The Role of Wearable Sensors to Monitor Physical Activity and Sleep Patterns in Older Adult Inpatients: A Structured Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Method
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
3. Results
3.1. Search Yield
3.2. Study Characteristics
3.3. Sensor Placement
3.4. Sensor Model
3.5. Monitoring Protocol
3.6. Physical Activity Outcomes
3.7. Sleep and Circadian Rhythm Outcomes
3.8. Acceptability and Tolerance
4. Discussion
4.1. Sensor Placements
4.2. Sensor Models
4.3. Sensor Outcomes
4.4. Acceptability
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Study | Study Setting (Admission Type) | Sample Size | Age: Mean [Standard Deviation] | Sensor Technology | Monitoring Duration | Sensor Characteristics/Signal Processing | Reported Measures | Recruitment and Retention | Sensor Removal/Missing Data (Participant Feedback) |
|---|---|---|---|---|---|---|---|---|---|
| Stroke | |||||||||
| Askim et al., 2013 [86] | Observational/Longitudinal: University Hospital/Norway (Emergency) | n = 28 | 78.7 [8.7] | ActivPAL2, with a tilt switch (1 axis) (Gorman Promed Pty Ltd., Victoria, Australia) | Continuous: Time 1—Hospital, 24 h; Time (2–4), post discharge, 3 days/nights. | Sampling frequency—10 Hz. | Volume: Time spent upright (standing/walking) ‘min’; Number of transitions; Sedentary Behaviour: Time spent lying and sitting ‘min’. | 64% retention (recruited to analysed) | Time 1: n = 5 died, n = 11 withdrew, n = 7 missing at random/short recording time: Wrong attachment and instrument failure *. |
| Gebruers et al., 2013 [84] | Observational/Prospective: Neurology Ward/Belgium (Emergency) | n = 148, Acute stroke = 129/Control = 19 | Acute stroke-74.0 [11.4]/Control—71.0 [14.0] | Octagonal basic motion loggers (Ambulatory Monitoring Inc.; Ardsley, NY, USA) | Continuous: 48 h (24 h analysed) | Data recorded in the proportional integrated mode (PIM), epoch (1 s), rebinned into 30 min epochs using a Java program (JBuilder version 3.0) | Volume: Counts; Ratios (activity of impaired arm/activity of nonimpaired arm) | NR | n = 5 accelerometer malfunction |
| Iacovelli et al., 2019 [83] | Observational/Prospective: Academic Hospital/Stroke Unit: (Emergency, control = Orthopedic disease) | n = 37, Stroke = 20/Control (Orthopedic) = 17 | Stroke—69.2 [10.1]/Control—70.4 [4.8] | EZ430-Chronos, Texas Instruments, Dallas, TX, USA | Continuous: 24 h | Acceleration at 33 Hz; Sampling rate (10-bit resolution over a 4 g full scale); epochs (1 min) | Volume: Epoch-related Motor Activity index 1 and index 2 (the standard deviation of the acceleration module and the module of the standard deviation of acceleration components, respectively); 24 h motor performance (mean values over 24 h); Asymmetry Rate Indices to show left or right motor activity prevalence. | NR | NR |
| Kerr et al., 2016 [82] | Observational/Prospective [2 sites]: Acute Hospital/Medical ward/Scotland: (Emergency) | n = 41 | 69 [11] | ActivPAL™ (1 axis) (PAL Technologies Ltd., Glasgow, UK) | Continuous: Time 1—hospital, 48 h; Time 2—home, 48 h. | Sampling frequency = 20 Hz | Volume: Time spent standing, stepping (minutes/day); Number of steps taken (per day). Sedentary behaviour: Time spent sitting/lying | 21% recruitment (eligible to recruited), 90% retention (recruited to analysed) | n = 2 skin irritation/feeling unwell. n = 1 technical fault |
| Kunkel et al., 2015 [87] | Observational/Prospective: University Hospital/UK: (Emergency) | n = 74 (at least one activity monitoring session)/Hospital only = 61 | At least one monitoring session—76, range = [44 to 95] NR for hospital only | ActivPAL™ physical activity logger (PAL Technologies Ltd., Glasgow, UK) | Discontinuous—Daytime hours | NR | Volume: Walking—steps/minute; Time spent standing or walking; Sedentary behaviour: Time spent sitting/lying | 76% retention (recruited to data collected) | n = 9 discharged, n = 7 death, n = 2 withdrawn, n = 1 moved, n = 1 differential diagnosis, n = 4 excluded/malpractice enquiry |
| Norvang et al., 2018 [81] | Observational/Prospective: University Hospital/Stroke Unit/Norway: (Emergency) | n = 58 | 75.1 [12.0] | ActivPALs from PAL Technologies Ltd., Glasgow, UK | Continuous: ≥3 days | Sampling frequency = 10 Hz; Battery capacity = 14 days | Volume: Time in upright positions (daily average)/duration of upright bouts/Sedentary behaviour: Time sitting and lying/duration of sitting bouts. (Threshold for transitions—1.5 s). Pattern: Daily variation of average time in lying, sitting, upright positions, and duration of sitting and upright bouts | [unclear] | n = 39 discharge less than 3 days, n = 6 technical error |
