Langmuir-Blodgett Films of Arachidic and Stearic Acids as Sensitive Coatings for Chloroform HF SAW Sensors
Abstract
1. Introduction
2. Materials and Methods
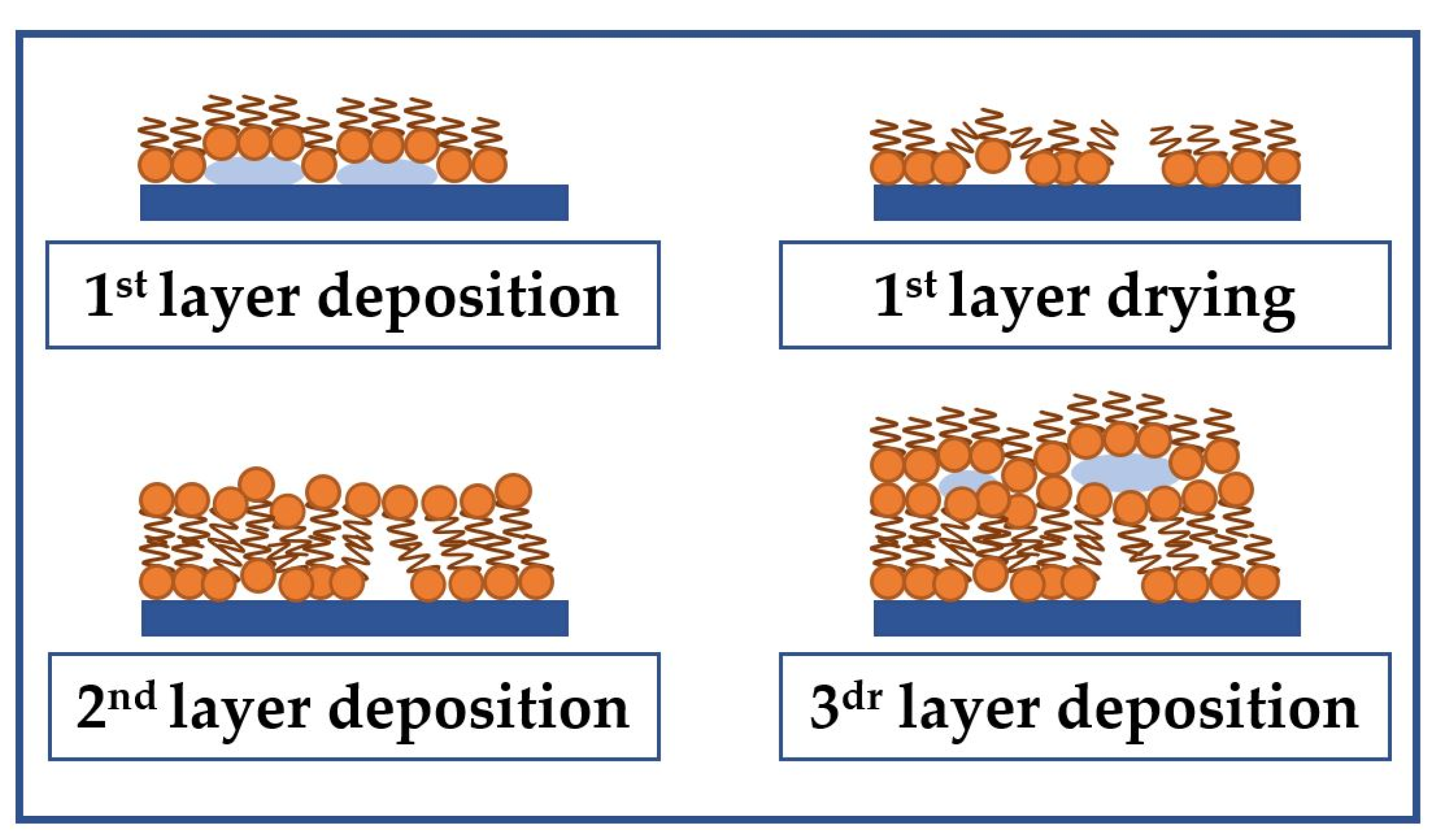
2.1. Formation of Sensitive Langmuir-Blodgett Films of Arachidic and Stearic Acids
2.2. Study of Surface Morphology of Obtained Langmuir-Blodgett Films of Stearic Acid
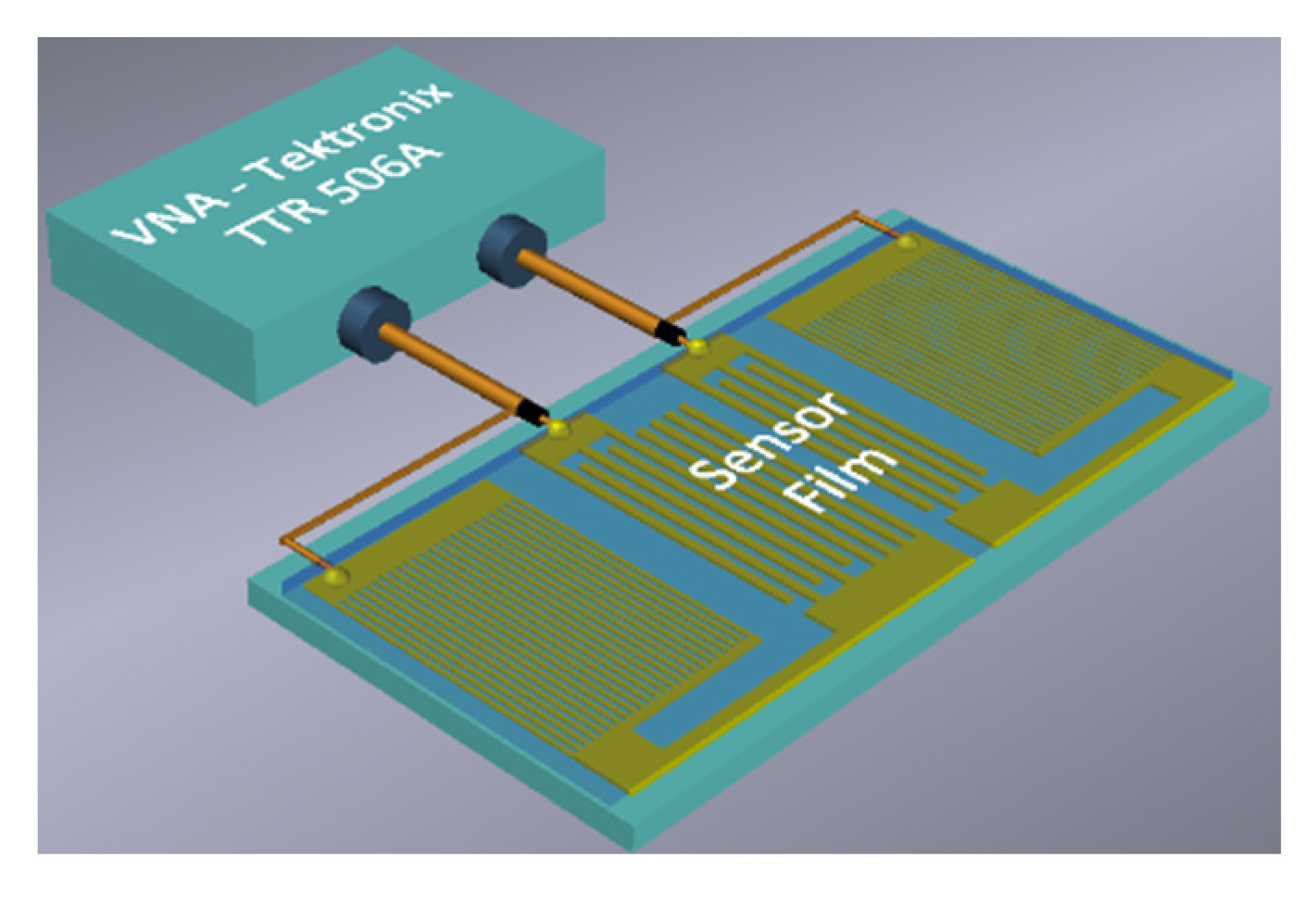
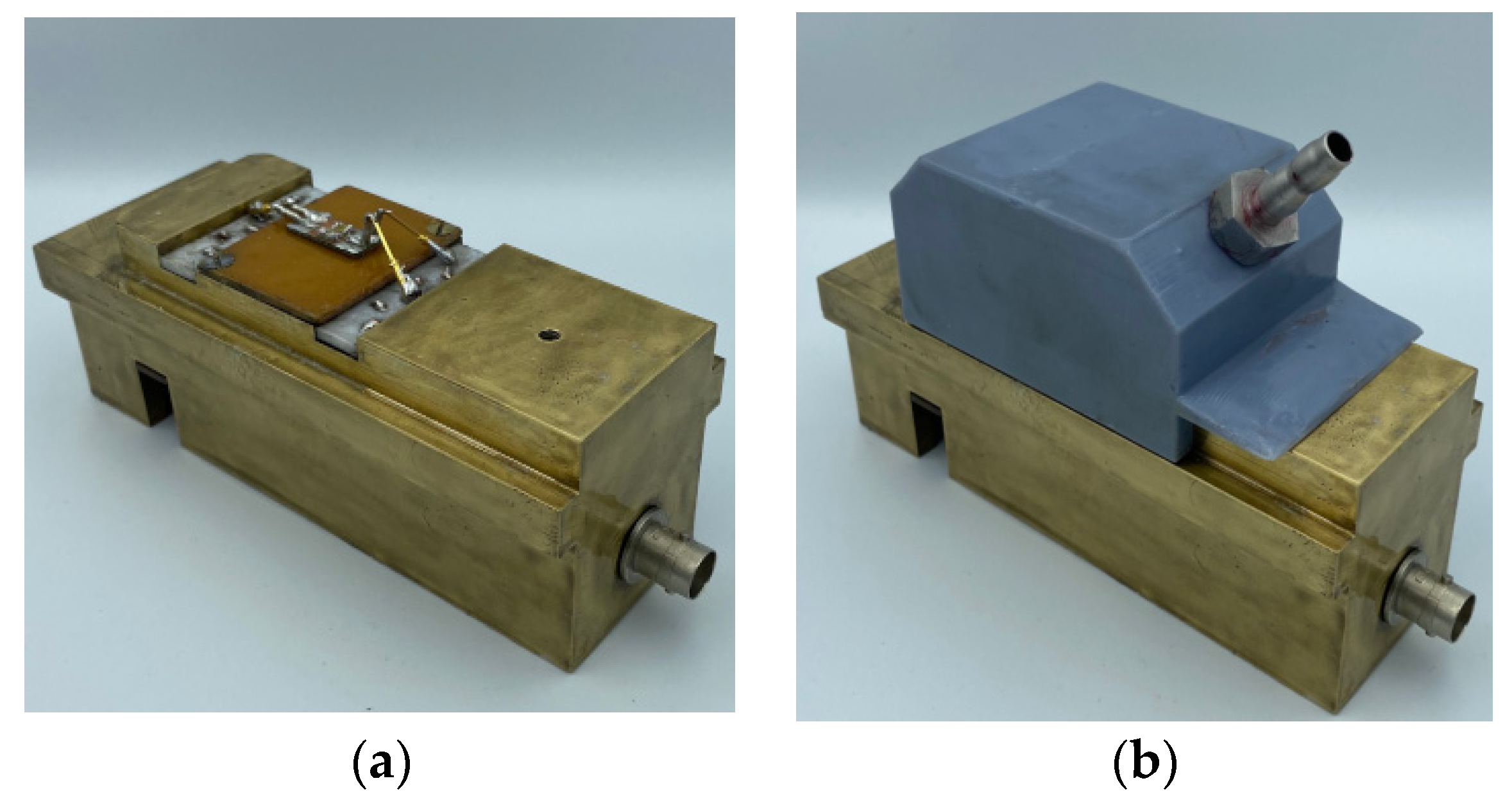
2.3. Two-Port Rayleigh SAW Resonator
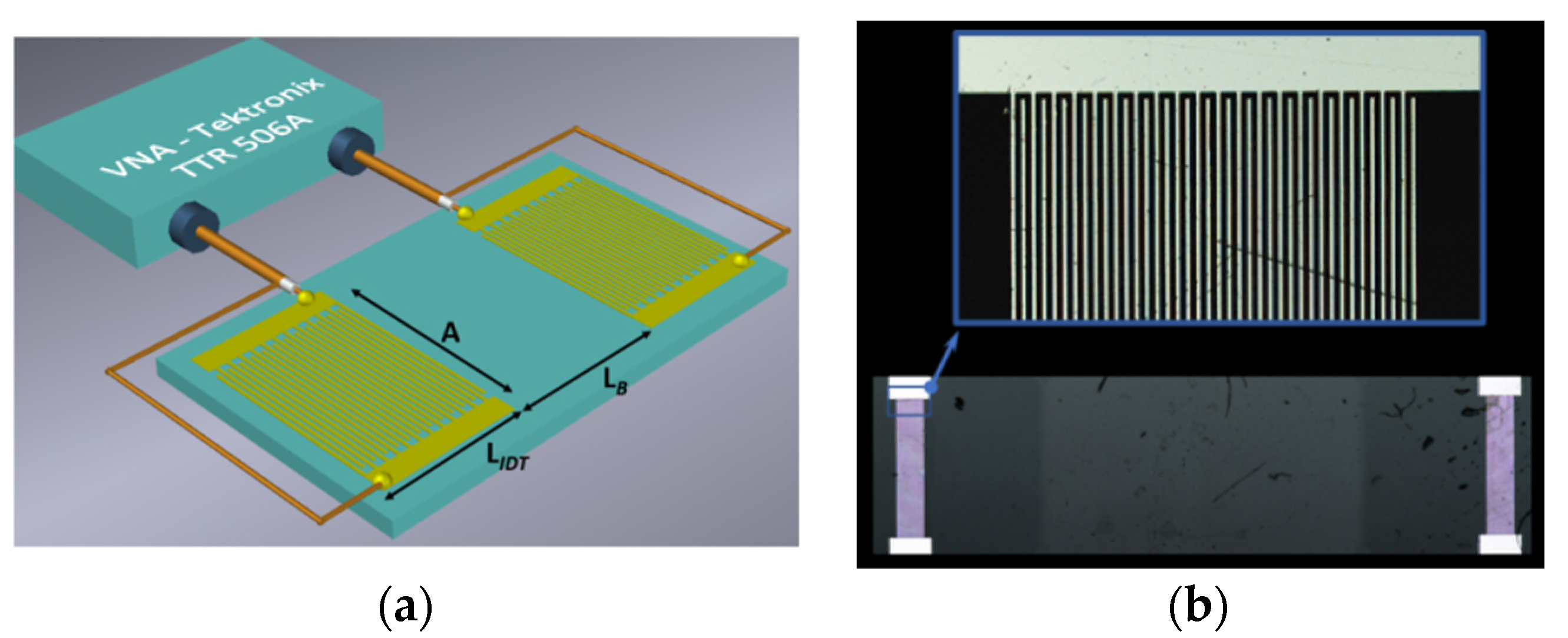
2.4. Rayleigh SAW Delay Line
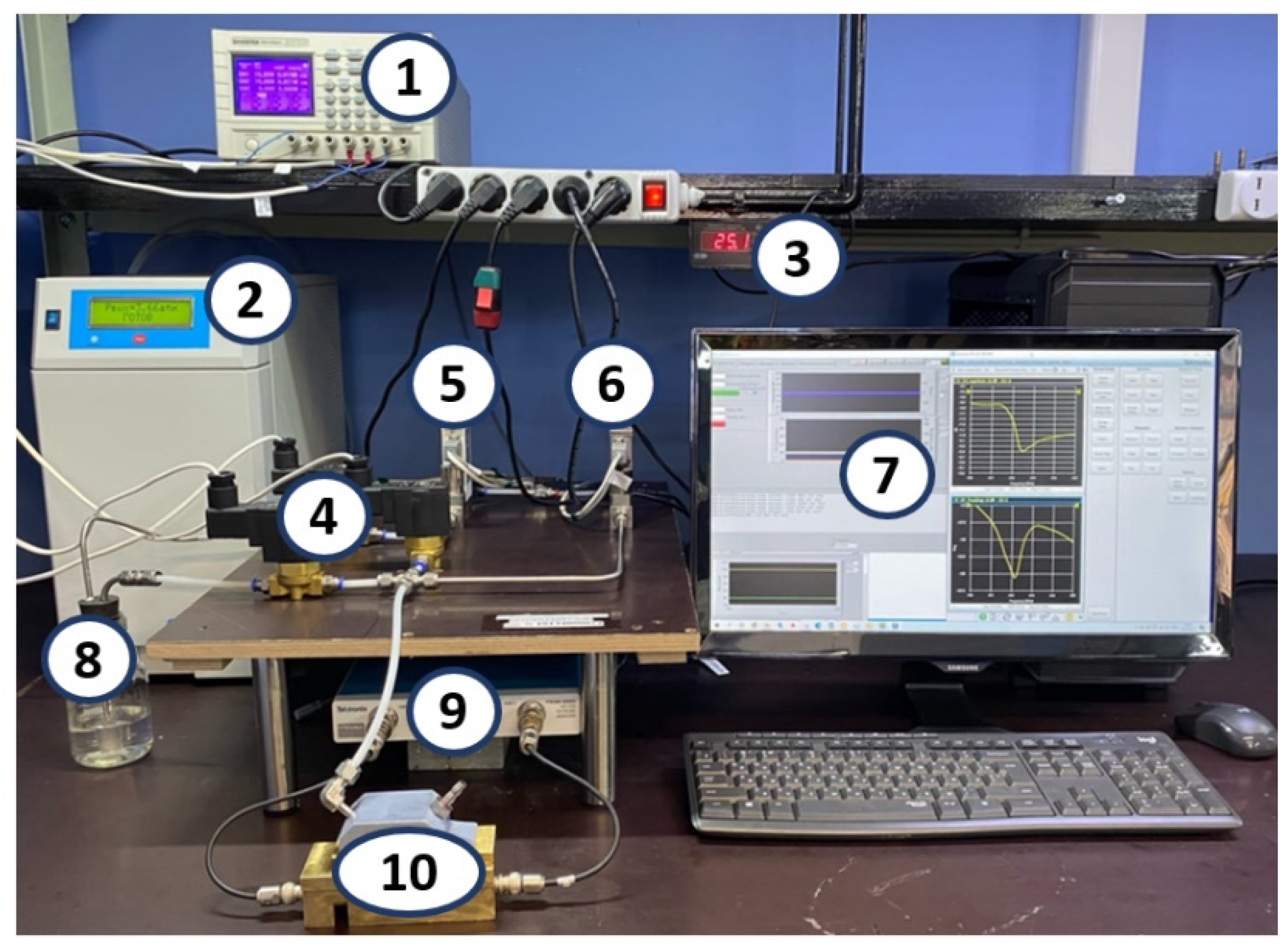
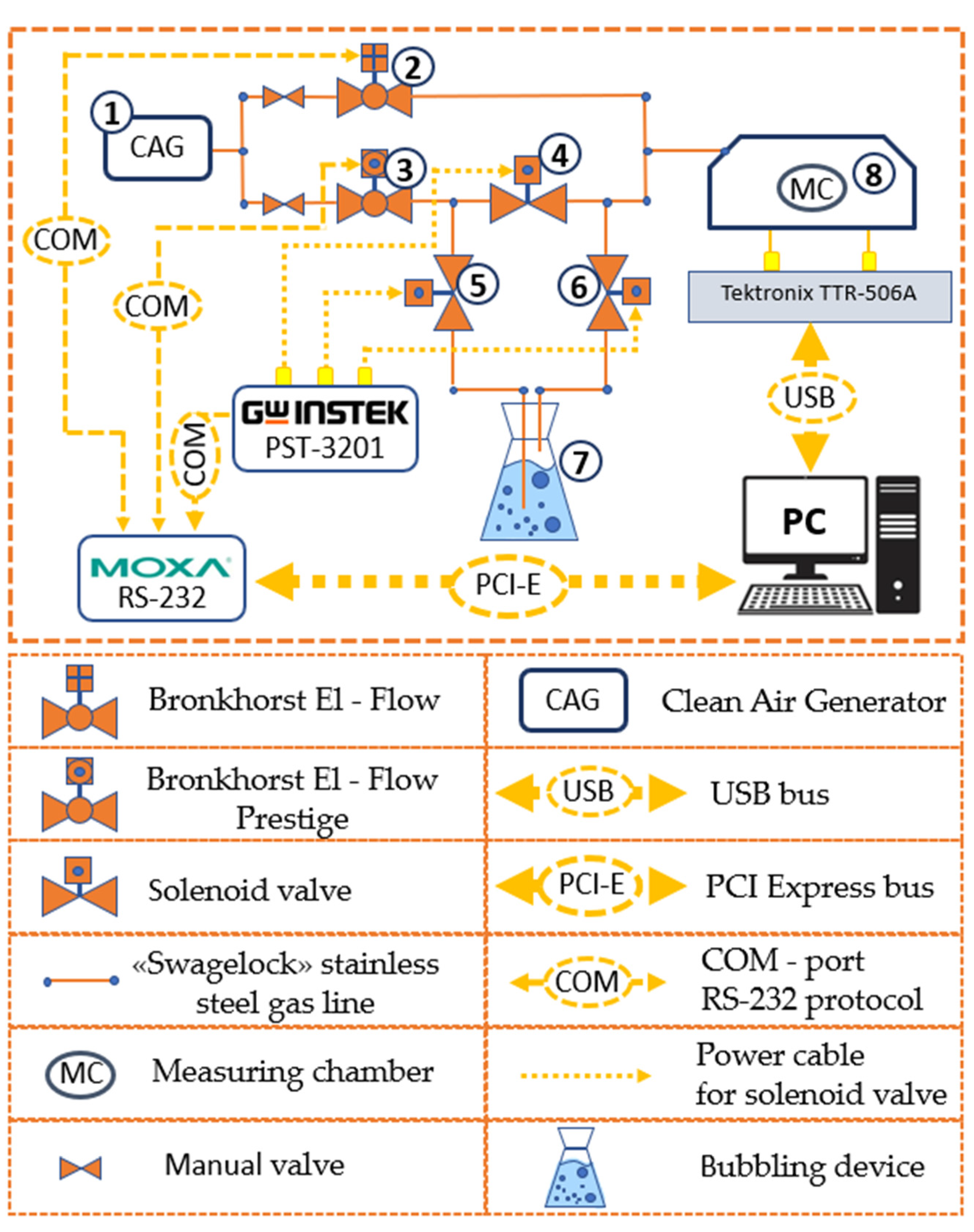
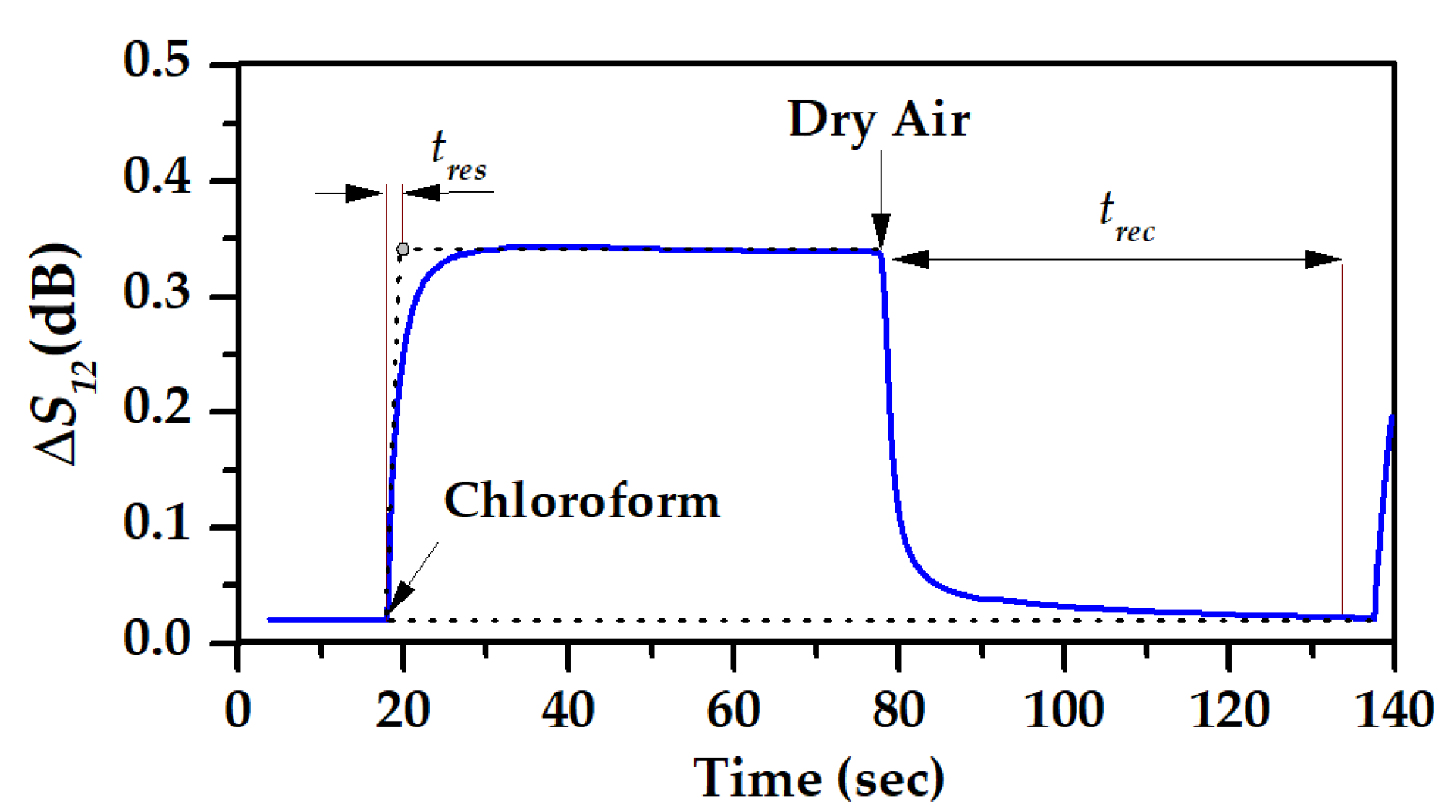
2.5. Set up and Methods for the Study of Gas-Sensitive Properties
2.6. Method for Calculation of Acoustoelectronic Devices Responses
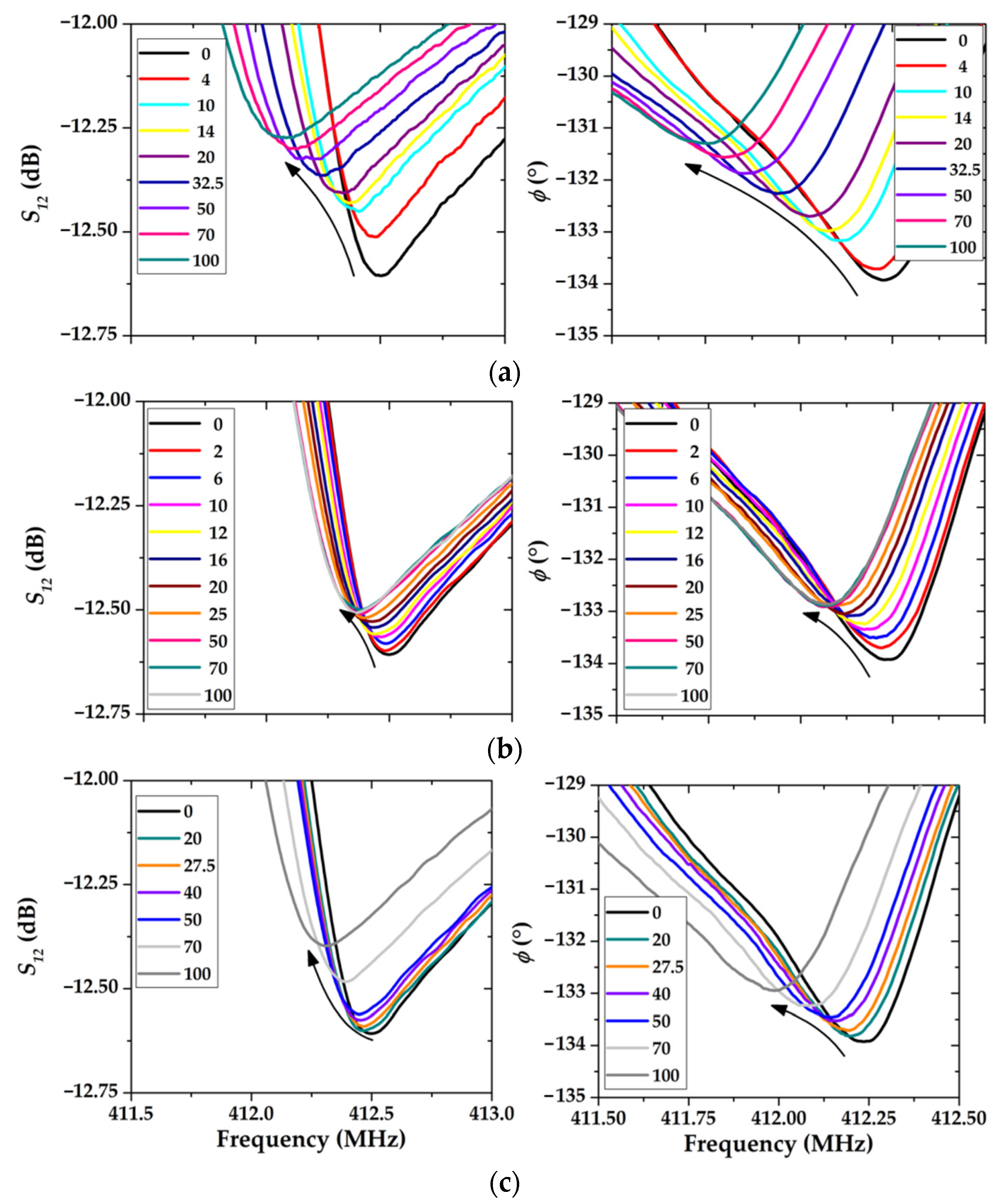
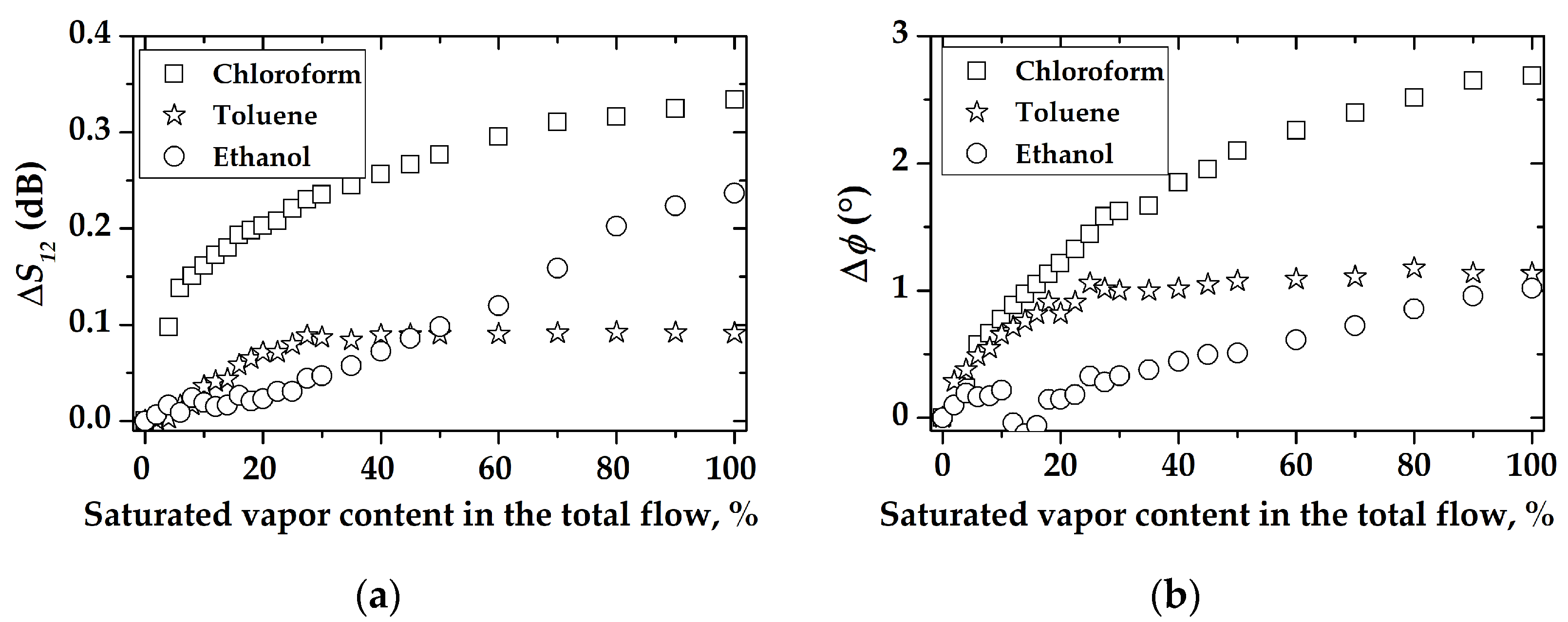
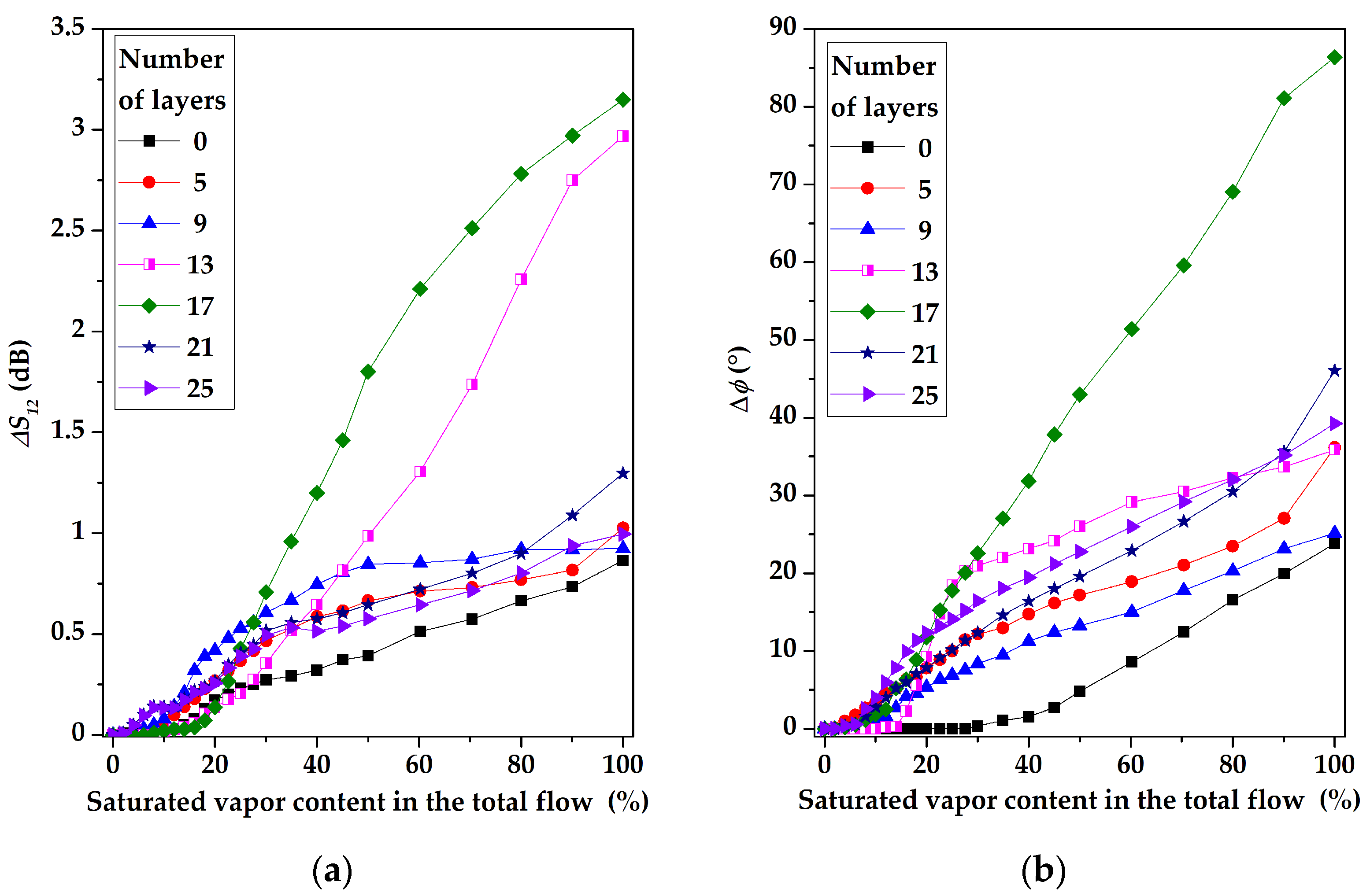
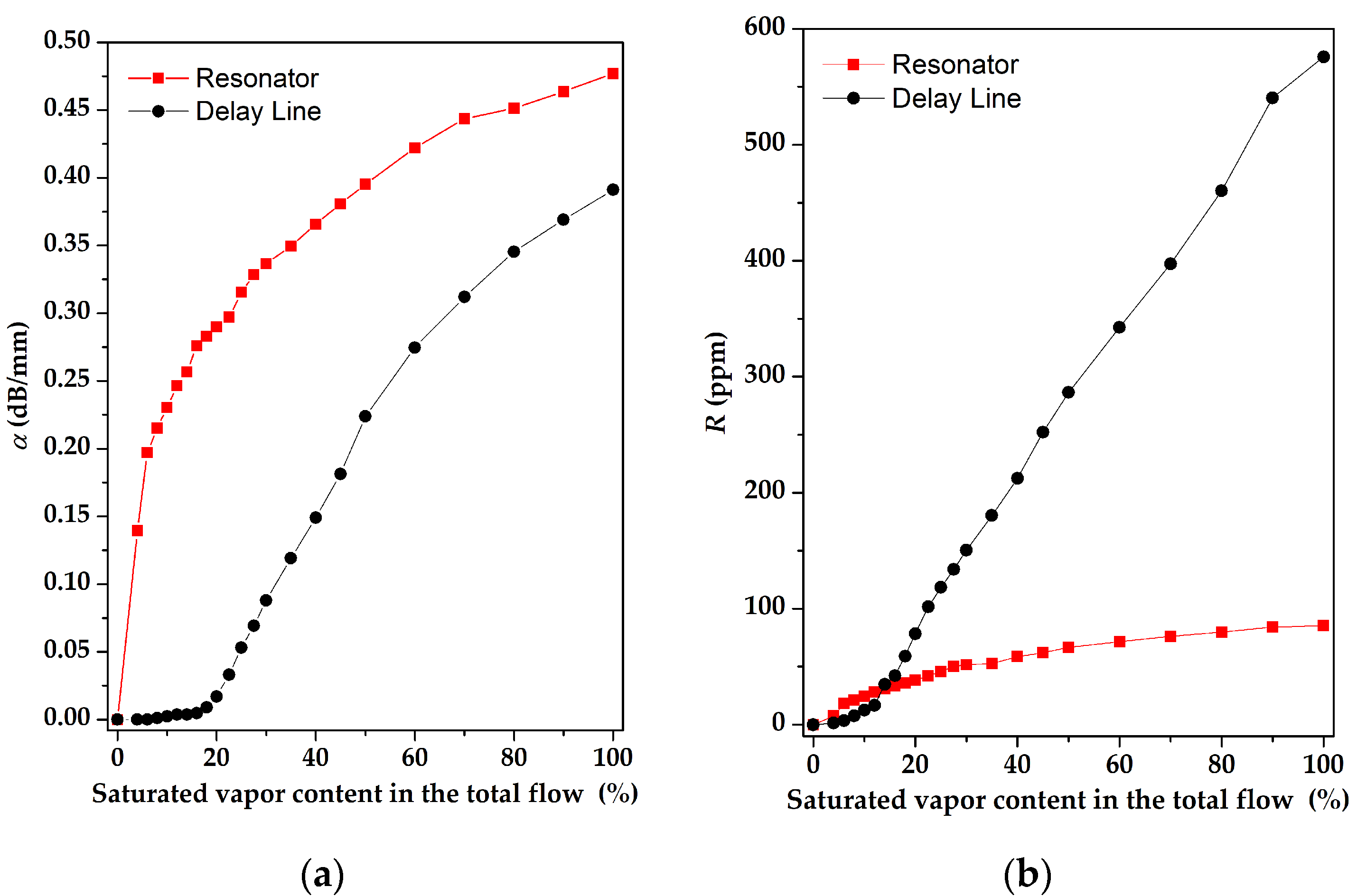
3. Results and Discussion
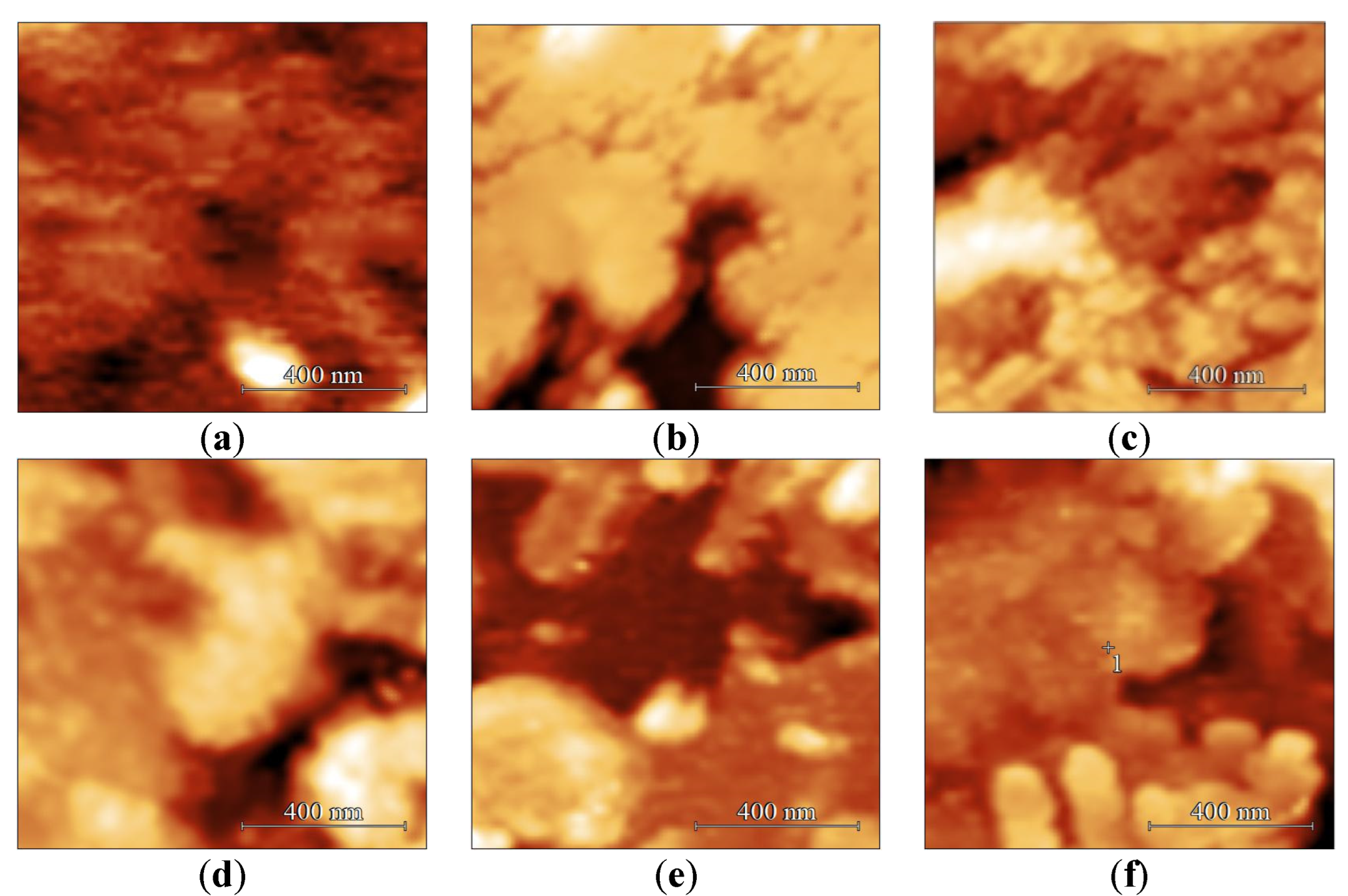
3.1. The Surface Morphology of Multilayer Coatings Based on LB Films of Stearic Acid
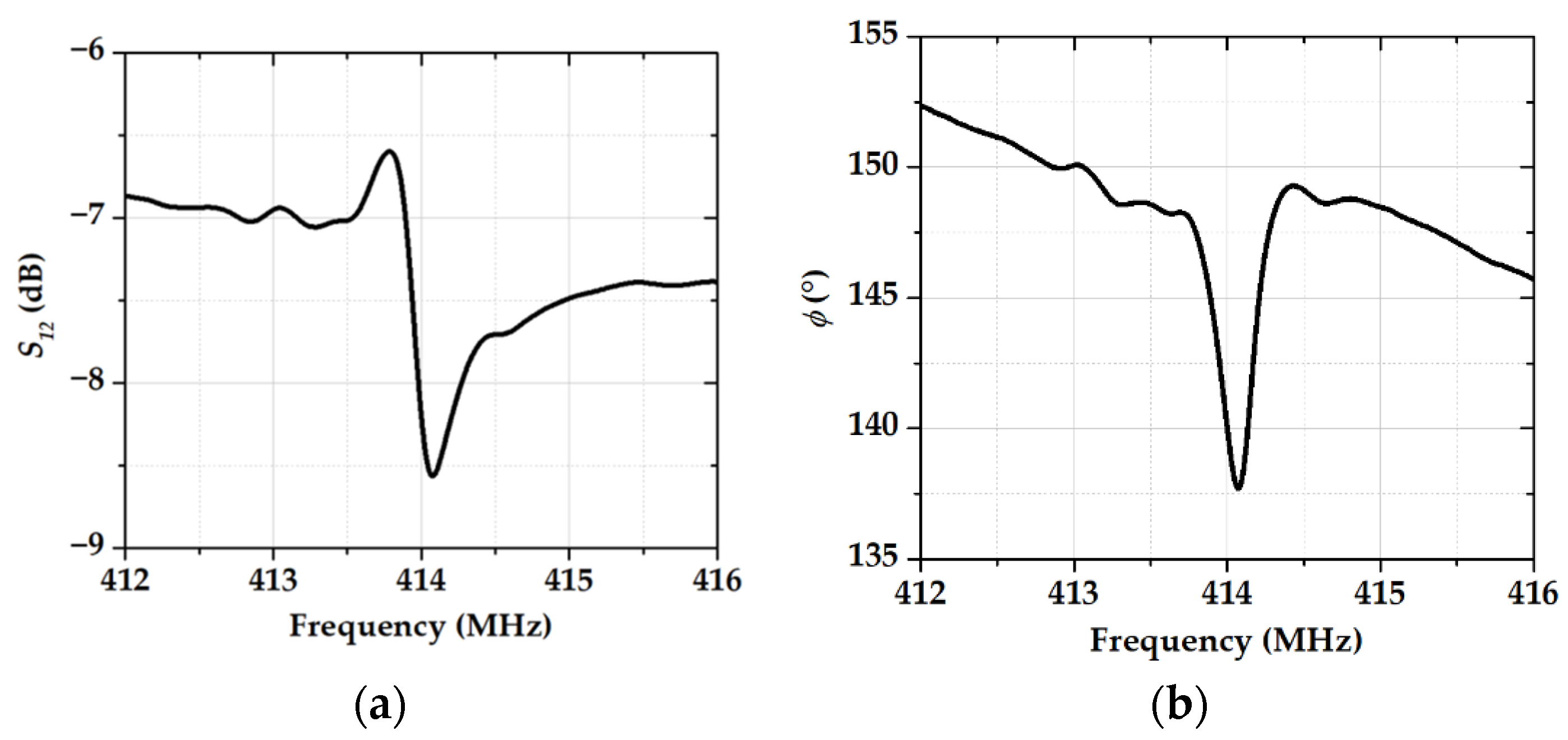
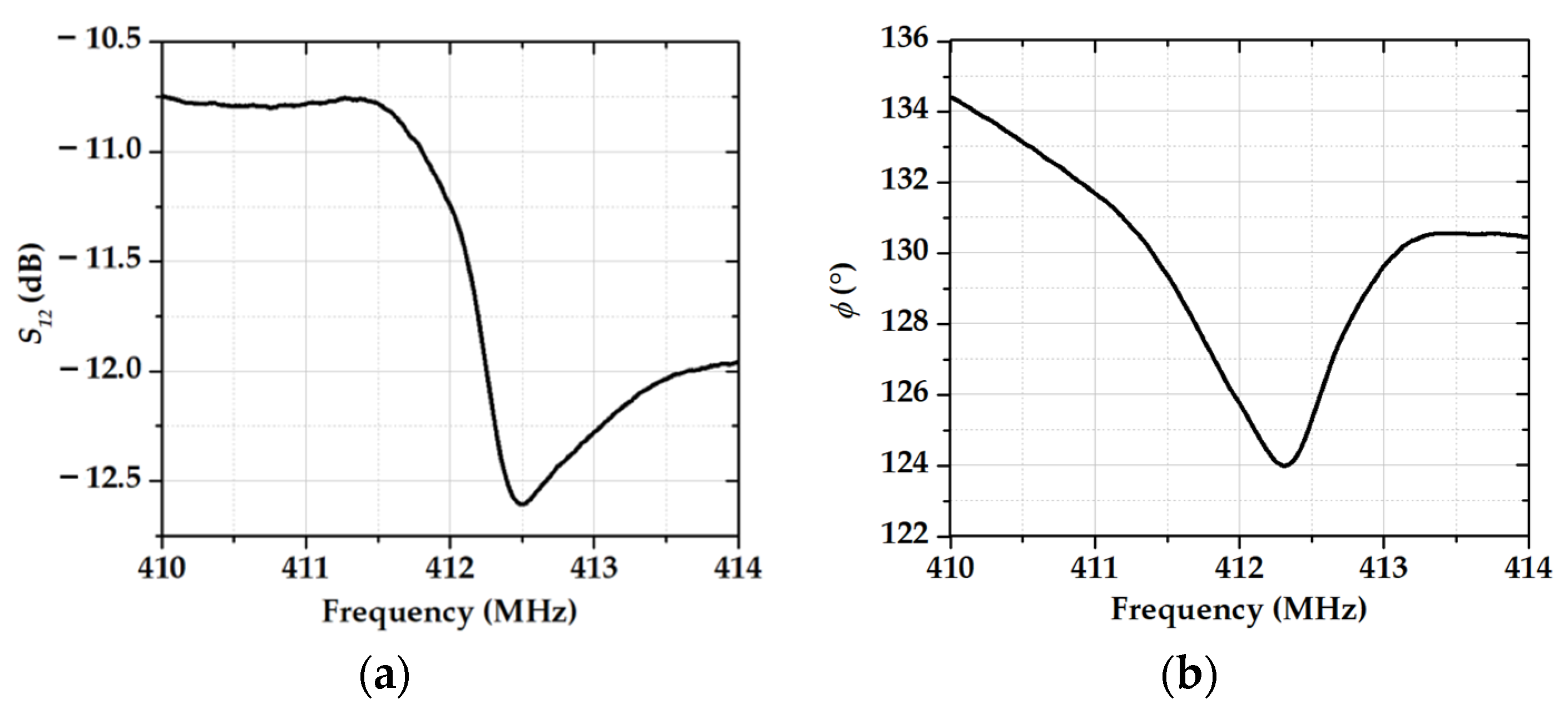
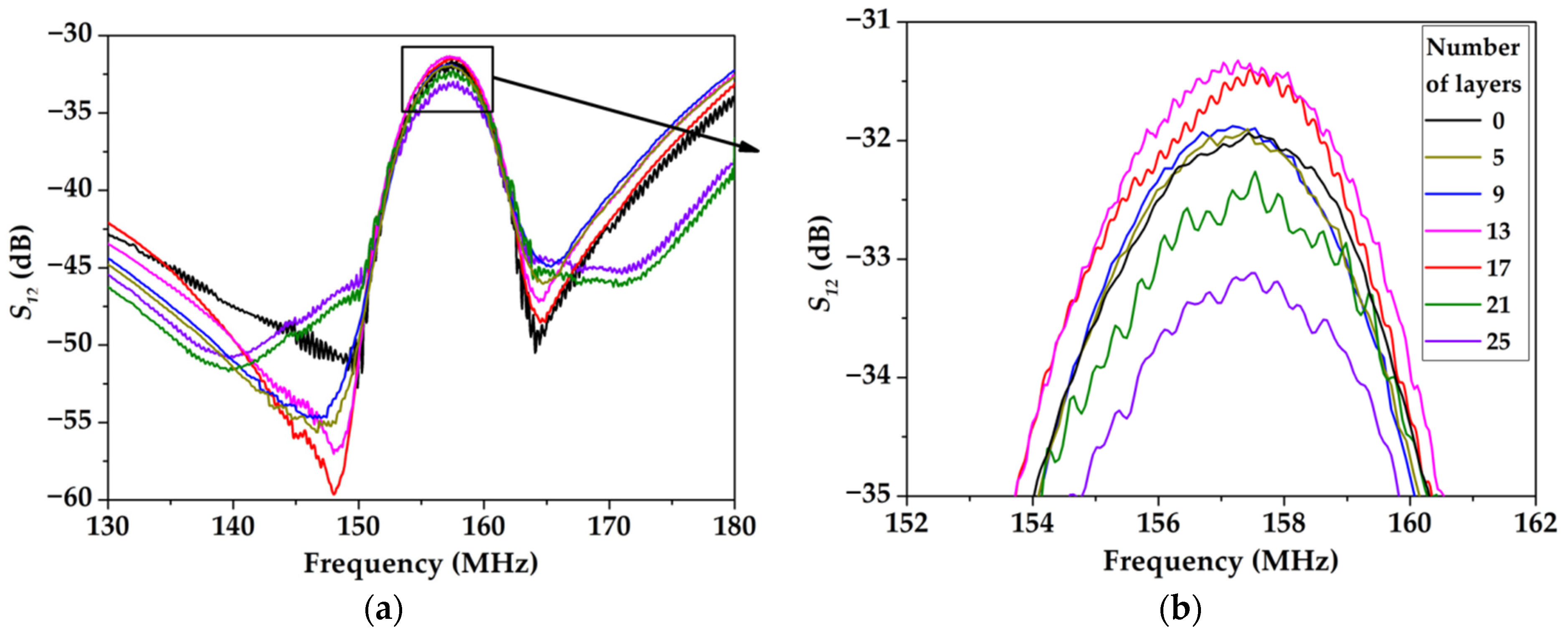
3.2. The Gas-Sensitive Properties of the Sensors Based on RSAW Resonator with LB Film of AA and SAW Delay Line with LB Film of SA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gavrilescu, M.; Demnerová, K.; Aamand, J.; Agathos, S.; Fava, F. Emerging pollutants in the environment: Present and future challenges in biomonitoring, ecological risks, and bioremediation. New Biotechnol. 