Adsorbed Oxygen Ions and Oxygen Vacancies: Their Concentration and Distribution in Metal Oxide Chemical Sensors and Influencing Role in Sensitivity and Sensing Mechanisms
Abstract
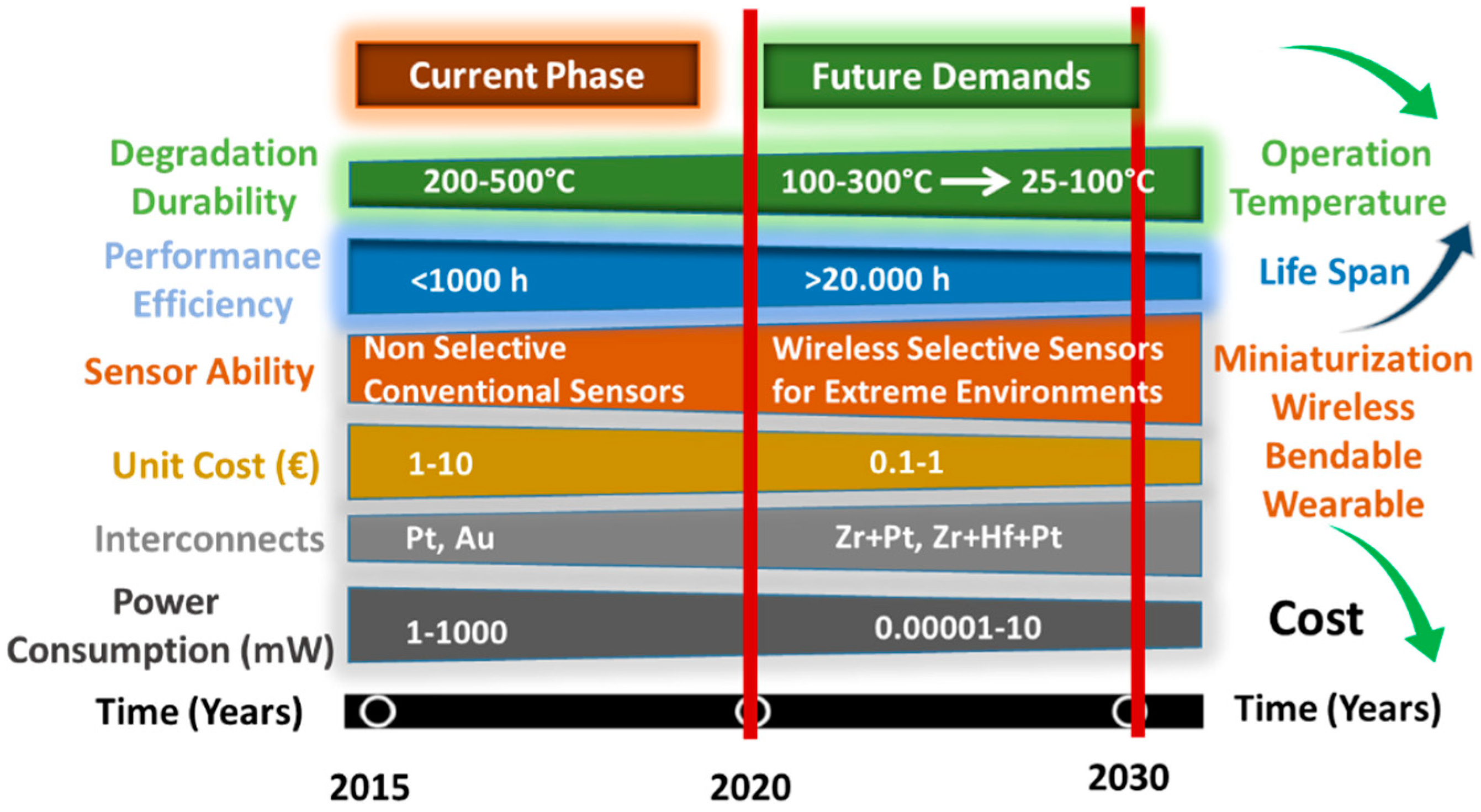
1. Introduction
2. Overview of Adsorbed Oxygen from the Viewpoint of Gas Sensor Designer
3. Review of Analytical-Spectral-Microscopic Tools for Semiconducting Metal Oxides (SMOs), Adsorbed-Chemisorbed Oxygen, and Chemical Gas Sensors Analysis
The Concerns with XPS Analysis of Chemisorbed-Adsorbed Oxygen Ions on SMOs’ Surfaces
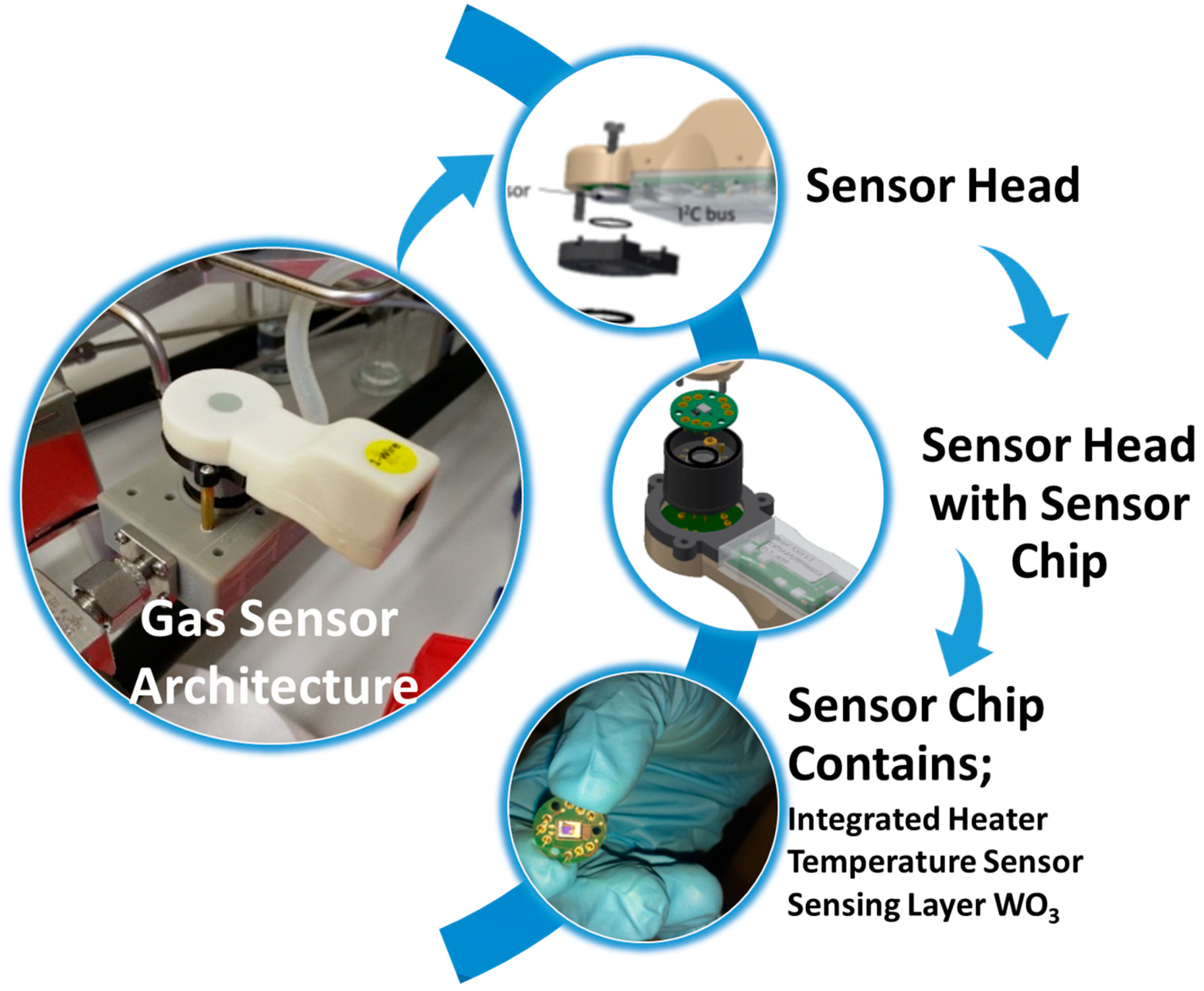
4. Experimental
5. Investigation of Adsorbed Oxygen Ions, Surface Chemistry-Homogeneity, and Work Function (Φ) of Semiconducting Metal Oxides (SMOs)
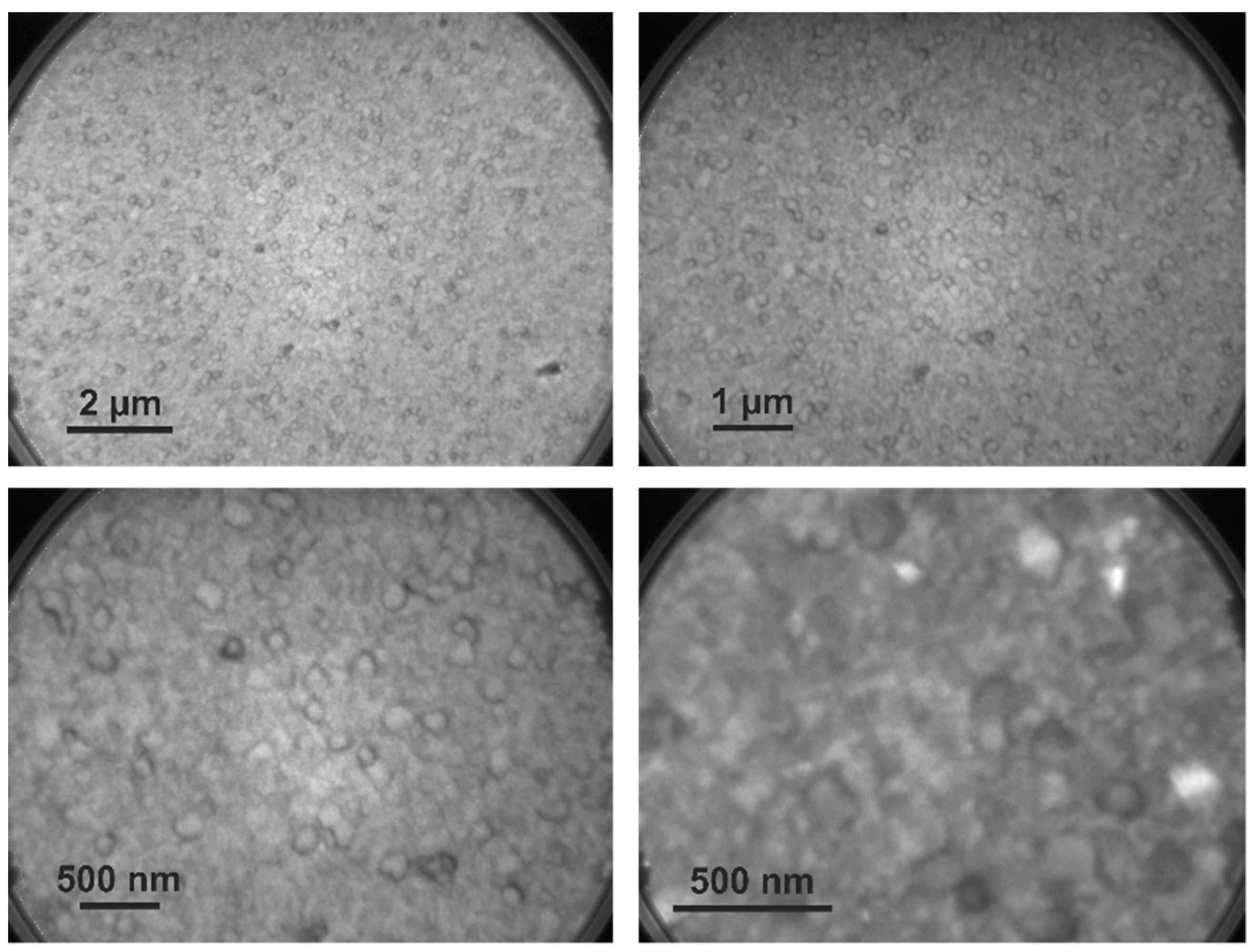
5.1. Surface Topography of WO3 through LEEM
5.2. Mapping Oxidation State Homogeneity on the WO3 Surface via XPEEM
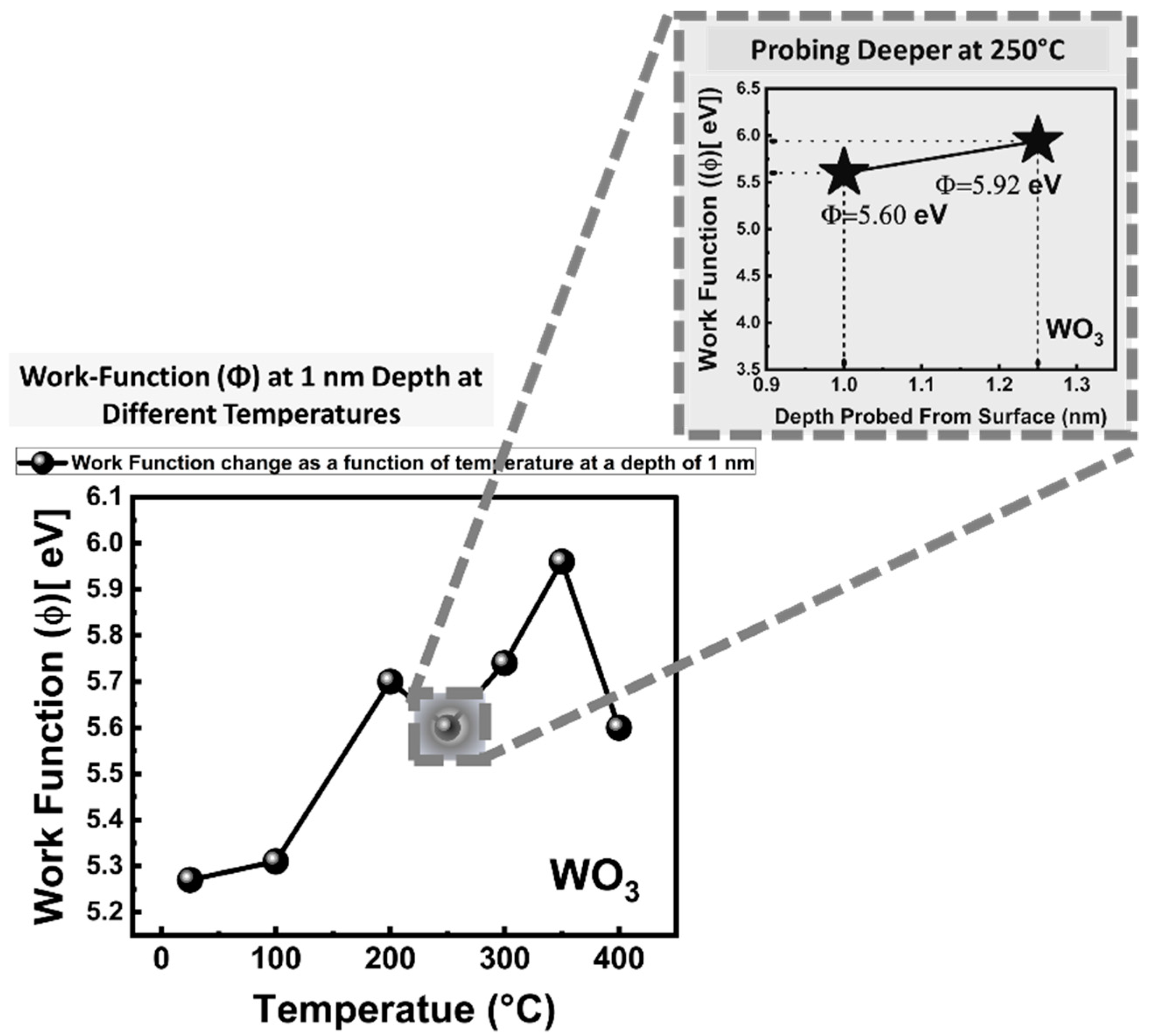
5.3. Work Function (Φ) Measurements
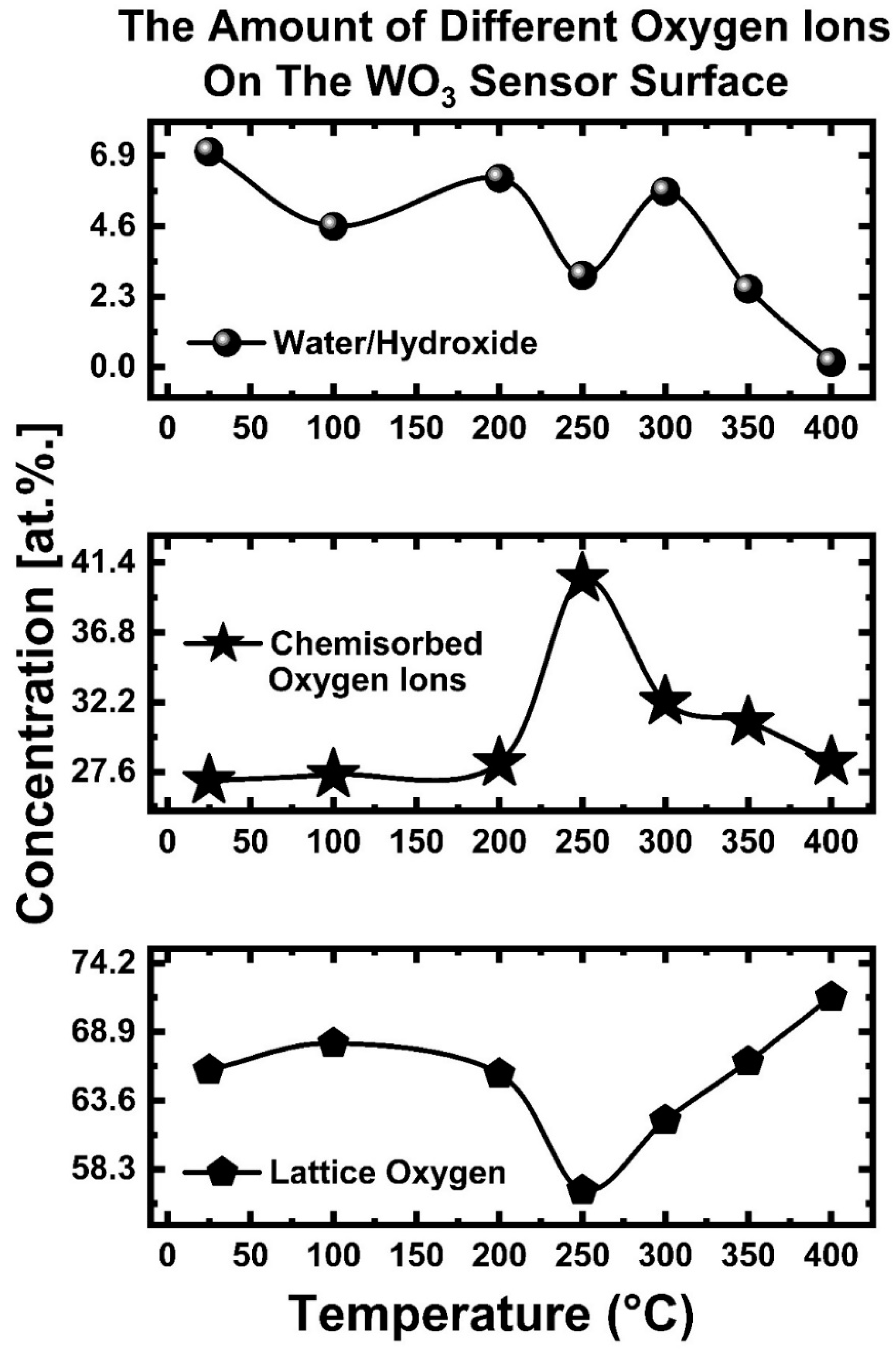
5.4. Amount of Adsorbed Oxygen Species on WO3 Sensor Surface
6. H2 Sensor Testing at 250 °C
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EXAFS | Extended X-Ray Absorption Fine Structure |
| SEXAFS | Surface Extended X-Ray Absorption Fine Structure |
| NEXAFS: | Near Edge X-Ray Absorption Fine Structure |
| XPS | X-Ray Photoelectron Spectroscopy |
| NAP-XPS | Near Ambient Pressure X-Ray Photoelectron Spectroscopy |
| UPS | Ultraviolet Photoelectron Spectroscopy |
| LEED | Low-Energy Electron Diffraction |
| AES | Auger Electron Spectroscopy |
| HR/-EELS | High-Resolution/Electron Energy-Loss Spectroscopy |
| ISS/RBS | Ion Scattering-Rutherford Backscattering Spectroscopy |
| FT-IR and Raman | Fourier Transform Infrared and Raman Spectroscopy |
| NMR | Nuclear Magnetic Resonance |
| LEEM | Low-Energy Electron Microscopy |
| XPEEM | X-ray photoemission electron Microscopy |
| UV-VIS | Ultraviolet–Visible Light Spectroscopy |
| CL/PL | Cathodoluminescence-Photoluminescence |
| XRF | X-Ray Fluorescence |
| EDS | Energy-Dispersive X-Ray Spectroscopy |
| SEM | Scanning Electron Microscopy |
| (GI)-XRD | (Grazing Incidence-) X-Ray Diffraction |
| TP(X) | Temperature Programmed (X:Reduction-Oxidation) |
References
- Çiftyürek, E.; Sabolsky, K.; Sabolsky, E.M. Molybdenum and tungsten oxide based gas sensors for high temperature detection of environmentally hazardous sulfur species. Sens. Actuators B Chem. 2016, 237, 262–274. [Google Scholar] [CrossRef]
- Çiftyürek, E.; Sabolsky, E.M.; Sabolsky, K. High temperature selective sensing of hydrogen with MgO-modified SrMoO4 micro-fibers. Sens. Actuators B Chem. 2017, 249, 296–310. [Google Scholar] [CrossRef]
- Cosandey, F.; Skandan, G.; Singhal, A. Materials and Processing Issues in Nanostructured Semiconductor Gas Sensors. JOM 2000, 52, 1–6. [Google Scholar]
- Sabolsky, E.M.; Gansor, P.; Çiftyürek, E.; Sabolsky, K.; Xu, C.; Zondlo, J.W. In situ formation of a solid oxide fuel cell (SOFC) cermet anode by NiWO4 reduction. J. Power Sources 2013, 237, 33–40. [Google Scholar] [CrossRef]
- Ross, T.; Zondlo, J.; Sabolsky, E.; Ciftyurek, E.; Koneru, A.; Thomas, T.; Celik, I.; Liu, X.; Sezer, H.; Damo, U. Performance and stability of large planar solid oxide fuel cells using phosphine contaminated hydrogen fuel. J. Power Sources 2018, 395, 185–194. [Google Scholar] [CrossRef]