| Sheedy et al., 2020 [80] | Observational/Prospective: Regional tertiary hospital/Stroke unit/Australia: (Emergency) | n = 78, <3 days monitoring = 24/>3 days = 54 | Overall median = 80.5, IQR = (70–86), >3 days monitored median = 82.5, IQR = (74–86) | ActivPAL™ (PAL Technologies Ltd.©, Glasgow, UK). | Continuous: ≥3 days up to 14 days (3 days analysed) | ActivPal proprietary software. | Volume: Standing time (min), % of time spent standing/stepping, number of steps; Sedentary behaviour: Time (min) spent inactive (lying or sitting) | 63% recruitment (screened to recruited), 95% retention (78 wore sensors) | n = 2 not tolerated, n = 2 device failed |
| Strommen et al., 2014 [79] | Observational/Prospective: University Hospital/Stroke Unit/Denmark: (Emergency—acute ischemic stroke or transient ischemic attack (TIA)) | n = 100, Transient ischemic attack (TIA) = 43/Ischemic stroke = 57 | TIA—68.9 [10.7]/Stroke—70.2 [13.4] | Actical accelerometers (Philips Respironics) | Continuous: up to 7 days | Epochs of 15 s | Volume: Activity counts (median total per day); Sedentary behaviour: Inactivity-activity count = 0 in all 5 accelerometers during 5-min periods. Pattern: 24-h pattern (Total Raw Activity Counts—from 5 sensors per hour) | 81% recruitment (eligible to recruited), 100% retention | 2.85% of the total file time is missing data—erroneous recording/non wear time/recording error |
| Respiratory Condition | |||||||||
| Borges and Carvalho, 2012 [78] | Observational/Prospective: General Hospital/Medical Unit/Brazil: (Emergency—exacerbation of COPD) | n = 20 | 68.6 [10.7] | DynaPort Moviemonitor (McRoberts, The Netherlands) | Discontinuous: 12 h day, 0800 to 2000; Time 1—(2 days) 3rd and 4th day following admission; Time 2–1 month post discharge | Manufacturer software | Volume: Number of steps and total active time (% spent standing, walking, others); Sedentary behaviour: Total inactive time, time spent, sitting and lying | 42% recruitment (eligible to recruited), 63% retention (recruited to analysed) | n = 4 early discharge, n = 3 transferred to ICU, died n = 2, n = 2 refused to appear in hospital 1 month after discharge, n = 1 another problem |
| Dall et al., 2019 [40] | Randomised: University Hospital/Pulmonary Ward/Denmark: (Asthma, Cancer, COPD, Dyspnoea, Pleural effusion, Pneumonia, Pneumothorax, Other) | n = 93, Feedback = 45/Non-feedback = 48 | Feedback 73.8 [12.8]/Non-feedback 71.9 [13.6] | Tri axial Accelerometer [no details] | Continuous: ≥24 h | Sampling frequency—12.5 Hz; Epochs of 10 s. (proprietary software) | Volume: Average daily time out of bed minutes/day (standing and walking); Sedentary behaviour: Average daily time spent in bed (lying down), sitting and inactive standing. (Visual feedback colour thresholds based on time in body positions) | 30% recruitment (eligible to recruited), 66% retention (recruited to analysed) | n = 3 withdrew, n = 1 allergic reaction to the BandAid, n = 25 (feedback group), n = 19 i(no feedback group) recordings of <24 h |
| Donaire-Gonzalez et al., 2011 [51] | Observational/Prospective/Validation study [8 sites]: Academic Hospital/Spain: (Rehab—COPD) | n = 172 | 70 [8] | SenseWear Pro 2 Armband (2 axis) (BodyMedia, Inc., Pittsburgh, PA, USA). | Discontinuous: 8 a.m.–10 p.m., ≥3 days to 8 days | NR | Volume: Number of steps per day. Intensity: Time of—any activity (>1.4 METs); Mild activity (>2.5 MET); Moderate activity (>3.6 MET); Energy expenditure during activity >1.4 MET, kcal/day Sedentary behaviour: <30 min/day of moderate activity | NR | NR |
| Orme et al., 2019 [77] | Observational/Prospective: Teaching Hospital/Cardiorespiratory unit/UK: (Emergency—exacerbation of chronic respiratory disease) | n = 259 | 70.0 [9.7] | SenseWear Pro 3 Armband (BodyMedia, Inc., Pittsburgh, PA, USA). | Continuous: —up to 14 days | Epochs of 60 s | Volume: Daily step count. Pattern: average step count—overnight, morning, afternoon and evening | NR | n = 7 incomplete data, device malfunction (number not provided) |
| Pitta et al., 2006 [88] | Observational/Prospective: University hospital/Respiratory ward/Belgium: (Emergency—deterioration in respiratory status) | n = 17 | Median = 69, IQR = [60–78] | DynaPort Moviemonitor (McRoberts, The Hague, The Netherlands) | Discontinuous: 12 h days; Time 1—day 2 and day 8 of admission; Time 2—post discharge. | Individual calibration: patient’s body characteristics (height, size of the abdomen). | Volume: Time spent standing, during the day/minutes; Sedentary behaviour: Time spent standing, sitting and lying during the day/minutes Intensity: Movement intensity m/s2 | 71% retention (recruited to analysed) | n = 1 early discharge, n = 3 required intensive care, n = 2 refused to continue |