2015, 32, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.; Stoy, P.; Sneddon, H.F. Chlorinated solvents: Their advantages, disadvantages, and alternatives in organic and medicinal chemistry. Chem. Rev. 2020, 121, 1582–1622. [Google Scholar] [CrossRef] [PubMed]
- Joshi, D.R.; Adhikari, N. An overview on common organic solvents and their toxicity. J. Pharm. Res. Int. 2019, 28, 1–18. [Google Scholar] [CrossRef]
- Dassi, M.; Madan, J.; Pandey, R.; Sharma, R. Chemical modulation of conducting polymer gate electrode work function based double gate Mg2Si TFET for gas sensing applications. J. Mater. Sci. Mater. Electron. 2022, 33, 23927–23936. [Google Scholar] [CrossRef]
- Onthath, H.; Maurya, M.R.; Bykkam, S.; Kasak, P.; Sadasivuni, K.K. Development and Fabrication of Carbon Nanotube (CNT)/CuO Nanocomposite for Volatile Organic Compounds (VOCs) Gas Sensor Application. Macromol. Symp. 2021, 402, 2270202. [Google Scholar] [CrossRef]
- Zhang, T.; Xing, Y.; Wang, G.; He, S. High sensitivity continuous monitoring of chloroform gas by using wavelength modulation photoacoustic spectroscopy in the near-infrared range. Appl. Sci. 2021, 11, 6992. [Google Scholar] [CrossRef]
- Tapanes, E.E.; Goode, J.R.; Rossiter, P.L.; Hill, A.J. Evanescent wave spectroscopy as an interface probe. In Materials Science Forum; Trans Tech Publications Ltd.: Zurich, Switzerland, 1995; Volume 189–190, pp. 205–210. [Google Scholar] [CrossRef]