- Potyrailo, R.A. Multivariable Sensors for Ubiquitous Monitoring of Gases in the Era of Internet of Things and Industrial Internet. Chem. Rev. 2016, 116, 11877–11923. [Google Scholar] [CrossRef]
- Çiftyürek, E.; Sabolsky, K.; Sabolsky, E.M. Platinum thin film electrodes for high-temperature chemical sensor applications. Sens. Actuators B Chem. 2013, 181, 702–714. [Google Scholar] [CrossRef]
- Çiftyürek, E.; McMillen, C.D.; Sabolsky, K.; Sabolsky, E.M. Platinum–zirconium composite thin film electrodes for high-temperature micro-chemical sensor applications. Sens. Actuators B Chem. 2015, 207, 206–215. [Google Scholar] [CrossRef]
- Crowder, B.L.; Sienko, M.J. Some Solid-State Studies of Tungsten Trioxide and Their Significance to Tungsten Bronze Theory. J. Chem. Phys. 1963, 38, 1576–1583. [Google Scholar] [CrossRef]
- Aguir, G.M.K.; Lemire, C.; Gillet, E.; Schierbaum, K. The structure and electrical conductivity of vacuum annealed WO3 thin films. Thin Solid Film. 2004, 467, 239–246. [Google Scholar]
- Uhlig, H.H.; King, P.F. The Flade Potential of Iron Passivated by Various Inorganic Corrosion Inhibitors. J. Electrochem. Soc. 1959, 106, 1–7. [Google Scholar] [CrossRef]
- Tseung, A.C.C. Semiconducting Oxide Oxygen Electrodes. J. Electrochem. Soc. 1978, 125, 1660–1664. [Google Scholar] [CrossRef]
- Wang, S.; Shi, W.; Lu, C. Chemisorbed Oxygen on the Surface of Catalyst-Improved Cataluminescence Selectivity. Anal. Chem. 2016, 88, 4987–4994. [Google Scholar] [CrossRef]
- Ciftyurek, E. Nano-derived sensors for high-temperature sensing of H2, SO2 and H2S. Ph.D. Thesis, West Virginia University, Morgantown, WV, USA, 2014. [Google Scholar] [CrossRef]
- Breiter, M.W. Reduction Mechanism of Chemisorbed Oxygen on Platinum Electrodes by Molecular Hydrogen. J. Electrochem. Soc. 1962, 109, 425. [Google Scholar] [CrossRef]
- Hammer, B.; Morikawa, Y.; Nørskov, J.K. CO Chemisorption at Metal Surfaces and Overlayers. Phys. Rev. Lett. 1996, 76, 2141–2144. [Google Scholar] [CrossRef] [PubMed]
- Masuda, K. Chemisorption of Ordered Overlayer on a Tight-Binding Metal Surface Two-Level Adsorbate. Z. Nat. Teil A 1982, 37, 1147–1164. [Google Scholar] [CrossRef]
- MacDougall, B.; Cohen, M. Breakdown of Oxide Films on Nickel. J. Electrochem. Soc. 1977, 124, 1185–1190. [Google Scholar] [CrossRef]
- Bielański, A.; Haber, J. Oxygen in Catalysis on Transition Metal Oxides. Catal. Rev. 1979, 19, 1–41. [Google Scholar] [CrossRef]
- Rothschild, A.; Komem, Y.; Cosandey, F. Low Temperature Reoxidation Mechanism in Nanocrystalline TiO2-δ Thin Films. J. Electrochem. Soc. 2001, 148, H85. [Google Scholar] [CrossRef]
- Che, M.; Tench, A. Characterization and Reactivity of Mononuclear Oxygen Species on Oxide Surfaces. Adv. Catal. 1982, 31, 77–133. [Google Scholar]
- Barteau, M.A.; Ko, E.I.; Madix, R.J. The Adsoprtion of CO, O2 and H2 on Pt(100)-(5x20). Surf. Sci. 1981, 102, 99–117. [Google Scholar] [CrossRef]
- Jones, T.E.; Rocha, T.C.; Knop-Gericke, A.; Stampfl, C.; Schlögl, R.; Piccinin, S. Thermodynamic and spectroscopic properties of oxygen on silver under an oxygen atmosphere. Phys. Chem. Chem. Phys. 2015, 17, 9288–9312. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.; Mather, P.G.; Perrella, A.C.; Read, J.C.; Buhrman, R.A. Oxygen stoichiometry and instability in aluminum oxide tunnel barrier layers. Phys. Rev. B 2005, 71, 161401. [Google Scholar] [CrossRef]
