and Multiple In2O3 Nanowire Devices. Nano Lett. 2004, 4, 1919–1924. [Google Scholar] [CrossRef]
- Wang, S.-H.; Chou, T.-C.; Liu, C.-C. Nano-Crystalline Tungsten Oxide NO2 Sensor. Sens. Actuators B Chem. 2003, 94, 343–351. [Google Scholar] [CrossRef]
- Oros, C.; Horprathum, M.; Wisitsoraat, A.; Srichaiyaperk, T.; Samransuksamer, B.; Limwichean, S.; Eiamchai, P.; Phokharatkul, D.; Nuntawong, N.; Chananonnawathorn, C.; et al. Ultra-Sensitive NO2 Sensor Based on Vertically Aligned SnO2 Nanorods Deposited by DC Reactive Magnetron Sputtering with Glancing Angle Deposition Technique. Sens. Actuators B Chem. 2016, 223, 936–945. [Google Scholar] [CrossRef]
- Kida, T.; Nishiyama, A.; Hua, Z.; Suematsu, K.; Yuasa, M.; Shimanoe, K. WO3 Nanolamella Gas Sensor: Porosity Control Using SnO2 Nanoparticles for Enhanced NO2 Sensing. Langmuir 2014, 30, 2571–2579. [Google Scholar] [CrossRef]
- Ponzoni, A.; Comini, E.; Sberveglieri, G.; Zhou, J.; Deng, S.Z.; Xu, N.S.; Ding, Y.; Wang, Z.L. Ultrasensitive and Highly Selective Gas Sensors Using Three-Dimensional Tungsten Oxide Nanowire Networks. Appl. Phys. Lett. 2006, 88, 203101. [Google Scholar] [CrossRef]
- Cai, Z.; Park, S. Enhancement Mechanisms of Ethanol-Sensing Properties Based on Cr2O3 Nanoparticle-Anchored SnO2 Nanowires. J. Mater. Res. Technol. 2020, 9, 271–281. [Google Scholar] [CrossRef]
- Park, J.Y.; Choi, S.-W.; Kim, S.S. Tailoring the Number of Junctions per Electrode Pair in Networked ZnO Nanowire Sensors. J. Am. Ceram. Soc. 2011, 94, 3922–3926. [Google Scholar] [CrossRef]
- Li, T.; Zeng, W.; Zhao, W. Gas Sensing Performance of Multiple SnO2 1D Nanostructures Based on Their Interconnect Manner. Mater. Lett. 2016, 167, 230–233. [Google Scholar] [CrossRef]
- Li, T.; Zeng, W. New Insight into the Gas Sensing Performance of SnO2 Nanorod-Assembled Urchins Based on Their Assembly Density. Ceram. Int. 2017, 43, 728–735. [Google Scholar] [CrossRef]
- Batzill, M.; Diebold, U. The Surface and Materials Science of Tin Oxide. Prog. Surf. Sci. 2005, 79, 47–154. [Google Scholar] [CrossRef]
- Diebold, U. The Surface Science of Titanium Dioxide. Surf. Sci. Rep. 2003, 48, 53–229. [Google Scholar] [CrossRef]
- Katsiev, K.; Kolmakov, A.; Fang, M.; Diebold, U. Characterization of Individual SnO2 Nanobelts with STM. Surf. Sci. 2008, 602, L112–L114. [Google Scholar] [CrossRef]
- Zhang, N.; Fan, Y.; Lu, Y.; Li, C.; Zhou, J.; Li, X.; Adimi, S.; Ruan, S. Synthesis of Au-Decorated SnO2 Crystallites with Exposed (221) Facets and Their Enhanced Acetylene Sensing Properties. Sens. Actuators B Chem. 2020, 307, 127629. [Google Scholar] [CrossRef]
- Chen, H.; Ma, S.Y.; Jin, W.X.; Li, W.Q. The Mode of Multi-Tier Nested Tin Dioxide Polyhedral Nanoparticles with Exposed High-Energy Facets and Their Gas Sensing Properties. Mater. Lett. 2016, 164, 627–630. [Google Scholar] [CrossRef]
- Shang, Y.; Guo, L. Facet-Controlled Synthetic Strategy of Cu2O-Based Crystals for Catalysis and Sensing. Adv. Sci. 2015, 2, 1500140. [Google Scholar] [CrossRef]
- Han, X.; Jin, M.; Xie, S.; Kuang, Q.; Jiang, Z.; Jiang, Y.; Xie, Z.; Zheng, L. Synthesis of Tin Dioxide Octahedral Nanoparticles with Exposed High-Energy {221} Facets and Enhanced Gas-Sensing Properties. Angew. Chem. Int. Ed. 2009, 48, 9180–9183. [Google Scholar] [CrossRef]
- Ouyang, J.; Pei, J.; Kuang, Q.; Xie, Z.; Zheng, L. Supersaturation-Controlled Shape Evolution of α-Fe2O3 Nanocrystals and Their Facet-Dependent Catalytic and Sensing Properties. ACS Appl. Mater. Interfaces 2014, 6, 12505–12514. [Google Scholar] [CrossRef]
- Yang, Y.; Hong, A.; Liang, Y.; Xu, K.; Yu, T.; Shi, J.; Zeng, F.; Qu, Y.; Liu, Y.; Ding, M.; et al. High-Energy {001} Crystal Facets and Surface Fluorination Engineered Gas Sensing Properties of Anatase Titania Nanocrystals. Appl. Surf. Sci. 2017, 423, 602–610. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, T. An Overview: Facet-Dependent Metal Oxide Semiconductor Gas Sensors. Sens. Actuators B Chem. 2018, 277, 604–633. [Google Scholar] [CrossRef]
- Ponzoni, A.; Baratto, C.; Bianchi, S.; Comini, E.; Ferroni, M.; Pardo, M.; Vezzoli, M.; Vomiero, A.; Faglia, G.; Sberveglieri, G. Metal Oxide Nanowire and Thin-Film-Based Gas Sensors for Chemical Warfare Simulants Detection. IEEE Sens. J. 2008, 8, 735–742. [Google Scholar] [CrossRef]