| Tsai et al., 2016 [76] | Observational/Prospective: Tertiary hospital/Respiratory ward/Australia: (Emergency—exacerbation of chronic obstructive pulmonary disease) | n = 50 | 71 [10] | SenseWear1 Armband (SWA; BodyMedia1, Pittsburgh, PA, USA | Continuous: Time 1—Hospital (3 days); Time (2, 3) home post discharge, 7 days; | NR | Volume: Total steps per day. Intensity: Total energy expenditure (calories/day), average (METs) per day, Active energy expenditure (>3.0 METs; calories/day); Physical activity duration (minutes/day) for light (1.5–3.0 METs), moderate (3.0–6.0 METs), vigorous (6.0–9.0 METs) and very vigorous (9.0 METs); Sedentary behaviour: Sedentary (0–1.5 METs) | 35% recruitment (screened to recruited), 95% retention (recruited to completion at time point 1) | n = 1 skin irritation, n = 3 dropouts, n = 2 removed sensor |
| Cardiac Medical/Surgical | |||||||||
| Cook et al., 2013 [39] | Observational/Prospective: Hospital/ICU discharge ward/USA: (Elective—cardiac surgery) | n = 149 | 67.8 [9], Range = [52–90] | Fitbit (Fitbit, Inc, San Francisco, CA) | NR | Configured to shortest stride length, (Fitbit website—proprietary software) | Volume: Steps per day (Median, IQR). Pattern: Step per day variation over each hospital day | NR | n = 2 deaths, n = 1 lost sensor |
| Floegel et al., 2019 [75] | Observational/Prospective/Pilot [2 sites]: Community hospital/Medical Unit/USA: (Emergency—heart failure) | n = 27 | 78.0 [9.8] | ActivPAL 3 (PAL Technologies Ltd.©, Glasgow, UK)/Tractivity (Kineteks Corp., Vancouver, BC, Canada) | Continuous: Hospital stay +30 days post discharge | NR | Volume: Time standing, Time ambulating (mean + SD); Hospital steps per 24 h; Sedentary behaviour: Time sitting, time lying % per 24 h | 45% recruitment (approached to recruited), 93% retention (recruited to completed) | n = 2 dislodged and removed by patient. Participant feedback: Semi-structured interview—participant themes during their hospital stay were ease of wear and compatibility with hospital technology |
| Izawa et al., 2015 [103] | Observational/Prospective: University hospital/Medical-Surgical Unit/Japan: (Emergency and Elective—Elderly Cardiac inpatients—myocardial infarction, CABG, valve replacement/heart failure) | n = 268, Female = 75/Male = 193 | Male—73.4 [6.2]/Female—73.1 [5.7] | Kenz Lifecorder (1 axis) (Suzuken Co, Ltd., Nagoya, Japan) | Continuous: 4 days (middle 2 days analysed) | Steps and PAEE based on pre-entered age, sex, height, and weight data. Proprietary software. | Volume: Total number of steps taken (average). Intensity: Average kcal expended over 2 days [Daily (PAEE) computed by the accelerometer every 4 s, using body weight (W) and a proprietary manufacturer’s factor Ka (exercise index) | 47% recruitment (screened to inclusion) Unclear retention—[27 excluded due to incomplete data] | n = 27 data incomplete * |
| Mungovan et al., 2017 [100] | Observational/Prospective: Private Hospital/Surgical/Australia: (Elective—cardiac surgery) | n = 83, CABG = 36, Valve = 35, CABG and valve = 12 | Overall -66 [12], CABG—67 [9]/Valve—63 [14]/CABG and valve—70 [10] | SenseWear Pro 3 Armband (2 axis) (BodyMedia, Inc., Pittsburgh, PA, USA). | Continuous: 5 days | SenseWear Professional Software (version 6.1). Proprietary software | Volume: Daily step count. Intensity: Physical activity intensity (METs); Duration of exercise >3 METS (min); Duration of exercise less than 3 METS (min) | Unclear recruitment/retention [106 screened, data available for 83] | n = 1 intolerance to the sensor. 90% met minimum compliance |
| § Redeker and Wykpisz, 1999 [118] | Observational/Prospective: University-affiliated Coronary Care Centre/Acute care/USA: (post coronary artery bypass surgery) | n = 22, middle age = 8, Older adults = 14 | Middle age—57.12 [6.62]/Older adults—72.36 [4.14] | The Mini Motion Logger (Ambulatory Monitoring Inc., Ardsley, NY, USA) | Continuous: during admission | Epochs of 60 s. Programmed for zero crossing mode. Proprietary software | Volume: Total activity counts. Pattern: Activity counts during 12-h intervals (day 0700–1900 h, night 1900–0700 h Circadian Rhythm: Acrophase (crest time of the fitted rhythmic function, or time of peak activity); Amplitude (half difference between peak and trough of the rhythm, or half maximum height of the oscillation); Mesor (rhythm adjusted mean); Percent rhythm (% variance in activity) | NR | NR |