- Ciftyürek, E.; Šmíd, B.; Li, Z.; Matolín, V.; Schierbaum, K. Spectroscopic Understanding of SnO2 and WO3 Metal Oxide Surfaces with Advanced Synchrotron Based; XPS-UPS and Near Ambient Pressure (NAP) XPS Surface Sensitive Techniques for Gas Sensor Applications under Operational Conditions. Sensors 2019, 19, 4737. [Google Scholar] [CrossRef] [PubMed]
- Puzzovio, D.; Carotta, M.; Cervi, A.; El Hachimi, A.; Joly, J.; Gaillard, F.; Guidi, V. TPD and ITPD study of materials used as chemoresistive gas sensors. Solid State Ion. 2009, 180, 1545–1552. [Google Scholar] [CrossRef]
- Galatsis, K.; Li, Y.; Wlodarski, W.; Comini, E.; Sberveglieri, G.; Cantalini, C.; Santucci, S.; Passacantando, M. Comparison of single and binary oxide MoO3, TiO2 and WO3 sol–gel gas sensors. Sens. Actuators B Chem. 2002, 83, 276–280. [Google Scholar] [CrossRef]
- Ciftyurek, E.; Wilken, M.; Zanders, D.; Mai, L.; Devi, A.; Schierbaum, K.D. Monitoring Surface Stoichiometry, Work Function and Valance Band of Tungsten Oxide (WO3), Molybdenum Oxide (MoO3) and Tin Oxide (SnO2) Thin Films as a Function of Temperature and Oxygen Partial Pressure with Advanced Surface Sensitive Techniques for Chemical Sensing Applications. Multidiscip. Digit. Publ. Inst. Proc. 2019, 14, 27. [Google Scholar] [CrossRef]
- Zhong, J.-Q.; Wang, M.; Akter, N.; Stacchiola, D.J.; Lu, D.; Boscoboinik, J.A. Room-Temperature in Vacuo Chemisorption of Xenon Atoms on Ru(0001) under Interface Confinement. J. Phys. Chem. C 2019, 123, 13578–13585. [Google Scholar] [CrossRef]
- Känzig, W.; Cohen, M.H. Paramagnetic Resonance of Oxygen in Alkali Halides. Phys. Rev. Lett. 1959, 3, 509–510. [Google Scholar] [CrossRef]
- Tench, A.J.; Holroyd, P. The identification of O2- adsorbed on magnesium oxide. Chem. Commun. 1968, 471–473. [Google Scholar] [CrossRef]
- Chang, S. Oxygen chemisorption on tin oxide: Correlation between electrical conductivity and EPR measurements. J. Vac. Sci. Technol. 1980, 17, 366–369. [Google Scholar] [CrossRef]
- Windischmann, H.; Mark, P. A Model for the Operation of a Thin-Film SnOx Conductance-Modulation Carbon Monoxide Sensor. J. Electrochem. Soc. 1978, 126, 627–633. [Google Scholar] [CrossRef]
- Clifford, P.K.; Tuma, D. Characteristics of semiconductor gas sensors II. transient response to temperature change. Sens. Actuators 1982, 3, 255–281. [Google Scholar] [CrossRef]
- Kim, D.; Pikhitsa, P.V.; Yang, H.; Choi, M. Room temperature CO and H2sensing with carbon nanoparticles. Nanotechnology 2011, 22, 485501. [Google Scholar] [CrossRef][Green Version]
- Simmons, G.W.; Beard, B.C. Characterization of acid-base properties of the hydrated oxides on iron and titanium metal surfaces. J. Phys. Chem. 1987, 91, 1143–1148. [Google Scholar] [CrossRef]
- Dang, T.A.; Gnanasekaran, R.; Deppe, D.D. Quantification of surface hydroxides using chemical labeling and XPS. Surf. Interface Anal. 1992, 18, 141–146. [Google Scholar] [CrossRef]
- Kurbatov, G.; Darque-Ceretti, E.; Aucouturier, M. Characterization of hydroxylated oxide film on iron surfaces and its acid-base properties using XPS. Surf. Interface Anal. 1992, 18, 811–820. [Google Scholar] [CrossRef]
- McCafferty, E.; Wightman, J. Determination of the Concentration of SurfaceHydroxyl Groups on Metal Oxide Films by aQuantitative XPS Method. Surf. Interface Anal. 1998, 26, 549–564. [Google Scholar] [CrossRef]