- Sze, S.M.; Ng, K.K. Chapter 1 Physics and Properties of Semiconductors—A review. In Physics of Semiconductor Devices, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2007; pp. 7–78. ISBN 978-0-471-14323-9. [Google Scholar]
- Carotta, M.C.; Ferroni, M.; Guidi, V.; Martinelli, G. Preparation and Characterization of Nanostructured Titania Thick Films. Adv. Mater. 1999, 11, 943–946. [Google Scholar] [CrossRef]
- Gribb, A.A.; Banfield, J.F. Particle Size Effects on Transformation Kinetics and Phase Stability in Nanocrystalline TiO2. Am. Mineral. 1997, 82, 717–728. [Google Scholar] [CrossRef]
- Ruiz, A.; Dezanneau, G.; Arbiol, J.; Cornet, A.; Morante, J.R. Study of the Influence of Nb Content and Sintering Temperature on TiO2 Sensing Films. Thin Solid Film. 2003, 436, 90–94. [Google Scholar] [CrossRef]
- Sysoev, V.V.; Schneider, T.; Goschnick, J.; Kiselev, I.; Habicht, W.; Hahn, H.; Strelcov, E.; Kolmakov, A. Percolating SnO2 Nanowire Network as a Stable Gas Sensor: Direct Comparison of Long-Term Performance versus SnO2 Nanoparticle Films. Sens. Actuators B Chem. 2009, 139, 699–703. [Google Scholar] [CrossRef]
- Afzal, A. β-Ga2O3 Nanowires and Thin Films for Metal Oxide Semiconductor Gas Sensors: Sensing Mechanisms and Performance Enhancement Strategies. J. Mater. 2019, 5, 542–557. [Google Scholar] [CrossRef]
- Fleischer, M.; Höllbauer, L.; Meixner, H. Effect of the Sensor Structure on the Stability of Ga2O3 Sensors for Reducing Gases. Sens. Actuators B Chem. 1994, 18, 119–124. [Google Scholar] [CrossRef]
- Bae, M.-S.; Kim, S.-H.; Baek, J.-S.; Koh, J.-H. Comparative Study of High-Temperature Annealed and RTA Process β-Ga2O3 Thin Film by Sol–Gel Process. Coatings 2021, 11, 1220. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Brinzari, V.; Ivanov, M.; Cerneavschi, A.; Rodriguez, J.; Cirera, A.; Cornet, A.; Morante, J. Structural Stability of Indium Oxide Films Deposited by Spray Pyrolysis during Thermal Annealing. Thin Solid Film. 2005, 479, 38–51. [Google Scholar] [CrossRef]
- Cuong, N.D.; Park, Y.W.; Yoon, S.G. Microstructural and Electrical Properties of Ga2O3 Nanowires Grown at Various Temperatures by Vapor–Liquid–Solid Technique. Sens. Actuators B Chem. 2009, 140, 240–244. [Google Scholar] [CrossRef]
- Krawczyk, M.; Suchorska-Woźniak, P.; Szukiewicz, R.; Kuchowicz, M.; Korbutowicz, R.; Teterycz, H. Morphology of Ga2O3 Nanowires and Their Sensitivity to Volatile Organic Compounds. Nanomaterials 2021, 11, 456. [Google Scholar] [CrossRef]
- Ruiz, A.; Cornet, A.; Sakai, G.; Shimanoe, K.; Morante, J.R.; Yamazoe, N. Preparation of Cr-Doped TiO2 Thin Film of P-Type Conduction for Gas Sensor Application. Chem. Lett. 2002, 31, 892–893. [Google Scholar] [CrossRef]
- Kwak, C.-H.; Kim, T.-H.; Jeong, S.-Y.; Yoon, J.-W.; Kim, J.-S.; Lee, J.-H. Humidity-Independent Oxide Semiconductor Chemiresistors Using Terbium-Doped SnO2 Yolk–Shell Spheres for Real-Time Breath Analysis. ACS Appl. Mater. Interfaces 2018, 10, 18886–18894. [Google Scholar] [CrossRef]
- Hammer, C.; Warmer, J.; Maurer, S.; Kaul, P.; Thoelen, R.; Jung, N. A Compact 16 Channel Embedded System with High Dynamic Range Readout and Heater Management for Semiconducting Metal Oxide Gas Sensors. Electronics 2020, 9, 1855. [Google Scholar] [CrossRef]
- Hijazi, Z.; Grassi, M.; Caviglia, D.D.; Valle, M. Time-Based Calibration-Less Read-out Circuit for Interfacing Wide Range MOX Gas Sensors. Integration 2018, 63, 232–239. [Google Scholar] [CrossRef]
- Vakifahmetoglu, C.; Buldu, M.; Karakuscu, A.; Ponzoni, A.; Assefa, D.; Soraru, G.D. High Surface Area Carbonous Components from Emulsion Derived SiOC and Their Gas Sensing Behavior. J. Eur. Ceram. Soc. 2015, 35, 4447–4452. [Google Scholar] [CrossRef]
- Bao, Y.; Wei, P.; Xia, X.; Huang, Z.; Homewood, K.; Gao, Y. Remarkably Enhanced H2 Response and Detection Range in Nb Doped Rutile/Anatase Heterophase Junction TiO2 Thin Film Hydrogen Sensors. Sens. Actuators B Chem. 2019, 301, 127143. [Google Scholar] [CrossRef]
- Galstyan, V.; Ponzoni, A.; Kholmanov, I.; Natile, M.M.; Comini, E.; Nematov, S.; Sberveglieri, G. Investigation of Reduced Graphene Oxide and a Nb-Doped TiO2 Nanotube Hybrid Structure To Improve the Gas-Sensing Response and Selectivity. ACS Sens. 2019, 4, 2094–2100. [Google Scholar] [CrossRef]
- Goto, K.; Konishi, K.; Murakami, H.; Kumagai, Y.; Monemar, B.; Higashiwaki, M.; Kuramata, A.; Yamakoshi, S. Halide Vapor Phase Epitaxy of Si Doped β-Ga2O3 and Its Electrical Properties. Thin Solid Film. 2018, 666, 182–184. [Google Scholar] [CrossRef]