- Dickert, F.L.; Bruckdorfer, T.; Feigl, H.; Haunschild, A.; Kuschow, V.; Obermeier, E.; Bulst, W.E.; Knauer, U.; Mages, G. Supramolecular detection vapours with QMB and SAW devices. Sens. Actuators B Chem. 1993, 13–14, 297–301. [Google Scholar] [CrossRef]
- Fernandes, D.L.A.; Gomes, M.T.S.R. Development of an electronic nose to identify and quantify volatile hazardous compounds. Talanta 2008, 77, 77–83. [Google Scholar] [CrossRef]
- Singh, P.; Yadava, R.D.S. Effect of film thickness and viscoelasticity on separability of vapour classes by wavelet and principal component analyses of polymer-coated surface acoustic wave sensor transients. Meas. Sci. Technol. 2011, 22, 025202. [Google Scholar] [CrossRef]
- Tasaltin, C.; Ebeoglu, M.A.; Ozturk, Z.Z. Acoustoelectric effect on the responses of SAW sensors coated with electrospun ZnO nanostructured thin film. Sensors 2012, 12, 12006–12015. [Google Scholar] [CrossRef]
- Siegal, M.P.; Mowry, C.D.; Pfeifer, K.B.; Gallis, D.F.S. Detecting trihalomethanes using nanoporous-carbon coated surface-acoustic-wave sensors. J. Electrochem. Soc. 2015, 162, B114–B120. [Google Scholar] [CrossRef]
- Urbańczyk, M.; Jakubik, W.; Kochowski, S. Investigation of sensor properties of copper phthalocyanine with the use of surface acoustic waves. Sens. Actuators B Chem. 1994, 22, 133–137. [Google Scholar] [CrossRef]
- Avramov, I.D.; Stahl, U. On the Mass Sensitivity of Rayleigh Surface Acoustic Wave (RSAW) Resonators. In Proceedings of the 2017 40th International Spring Seminar on Electronics Technology (ISSE), Sofia, Bulgaria, 10–14 May 2017; p. 8000982. [Google Scholar]