- Barteau, M.A.; Madix, R.J. A Photelectron Spectroscopic Investigation of The Interaction Between H2O and Oxygen on Ag(110). Surf. Sci. 1984, 140, 108–122. [Google Scholar] [CrossRef]
- Barteau, M.; Ko, E.; Madix, R. The oxidation of CO on the Pt(100)-(5 × 20) surface. Surf. Sci. 1981, 104, 161–180. [Google Scholar] [CrossRef]
- Tan, B.J.; Klabunde, K.J.; Sherwood, P.M.A. X-ray photoelectron spectroscopy studies of solvated metal atom dispersed catalysts. Monometallic iron and bimetallic iron-cobalt particles on alumina. Chem. Mater. 1990, 2, 186–191. [Google Scholar] [CrossRef]
- McIntyre, N.S.; Zetaruk, D.G. X-ray photoelectron spectroscopic studies of iron oxides. Anal. Chem. 1977, 49, 1521–1529. [Google Scholar] [CrossRef]
- Sanjnes, R.; Coluzza, C.; Rosenfeld, D.; Gozzo, F.; Almeras, P.; Levy, F.; Margaritondo, G. Photoemission spectromicroscopy: A new insight in the chemistry of SnOx films for gas sensors. J. Appl. Phys. 1992, 73, 3997. [Google Scholar] [CrossRef]
- Drawdy, J.E.; Hoflund, G.B.; Davidson, M.R.; Upchurch, B.T.; Schryer, D.R. Characterization study of polycrystalline tin oxide surfaces before and after reduction in CO. Surf. Interface Anal. 1992, 19, 559–564. [Google Scholar] [CrossRef]
- Cox, D.F.; Hoflund, G.B.; Hocking, W.H. A SIMS depth profiling study of the hydration layer formed at polycrystalline tin oxide surfaces by atmospheric exposure. Appl. Surf. Sci. 1986, 26, 239–245. [Google Scholar] [CrossRef]
- Gaggiotti, G.; Galdikas, A.; Kačiulis, S.; Mattogno, G.; Šetkus, A. Surface chemistry of tin oxide based gas sensors. J. Appl. Phys. 1994, 76, 4467–4471. [Google Scholar] [CrossRef]
- Shoji, R.; Mochizuki, Y.; Kobayashi, Y.; Yamauchi, N.; Sato, K. Adsorption and Photoelectrodeposition of Heavy Metal Ions from Wastewater using SnOx(1 < x < 2)/CeO2 Photocatalysts. Glob. J. Res. Eng. 2018, 18, 23–35. [Google Scholar]
- Haddad, K.; Abokifa, A.; Kavadiya, S.; Lee, B.; Banerjee, S.; Raman, B.; Banerjee, P.; Lo, C.; Fortner, J.; Biswas, P. SnO2 Nanostructured Thin Films for Room-Temperature Gas Sensing of Volatile Organic Compounds. ACS Appl. Mater. Interfaces 2018, 10, 29972–29981. [Google Scholar] [CrossRef]
- Caruso, T.; Lenardi, C.; Agostino, R.G.; Amati, M.; Bongiorno, G.; Mazza, T.; Policicchio, A.; Formoso, V.; Maccallini, E.; Colavita, E.; et al. Electronic structure of cluster assembled nanostructured TiO2 by resonant photoemission at the Ti L2,3 edge. J. Chem. Phys. 2008, 128, 094704. [Google Scholar] [CrossRef]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2Photocatalysis: A Historical Overview and Future Prospects. Jpn. J. Appl. Phys. 2005, 44, 8269–8285. [Google Scholar] [CrossRef]
- Choi, J.Y.; Heo, K.; Cho, K.-S.; Hwang, S.W.; Kim, S.; Lee, S.Y. Engineering of band gap states of amorphous SiZnSnO semiconductor as a function of Si doping concentration. Sci. Rep. 2016, 6, 36504. [Google Scholar] [CrossRef] [PubMed]
- Eloirdi, R.; Cakir, P.; Huber, F.; Seibert, A.; Konings, R.; Gouder, T. X-ray photoelectron spectroscopy study of the reduction and oxidation of uranium and cerium single oxide compared to (U-Ce) mixed oxide films. Appl. Surf. Sci. 2018, 457, 566–571. [Google Scholar] [CrossRef]