- Mazeina, L.; Picard, Y.N.; Maximenko, S.I.; Perkins, F.K.; Glaser, E.R.; Twigg, M.E.; Freitas, J.A.; Prokes, S.M. Growth of Sn-Doped β-Ga2O3 Nanowires and Ga2O3−SnO2 Heterostructures for Gas Sensing Applications. Cryst. Growth Des. 2009, 9, 4471–4479. [Google Scholar] [CrossRef]
- Li, Y.; Trinchi, A.; Wlodarski, W.; Galatsis, K.; Kalantar-zadeh, K. Investigation of the Oxygen Gas Sensing Performance of Ga2O3 Thin Films with Different Dopants. Sens. Actuators B Chem. 2003, 93, 431–434. [Google Scholar] [CrossRef]
- Wang, D.; Lou, Y.; Wang, R.; Wang, P.; Zheng, X.; Zhang, Y.; Jiang, N. Humidity Sensor Based on Ga2O3 Nanorods Doped with Na+ and K+ from GaN Powder. Ceram. Int. 2015, 41, 14790–14797. [Google Scholar] [CrossRef]
- Singh, N.; Ponzoni, A.; Comini, E.; Lee, P.S. Chemical Sensing Investigations on Zn–In2O3 Nanowires. Sens. Actuators B Chem. 2012, 171, 244–248. [Google Scholar] [CrossRef]
- Sun, X.; Liu, X.; Deng, X.; Xu, X. Synthesis of Zn-Doped In2O3 Nano Sphere Architectures as a Triethylamine Gas Sensor and Photocatalytic Properties. RSC Adv. 2016, 6, 89847–89854. [Google Scholar] [CrossRef]
- Li, P.; Fan, H.; Cai, Y.; Xu, M.; Long, C.; Li, M.; Lei, S.; Zou, X. Phase Transformation (Cubic to Rhombohedral): The Effect on the NO2 Sensing Performance of Zn-Doped Flower-like In2O3 Structures. RSC Adv. 2014, 4, 15161–15170. [Google Scholar] [CrossRef]
- Zhao, Q.; Ju, D.; Deng, X.; Huang, J.; Cao, B.; Xu, X. Morphology-Modulation of SnO2 Hierarchical Architectures by Zn Doping for Glycol Gas Sensing and Photocatalytic Applications. Sci. Rep. 2015, 5, 7874. [Google Scholar] [CrossRef] [Green Version]
- Rumyantseva, M.N.; Safonova, O.V.; Boulova, M.N.; Ryabova, L.I.; Gas’kov, A.M. Dopants in Nanocrystalline Tin Dioxide. Russ. Chem. Bull. 2003, 52, 1217–1238. [Google Scholar] [CrossRef]
- Degler, D.; Weimar, U.; Barsan, N. Current Understanding of the Fundamental Mechanisms of Doped and Loaded Semiconducting Metal-Oxide-Based Gas Sensing Materials. ACS Sens. 2019, 4, 2228–2249. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.M.; Akbar, S.A.; Morris, P.A. Synergistic Effects in Gas Sensing Semiconducting Oxide Nano-Heterostructures: A Review. Sens. Actuators B Chem. 2019, 286, 624–640. [Google Scholar] [CrossRef]
- Lin, T.; Lv, X.; Li, S.; Wang, Q. The Morphologies of the Semiconductor Oxides and Their Gas-Sensing Properties. Sensors 2017, 17, 2779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sze, S.M.; Ng, K.K. Chapter 3 Metal-Semiconductors Contacts. In Physics of Semiconductor Devices, 3rd ed.; John Wiley & Sons: Hoboken NJ, USA, 2007; pp. 134–196. ISBN 978-0-471-14323-9. [Google Scholar]
- Roldán, A.; González, S.; Ricart, J.M.; Illas, F. Critical Size for O2 Dissociation by Au Nanoparticles. ChemPhysChem 2009, 10, 348–351. [Google Scholar] [CrossRef]
- Tofighi, G.; Degler, D.; Junker, B.; Müller, S.; Lichtenberg, H.; Wang, W.; Weimar, U.; Barsan, N.; Grunwaldt, J.-D. Microfluidically Synthesized Au, Pd and AuPd Nanoparticles Supported on SnO2 for Gas Sensing Applications. Sens. Actuators B Chem. 2019, 292, 48–56. [Google Scholar] [CrossRef] [Green Version]
- Kolmakov, A.; Klenov, D.O.; Lilach, Y.; Stemmer, S.; Moskovits, M. Enhanced Gas Sensing by Individual SnO2 Nanowires and Nanobelts Functionalized with Pd Catalyst Particles. Nano Lett. 2005, 5, 667–673. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, F.-X.; Lian, X.-X.; Zou, Y.-L.; Wang, Q.; Zhou, Q.-J. Highly Sensitive Ethanol Sensor Based on Au-Decorated SnO2 Nanoparticles Synthesized Through Precipitation and Microwave Irradiation. J. Electron. Mater. 2016, 45, 3149–3156. [Google Scholar] [CrossRef]
- Jin, C.; Kim, H.; Park, S.; Kim, H.W.; Lee, S.; Lee, C. Enhanced Ethanol Gas Sensing Properties of SnO2 Nanobelts Functionalized with Au. Ceram. Int. 2012, 38, 6585–6590. [Google Scholar] [CrossRef]
- Kim, B.-Y.; Cho, J.S.; Yoon, J.-W.; Na, C.W.; Lee, C.-S.; Ahn, J.H.; Kang, Y.C.; Lee, J.-H. Extremely Sensitive Ethanol Sensor Using Pt-Doped SnO2 Hollow Nanospheres Prepared by Kirkendall Diffusion. Sens. Actuators B Chem. 2016, 234, 353–360. [Google Scholar] [CrossRef]
- Xue, X.; Chen, Z.; Ma, C.; Xing, L.; Chen, Y.; Wang, Y.; Wang, T. One-Step Synthesis and Gas-Sensing Characteristics of Uniformly Loaded Pt@SnO2 Nanorods. J. Phys. Chem. C 2010, 114, 3968–3972. [Google Scholar] [CrossRef]