| Takahashi et al., 2015 [117] | Observational/Prospective: Cardiovascular centre/Japan: (Cardiac surgery) | n = 133 | Overall—66.4, range [38–84], Cardiac re-hospitalization—71.6 [5.6]/No Cardiac re-hospitalization—65.7 [9.5] | Active Style Pro HJA-350IT (Omron Healthcare, Kyoto, Japan) | Discontinuous—>8 h/day, during admission; 3 days analysed | NR | Volume: Number of steps walked during last three days of admission (Mean/SD) | 83% retention (recruited to analysed) | n = 6 did not wear activity monitor as required |
| Thorup et al., 2017 [53] | Observational/Prospective/Validation study [2 sites]: University Hospital/Cardiothoracic Surgery and Cardiology Department/Denmark: (Elective and Emergency admission—cardiac disease) | n = 24 (hospital) | Hospitalised—67 [10.03] | The Zip (FITBIT, 405 Howard Street San Francisco, CA 94105, USA)/Shimmer3 (Gyroscope) (Shimmer Research, Dublin, Ireland). | Continuous: Time 1—inpatient, 24 h; Time 2—home 4 weeks later, 24 h | The Zip (proprietary software). Shimmer (Sample rate = 50 Hz) | Volume: Total steps per day; % relative error (between sensor models) for step count time periods of 24 h and time periods of 3 min | 85% recruitment (approached to recruited) 73% retention (recruited to completion timepoint 1) | n = 1 early discharge, n = 7 withdrew |
| Orthopedic Surgery/Fractures | |||||||||
| Davenport et al., 2015 [73] | Observational/Prospective: Metropolitan Hospital/Acute orthopaedic ward/Australia: (Emergency—Hip Fracture requiring surgical management) | n = 20 | 79.1 [9.3] | ActivPAL™ (PAL Technologies Ltd.©, Glasgow, UK). | Discontinuous: 9 a.m.—6 p.m.; Time 1—on admission, 1 day; Time 2—2 weeks later, 1 day. | NR | Volume: Time spent standing and walking (%), Average steps per day. Sedentary Behaviour: Time spent lying/sitting (%); | 100% retention [timepoint 1], (93% timepoint 2) | n = 1 device removed <24 h *, n = 1 device malfunctioned |
| Denkinger et al., 2014 [52] | Observational/Prospective/Validation study: Rehab facility/Geriatric rehab ward/Germany:(Hip fracture) | n = 70 | Median = 83, IQR = (79.0–87.3) | Physilog ® (BioAGM, CH)—with single axis gyroscope | Discontinuous: 9 am-6 p.m., 1 day | Sampling rate = 40-Hz | Volume: Time spent walking ‘defined as 3 steps or more’ (min); Time spent upright (min) | NR | n = 2 inter-current illness, n = 2 refusal, n = 1 early discharge |
| Hayashi et al., 2018 [99] | Observational/Prospective: University Hospital/Japan: (Elective—TKA and THA) | n = 72, TKA = 40/THA = 32 | Overall—69.0 [10.5]/TKA—72.4 [7.4]/THA—64.7 [12.4] | Lifecorder GS; Suzuken, Nagoya, Japan | Continuous: 8 days (postop day 3 to 10) | NR | Volume: Total number of daily steps | ||
| Keppler et al., 2020 [72] | Observational/Prospective: University Hospital/Surgical-Trauma Unit/Germany: (Emergency—Orthogeriatric patients with PFF and PHF) | n = 31, PFF = 21, PHF = 10 | PFF—80.86 [6.75]/PHF—75.20 [6.86] | Actibelt ®, Trium Analysis Online GmbH, Munich | Continuous: Up to 10 days | NR | Volume: Average number of daily steps. Intensity: Walking speed (m/s) | NR | n = 6 dropped out * |
| § Krenk et al., 2013 [98] | Observational/Prospective: Denmark: Elective—fast track THA and TKA) | n = 20 | Overall—70.5, range (61–89) | Actiwatch spectrum ambulatory activity device (Philips Respironics, Murrysville, PA, USA) | Continuous: Time 1, 3 days prior to surgery; Time 2, 7 days postoperatively. | (Proprietary—Respironics) | Volume: Maximum activity count per day; Mean activity count per minute; Total activity count (24 h—6 a.m. to 6 a.m.). Sleep: Mean day-time sleep (min); Mean night-time sleep (min); (Measurement for night-time taken from patients recorded lights-off and lights-on) | 83% recruitment (approached to recruited), 95% retention (recruited to analysed)1 excluded | Sensor never removed for more than 20 min |
| Kronborg et al., 2016 [71] | Observational/Prospective: University Hospital/Orthopedic ward/Denmark: (Emergency—Hip fracture surgery) | n = 37 | 80 [8.4] | ActivPAL3™ (PAL Technologies Ltd.©, Glasgow, UK) | Continuous: —Up to 10 days | NR | Volume: Time spent upright ‘min’ (standing and walking); Number of daily upright events (sitting to standing) per 24 h (Walking defined as an activity in the Z-axis with a cadence of more than 20 steps per minute) | NR | n = 8 transfer to different unit, isolation, death or discharge, n = 2 technical error |