- Zanders, D.; Ciftyurek, E.; Subaşı, E.; Huster, N.; Bock, C.; Kostka, A.; Rogalla, D.; Schierbaum, K.; Devi, A. PEALD of HfO2 Thin Films: Precursor Tuning and a New Near-Ambient-Pressure XPS Approach to in Situ Examination of Thin-Film Surfaces Exposed to Reactive Gases. ACS Appl. Mater. Interfaces 2019, 11, 28407–28422. [Google Scholar] [CrossRef] [PubMed]
- Kawabe, T.; Tabata, K.; Suzuki, E.; Yamaguchi, A.Y.; Nagasawa, Y. Electronic States of Chemisorbed Oxygen Species and Their Mutually Related Studies on SnO2 Thin Film. J. Phys. Chem. B 2001, 105, 4239–4244. [Google Scholar] [CrossRef]
- Carbonio, E.A.; Rocha, T.C.R.; Klyushin, A.Y.; Píš, I.; Magnano, E.; Nappini, S.; Piccinin, S.; Knop-Gericke, A.; Schlögl, R.; Jones, T.E. Are multiple oxygen species selective in ethylene epoxidation on silver? Chem. Sci. 2018, 9, 990–998. [Google Scholar] [CrossRef]
- Hirsch, O.; Kvashnina, K.O.; Luo, L.; Süess, M.J.; Glatzel, P.; Koziej, D. High-energy resolution X-ray absorption and emission spectroscopy reveals insight into unique selectivity of La-based nanoparticles for CO2. Proc. Natl. Acad. Sci. USA 2015, 112, 15803–15808. [Google Scholar] [CrossRef]
- Prabhu, E.; Gnanasekar, K.I.; Ravindran, T.R.; Jayaraman, V.; Gnanasekaran, T. Changes in Carrier Concentration and Debye Length: Experimental Evidence from van der Pauw Hall Measurements on NOx Sensing of In2O3. J. Electrochem. Soc. 2014, 161, B176. [Google Scholar] [CrossRef]
- Piersma, B.J.; Warner, T.B.; Schuldiner, S. Interaction of Carbon Dioxide with Hydrogen Chemisorbed on a Platinum Electrodo. J. Electrochem. Soc. 1966, 112, 841–846. [Google Scholar] [CrossRef]
- Ciftyurek, E.; Bragg, D.; Oginni, O.; Levelle, R.; Singh, K.; Sivanandan, L.; Sabolsky, E.M. Performance of activated carbons synthesized from fruit dehydration biowastes for supercapacitor applications. Environ. Prog. Sustain. Energy 2019, 38, e13030. [Google Scholar] [CrossRef]
- Sun, Y.; Suematsu, K.; Watanabe, K.; Nishibori, M.; Hu, J.; Zhang, W.; Shimanoe, K. Determination of Effective Oxygen Adsorption Species for CO Sensing Based on Electric Properties of Indium Oxide. J. Electrochem. Soc. 2018, 165, B275–B280. [Google Scholar] [CrossRef]
- Zanders, D.; Ciftyurek, E.; Hoppe, C.; Arcos, T.; Kostka, A.; Rogalla, D.; Grundmeier, G.; Schierbaum, K.; Devi, A. Validation of a Terminally Amino Functionalized Tetra-Alkyl Sn(IV) Precursor in Metal–Organic Chemical Vapor Deposition of SnO2 Thin Films: Study of Film Growth Characteristics, Optical, and Electrical Properties. Adv. Mater. Interfaces 2019, 6, 1801540. [Google Scholar] [CrossRef]
- Lamberti, C.; Groppo, E.; Spoto, G.; Bordiga, S.; Zecchina, A. Infrared Spectroscopy of Transient Surface Species. Adv. Catal. 2007, 51, 1–74. [Google Scholar] [CrossRef]
- Stair, P.C. The Application of UV Raman Spectroscopy for the Characterization of Catalysts and Catalytic Reactions. Adv. Catal. 2007, 51, 75–98. [Google Scholar]
- Arijs, E.; Cardon, F.; der Vorst, W.M.-V. The influence of surface donor states on the chemisorption kinetics of oxygen at the surface of ZnO single crystals. II. Experimental results. J. Solid State Chem. 1973, 6, 319–326. [Google Scholar] [CrossRef]
- Morrison, S.R. Surface Barrier Effects in Adsorption, Illustrated by Zinc Oxide. Adv. Catal. 1955, 7, 259–301. [Google Scholar]
- Komuro, M. Kinetic Studies of Oxygen Chemisorption on the Rutile Single-crystal Surface by Means of Electrical Conductivity. Bull. Chem. Soc. Jpn. 1975, 48, 756–761. [Google Scholar] [CrossRef]