- Punginsang, M.; Zappa, D.; Comini, E.; Wisitsoraat, A.; Sberveglieri, G.; Ponzoni, A.; Liewhiran, C. Selective H2S Gas Sensors Based on Ohmic Hetero-Interface of Au-Functionalized WO3 Nanowires. Appl. Surf. Sci. 2022, 571, 151262. [Google Scholar] [CrossRef]
- Lee, I.; Choi, S.-J.; Park, K.-M.; Lee, S.S.; Choi, S.; Kim, I.-D.; Park, C.O. The Stability, Sensitivity and Response Transients of ZnO, SnO2 and WO3 Sensors under Acetone, Toluene and H2S Environments. Sens. Actuators B Chem. 2014, 197, 300–307. [Google Scholar] [CrossRef]
- Krivetskiy, V.; Zamanskiy, K.; Beltyukov, A.; Asachenko, A.; Topchiy, M.; Nechaev, M.; Garshev, A.; Krotova, A.; Filatova, D.; Maslakov, K.; et al. Effect of AuPd Bimetal Sensitization on Gas Sensing Performance of Nanocrystalline SnO2 Obtained by Single Step Flame Spray Pyrolysis. Nanomaterials 2019, 9, 728. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Cheng, Z.; Xiang, Q.; Yan, L.; Wang, X.; Xu, J. Bimetal PdAu Decorated SnO2 Nanosheets Based Gas Sensor with Temperature-Dependent Dual Selectivity for Detecting Formaldehyde and Acetone. Sens. Actuators B Chem. 2019, 283, 590–601. [Google Scholar] [CrossRef]
- Zeb, S.; Peng, X.; Shi, Y.; Su, J.; Sun, J.; Zhang, M.; Sun, G.; Nie, Y.; Cui, Y.; Jiang, X. Bimetal Au-Pd Decorated Hierarchical WO3 Nanowire Bundles for Gas Sensing Application. Sens. Actuators B Chem. 2021, 334, 129584. [Google Scholar] [CrossRef]
- Wei, T.-Y.; Yeh, P.-H.; Lu, S.-Y.; Wang, Z.L. Gigantic Enhancement in Sensitivity Using Schottky Contacted Nanowire Nanosensor. J. Am. Chem. Soc. 2009, 131, 17690–17695. [Google Scholar] [CrossRef]
- Navale, S.; Shahbaz, M.; Majhi, S.M.; Mirzaei, A.; Kim, H.W.; Kim, S.S. CuxO Nanostructure-Based Gas Sensors for H2S Detection: An Overview. Chemosensors 2021, 9, 127. [Google Scholar] [CrossRef]
- Shao, F.; Hoffmann, M.W.G.; Prades, J.D.; Zamani, R.; Arbiol, J.; Morante, J.R.; Varechkina, E.; Rumyantseva, M.; Gaskov, A.; Giebelhaus, I.; et al. Heterostructured P-CuO (Nanoparticle)/n-SnO2 (Nanowire) Devices for Selective H2S Detection. Sens. Actuators B Chem. 2013, 181, 130–135. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, S.; Yu, Q.; Wang, S.; Sun, P.; Lu, H.; Liu, F.; Yan, X.; Lu, G. Novel Self-Assembly Route Assisted Ultra-Fast Trace Volatile Organic Compounds Gas Sensing Based on Three-Dimensional Opal Microspheres Composites for Diabetes Diagnosis. ACS Appl. Mater. Interfaces 2018, 10, 32913–32921. [Google Scholar] [CrossRef]
- Zhao, S.; Shen, Y.; Maboudian, R.; Carraro, C.; Han, C.; Liu, W.; Wei, D. Facile Synthesis of ZnO-SnO2 Hetero-Structured Nanowires for High-Performance NO2 Sensing Application. Sens. Actuators B Chem. 2021, 333, 129613. [Google Scholar] [CrossRef]
- Choi, S.-B.; Lee, W.S.; Lee, C.; Lee, S. Enhanced NO2 Gas-Sensing Performance of Pd/ZnO-Codecorated SnO2 Nanorod Sensors. Appl. Phys. A 2018, 124, 817. [Google Scholar] [CrossRef]
- Kim, J.-H.; Katoch, A.; Kim, S.S. Optimum Shell Thickness and Underlying Sensing Mechanism in p–n CuO–ZnO Core–Shell Nanowires. Sens. Actuators B Chem. 2016, 222, 249–256. [Google Scholar] [CrossRef]
- Kim, J.-H.; Katoch, A.; Kim, S.-H.; Kim, S.S. Chemiresistive Sensing Behavior of SnO2 (n)–Cu2O (p) Core–Shell Nanowires. ACS Appl. Mater. Interfaces 2015, 7, 15351–15358. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-W.; Katoch, A.; Sun, G.-J.; Kim, J.-H.; Kim, S.-H.; Kim, S.S. Dual Functional Sensing Mechanism in SnO2–ZnO Core–Shell Nanowires. ACS Appl. Mater. Interfaces 2014, 6, 8281–8287. [Google Scholar] [CrossRef] [PubMed]
- Tharsika, T.; Haseeb, A.S.M.A.; Akbar, S.A.; Sabri, M.F.; Hoong, W.Y. Enhanced Ethanol Gas Sensing Properties of SnO2-Core/ZnO-Shell Nanostructures. Sensors 2014, 14, 14586–14600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ju, D.-X.; Xu, H.-Y.; Qiu, Z.-W.; Zhang, Z.-C.; Xu, Q.; Zhang, J.; Wang, J.-Q.; Cao, B.-Q. Near Room Temperature, Fast-Response, and Highly Sensitive Triethylamine Sensor Assembled with Au-Loaded ZnO/SnO2 Core–Shell Nanorods on Flat Alumina Substrates. ACS Appl. Mater. Interfaces 2015, 7, 19163–19171. [Google Scholar] [CrossRef]
- Jang, Y.-G.; Kim, W.-S.; Kim, D.-H.; Hong, S.-H. Fabrication of Ga2O3/SnO2 Core–Shell Nanowires and Their Ethanol Gas Sensing Properties. J. Mater. Res. 2011, 26, 2322–2327. [Google Scholar] [CrossRef]