- Stahl, U.; Voigt, A.; Dirschka, M.; Barié, N.; Richter, C.; Waldbaur, A.; Gruhl, F.J.; Rapp, B.E.; Rapp, M.; Länge, K. Long-term stability of polymer-coated surface transverse wave sensors for the detection of organic solvent vapors. Sensors 2017, 17, 2529. [Google Scholar] [CrossRef] [PubMed]
- Kus, F.; Altinkok, C.; Zayim, E.; Erdemir, S.; Tasaltin, C.; Gurol, I. Surface acoustic wave (SAW) sensor for volatile organic compounds (VOCs) detection with calix[4]arene functionalized Gold nanorods (AuNRs) and silver nanocubes (AgNCs). Sens. Actuators B Chem. 2021, 330, 129402. [Google Scholar] [CrossRef]
- Avramov, I.D.; Ivanov, G.R. Layer by layer optimization of Langmuir–Blodgett films for surface acoustic wave (SAW) based sensors for volatile organic compounds (VOC) detection. Coatings 2022, 12, 669. [Google Scholar] [CrossRef]
- Guo, H.; Gao, Y.; Liu, T. A theoretical study of the VOC sensor based on polymer-coated diaphragm embedded with FBAR. Measurement 2018, 129, 206–210. [Google Scholar] [CrossRef]
- Viespe, C.; Dinca, V.; Popescu-Pelin, G.; Miu, D. Love wave surface acoustic wave sensor with laser-deposited nanoporous gold sensitive layer. Sensors 2019, 19, 4492. [Google Scholar] [CrossRef]
- Kılınc, N.; Atilla, D.; Gurek, A.G.; Ozturka, Z.Z.; Ahsen, V. Volatile organic compounds sensing properties of tetrakis(alkylthio)-substituted lutetium(III) bis phthalocyanines thin films. Talanta 2009, 80, 263–268. [Google Scholar] [CrossRef]
