Application of Near Infrared Spectroscopy to Monitor the Quality Change of Sour Cherry Stored under Modified Atmosphere Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment I
2.1.1. Sour Cherry Samples and Their Treatment
Materials
Sample Preparation
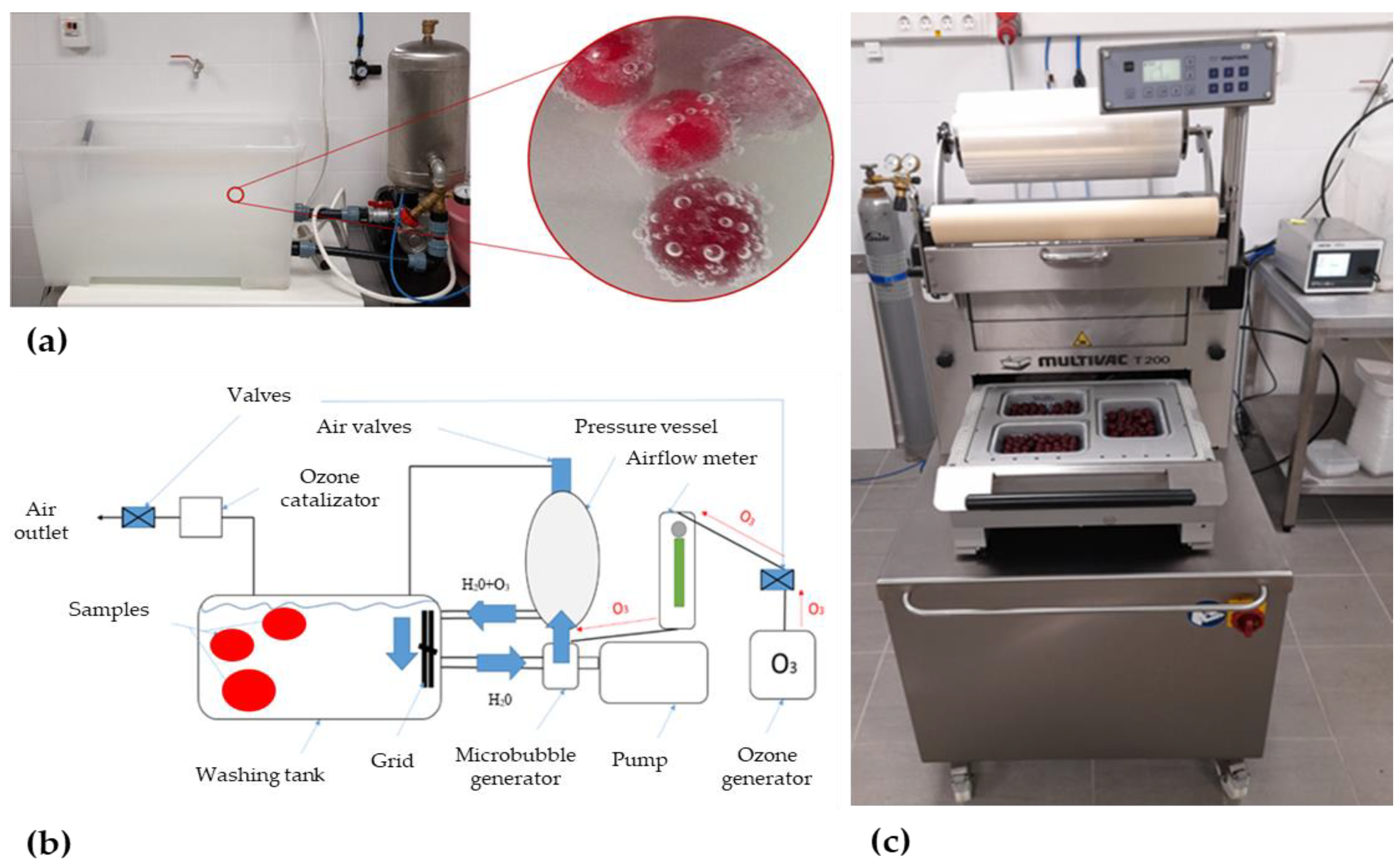
0.4 g O3/m3 = 467 ×0.4 = 187~190 ppm O3
100 L h−1 × 190 ppm = 19 mg O3/h
Sample Packaging
2.1.2. Reference Measurements
Determination of Headspace Gas Concentration
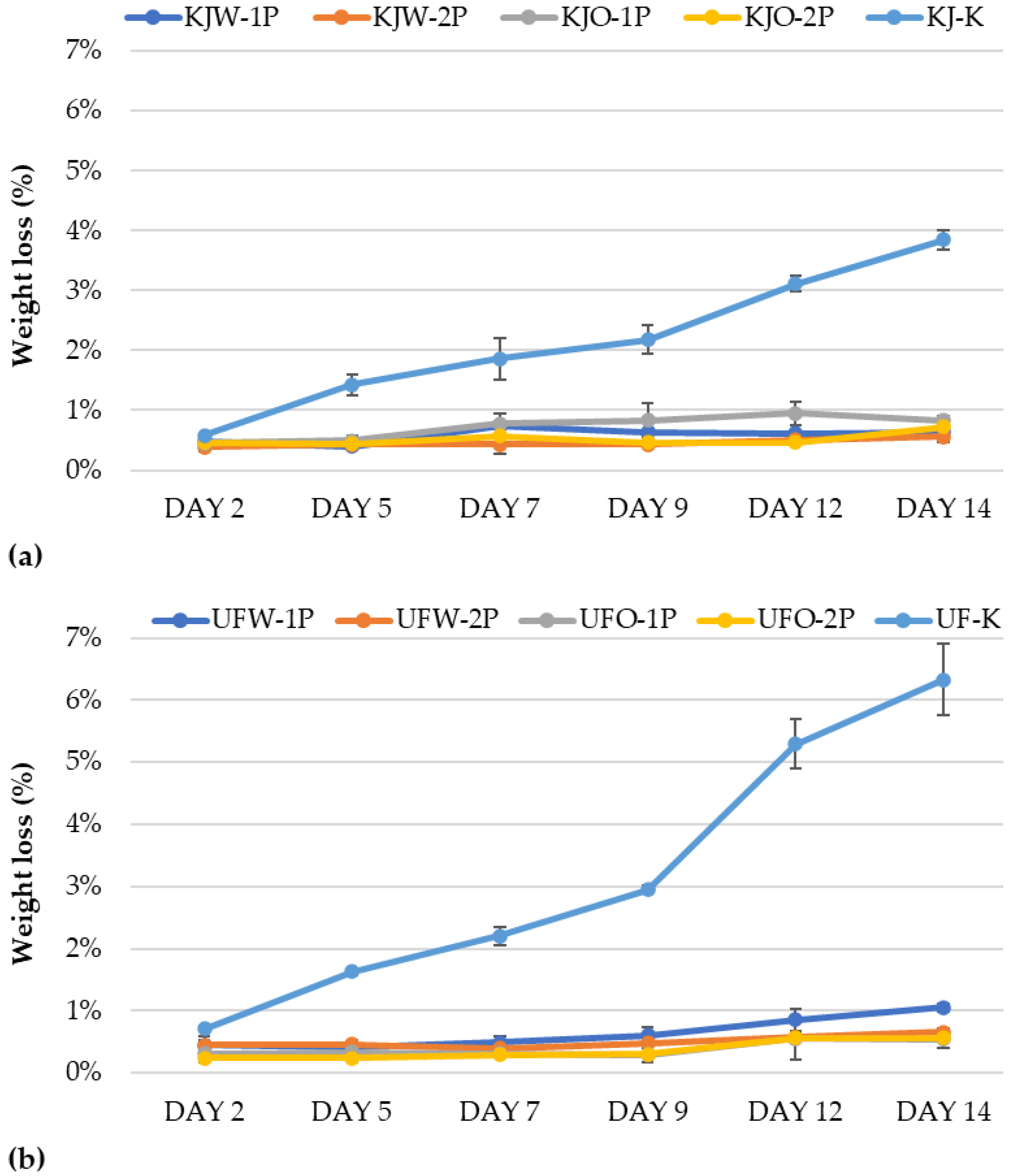