- Marley, J.A.; Dockerty, R.C. Electrical Properties of Stannic Oxide Single Crystals. Phys. Rev. (Ser. I) 1965, 140, A304–A310. [Google Scholar] [CrossRef]
- Samson, S.; Fonstad, C.G. Defect structure and electronic donor levels in stannic oxide crystals. J. Appl. Phys. 1973, 44, 4618–4621. [Google Scholar] [CrossRef]
- Kaliaguine, S.; Shelimov, B.; Kazansky, V. Reactions of methane and ethane with hole centers O−. J. Catal. 1978, 55, 384–393. [Google Scholar] [CrossRef]
- Bevan, D.J.M.; Shelton, J.P.; Anderson, J.S. 351. Properties of some simple oxides and spinels at high temperatures. J. Chem. Soc. 1948, 1729–1741. [Google Scholar] [CrossRef]
- Heiland, G.; Kohl, D. Interpretation of surface phenomena on ZnO by the compensation model. Phys. Status Solidi 1978, 49, 27–37. [Google Scholar] [CrossRef]
- Pöppl, A.; Völkel, G. ESR and Photo-ESR Investigations of Zinc Vacancies and Interstitial Oxygen Ions in Undoped ZnO Ceramics. Phys. Status Solidi 1991, 125, 571–581. [Google Scholar] [CrossRef]
- Janotti, A.; Van de Walle, C.G. Native point defects in ZnO. Phys. Rev. B 2007, 76, 165202. [Google Scholar] [CrossRef]
- Barsan, N.; Klaus, S. Basiscs of semiconducting metal oxide-based gas sensors. In Metal Oxides Series: Gas Sensors Based on Conducting Metal Oxides; Elseiver: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Brundle, C.R.; Evans, C.A., Jr.; Wilson, S. Encyclopedia of Materials Charecterization; Gulf Professional Publishing: Oxford, UK, 1992. [Google Scholar]
- Cox, D.F.; Fryberger, T.B.; Semancik, S. Oxygen vacancies and defect electronic states on the SnO2 (110)-1×1 Surface. Phys. Rev. B 1988, 38, 2072. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.F.; Fryberger, T.B.; Semancik, S. Surface reconstructions of oxygen deficient SnO2(110). Surf. Sci. 1989, 224, 121–142. [Google Scholar] [CrossRef]
- Cox, D.F.; Fryberger, T.B. Preferential isotopic labeling of lattice oxygen positions on the SnO2(110) surface. Surf. Sci. 1990, 227, L105–L108. [Google Scholar] [CrossRef]
- Melaet, G.; Ralston, W.T.; Liu, C.W.; Somorjai, A.G. Time-Resolved (2 s) Study of the Initial Steps of the Catalytic Hy-drogenation of CO: From Branched Isomers to Unsaturated Molecules. J. Phys. Chem. C 2014, 118, 26921–26925. [Google Scholar] [CrossRef]
- Kaichev, V.V.; Bukhtiyarov, V.; Hävecker, M.; Knop-Gercke, A.; Mayer, R.W.; Schlögl, R. The Nature of Electrophilic and Nucleophilic Oxygen Adsorbed on Silver. Kinet. Catal. 2003, 44, 432–440. [Google Scholar] [CrossRef]
- Köck, E.V.; Kogler, M.; Bielz, T.; Klötzer, B.; Penner, S. In Situ FT-IR Spectroscopic Study of CO2 and CO Adsorption on Y2O3, ZrO2, and Yttria-Stabilized ZrO2. J. Phys. Chem. C 2013, 117, 17666–17673. [Google Scholar] [CrossRef]
- Yang, C.; Wöll, C. IR spectroscopy applied to metal oxide surfaces: Adsorbate vibrations and beyond. Adv. Phys. X 2017, 2, 373–408. [Google Scholar] [CrossRef]
- Gao, F.; Wang, Y.; Goodman, D.W. CO Oxidation over AuPd(100) from Ultrahigh Vacuum to Near-Atmospheric Pressures: The Critical Role of Contiguous Pd Atoms. J. Am. Chem. Soc. 2009, 131, 5734–5735. [Google Scholar] [CrossRef] [PubMed]
- Freund, H.-J.; Meijer, G.; Scheffler, M.; Schlögl, R.; Wolf, M. CO Oxidation as a Prototypical Reaction for Heterogeneous Processes. Angew. Chem. Int. Ed. 2011, 50, 10064–10094. [Google Scholar] [CrossRef] [PubMed]