| Marsault et al., 2020 [70] | Observational/Prospective: Academic Hospital/Denmark: (Emergency—PFF) | n = 64, Fall hip fracture = 52/fall no fracture = 12 | Overall—81.2 [7.8], Hip frac—81.29 [7.45]/Fall group no hip frac—80.83 [9.54] | Axivity™ AX3 tracker (Newcastle upon Tyne, UK) | Continuous: —Time 1, Day 1–3 after operation or admission to the department; Time 2, at discharge and home | Epochs of 60 s; Filter between 0.5 and 20 Hz (wear time analysis); Each minute categorized into “active” or “not active” threshold of SVM > 0.005 (SVM > 0.01 categorized as very high active minutes) | Volume: Signal Vector Magnitude (SVM) (Threshold of SVM > 0.005 for ‘active’ minutes). Intensity: 10-min periods categorized (Sedentary behaviour) 0–10% active minutes, low activity >10–25% active minutes, medium >25–60%, high >60%; Threshold of SVM >0.01 ‘very active’ minutes | 97% retention (recruited to analysed) | n = 2 removed |
| Peiris et al., 2013 [102] | Observational/Prospective: Rehabilitation hospital/Orthopaedic ward/Australia: (lower limb orthopaedic condition, hip or knee replacement, hip fracture) | n = 54 | 74 [11] | ActivPAL™ (1 axis) (PAL Technologies Ltd.©, Glasgow, UK). | Continuous: 5 days | NR | Volume: steps per day, time spent in upright activities per day (minutes); Time spent walking per day (minutes); Sedentary behaviour: Time spent inactive per day (hours). Intensity: Moderate intensity activity per day (>60 steps/minute), (METs) >3.0, Activity counts > 1075 counts | 50% recruitment (screened to recruited), 100% retention | n = 1 redness/minor itching around the dressing that secured the monitor (did not withdraw) |
| Schmal et al., 2018 [69] | Observational/Prospective: Denmark: (Emergency—post op—PFF) | n = 22 | 81 [8] | Misfit Shine (Burlingame, CA, USA)/Axivity AX3 (Newcastle upon Tyne, UK) | Continuous: Time 1—24 h, day 2 ± 1 (shortly after operation); Time 2—24 h, 8 ± 3 (shortly before discharge) | Epochs of 60 s; Filter between 0.5 and 20 Hz subjected to a wear time analyses; | Volume: Signal Vector Magnitude (SVM) (Threshold of SVM > 0.005 for ‘active’ minutes). Intensity: Frequency of active minutes grouped as category 1 (“no activity, 0–10%, category 2 (“low activity”) >10–25%, category 3 (“middle activity”) >25–60%, category 4 (“high activity”) >60%; Sedentary behaviour: category 1 (“no activity”) | NR | NR |
| Twiggs et al., 2018 [96] | Observational/Prospective (Pre-post op): Surgical department/Australia: (Elective—scheduled for TKR) | n = 91, post op ‘in hospital’ = 68 | Overall—67.5 [13.1] (NR for post op) | Fitbit Flex | Continuous: Time 1, (2 weeks before op); Time 2, (1 day after op) (analysed days 2–4); Time 3, (6 weeks after op) (7 days for each). | NR | Volume: Daily step counts | 72% retention (68 analysed, day 2–4 post-operative period) | Data loss maybe—patient non-compliance, protocol failure with regards to fully charged devices, technical failures of the devices * |
| van Dijk-Huisman et al., 2020 [41] | Observational/Prospective/Pilot study/Quasi experimental/Interventional: University medical centre/Orthopedic ward/Netherlands: (Elective—post orthopaedic surgery) | n = 97, control group = 64/intervention group = 33; (Analysed overall = 88, control = 61/intervention = 27) | Intervention group (analysed) median = 63.73, IQR = 16.62/Control group (analysed) median = 67.19, IQR = 11.35, | MOX activity monitor (MOX; Maastricht Instruments B.V., Maastricht, The Netherlands./(ADXL362; Analog Devices, Norwood, MA, USA) wireless device: Hospital Fit (HFITAPP0, Maastricht Instruments B.V., Maastricht, The Netherlands). | Continuous: post op to discharge | Sampling frequency = 25 Hz; Range = 8 g; Segmented in to one-second-long windows, fixed non-overlapping sliding window each classified as dynamic or static; Static windows -sensor orientation assessed; Static window—cut-off value of 0.8 g to classify standing or sedentary | Volume: Minutes spent standing and walking per day and per week. Pattern: Variation in minutes spent standing and walking per day. (Days with ≥20 h of wear time were considered valid measurement) | 90.7% retention | n = 9 missing data, n = 5 delayed sensor fixation, n = 3 accelerometer malfunctioning |
| Mixed Admissions (Delirium and Dementia) | |||||||||