- Adimule, V.; Bhowmik, D.; Gowda, A.H.J. Morphology, Characterization, and Gas Sensor Properties of Sr Doped WO3 Thin Film Nanostructures. In Macromolecular Symposia; Wiley-VCH GmbH: Weinheim, Germany, 2021; Volume 400, p. 2100065. [Google Scholar] [CrossRef]
- Schierbaum, K.-D.; Gerlach, A.; Gopel, W.; Muller, W.M.; Vogtle, F.; Dominik, A.; Roth, H.J. Surface and bulk interactions of organic molecules with calixarene layers. Fresenius’ J. Anal. Chem. 1994, 349, 372–379. [Google Scholar] [CrossRef]
- Ivanov, G.R.; Avramov, I.D.; Strijkova, V.J.; Marinov, Y.G.; Vlakhov, T.E.; Bogdanova, E.; Hadjichristov, G.B. Mass sensitivity of Langmuir-Blodgett monolayer film coated surface acoustic wave resonators to volatile organic solvents. J. Phys. Conf. Ser. 2021, 1762, 012002. [Google Scholar] [CrossRef]
- Ivanov, G.R.; Avramov, I.D. Langmuir-Blodgett Films from Fluorescently Labelled Phospholipids Deposited on Surface Acoustic Wave Devices. J. Phys. Conf. Ser. 2019, 1186, 012007. [Google Scholar] [CrossRef]
- Ivanov, G.R.; Polevska, Z. First observation of 3D aggregates in a single-component Langmuir film below the equilibrium spreading pressure. MATEC Web Conf. 2017, 98, 01003. [Google Scholar] [CrossRef]
- Ivanov, G.R.; Kostadinov, K.G.; Song, Z. Formation of nanosized needle structures in ultra-thin organic film for biosensor application. In Proceedings of the IEEE International Conference on Manipulation, Manufacturing and Measurement on the Nanoscale, Zhenjiang, China, 4–8 August 2019; pp. 350–353. [Google Scholar]