Determination of Weight Loss
Determination of Decay Rate
Determination of Color
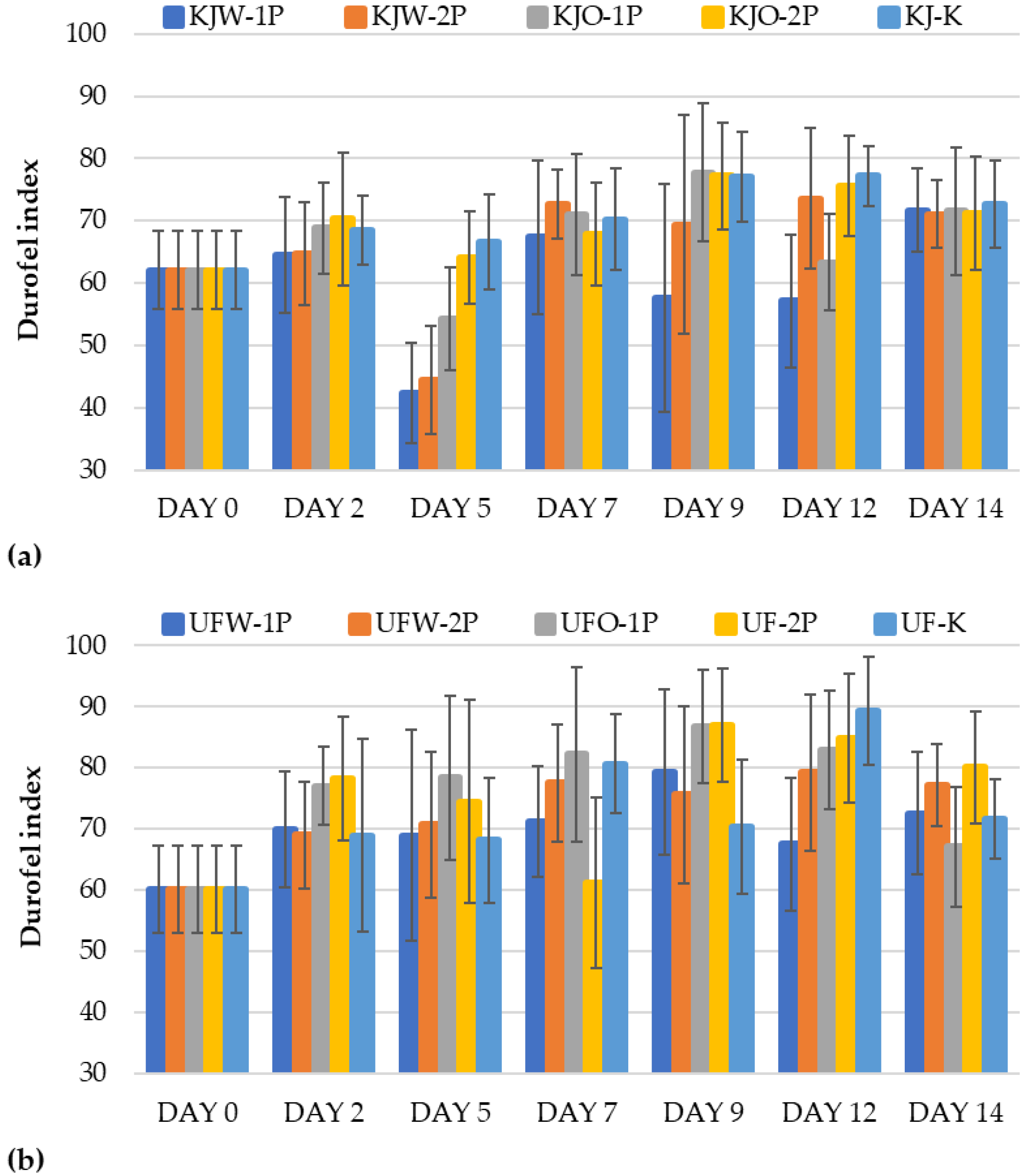
Determination of Firmness
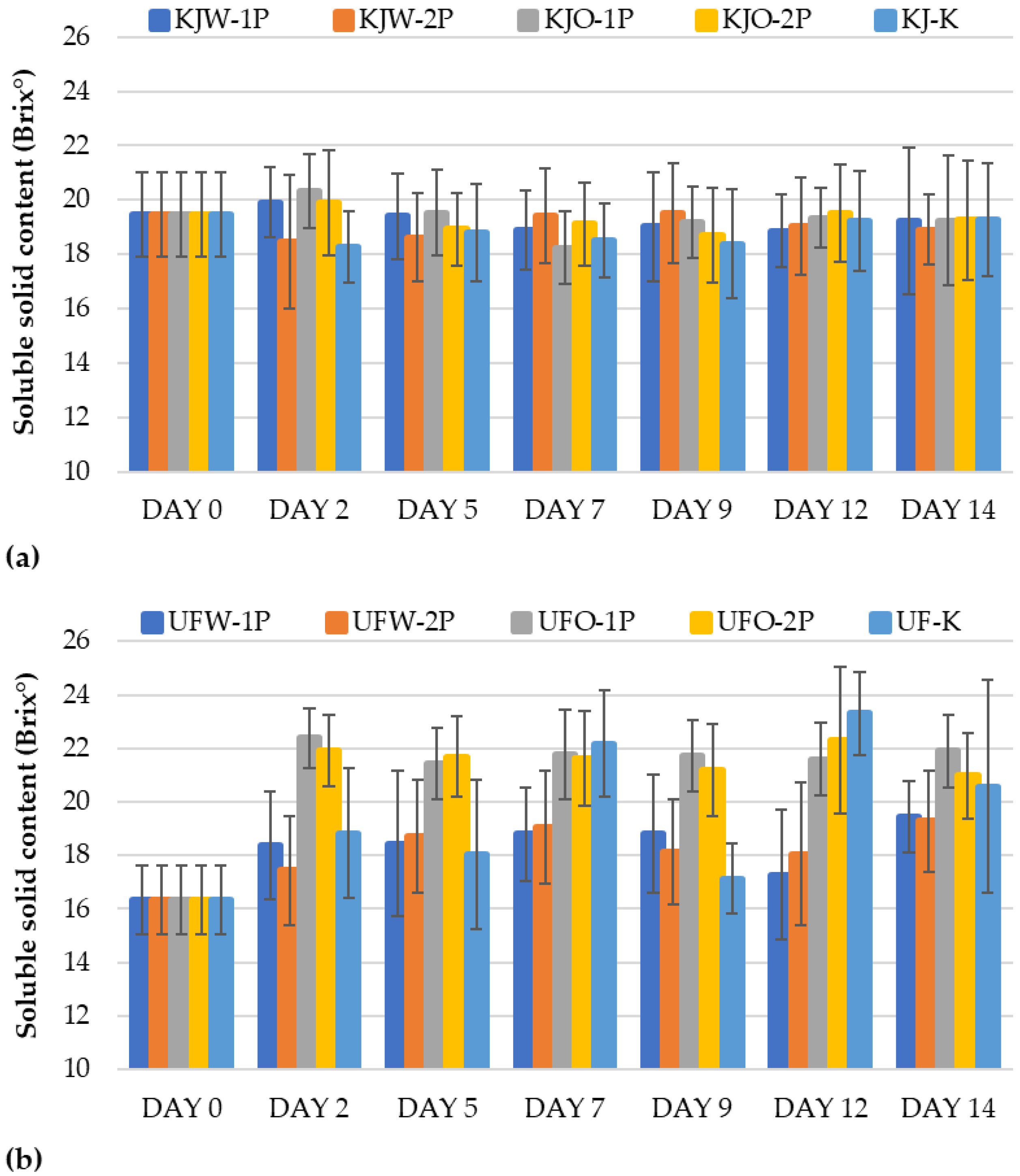
Determination of Total Soluble Solid Content
Determination of Total Plate Count
- A metal ring with an inner diameter of 1 cm was sprayed with a 70 v/v% alcohol solution using tweezers and then sterilized over a flame.
- The ring was placed on the surface of the sour cherry so that the sampling area was a constant, and a sterile piece of gauze (Mulltupfer EN 14079 Typ 20, NOBA Verbandmittel Danz GmbH, Wetter (Ruhr), Germany) was pressed onto the area inside the ring using sterilized forceps.
- The samples (pieces of gauze) were soaked in 9 mL of peptone water for 5 min, shaking the solution at intervals to transfer bacteria and fungi into the solution as efficiently as possible.
- After preparing a decimating dilution series from the resulting sample solution, plate casting was performed with Nutrient Agar (Biokar Diagnostics, Allonne, France).