| § Davoudi et al. 2019 [68] | Observational/Prospective: University Hospital/ICU/USA: (Emergency—post surgery delirium) | n = 17/Delirious = 4/Non-delirious = 8 | Overall Median = 69, IQR = (54.0–73.0)/Delirious Median = 72.5, IQR = (64.5 -74.5)/Non-delirious Median = 62.5, IQR = (37.7–73.0) | Actigraph GT3X (GT3X) devices (ActiGraph, LLC. Pensacola, FL, USA) | Continuous: Up to 7 days | Sampling frequency = 100 Hz; Analysed as 1-min activity counts | Volume: Activity counts (mean/SD); Root Mean Square of Sequential Differences: Root Mean Square of Sequential Differences/Standard Deviation. PATTERN: Activity counts (daytime 7 a.m.–7 p.m.—night-time 7 p.m.–7 a.m.) Mean/SD Sleep: Number of immobile minutes (day and night). Circadian Rhythm: M10—Activity intensity of 10-h window with highest sum of activity intensity; L5—Activity intensity 5-h window with lowest sum of activity; Relative amplitude—Difference between M10 and L5. | 55% retention (recruited to analysed) | n = 1 or 2 removed at patient’s request, during bathing, or during clinical routines Moved to different ward * |
| Evensen et al., 2019 [67] | Observational/Cross sectional: University Hospital/Geriatric Ward/Norway: (Emergency–patients with delirium) | n = 60, Hyperactive = 15/hypoactive = 20/Mixed = 17/No-subtype = 8 | Hyperactive—86.3 [6.3]/Hypoactive—85.5 [4.4]/Mixed—88.7 [4.7]/No-subtype—86.1 [5.8] | ActivPAL™ (PAL Technologies Ltd., Glasgow, UK)/Actigraph GT3X (GT3X) devices (ActiGraph, LLC. Pensacola, FL, USA) | Continuous: >24 h | Filtered; Epochs into 1 s non-overlapping (using ActiLife software (V.6.13.3)) Threshold of 0.5 (counts s-1) to separate static from dynamic behaviour. | Volume: Activity measured as upright time (minutes) (upright event minimum 10 s); Sit-to-stand transitions (numbers); Total wrist activity (counts); Sedentary behaviour: Wrist activity in a sedentary position (%) (Threshold of 0.5 (counts s-1) to separate static from dynamic behaviour of wrist) | 33% recruitment (eligible to recruited), 58% retention (recruited to analysed) | n = 23 not worn, n = 5 removed sensors, n = 3 worn less than 24 h, n = 5 technical failure, n = 7 sensors lost |
| Fleiner et al., 2019 [66] | Observational/Cross sectional/Prospective: Academic Hospital/Dementia wards/Germany: (Emergency—Geriatric Psychiatry—Dementia) | n = 87 | 81 [6.2] | uSense (hybrid motion sensor) | Continuous: 72 h | Software for signal processing and activity recognition: outcome of the FARSEEING EU project | Volume: Time sitting/standing/walking. Daily step counts (mean h/day %); Gait, h/day (%); Sedentary behaviour: Lying, Sedentary sitting/standing, h/day (%) | 74% retention (recruited to analysed) | n = 1 refused the sensor attachment, n = 5 removed the device, n = 4 incomplete or missing, n = 1 excluded (used four wheeled walker) |
| Mahlberg et al., 2007 [42] | Observational/Prospective/Pilot/Interventional: Geriatric Psychiatry Dep/Germany: (probable dementia of the Alzheimer type) | n = 20, Rivastigmine = 10/Placebo = 10 | Overall—80.4 [9.1], Rivastigmine—82.6 [7.2]/Placebo—78.2 [10.3] | Actiwatch, Cambridge Neurotechnology Co., Cambridge, UK | Continuous: 2 weeks (First 3 days and last 3 days analysed) | NR | Volume: Activity counts 103. Pattern: Activity counts (diurnal 6 a.m.–9 p.m., nocturnal 9 p.m.–6 a.m., evening 3 p.m.–9 p.m.) | 95% retention (recruited to completed) | n = 1 withdrew, n = 2 technical reasons |
| Maybrier et al., 2019 [94] | Observational/Prospective: Academic Hospital/USA: (Elective-surgical—post operative delirium) | n = 83, no delirium postoperative days (POD) 0–5 = 51/delirium during POD 0–1 = 24/delirium during POD 2–5 = 13 [overlap of subjects for outcome groups] | Intact POD 0–5 Median = 68, IQR = 10/Delirium POD 0–1 Median = 72 IQR = 16/Delirium POD 2–5 Median = 69, IQR = 14 | ASPW wActiSleep Plus, ASPB, and wGT3XBT (ActiGraph Corp., Pensacola, FL, USA). | Continuous: 24 h after surgery up to discharge | Sampling frequency = 30 Hz; Counts binned in 1-min intervals across three accelerometer axes; | Volume: Root mean-squared activity (RMSactivity); Median activity counts (MAC); Sedentary behaviour: Number of immobile minutes (NOIM), defined as—total number of minutes with an RMSactivity count of zero. Pattern: MACDay; MACNight; MACDay-Night activity between day and night | 55% retention (recruited to analysed), 83 patients data analysed—due to exclusion | n = 6 device error, n = 32 incomplete activity data during 16:00–6:00 |
| § Osse et al., 2009 [38] | Observational/Prospective: Medical centre/Cardiothoracic surgery dept/Holland: (Elective—Postcardiotomy delirium) | n = 79, ‘Non-cr-Del’(non-clinically relevant delirium) = 46, ‘Short-Del’ = 16, ’Sustained-Del’ = 17 | ‘Non-cr-Del—72.9 [4.9]/Short-Del—75.2 [4.1]/Sustained Del—75.2 [4.5] | Actiwatch (Cambridge Neurotechnology Ltd., Cambridge, UK) | Continuous: post-surgery for up to 6 days | Epochs of 60 s | Volume: Activity per minute (mean); Sedentary behaviour: Number of minutes immobile (based on the total number of minutes with a score of zero). Pattern: Sedentary behaviour: number of minutes Immobile (night-time 23.00–06.00 h and daytime 06.00 h–23.00 h) Circadian Rhythm: Restlessness Index (addition of the percentage of time spent moving and the percentage immobility phases of 1 min); Activity Amplitude (difference between the least active 5 h period and the most active 10 h period within each 24-h period) | 90% recruitment (eligible to recruited), 91% retention (recruited to analysed) | n = 7 technical problems |