- Chang, Y.; Tang, N.; Qu, H.; Liu, J.; Zhang, D.; Zhang, H.; Pang, W.; Duan, X. Detection of volatile organic compounds by self-assembled monolayer coated sensor array with concentration-independent fingerprints. Sci. Rep. 2016, 6, 23970. [Google Scholar] [CrossRef] [PubMed]
- Brondz, I. Fatty Acids. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2005; pp. 76–88. [Google Scholar] [CrossRef]
- Deng, Y.; Xu, L.; Lu, H.; Wang, H.; Shi, Y. Direct measurement of the contact angle of water droplet on quartz in a reservoir rock with atomic force microscopy. Chem. Eng. Sci. 2018, 177, 445–454. [Google Scholar] [CrossRef]
- Erb, R.A. Wettability of gold. J. Phys. Chem. 1968, 72, 2412–2417. [Google Scholar] [CrossRef]
- Gretic, Z.H.; Mioc, E.K.; Cadez, V.; Segota, S.; Curkovic, H.O.; Hosseinpour, S. The Influence of Thickness of Stearic Acid Self-Assembled Film on Its Protective Properties. J. Electrochem. Soc. 2016, 163, C937–C944. [Google Scholar] [CrossRef]
- Oncins, G.; Torrent-Burgues, J.; Sanz, F. Nanomechanical Properties of Arachidic Acid Langmuir-Blodgett Films. J. Phys. Chem. C 2008, 112, 1967–1974. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P.; Valtr, M. Estimation of roughness measurement bias originating from background subtraction. Meas. Sci. Technol. 2020, 31, 094010. [Google Scholar] [CrossRef]
- Nečas, D.; Valtr, M.M.; Klapetek., P. How levelling and scan line corrections ruin roughness measurement and how to prevent it. Sci. Rep. 2020, 10, 15294. [Google Scholar] [CrossRef]