- After the solidification of the agar, the Petri dishes were incubated at 30 °C for 24 h in a thermostat and the total aerobic mesophilic colony count was determined from the dishes with a colony count between 30 and 300 using a colony counter (BZG 30, WTW, Weilheim in Oberbayern, Germany).
2.1.3. Univariate Data Analysis
2.2. Experiment II
2.2.1. Fruits and Their Pretreatment
- L1: fruits considered as less ripe;
- L2: fruits considered fairly ripe;
- L3: fruits considered as ripe.
2.2.2. Acquisition of the Spectra
2.2.3. Data Analysis
3. Results and Discussion
3.1. Results of Experiment I
3.1.1. Results of Headspace Gas Concertation
3.1.2. Results of Weight Loss
3.1.3. Results of the Decay Rate
3.1.4. Results of Color Measurements
3.1.5. Results of Firmness
3.1.6. Results of Soluble Solid Content
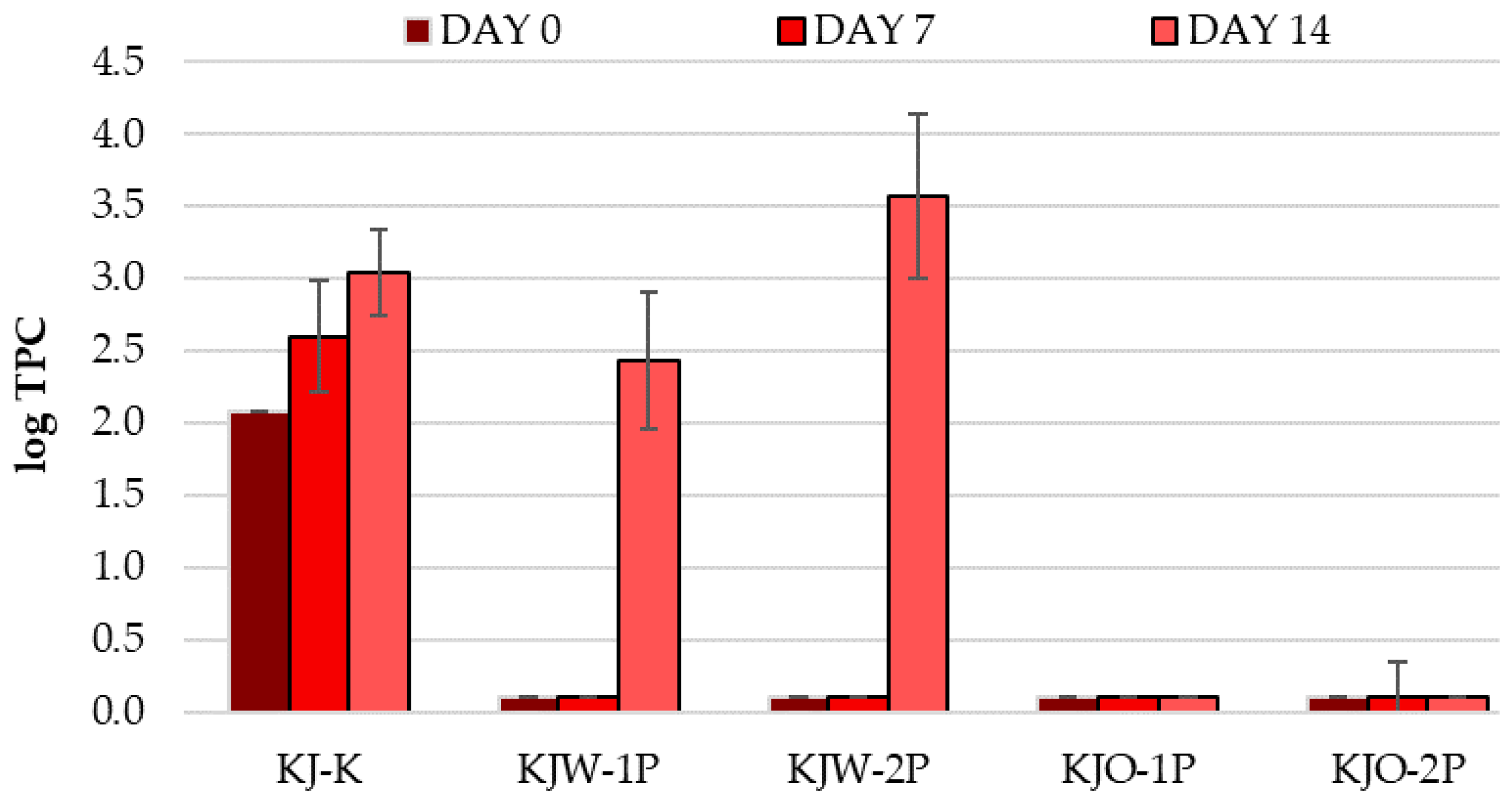
3.1.7. Results of Total Plate Count
3.2. Results of Experiment II
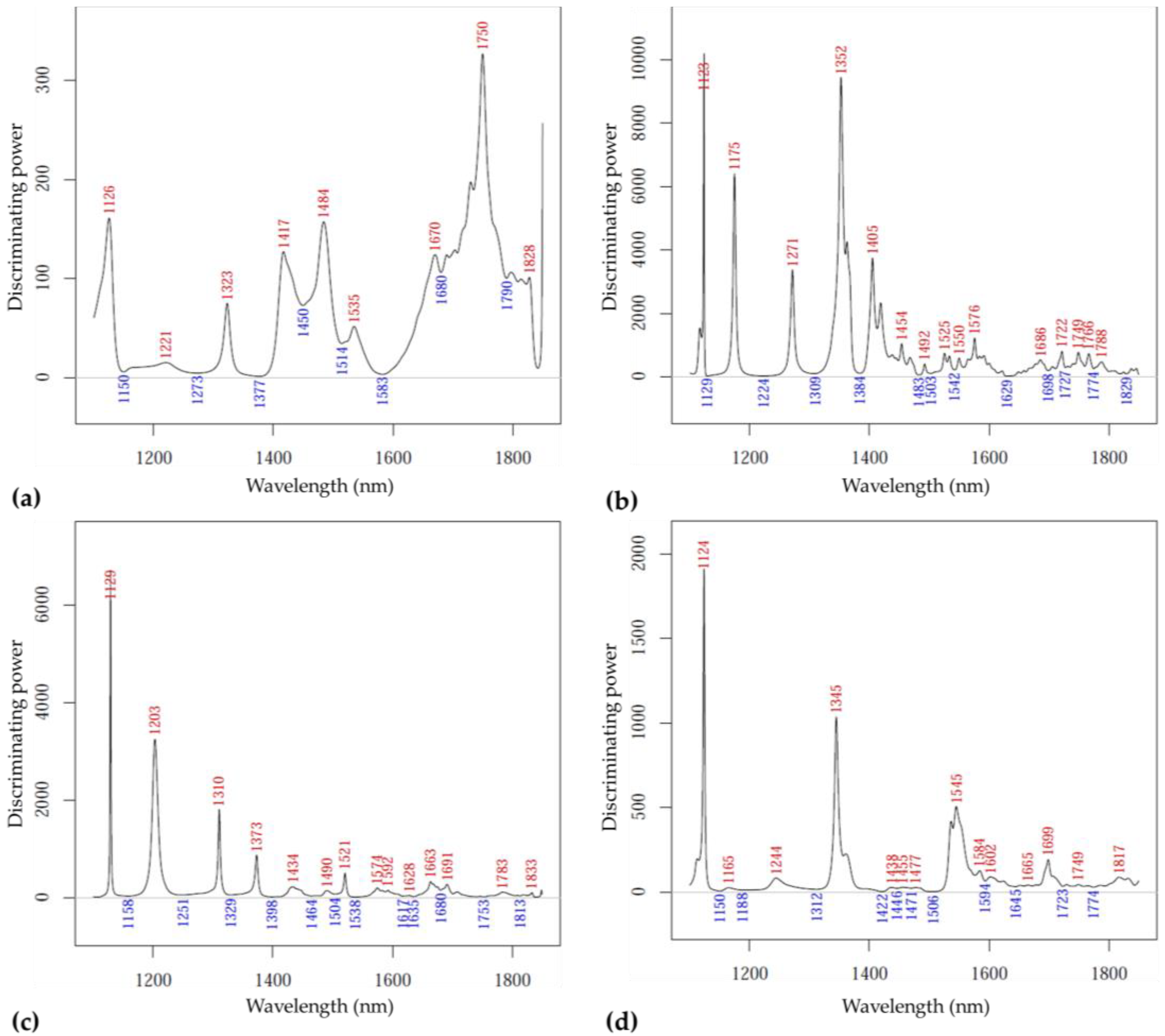
3.2.1. Inspection of the Difference Spectra
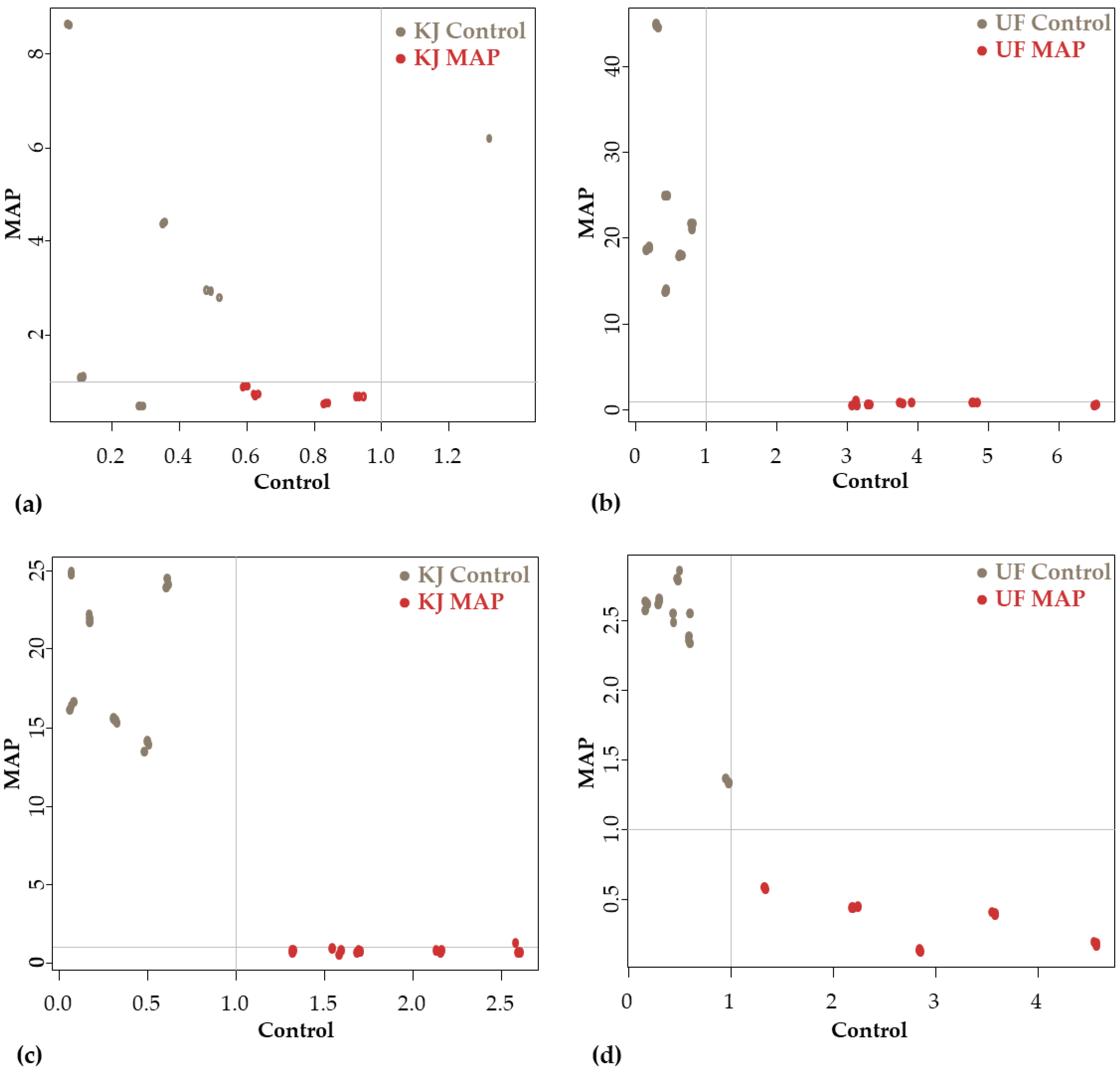
3.2.2. Analysis of Differences between Sample Groups with SIMCA