| Stubbs et al., 2007 [43] | Observational/Prospective/Intervention (pre-post): Independent psychiatric hospital/Medical psychiatric ward/UK: (Older psychiatric inpatients) | n = 53 | 71.6, range [51–85] | Pedometer * | Discontinuous: 8.30 a.m.–5.30 p.m., 3 days | NR | Volume: Step count per day (mean) | 89% retention (recruited to completion) | n = 3 discharged, n = 2 refused to wear |
| § Valembois et al., 2015 [33] | Observational/Prospective/Cross sectional: Intermediate care unit/Geriatric ward/France: (Emergency—Dementia and apathy or aberrant motor behaviour) | n = 183 | 84.9 [6.8] | Vivago (Vivago®, Vivago Oy, Espoo, Finland) | Continuous: 10 days | Proprietary software (Vista, Vivago, Finland) | Pattern: Mean motor activity (grouped by pre-defined 3-h periods during the day 0:00 to 2:59, 3.00 to 05.59, 06.00 to 8.59, 9.00 to 11.59, 12.00 to 14.59; 15.00 to 17.59; 18.00 to 20.59 and 21.00 to 23.59) Sleep: Total sleep time (min); Number night awakenings (n); (Proprietary software) | 100% retention (obtained analysable actigraphy data for all the patients included) | impaired sensor contact with skin/long period of time out of the detection zone * |
| Patients In Intensive Care | |||||||||
| Estrup et al., 2019 [64] | Observational/Prospective: University Hospital/ICU/Denmark: (Emergency) | n = 44 | 72 [10] | Micro SleepWatch® (Ambulatory Monitoring, Ardsley, NY, USA) | Continuous: up to 7 days | Proprietary software—activity as a count of movements per time unit in zero crossing mode | Volume: Activity counts per day (mean); Maximum activity in 1 h interval on day 2. Pattern: Mean activity per hour during daytime (7 a.m.–4 p.m.); Mean difference between day and night | 67% recruitment (screened to recruited), 93% retention (recruited to analysed). | n = 2 transferred, n = 1 died, non-wear time (readings <50 counts) number not defined |
| Other Surgery | |||||||||
| Jonsson et al., 2019 [44] | Randomised control trial: University Hospital/Surgical/Sweden: (Elective—thoracic surgery—confirmed or suspected lung cancer) | n = 94, intervention = 50/Control = 44 | Intervention—69 [8]/Control—68 [8] | ActiGraph, model GT3X+, Manufacturing Technology Inc., Pensacola, FL, USA | Continuous: hospital ≥3 days | Sample frequency = 30 Hz; Epochs of 10 s | Volume: Average counts; Steps per hour | 81% recruitment (eligible to recruited—intervention: control, 54:53), 71% retention (recruited to analysed 50:44) | n = 10 early discharge, n = 3 accelerometer malfunction |
| Matsuo et al., 2015 [108] | Observational/Prospective: Rehab Unit/Japan: (Rehab—lower extremity bypass surgery) | n = 13, active group = 6/inactive group = 7 | 72.8 [5.9] | Active Style Pro. HJA-350IT, Omron Healthcare | Continuous: 2 days prior to surgery to discharge (except on the day of surgery) | BI-LINK-analysis software (Omron Healthcare) | Volume: Daily steps; Maximum walking distance. Intensity: METs-hours/day (calculated using BI-LINK-analysis software (Omron Healthcare) | NR | NR |
| Older Adults | |||||||||
| § Beveridge et al., 2015 [116] | Observational/Prospective: Medical Centre/Medical Ward/USA | n = 120 Subgroup activity (with sleep data) (Overall = 300) | Subgroup activity (with sleep data)—65.5 [11.1] | Actiwatch2, Respironics, Inc., Murrysville, PA, USA | Continuous: during admission | Proprietary software (Actiware 5 ‘respironics inc’—summed over 15 and 60 s intervals | Volume: Average and maximum Activity Counts (min); Total Activity Counts. (Calculated from reported wake up on morning to bed that night) Sleep: Total time spent asleep (night-time); Sleep efficiency. (Assumed sleep period based on reported bedtimes and morning wake times) | 42% recruitment (consented to larger study to recruited), 71% retention rate [recruited to analysed] | n = 104 removed sensor at night or sensor failure, n = 49 removed during the day, |