- Avramov, I.D. High-performance surface transverse wave resonators in the lower GHz frequency range. Int. J. High Speed Electron. Syst. 2000, 10, 735–792. [Google Scholar] [CrossRef]
- Avramov, I.D. Design of Rayleigh SAW resonators for applications as gas sensors in highly reactive chemical environments. In Proceedings of the 2006 IEEE International Frequency Control Symposium and Exposition, Miami, FL, USA, 4–7 June 2006; pp. 381–388. [Google Scholar]
- Cross, P.S.; Shreve, W.R. Synchronous IDT SAW resonators with Q above 10,000. In Proceedings of the 1979 Ultrasonics Symposium, New Orleans, LA, USA, 26–28 September 1979; pp. 824–829. [Google Scholar]
- Avramov, I.D.; Voigt, A.; Rapp, M. Rayleigh SAW resonators using gold electrode structure for gas sensor applications in chemically reactive environments. Electron. Lett. 2005, 41, 450–452. [Google Scholar] [CrossRef]
- Martin, S.J.; Ricco, A.J.; Niemczyk, T.M.; Frye, G.C. Characterization of SH acoustic plate mode liquid sensors. Sens. Actuators 1989, 20, 253–268. [Google Scholar] [CrossRef]
- Adamson, A.W.; Gast, A.P. Physical Chemistry of Surfaces, 6th ed.; A Wiley-Interscience publication John Wiley& Sons, Inc.: New York, NY, USA, 1997; p. 784. Available online: https://www.eng.uc.edu/~beaucag/Classes/AdvancedMaterialsThermodynamics/Books/Arthur%20W.%20Adamson,%20Alice%20P.%20Gast%20-%20Physical%20chemistry%20of%20surfaces-Wiley%20(1997).pdf (accessed on 22 November 2022).