- Rameshan, C.; Stadlmayr, W.; Weilach, C.; Penner, S.; Zemlyanov, D.; Rupprechter, G.; Klötzer, B. Subsurface-gesteuerte CO2-Selektivität von PdZn-Oberflächenlegierungen in der H2-Erzeugung durch Methanoldampfreformierung. Angew. Chem. 2010, 1222, 3292–3296. [Google Scholar] [CrossRef]
- Kuhn, W.; Szanyi, J.; Goodman, D. CO adsorption on Pd(111): The effects of temperature and pressure. Surf. Sci. 1992, 274, L611–L618. [Google Scholar] [CrossRef]
- Conant, T.; Karim, A.M.; Lebarbier, V.; Wang, Y.; Girgsdies, F.; Schlögl, R.; Datye, A. Stability of bimetallic Pd–Zn catalysts for the steam reforming of methanol. J. Catal. 2008, 257, 64–70. [Google Scholar] [CrossRef]
- Rameshan, C.; Stadlmayr, W.; Weilach, C.; Penner, S.; Lorenz, H.; Hävecker, M.; Blume, R.; Rocha, T.; Teschner, D.; Knop-Gericke, A.; et al. Subsurface-Controlled CO2 Selectivity of PdZn Near-Surface Alloys in H2Generation by Methanol Steam Reforming. Angew. Chem. Int. Ed. 2010, 49, 3224–3227. [Google Scholar] [CrossRef]
- Thajudheen, T.; Dixon, A.; Gardonio, S.; Arčon, I.; Valant, M. Oxygen Vacancy-Related Cathodoluminescence Quenching and Polarons in CeO2. J. Phys. Chem. C 2020, 124, 19929–19936. [Google Scholar] [CrossRef]
- Han, H.-L.; Melaet, G.; Alayoglu, S.; Somorjai, G.A. In Situ Microscopy and Spectroscopy Applied to Surfaces at Work. ChemCatChem 2015, 7, 3625–3638. [Google Scholar] [CrossRef]
- Kwoka, M.; Ottaviano, L.; Passacantando, M.; Santucci, S.; Czempik, G.; Szuber, J. XPS study of the surface chemistry of L-CVD SnO2 thin films after oxidation. Thin Solid Film. 2005, 490, 36–42. [Google Scholar] [CrossRef]
- Lančok, J.; Santoni, A.; Penza, M.; Loreti, S.; Menicucci, I.; Minarini, C.; Jelinek, M. Tin oxide thin films prepared by laser-assisted metal–organic CVD: Structural and gas sensing properties. Surf. Coat. Technol. 2005, 200, 1057–1060. [Google Scholar] [CrossRef]
- Dupin, J.-C.; Gonbeau, D.; Vinatier, P.; Levasseur, A. Systematic XPS studies of metal oxides, hydroxides and peroxides. Phys. Chem. Chem. Phys. 2000, 2, 1319–1324. [Google Scholar] [CrossRef]
- Kelley, R.; Song, K.; Van Leer, B.; Wall, D.; Kwakman, L. Xe+ FIB Milling and Measurement of Amorphous Silicon Damage. Microsc. Microanal. 2013, 19, 862–863. [Google Scholar] [CrossRef]
- Wilken, M.; Ciftyürek, E.; Cwik, S.; Mai, L.; Mallick, B.; Rogalla, D.; Schierbaum, K.; Devi, A. CVD Grown Tungsten Oxide for Low Temperature Hydrogen Sensing: Tuning Surface Characteristics via Materials Processing for Sensing Applications. Small 2022, 2204636. [Google Scholar] [CrossRef] [PubMed]
- Wildfire, C.; Ciftyurek, E.; Sabolsky, K.; Sabolsky, E.M. Fabrication and Testing of High-Temperature Nano-Derived Resistive-Type Microsensors for Hydrogen Sensing. J. Electrochem. Soc. 2014, 161, B3094–B3102. [Google Scholar] [CrossRef]
- Mews, M.; Korte, L.; Rech, B. Oxygen Vacancies in Tungsten Oxide and Their Influence on Tungsten Oxide/Silicon Het-erojunction Solar Cells. Sol. Energy Mater. Sol. Cells 2016, 158, 77–83. [Google Scholar] [CrossRef]
- Azad, A.M.; Akbar, S.A.; Mhaisalkar, S.G.; Birkefeld, L.D.; Goto, K.S. Solid State Gas Sensors A review. J. Electrochem. Soc. 1992, 139, 3690–3701. [Google Scholar] [CrossRef]