- Xue, X.-T.; Zhu, L.-Y.; Yuan, K.-P.; Zeng, C.; Li, X.-X.; Ma, H.-P.; Lu, H.-L.; Zhang, D.W. ZnO Branched P-CuxO @n-ZnO Heterojunction Nanowires for Improving Acetone Gas Sensing Performance. Sens. Actuators B Chem. 2020, 324, 128729. [Google Scholar] [CrossRef]
- Bang, J.H.; Mirzaei, A.; Han, S.; Lee, H.Y.; Shin, K.Y.; Kim, S.S.; Kim, H.W. Realization of Low-Temperature and Selective NO2 Sensing of SnO2 Nanowires via Synergistic Effects of Pt Decoration and Bi2O3 Branching. Ceram. Int. 2021, 47, 5099–5111. [Google Scholar] [CrossRef]
- Choi, M.S.; Bang, J.H.; Mirzaei, A.; Oum, W.; Na, H.G.; Jin, C.; Kim, S.S.; Kim, H.W. Promotional Effects of ZnO-Branching and Au-Functionalization on the Surface of SnO2 Nanowires for NO2 Sensing. J. Alloy. Compd. 2019, 786, 27–39. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Kang, S.Y.; Mirzaei, A.; Choi, M.S.; Bang, J.H.; Kim, S.S.; Kim, H.W. Enhancement of Gas Sensing Properties by the Functionalization of ZnO-Branched SnO2 Nanowires with Cr2O3 Nanoparticles. Sens. Actuators B Chem. 2017, 249, 656–666. [Google Scholar] [CrossRef]
- Wan, Q.; Huang, J.; Xie, Z.; Wang, T.; Dattoli, E.N.; Lu, W. Branched SnO2 Nanowires on Metallic Nanowire Backbones for Ethanol Sensors Application. Appl. Phys. Lett. 2008, 92, 102101. [Google Scholar] [CrossRef]
- Li, X.; Tao, L.; Chen, Z.; Fang, H.; Li, X.; Wang, X.; Xu, J.-B.; Zhu, H. Graphene and Related Two-Dimensional Materials: Structure-Property Relationships for Electronics and Optoelectronics. Appl. Phys. Rev. 2017, 4, 021306. [Google Scholar] [CrossRef]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of Individual Gas Molecules Adsorbed on Graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef]
- Robinson, J.T.; Perkins, F.K.; Snow, E.S.; Wei, Z.; Sheehan, P.E. Reduced Graphene Oxide Molecular Sensors. Nano Lett. 2008, 8, 3137–3140. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Wang, J.; Xu, J.-L.; Li, X.; Xie, D.; Xiang, L.; Komarneni, S. Confined Formation of Ultrathin ZnO Nanorods/Reduced Graphene Oxide Mesoporous Nanocomposites for High-Performance Room-Temperature NO2 Sensors. ACS Appl. Mater. Interfaces 2016, 8, 35454–35463. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, L.; Guo, F.; Liu, S.; Qi, L.; Shan, M.; Fan, X. Facial Development of High Performance Room Temperature NO2 Gas Sensors Based on ZnO Nanowalls Decorated RGO Nanosheets. Appl. Surf. Sci. 2017, 423, 721–727. [Google Scholar] [CrossRef]
- Cao, P.; Cai, Y.; Pawar, D.; Navale, S.T.; Rao, C.N.; Han, S.; Xu, W.; Fang, M.; Liu, X.; Zeng, Y.; et al. Down to Ppb Level NO2 Detection by ZnO/RGO Heterojunction Based Chemiresistive Sensors. Chem. Eng. J. 2020, 401, 125491. [Google Scholar] [CrossRef]
- Sun, Z.; Huang, D.; Yang, Z.; Li, X.; Hu, N.; Yang, C.; Wei, H.; Yin, G.; He, D.; Zhang, Y. ZnO Nanowire-Reduced Graphene Oxide Hybrid Based Portable NH3 Gas Sensing Electron Device. IEEE Electron Device Lett. 2015, 36, 1376–1379. [Google Scholar] [CrossRef]
- Chen, Z.; Guo, H.; Zhang, F.; Li, X.; Yu, J.; Chen, X. Porous ZnO/RGO Nanosheet-Based NO2 Gas Sensor with High Sensitivity and Ppb-Level Detection Limit at Room Temperature. Adv. Mater. Interfaces 2021, 8, 2101511. [Google Scholar] [CrossRef]
- Feng, Q.; Li, X.; Wang, J. Percolation Effect of Reduced Graphene Oxide (RGO) on Ammonia Sensing of RGO-SnO2 Composite Based Sensor. Sens. Actuators B Chem. 2017, 243, 1115–1126. [Google Scholar] [CrossRef]
- Song, Z.; Wei, Z.; Wang, B.; Luo, Z.; Xu, S.; Zhang, W.; Yu, H.; Li, M.; Huang, Z.; Zang, J.; et al. Sensitive Room-Temperature H2S Gas Sensors Employing SnO2 Quantum Wire/Reduced Graphene Oxide Nanocomposites. Chem. Mater. 2016, 28, 1205–1212. [Google Scholar] [CrossRef]
- Shewale, P.S.; Yun, K.-S. Synthesis and Characterization of Cu-Doped ZnO/RGO Nanocomposites for Room-Temperature H2S Gas Sensor. J. Alloy. Compd. 2020, 837, 155527. [Google Scholar] [CrossRef]
- Fang, W.; Yang, Y.; Yu, H.; Dong, X.; Wang, R.; Wang, T.; Wang, J.; Liu, Z.; Zhao, B.; Wang, X. An In2O3 Nanorod-Decorated Reduced Graphene Oxide Composite as a High-Response NOx Gas Sensor at Room Temperature. New J. Chem. 2017, 41, 7517–7523. [Google Scholar] [CrossRef]
- Deng, S.; Tjoa, V.; Fan, H.M.; Tan, H.R.; Sayle, D.C.; Olivo, M.; Mhaisalkar, S.; Wei, J.; Sow, C.H. Reduced Graphene Oxide Conjugated Cu2O Nanowire Mesocrystals for High-Performance NO2 Gas Sensor. J. Am. Chem. Soc. 2012, 134, 4905–4917. [Google Scholar] [CrossRef] [PubMed]