Discrimination according to Storage Time
Discrimination according to Ripeness on the First and Last Days of Storage
Discrimination according to the Packaging on the First and Last Day of Storage
Discrimination according to Storage Condition on the First and Last Days of Storage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blando, F.; Oomah, B.D. Sweet and sour cherries: Origin, distribution, nutritional composition and health benefits. Trends Food Sci. Technol. 2019, 86, 517–529. [Google Scholar] [CrossRef]
- Ricardo-Rodrigues, S.; Laranjo, M.; Agulheiro-Santos, A.C. Methods for quality evaluation of sweet cherry. J. Sci. Food Agric. 2022, 103, 463–478. [Google Scholar] [CrossRef] [PubMed]
- Homoki, J.R.; Nemes, A.; Fazekas, E.; Gyémánt, G.; Balogh, P.; Gál, F.; Al-Asri, J.; Mortier, J.; Wolber, G.; Babinszky, L. Anthocyanin composition, antioxidant efficiency, and α-amylase inhibitor activity of different Hungarian sour cherry varieties (Prunus cerasus L.). Food Chem. 2016, 194, 222–229. [Google Scholar] [CrossRef]
- Alba C, M.-A.; Daya, M.; Franck, C. Tart Cherries, and health: Current knowledge and need for a better understanding of the fate of phytochemicals in the human gastrointestinal tract. Crit. Rev. Food Sci. Nutr. 2019, 59, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Aryanpooya, Z.; Davarynejad, G.H. The effect of modified atmosphere packaging on sour cherry fruits quality. In Proceedings of the VI International Postharvest Symposium 877, Antalya, Turkey, 8–12 April 2009; pp. 449–453. [Google Scholar]
- Nguyen, L.P.L.; Hitka, G.; Zsom, T.; Kókai, Z. Application of 1-MCP on apricots at different temperatures and days after harvest. Acta Aliment. 2016, 45, 542–550. [Google Scholar] [CrossRef] [Green Version]
- Wills, R.; Golding, J. Postharvest: An Introduction to the Physiology and Handling of Fruit and Vegetables; UNSW Press: Sydney, Australia, 2016; ISBN 1742247857. [Google Scholar]
- Andor, D.; Bubán, T.; Gonda, I.; Tóth, M.G.; Göndör, J.; Hrotkó, K.; Jenser, G.; Ligetvári, F.; Nyéki, J.; Papp, J.; et al. Gyümölcstermesztési Alapismeretek. Available online: https://docplayer.hu/5314735-Gyumolcstermesztesi-alapismeretek.html (accessed on 8 November 2022).