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook; CRC: Boca Raton, FL, USA, 2007. [Google Scholar]
- Barton, A. Handbook of Solubility Parameters and Other Cohesion Parameters; CRC: Baton Rouge, FL, USA, 1983; p. 82. [Google Scholar]
- Lee, W.J.; Goh, P.S.; Lau, W.J.; Ismail, A.F.; Hilal, H. Green Approaches for Sustainable Development of Liquid Separation Membrane. Membranes 2021, 11, 235. [Google Scholar] [CrossRef] [PubMed]




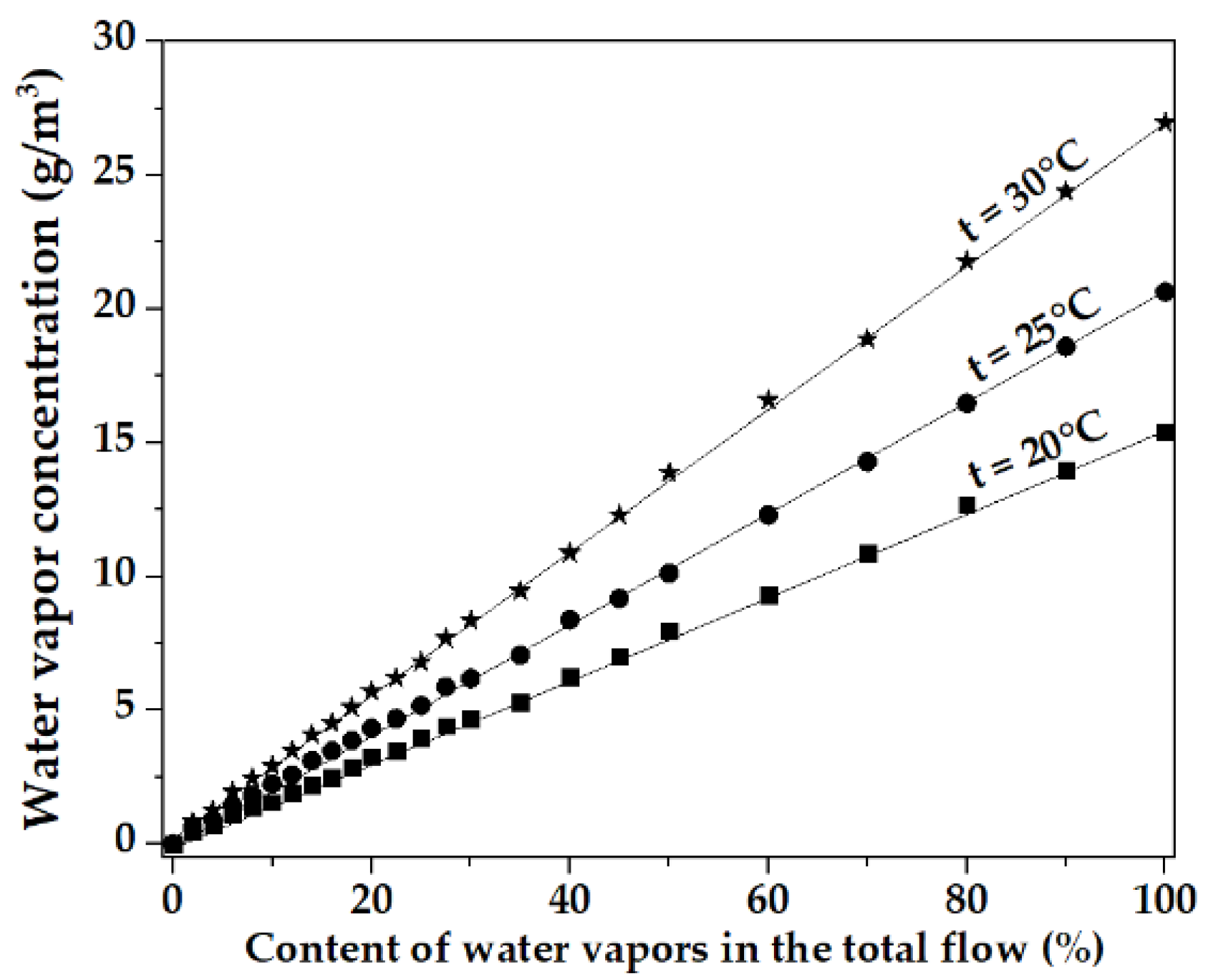

| Content Probe in Total Flow, % | Chloroform (CHCl3), g/m3 | Ethanol (C2H5OH), g/m3 | Toluene (CH), g/m3 |
|---|---|---|---|
| 2 | 18.24 | 1.99 | 1.93 |
| 4 | 36.48 | 3.99 | 3.87 |
| 6 | 54.72 | 5.98 | 5.81 |
| 8 | 72.97 | 7.98 | 7.75 |
| 10 | 91.21 | 9.98 | 9.69 |
| 12 | 109.45 | 11.97 | 11.62 |
| 14 | 127.69 | 13.97 | 13.56 |
| 16 | 145.94 | 15.96 | 15.51 |
| 18 | 164.18 | 17.96 | 17.44 |
| 20 | 182.42 | 19.96 | 19.38 |
| 22.5 | 205.22 | 22.45 | 21.81 |
| 25 | 228.03 | 24.95 | 24.22 |
| 27.5 | 250.56 | 27.54 | 26.75 |
| 30 | 274.67 | 30.27 | 29.25 |
| 35 | 319.35 | 35.08 | 34.16 |
| 40 | 364.85 | 39.92 | 38.76 |
| 45 | 410.54 | 45.24 | 43.89 |
| 50 | 456.06 | 49.91 | 48.45 |
| 60 | 545.58 | 60.12 | 58.24 |
| 70 | 639.89 | 70.17 | 68.09 |
| 80 | 731.63 | 80.14 | 77.87 |
| 90 | 822.74 | 90.16 | 87.45 |
| 100 | 912.12 | 99.81 | 96.91 |
| Number of Layers, pcs. | Ra, nm | Surface Area, μm2 |
|---|---|---|
| 5 | 5.1 | 27.8 |
| 9 | 5.3 | 28.1 |
| 13 | 6.7 | 28.4 |
| 17 | 9.3 | 29.7 |
| 21 | 7.6 | 27.5 |
| 25 | 7.3 | 26.2 |
| Material | δd, MPa1/2 | δp, MPa1/2 | δH, MPa1/2 |
|---|---|---|---|
| Chloroform | 17.8 | 3.1 | 5.7 |
| Ethanol | 15.8 | 8.8 | 19.4 |
| Toluene | 18 | 1.4 | 2 |
| Arachidic acid | 16.3 | 2.9 | 5 |
| Stearic acid | 16.3 | 3.3 | 5.5 |
| RED | |||||
|---|---|---|---|---|---|
| Acid | SA | AA | SA | AA | |
| Vapor | |||||
| Chloroform | 3 | 3.1 | 0.6 | 0.8 | |
| Ethanol | 14.9 | 15.5 | 3.2 | 3.9 | |
| Toluene | 5.2 | 4.8 | 1.1 | 1.2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorbachev, I.; Smirnov, A.; Ivanov, G.; Avramov, I.; Datsuk, E.; Venelinov, T.; Bogdanova, E.; Anisimkin, V.; Kolesov, V.; Kuznetsova, I. Langmuir-Blodgett Films of Arachidic and Stearic Acids as Sensitive Coatings for Chloroform HF SAW Sensors. Sensors 2023, 23, 100. https://doi.org/10.3390/s23010100
Gorbachev I, Smirnov A, Ivanov G, Avramov I, Datsuk E, Venelinov T, Bogdanova E, Anisimkin V, Kolesov V, Kuznetsova I. Langmuir-Blodgett Films of Arachidic and Stearic Acids as Sensitive Coatings for Chloroform HF SAW Sensors. Sensors. 2023; 23(1):100. https://doi.org/10.3390/s23010100
Chicago/Turabian StyleGorbachev, Ilya, Andrey Smirnov, George Ivanov, Ivan Avramov, Elizaveta Datsuk, Tony Venelinov, Evgenija Bogdanova, Vladimir Anisimkin, Vladimir Kolesov, and Iren Kuznetsova. 2023. "Langmuir-Blodgett Films of Arachidic and Stearic Acids as Sensitive Coatings for Chloroform HF SAW Sensors" Sensors 23, no. 1: 100. https://doi.org/10.3390/s23010100
APA StyleGorbachev, I., Smirnov, A., Ivanov, G., Avramov, I., Datsuk, E., Venelinov, T., Bogdanova, E., Anisimkin, V., Kolesov, V., & Kuznetsova, I. (2023). Langmuir-Blodgett Films of Arachidic and Stearic Acids as Sensitive Coatings for Chloroform HF SAW Sensors. Sensors, 23(1), 100. https://doi.org/10.3390/s23010100