- Kampara, R.K.; Rai, P.K.; Jeyaprakash, B.G. Highly Sensitive Graphene Oxide Functionalized ZnO Nanowires for Ammonia Vapour Detection at Ambient Temperature. Sens. Actuators B Chem. 2018, 255, 1064–1071. [Google Scholar] [CrossRef]
- Van Quang, V.; Van Dung, N.; Sy Trong, N.; Duc Hoa, N.; Van Duy, N.; Van Hieu, N. Outstanding Gas-Sensing Performance of Graphene/SnO2 Nanowire Schottky Junctions. Appl. Phys. Lett. 2014, 105, 013107. [Google Scholar] [CrossRef]
- Tyagi, P.; Sharma, A.; Tomar, M.; Gupta, V. A Comparative Study of RGO-SnO2 and MWCNT-SnO2 Nanocomposites Based SO2 Gas Sensors. Sens. Actuators B Chem. 2017, 248, 980–986. [Google Scholar] [CrossRef]
- Robinson, J.A.; Snow, E.S.; Bǎdescu, Ş.C.; Reinecke, T.L.; Perkins, F.K. Role of Defects in Single-Walled Carbon Nanotube Chemical Sensors. Nano Lett. 2006, 6, 1747–1751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, P.-X.; Liu, C.; Cheng, H.-M. Purification of Carbon Nanotubes. Carbon 2008, 46, 2003–2025. [Google Scholar] [CrossRef]
- Waclawik, E.R.; Chang, J.; Ponzoni, A.; Concina, I.; Zappa, D.; Comini, E.; Motta, N.; Faglia, G.; Sberveglieri, G. Functionalised Zinc Oxide Nanowire Gas Sensors: Enhanced NO2 Gas Sensor Response by Chemical Modification of Nanowire Surfaces. Beilstein J. Nanotechnol. 2012, 3, 368–377. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.; Kaur, N.; Drera, G.; Casotto, A.; Sangaletti, L.; Comini, E. SAM Functionalized ZnO Nanowires for Selective Acetone Detection: Optimized Surface Specific Interaction Using APTMS and GLYMO Monolayers. Adv. Funct. Mater. 2020, 30, 2003217. [Google Scholar] [CrossRef]
- Hoffmann, M.W.G.; Prades, J.D.; Mayrhofer, L.; Hernandez-Ramirez, F.; Järvi, T.T.; Moseler, M.; Waag, A.; Shen, H. Highly Selective SAM–Nanowire Hybrid NO2 Sensor: Insight into Charge Transfer Dynamics and Alignment of Frontier Molecular Orbitals. Adv. Funct. Mater. 2014, 24, 595–602. [Google Scholar] [CrossRef] [Green Version]
- Paolesse, R.; Nardis, S.; Monti, D.; Stefanelli, M.; Di Natale, C. Porphyrinoids for Chemical Sensor Applications. Chem. Rev. 2017, 117, 2517–2583. [Google Scholar] [CrossRef] [Green Version]
- Magna, G.; Catini, A.; Kumar, R.; Palmacci, M.; Martinelli, E.; Paolesse, R.; Di Natale, C. Conductive Photo-Activated Porphyrin-ZnO Nanostructured Gas Sensor Array. Sensors 2017, 17, 747. [Google Scholar] [CrossRef] [Green Version]
- Sivalingam, Y.; Martinelli, E.; Catini, A.; Magna, G.; Pomarico, G.; Basoli, F.; Paolesse, R.; Di Natale, C. Gas-Sensitive Photoconductivity of Porphyrin-Functionalized ZnO Nanorods. J. Phys. Chem. C 2012, 116, 9151–9157. [Google Scholar] [CrossRef]
- Magna, G.; Muduganti, M.; Stefanelli, M.; Sivalingam, Y.; Zurlo, F.; Di Bartolomeo, E.; Catini, A.; Martinelli, E.; Paolesse, R.; Di Natale, C. Light-Activated Porphyrinoid-Capped Nanoparticles for Gas Sensing. ACS Appl. Nano Mater. 2021, 4, 414–424. [Google Scholar] [CrossRef]
- Salehi, A. A Highly Sensitive Self Heated SnO2 Carbon Monoxide Sensor. Sens. Actuators B Chem. 2003, 96, 88–93. [Google Scholar] [CrossRef]
- Majhi, S.M.; Mirzaei, A.; Kim, H.W.; Kim, S.S.; Kim, T.W. Recent Advances in Energy-Saving Chemiresistive Gas Sensors: A Review. Nano Energy 2021, 79, 105369. [Google Scholar] [CrossRef]
- Meng, G.; Zhuge, F.; Nagashima, K.; Nakao, A.; Kanai, M.; He, Y.; Boudot, M.; Takahashi, T.; Uchida, K.; Yanagida, T. Nanoscale Thermal Management of Single SnO2 Nanowire: Pico-Joule Energy Consumed Molecule Sensor. ACS Sens. 2016, 1, 997–1002. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, L.; Li, K.H.; Tan, O.K. Microhotplates for Metal Oxide Semiconductor Gas Sensor Applications—Towards the CMOS-MEMS Monolithic Approach. Micromachines 2018, 9, 557. [Google Scholar] [CrossRef] [Green Version]
- Lahlalia, A.; Le Neel, O.; Shankar, R.; Selberherr, S.; Filipovic, L. Improved Sensing Capability of Integrated Semiconducting Metal Oxide Gas Sensor Devices. Sensors 2019, 19, 374. [Google Scholar] [CrossRef] [Green Version]
- Prades, J.D.; Jimenez-Diaz, R.; Hernandez-Ramirez, F.; Cirera, A.; Romano-Rodriguez, A.; Morante, J.R. Harnessing Self-Heating in Nanowires for Energy Efficient, Fully Autonomous and Ultra-Fast Gas Sensors. Sens. Actuators B Chem. 2010, 144, 1–5. [Google Scholar] [CrossRef]