- Aslantas, R.; Angin, I.; Gokalp Boydas, M.; Ozkan, G.; Kara, M. Fruit characteristics and detachment parameters of sour cherry (Prunus cerasus L. cv.‘Kütahya’) as affected by various maturity stages. Erwerbs-Obstbau 2016, 58, 127–134. [Google Scholar] [CrossRef]
- Chełpiński, P.; Ochmian, I.; Forczmański, P. Sweet cherry skin colour measurement as an non-destructive indicator of fruit maturity. Acta Univ. Cibiniensis, Ser. E Food Technol 2019, 23, 157–166. [Google Scholar] [CrossRef] [Green Version]
- Pedisić, S.; Levaj, B.; Dragović-Uzelac, V.; Kos, K. Physicochemical composition, phenolic content and antioxidant activity of sour cherry cv. Marasca during ripening. Agric. Conspec. Sci. 2007, 72, 295–300. [Google Scholar]
- Le Nguyen, L.P.; Zsom, T.; Sao Dam, M.; Baranyai, L.; Hitka, G. Evaluation of the 1-MCP microbubbles treatment for shelf-life extension for melons. Postharvest Biol. Technol. 2019, 150, 89–94. [Google Scholar] [CrossRef]
- Burns, S.E.; Yiacoumi, S.; Tsouris, C. Microbubble generation for environmental and industrial separations. Sep. Purif. Technol. 1997, 11, 221–232. [Google Scholar] [CrossRef]
- Ikeura, H.; Kobayashi, F.; Tamaki, M. Removal of residual pesticides in vegetables using ozone microbubbles. J. Hazard. Mater. 2011, 186, 956–959. [Google Scholar] [CrossRef] [PubMed]
- Kader, A.A.; Zagory, D.; Kerbel, E.L.; Wang, C.Y. Modified atmosphere packaging of fruits and vegetables. Crit. Rev. Food Sci. Nutr. 1989, 28, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Davarynejad, G.H.; Aryanpooya, Z.; Persely, S.Z. Effect of modified atmosphere packaging on fresh sour cherry fruit quality. In Proceedings of the VI International Cherry Symposium 1020, Reñaca-Viña del Mar, Chile, 15–19 November 2009; pp. 143–147. [Google Scholar]
- Ahmadi, M.; Davarinezhad, G.H.H.; Azizi, M.; Sedaghat, N.; Tehranifar, A. Effect of Modified Atmosphere Packaging on Quality Properties and Storability Extending in Two Sour Cherry Cultivars. In Proceedings of the 6th International Postharvest Symposium, Antalya, Turkey, 8–12 April 2009. [Google Scholar]
- Daş, E.; Gürakan, G.C.; Bayındırlı, A. Effect of controlled atmosphere storage, modified atmosphere packaging and gaseous ozone treatment on the survival of Salmonella Enteritidis on cherry tomatoes. Food Microbiol. 2006, 23, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Peng, J.; Xie, C.; Bao, Y.; He, Y. Fruit quality evaluation using spectroscopy technology: A review. Sensors 2015, 15, 11889–11927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitalis, F.; Bósquez, J.-P.A.; Gillay, Z.; Fodor, M.; Kovacs, Z. Development of fingerprint-based sorting models to determine the ripeness of differ-ent stone fruits. In Proceedings of the PTEP XXXIV, Scientific and Professional Conference, Sokobanja, Serbia, 3–8 April 2022. [Google Scholar]
- Li, X.; Wei, Y.; Xu, J.; Feng, X.; Wu, F.; Zhou, R.; Jin, J.; Xu, K.; Yu, X.; He, Y. SSC and pH for sweet assessment and maturity classification of harvested cherry fruit based on NIR hyperspectral imaging technology. Postharvest Biol. Technol. 2018, 143, 112–118. [Google Scholar] [CrossRef]
- Escribano, S.; Biasi, W.V.; Lerud, R.; Slaughter, D.C.; Mitcham, E.J. Non-destructive prediction of soluble solids and dry matter content using NIR spectroscopy and its relationship with sensory quality in sweet cherries. Postharvest Biol. Technol. 2017, 128, 112–120. [Google Scholar] [CrossRef]
- Fodor, M. Development of FT-NIR Technique to Determine the Ripeness of Sweet Cherries and Sour Cherries. Processes 2022, 10, 2423. [Google Scholar] [CrossRef]
- Khorshidi, S.; Davarynejad, G.; Tehranifar, A.; Fallahi, E. Effect of modified atmosphere packaging on chemical composition, antioxidant activity, anthocyanin, and total phenolic content of cherry fruits. Hortic. Environ. Biotechnol. 2011, 52, 471–481. [Google Scholar]
- Xing, S.; Zhang, X.; Gong, H. The effect of CO2 concentration on sweet cherry preservation in modified atmosphere packaging. Czech J. Food Sci. 2020, 38, 103–108. [Google Scholar] [CrossRef]
- Lara, I.; Camats, J.A.; Comabella, E.; Ortiz, A. Eating quality and health-promoting properties of two sweet cherry (Prunus avium L.) cultivars stored in passive modified atmosphere. Food Sci. Technol. Int. 2015, 21, 133–144. [Google Scholar] [CrossRef] [Green Version]
- Pinto de Andrade, L.; Veloso, A.; Espírito Santo, C.; Dinis Gaspar, P.; Silva, P.D.; Resende, M.; Beato, H.; Baptista, C.; Pintado, C.M.; Paulo, L. Effect of Controlled Atmospheres and Environmental Conditions on the Physicochemical and Sensory Characteristics of Sweet Cherry Cultivar Satin. Agronomy 2022, 12, 188. [Google Scholar] [CrossRef]
- Guo, H.; Yan, F.; Li, P.; Li, M. Determination of storage period of harvested plums by near-infrared spectroscopy and quality attributes. J. Food Process. Preserv. 2022, 46, e16504. [Google Scholar] [CrossRef]