- Zywitzki, D.; Schaper, R.; Ciftyürek, E.; Wree, J.; Taffa, D.H.; Baier, D.M.; Rogalla, D.; Li, Y.; Meischein, M.; Ludwig, A.; et al. Chemical Vapor Deposition of Cobalt and Nickel Ferrite Thin Films: Investigation of Structure and Pseudocapacitive Properties. Adv. Mater. Interfaces 2021, 8, 2100949. [Google Scholar] [CrossRef]







| Technique | Elemental Identification | Chemical State | Structure | Surface Defects | Bulk Defects | Morphology | Imaging | Depth Probed (nm) | Lateral Resolution (µm) | Quantification | In-Situ Applicability | Chemical State Mapping | Elemental Mapping | Electronic Properties |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (GI-)XRD | ✓/X | ✓/X | ✓ | ✓(GI) | ✓ | X | ✓ | 10-Bulk | ≥10000 | ✓ | ✓ | ✓/X | ✓/X | X |
| EXAFS | ✓ | X | ✓ | X | X | X | X | ≥1000 | >1000 | ✓/X | ✓ | X | X | ✓ |
| SEXAFS | ✓ | X | ✓ | X | X | X | X | 1–10 | >1000 | ✓/X | ✓ | X | X | ✓ |
| NEXAFS | ✓ | ✓/X | ✓ | X | X | X | X | 1–10 | >1000 | ✓/X | ✓ | X | X | ✓ |
| XPS | ✓ | ✓ | X | ✓ | ✓* | X | ✓ | 0.5–10 | 50–100 | ✓ | ✓ | ✓ | ✓ | ✓ |
| NAP-XPS | ✓ | ✓ | X | ✓ | ✓* | X | ✓ | 0.5–10 | 50–100 | ✓ | ✓ | ✓ | ✓ | ✓ |
| UPS | X | ✓ | X | ✓ | X | X | X | 0.5–5 | 150 | ✓ | X | X | X | ✓ |
| LEED | X | X | ✓ | ✓ | X | X | ✓ | 1–5 | <0.1 | X | ✓ | X | X | X |
| AES | ✓ | ✓/X | X | ✓ | ✓* | X | ✓ | 0.5–10 | <0.1 | X | ✓ | X | ✓ | X |
| ISS/RBS | ✓ | ✓/X | ✓ | X | X | X | X | 0.3–3 | 150 | ✓ | X | X | X | X |
| FT-IR | ✓ | ✓/X | X | X | ✓ | X | ✓ | ≥1000 | ≥5000 | ✓/X | ✓ | X | X | X |
| Raman | ✓ | ✓/X | ✓ | X | ✓ | X | ✓ | ≥1000 | 1–10 | ✓/X | ✓ | X | X | X |
| NMR | X | ✓ | ✓ | X | ✓ | X | X | >104 | >1000 | ✓ | ✓ | X | X | X |
| LEEM | X | X | X | X | X | ✓ | ✓ | 1–3 | >1000 | X | X | X | X | ✓/X |
| XPEEM | ✓ | ✓ | X | ✓ | ✓* | ✓ | ✓ | 1–10 | <0.1 | ✓ | ✓ | ✓ | ✓ | ✓ |
| UV-VIS | X | X | X | X | ✓ | X | X | ~1000 | 5–100 | ✓ | X | X | X | ✓ |
| CL/PL | X | ✓ | X | ✓/X | ✓ | X | ✓ | 10–1000 | ≥1 | ✓/X | X/✓ | ✓ | X | ✓ |
| XRF | ✓ | X | X | X | X | X | ✓ | ≥1000 | 1000 | ✓ | ✓ | X | ✓ | X |
| EDS-SEM | ✓ | X | X | X | X | ✓ | ✓ | >100 | 0.5 | ✓ | ✓/X | X | ✓ | X |
| H/R-EELS | ✓ | ✓ | X | ✓ | X | X | X | 2–20 | <0.1 | ✓/X | ✓/X | X | ✓/X | ✓ |
| TP(X) | X | ✓/X | X | ✓ | X | X | X | >104 | >1000 | ✓ | ✓ | X | X | X |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciftyurek, E.; Li, Z.; Schierbaum, K. Adsorbed Oxygen Ions and Oxygen Vacancies: Their Concentration and Distribution in Metal Oxide Chemical Sensors and Influencing Role in Sensitivity and Sensing Mechanisms. Sensors 2023, 23, 29. https://doi.org/10.3390/s23010029
Ciftyurek E, Li Z, Schierbaum K. Adsorbed Oxygen Ions and Oxygen Vacancies: Their Concentration and Distribution in Metal Oxide Chemical Sensors and Influencing Role in Sensitivity and Sensing Mechanisms. Sensors. 2023; 23(1):29. https://doi.org/10.3390/s23010029
Chicago/Turabian StyleCiftyurek, Engin, Zheshen Li, and Klaus Schierbaum. 2023. "Adsorbed Oxygen Ions and Oxygen Vacancies: Their Concentration and Distribution in Metal Oxide Chemical Sensors and Influencing Role in Sensitivity and Sensing Mechanisms" Sensors 23, no. 1: 29. https://doi.org/10.3390/s23010029
APA StyleCiftyurek, E., Li, Z., & Schierbaum, K. (2023). Adsorbed Oxygen Ions and Oxygen Vacancies: Their Concentration and Distribution in Metal Oxide Chemical Sensors and Influencing Role in Sensitivity and Sensing Mechanisms. Sensors, 23(1), 29. https://doi.org/10.3390/s23010029