- Ngoc, T.M.; Van Duy, N.; Hung, C.M.; Hoa, N.D.; Trung, N.N.; Nguyen, H.; Van Hieu, N. Ultralow Power Consumption Gas Sensor Based on a Self-Heated Nanojunction of SnO2 Nanowires. RSC Adv. 2018, 8, 36323–36330. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-H.; Kim, H.W.; Kim, S.S. Self-Heating Effects on the Toluene Sensing of Pt-Functionalized SnO2–ZnO Core–Shell Nanowires. Sens. Actuators B Chem. 2017, 251, 781–794. [Google Scholar] [CrossRef]
- Fàbrega, C.; Casals, O.; Hernández-Ramírez, F.; Prades, J.D. A Review on Efficient Self-Heating in Nanowire Sensors: Prospects for Very-Low Power Devices. Sens. Actuators B Chem. 2018, 256, 797–811. [Google Scholar] [CrossRef] [Green Version]
- Kunt, T.A.; McAvoy, T.J.; Cavicchi, R.E.; Semancik, S. Optimization of Temperature Programmed Sensing for Gas Identification Using Micro-Hotplate Sensors. Sens. Actuators B Chem. 1998, 53, 24–43. [Google Scholar] [CrossRef]
- Meier, D.C.; Evju, J.K.; Boger, Z.; Raman, B.; Benkstein, K.D.; Martinez, C.J.; Montgomery, C.B.; Semancik, S. The Potential for and Challenges of Detecting Chemical Hazards with Temperature-Programmed Microsensors. Sens. Actuators B Chem. 2007, 121, 282–294. [Google Scholar] [CrossRef]
- Krivetskiy, V.; Efitorov, A.; Arkhipenko, A.; Vladimirova, S.; Rumyantseva, M.; Dolenko, S.; Gaskov, A. Selective Detection of Individual Gases and CO/H2 Mixture at Low Concentrations in Air by Single Semiconductor Metal Oxide Sensors Working in Dynamic Temperature Mode. Sens. Actuators B Chem. 2018, 254, 502–513. [Google Scholar] [CrossRef]
- Lee, A.P.; Reedy, B.J. Temperature Modulation in Semiconductor Gas Sensing. Sens. Actuators B Chem. 1999, 60, 35–42. [Google Scholar] [CrossRef]
- Prades, J.D.; Hernández-Ramírez, F.; Fischer, T.; Hoffmann, M.; Müller, R.; López, N.; Mathur, S.; Morante, J.R. Quantitative Analysis of CO-Humidity Gas Mixtures with Self-Heated Nanowires Operated in Pulsed Mode. Appl. Phys. Lett. 2010, 97, 243105. [Google Scholar] [CrossRef]





| Core MOX, Morphology | Shell MOX | T (°C) | Gas, Concentration | S (type) | Ref. |
|---|---|---|---|---|---|
| CuO, nanowire network | -- | 300 | CO, 1 ppm | 2.2 (p) | [145] |
| CuO, nanowire network | ZnO | 300 | CO, 1 ppm | 6 (p) | [145] |
| ZnO, nanowire network | -- | 300 | CO, 1 ppm | 1.3 (n) | [145] |
| CuO, nanowire network | -- | 300 | C6H6, 1 ppm | 2.3 (p) | [145] |
| CuO, nanowire network | ZnO | 300 | C6H6, 1 ppm | 5.8 (p) | [145] |
| ZnO, nanowire network | -- | 300 | C6H6, 1 ppm | 2.3 (n) | [145] |
| SnO2, nanowire network | -- | 300 | C7H8, 10 ppm | 2 (n) | [146] |
| SnO2, nanowire network | Cu2O | 300 | C7H8, 10 ppm | 12 (p) | [146] |
| SnO2, nanowire network | -- | 300 | C6H6, 10 ppm | 2 (n) | [146] |
| SnO2, nanowire network | Cu2O | 300 | C6H6, 10 ppm | 13 (p) | [146] |
| SnO2, nanowire network | -- | 300 | NO2, 10 ppm | 130 (n) | [146] |
| SnO2, nanowire network | Cu2O | 300 | NO2, 10 ppm | 2 (p) | [146] |
| SnO2, nanowire network | -- | 300 | CO, 10 ppm | 5 (n) | [147] |
| SnO2, nanowire network | ZnO | 300 | CO, 10 ppm | 80 (n) | [147] |
| SnO2, nanowire network | -- | 300 | C6H6, 10 ppm | 5 (n) | [147] |
| SnO2, nanowire network | ZnO | 300 | C6H6, 10 ppm | 80 (n) | [147] |
| SnO2, nanowire network | -- | 300 | NO2, 10 ppm | 160 (n) | [147] |
| SnO2, nanowire network | ZnO | 300 | NO2, 10 ppm | 25 (n) | [147] |
| ZnO, nanowire network | -- | 40 | Triethylamine, 50 ppm | 4 (n) | [149] |
| ZnO, nanowire network | SnO2 | 40 | Triethylamine, 50 ppm | 7 (n) | [149] |
| ZnO, nanowire network | SnO2 + Au | 40 | Triethylamine, 50 ppm | 12 (n) | [149] |
| ZnO, nanowire network | -- | 40 | Acetone, 500 ppm | 2 (n) | [149] |
| ZnO, nanowire network | SnO2 | 40 | Acetone, 500 ppm | 5 (n) | [149] |
| ZnO, nanowire network | SnO2 + Au | 40 | Acetone, 500 ppm | 6 (n) | [149] |
| ZnO, nanowire network | -- | 40 | Ethanol, 50 ppm | 2 (n) | [149] |
| ZnO, nanowire network | SnO2 | 40 | Ethanol, 50 ppm | 4 (n) | [149] |
| ZnO, nanowire network | SnO2 + Au | 40 | Ethanol, 50 ppm | 6 (n) | [149] |
| Ga2O3, nanowire network | -- | 600 | Ethanol, 1000 ppm | 100 (n) | [150] |
| Ga2O3, nanowire network | SnO2 | 400 | Ethanol, 1000 ppm | 65 (n) | [150] |
| Backbone MOX | Coating, Morphology | T (°C) | Gas, Concentration | S (type) | Ref. |
|---|---|---|---|---|---|
| SnO2 | ZnO, shell | 400 | Ethanol, 20 ppm | 20 (n) | [148] |
| SnO2 | ZnO, shell + branch | 400 | Ethanol, 20 ppm | 32 (n) | [148] |
| CuxO | -- | 250 | Acetone, 50 ppm | 1.2 (p) | [151] |
| CuxO | ZnO, shell | 250 | Acetone, 50 ppm | 1.5 (n) | [151] |