- Vitalis, F.; Tjandra Nugraha, D.; Aouadi, B.; Aguinaga Bósquez, J.P.; Bodor, Z.; Zaukuu, J.-L.Z.; Kocsis, T.; Zsom-Muha, V.; Gillay, Z.; Kovacs, Z. Detection of monilia contamination in plum and plum juice with NIR spectroscopy and electronic tongue. Chemosensors 2021, 9, 355. [Google Scholar] [CrossRef]
- Vitalis, F.; Bósquez, J.P.A.; Aouadi, B.; Bodor, Z.; Zaukuu, J.-L.Z.; Kovacs, Z. Monitoring of brown rot caused by Monilia fructigena on plums with the aquaphotomics approach. In Proceedings of the 4th Aquaphotomics International Conference, Kobe, Japan, 20–22 March 2021; Tsenkova, R., Yasui, M., Kovacs, Z., Muncan, J., Eds.; 2021; pp. 122–123. [Google Scholar]
- Vitális, F.; Nici, V.; Aguinaga Bósquez, J.-P.; Gillay, Z.; Petróczy, M.; Kovács, Z. Investigation of Monilinia fructigena fungal infection in sour cherries with hyperspectral imaging. In Proceedings of the IV International Conference on Food Science and Technology, Budapest, Hungary, 9–11 June 2022; p. 65. [Google Scholar]
- Le Nguyen, L.P.; Visy, A.; Baranyai, L.; Friedrich, L.; Mahajan, P.V. Application of hue spectra fingerprinting during cold storage and shelf-life of packaged sweet cherry. J. Food Meas. Charact. 2020, 14, 2689–2702. [Google Scholar] [CrossRef]
- Kovacs, Z.; Pollner, B. Aquaphotomics-Software R-Package “aquap2”. In Proceedings of the Understanding Water in Biology 2nd International Symposium, Kobe, Japan, 26–29 November 2016; pp. 26–29. [Google Scholar]
- Savitzky, A.; Golay, M.J.E. Smoothing and Differentiation of Data by Simplified Least Squares Procedures. Anal. Chem. 1951, 36, 1627–1639. [Google Scholar] [CrossRef]
- Wold, S.; Sjöström, M. SIMCA: A method for analyzing chemical data in terms of similarity and analogy. In Chemometrics: Theory and Application; Kowalski, B.R., Ed.; American Chemical Society: Washington DC, USA, 1977; pp. 243–282. [Google Scholar]
- Zeng, J.; Guo, Y.; Han, Y.; Li, Z.; Yang, Z.; Chai, Q.; Wang, W.; Zhang, Y.; Fu, C. A Review of the Discriminant Analysis Methods for Food Quality Based on Near-Infrared Spectroscopy and Pattern Recognition. Molecules 2021, 26, 749. [Google Scholar] [CrossRef]
- Wang, X. 7—Near-infrared spectroscopy for food quality evaluation. In Woodhead Publishing Series in Food Science, Technology and Nutrition; Zhong, J., Wang, X., Eds.; Woodhead Publishing: Sawsto, UK, 2019; pp. 105–118. ISBN 978-0-12-814217-2. [Google Scholar]
- Malegori, C.; Muncan, J.; Mustorgi, E.; Tsenkova, R.; Oliveri, P. Analysing the water spectral pattern by near-infrared spectroscopy and chemometrics as a dynamic multidimensional biomarker in preservation: Rice germ storage monitoring. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 265, 120396. [Google Scholar] [CrossRef]







| Variety | Treatment | Perforation Number | Marking |
|---|---|---|---|
| ‘Kántorjánosi’ (KJ) | Control (K) | Without perf. (WOP) | KJ–K |
| ‘Kántorjánosi’ (KJ) | Tap Water (W) | One piece of perf. (1P) | KJW-1P |
| ‘Kántorjánosi’ (KJ) | Tap Water (W) | Two pieces of perf. (2P) | KJW-2P |
| ‘Kántorjánosi’ (KJ) | Ozone Microbubble (O) | One piece of perf. (1P) | KJO-1P |
| ‘Kántorjánosi’ (KJ) | Ozone Microbubble (O) | Two pieces of perf. (2P) | KJO-2P |
| ‘Újfehértói fürtös’ (UF) | Control (K) | Without perf. (WOP) | UF–K |
| ‘Újfehértói fürtös’ (UF) | Tap Water (W) | One piece of perf. (1P) | UFW-1P |
| ‘Újfehértói fürtös’ (UF) | Tap Water (W) | Two pieces of perf. (2P) | UFW-2P |
| ‘Újfehértói fürtös’ (UF) | Ozone Microbubble (O) | One piece of perf. (1P) | UFO-1P |
| ‘Újfehértói fürtös’ (UF) | Ozone Microbubble (O) | Two pieces of perf. (2P) | UFO-2P |
| Variety | Packaging | Storage Temperature | Ripeness |
|---|---|---|---|
| ‘Kántorjánosi’ | Control | ~3 °C | L1 |
| L2 | |||
| L3 | |||
| ~5 °C | L1 | ||
| L2 | |||
| L3 | |||
| MAP | ~3 °C | L1 | |
| L2 | |||
| L3 | |||
| ~5 °C | L1 | ||
| L2 | |||
| L3 | |||
| ‘Újfehértói fürtös’ | Control | ~3 °C | L1 |
| L2 | |||
| L3 | |||
| ~5 °C | L1 | ||
| L2 | |||
| L3 | |||
| MAP | ~3 °C | L1 | |
| L2 | |||
| L3 | |||
| ~5 °C | L1 | ||
| L2 | |||
| L3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabo, G.; Vitalis, F.; Horvath-Mezofi, Z.; Gob, M.; Aguinaga Bosquez, J.P.; Gillay, Z.; Zsom, T.; Nguyen, L.L.P.; Hitka, G.; Kovacs, Z.; et al. Application of Near Infrared Spectroscopy to Monitor the Quality Change of Sour Cherry Stored under Modified Atmosphere Conditions. Sensors 2023, 23, 479. https://doi.org/10.3390/s23010479
Szabo G, Vitalis F, Horvath-Mezofi Z, Gob M, Aguinaga Bosquez JP, Gillay Z, Zsom T, Nguyen LLP, Hitka G, Kovacs Z, et al. Application of Near Infrared Spectroscopy to Monitor the Quality Change of Sour Cherry Stored under Modified Atmosphere Conditions. Sensors. 2023; 23(1):479. https://doi.org/10.3390/s23010479
Chicago/Turabian StyleSzabo, Gergo, Flora Vitalis, Zsuzsanna Horvath-Mezofi, Monika Gob, Juan Pablo Aguinaga Bosquez, Zoltan Gillay, Tamás Zsom, Lien Le Phuong Nguyen, Geza Hitka, Zoltan Kovacs, and et al. 2023. "Application of Near Infrared Spectroscopy to Monitor the Quality Change of Sour Cherry Stored under Modified Atmosphere Conditions" Sensors 23, no. 1: 479. https://doi.org/10.3390/s23010479