| CuxO | ZnO, shell + branch | 250 | Acetone, 50 ppm | 6.5 (n) | [151] |
| SnO2 | -- | 50 | NO2, 1 ppm | -- | [152] |
| SnO2 | Bi2O3 branch | 50 | NO2, 1 ppm | 3 (n) | [152] |
| SnO2 | Bi2O3 branch + Pt nanoparticles | 50 | NO2, 1 ppm | 28 (n) | [152] |
| SnO2 | -- | 250 | NO2, 1 ppm | 10 (n) | [152] |
| SnO2 | Bi2O3 branch | 250 | NO2, 1 ppm | 50 (n) | [152] |
| SnO2 | Bi2O3 branch + Pt nanoparticles | 250 | NO2, 1 ppm | -- | [152] |
| SnO2 | -- | 300 | NO2, 20 ppm | 2 (n) | [153] |
| SnO2 | ZnO branch | 300 | NO2, 20 ppm | 4 (n) | [153] |
| SnO2 | ZnO branch + Au nanoparticles | 300 | NO2, 20 ppm | 13 (n) | [153] |
| SnO2 | -- | 300 | NO2, 10 ppm | 2 (n) | [154] |
| SnO2 | ZnO branch | 300 | NO2, 10 ppm | 5 (n) | [154] |
| SnO2 | ZnO branch + Cr2O3 nanoparticles | 300 | NO2, 10 ppm | 58 (n) | [154] |
| Sb-doped SnO2 | SnO2, branched | 300 | Ethanol, 100 ppm | 51 (n) | [155] |
| MOX, Morphology | 2D Carbon Material | T (°C) | Gas, Concentration | S (type) | Ref. |
|---|---|---|---|---|---|
| ZnO, nanorods | -- | RT | NO2, 1 ppm | 1.8 (n) | [159] |
| -- | RGO | RT | NO2, 1 ppm | 1.2 (p) | [159] |
| ZnO, nanorods | RGO | RT | NO2, 1 ppm | 2.2 (p) | [159] |
| ZnO, nanosheets | -- | RT | NO2, 50 ppm | 6 (n) | [160] |
| ZnO, nanosheets | RGO | RT | NO2, 50 ppm | 9 (n) | [160] |
| ZnO, hierarchical spheres | -- | 110 | NO2, 1 ppm | 4 (n) | [161] |
| ZnO, hierarchical spheres | RGO | 110 | NO2, 1 ppm | 20 (n) | [161] |
| ZnO, hierarchical porous sheets | RGO | RT | NO2, 1 ppm | 10 (p) | [163] |
| ZnO, nanoparticles | RGO | RT | NH3, 1 ppm | 1.07 (p) | [162] |
| Cu doped SnO2, nanowires | RGO | RT | H2S, 50 ppm | 33 (n) | [165] |
| Cu doped SnO2, nanowires | RGO | RT | NH3, 50 ppm | 1.25 (n) | [165] |
| Cu doped SnO2, nanowires | RGO | RT | NO2, 50 ppm | 1.5 (n) | [165] |
| Cu doped ZnO, nanorods | RGO | RT | H2S, 50 ppm | 1.05 (p) | [166] |
| In2O3, nanorods | RGO | RT | NO2, 97 ppm | 2.5 (n) | [167] |
| Cu2O, hierarchical mesocrystals | -- | RT | NO2, 2 ppm | 1.4 (p) | [168] |
| -- | RGO | RT | NO2, 2 ppm | 1.2 (p) | [168] |
| Cu2O, hierarchical mesocrystals | RGO | RT | NO2, 2 ppm | 1.7 (p) | [168] |
| SnO2, nanowire | G | RT | NO2, 0.1 ppm | 11 (sb) | [170] |
| MOX, Morphology | Organic Coating | T (°C) | Gas, Concentration | S (type) | Ref. |
|---|---|---|---|---|---|
| ZnO, nanowires | -- | 190 | NO2, 2 ppm | 1.3 (n) | [174] |
| ZnO, nanowires | THMA | 190 | NO2, 2 ppm | 1.2 (n) | [174] |
| ZnO, nanowires + nanoparticles | -- | 190 | NO2, 2 ppm | 1.22 (n) | [174] |
| ZnO, nanowires + nanoparticles | THMA | 190 | NO2, 2 ppm | 1.44 (n) | [174] |
| ZnO, nanowires | -- | 300 | Acetone, 50 ppm | 30 (n) | [175] |
| ZnO, nanowires | GLYMO | 300 | Acetone, 50 ppm | 90 (n) | [175] |
| ZnO, nanowires | APTMS | 300 | Acetone, 50 ppm | 160 (n) | [175] |
| SnO2, nanowires | en-APTAS 1 | RT (*) | NO2, 250 ppb | 10 (n) | [176] |
| ZnO, nanoparticles | H2TPPCOOH porphyrin | RT | Pentanol, 60 ppm | 1.1 (n) | [178] |
| ZnO, nanorods | -- | RT (+) | Ethanol, 104 ppm | 1.01 (p) | [179] |
| ZnO, nanorods | H2TPPCOOH porphyrin | RT (§) | Ethanol, 104 ppm | 1.002 (n) | [179] |
| ZnO, nanorods | -- | RT (+) | Triethylamine, 104 ppm | 1.01 (p) | [179] |
| ZnO, nanorods | H2TPPCOOH porphyrin | RT (§) | Triethylamine, 104 ppm | 1.8 (n) | [179] |
| Single-Phase Materials | ||
|---|---|---|
| Feature | Nanowires (NWs) | Nanoparticles (NPs) |
| Surface termination | Well-defined crystalline planes:
| Rounded shape:
|
| Length-to-diameter aspect ratio | Large, often >10:
| Almost unitary, ≈1:
|
| Doping |
| |
|
| |
| Hierarchical structures |
| |
| Heterostructure-based materials | ||
| Surface functionalization | Nanowires (NWs) | Nanoparticles (NPs) |
| metallic nanoparticles |
| |
| MOX nanostructures |
| |
| -- | |
| Organic materials |
| |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponzoni, A. Metal Oxide Chemiresistors: A Structural and Functional Comparison between Nanowires and Nanoparticles. Sensors 2022, 22, 3351. https://doi.org/10.3390/s22093351
Ponzoni A. Metal Oxide Chemiresistors: A Structural and Functional Comparison between Nanowires and Nanoparticles. Sensors. 2022; 22(9):3351. https://doi.org/10.3390/s22093351
Chicago/Turabian StylePonzoni, Andrea. 2022. "Metal Oxide Chemiresistors: A Structural and Functional Comparison between Nanowires and Nanoparticles" Sensors 22, no. 9: 3351. https://doi.org/10.3390/s22093351