| Brown et al., 2009 [3] | Observational/Prospective: Veterans Affairs Medical Centre/Medical ward/USA | n = 45 | 74.0 [6.5] | AugmenTech, Inc; Pittsburgh, Pennsylvania] * Only 1 axis used | Continuous: Up to 7 days | Epochs of 20 s | Volume: Mean % of 1 hr intervals each day spent, standing/walking. Sedentary behaviour: Mean % of 1 hr intervals each day spent lying + sitting | 90% retention (recruited to analysed) | n = 2 declined health, n = 2 withdrew, lack of data within 48 h of admission or having less than 23 h of data * |
| Cohen et al., 2019 [45] | Quasi-experimental/Interventional/pre-post: Medical centre/Internal medical unit/Northern Israel | n = 377, Control = 188/Intervention group = 189 | 75.1 [7] | Actical accelerometers (Philips Respironics) | Continuous: Up to 3 days | NR | Volume: Mean number of steps per day. | 45% recruitment (approached to recruited), 94% retention (recruited to completion) | n = 6 died, n = 3 became delirious, n = 9 withdrew, n = 1 transferred, n = 1 deterioration, n = 8 early discharge, |
| Evensen et al., 2017 [101] | Observational/Prospective: Academic Hospital/Geriatric Ward/Norway (90% emergency) | n = 38 | 82.9 [6], Range = [67.6–92.5] | ActivPAL™ (PAL Technologies Ltd., Glasgow, UK) | Continuous: During admission, 24 h analysed | NR | Volume: Number of upright events (minimum event 9.9 s); Lengths of upright events (minutes); Maximum length of upright events (minutes); Upright event variability (IQR). Pattern: time in upright position night (00–06), morning (06–12), afternoon (12–18), evening (18–24) | 88% retention (recruited to analysed) | n = 5 missing data * |
| Fisher et al., 2011 [63] | Observational/Prospective: Teaching Hospital/Acute care for elderly/USA (Emergency) | n = 239 | 76 [6], Range [65–100] | StepWatch Activity Monitor (SAM) (2 axis) (Modus health, Washington, DC, USA) | Continuous: ≥24 h up to discharge | NR | Volume: Total number of steps per day; Mean daily steps; Total minutes of ambulatory steps (defined as stride counts recorded by the monitor times two); Total minutes of ambulatory activity (number of 1-min intervals recorded by the monitor with a stride count greater than 0) | 74% retention (recruited to analysed) | n = 28 removed *, n = 36 < 1 complete 24-h day (midnight to midnight), n = 18 medical reasons/tests, n = 84 incomplete data *, n = 2 wore SAM home data lost |
| Hartley et al., 2018 [114] | Observational/Prospective/Feasibility:University Hospital/Department of medicine for the Elderly wards/UK: | n = 24, Men = 12/Women = 12 | Overall Median = 80.5, Range = (70.0–95.0), Male Median = 79.5, Range = (70–95])/Female Median = 81.5, Range = (71–89) | Axivity™ AX3 tracker (Newcastle upon Tyne, UK) | Continuous: 48 h | Sampling frequency = 100 Hertz; Range of ± 4 g; Autocalibrated to local gravity; Low-pass filter at 20 Hertz; Epochs of 5 s (summarised from three signals). | Volume: Euclidean Norm Minus One (ENMO)/Time spent moving upright/sitting/standing/lying (%); (cutoff threshold ENMO value of >13 milligravity units (mg) for moving) Sedentary behaviour: Time spent sitting and lying (%) | 79% retention (recruited to analysed) | n = 1 sensor fell off, n = 4 early discharge, n = 1 removed for MRI scan. Participant feedback: Questionnaire–All participants report ‘no discomfort’, with sleep not affected. |
| Klenk et al., 2019 [107] | Observational/Prospective: Geriatric Hospital/Germany: (Rehab) | n = 647 | 82.0 [7.19] | ActivPAL3™ (PAL Technologies Ltd.©, Glasgow, UK) | Continuous: day 2 and day 15 of admission (24 h x2) | NR | Volume: Mean walking duration; Walking bout duration (interval between two periods of standing); Number of sit-to-stand transfers | 52% retention (recruited to analysed) | n = 555 missing data on day 2 or 15, n = 49 incomplete 24 h * |
| Kolk et al., 2021 [62] | Observational/Prospective/[6 sites]: General Hospital/Internal;cardiology;geriatric wards/Holland: (Emergency) | n = 188 | 79.1 [6.7] | Fitbit Flex activity tracker (Fitbit, Inc., San Francisco, CA, USA) | Continuous: hospital admission to 1 week post discharge. | NR | Volume: number of steps per day | Unclear | n = 12 medical, n = 11 died, n = 46 technical/logistic reason, n = 17 lost to follow-up, n = 72 unknown |
| Lim et al., 2018 [61] | Observational/Prospective/Cross sectional:Acute medical ward/England (Emergency) | n = 38, Men = 18/Women = 20 | Overall—87.8 [4.8], Men—88.3 [5.1]/Women—87.5 [4.5] | StepWatch Activity Monitor (SAM) (2 axis) (Modus health, Washington, US) and GENEActiv (Activinsights, Kimbolton, UK) | Continuous: ≥24 h, up to 7 days | Frequency = 100 Hz (GENEActiv) | Volume: Steps per day; Total minutes per day stepping; Minutes spent in different bout lengths Intensity: Minutes per day with acceleration ≥12 milli-g. Pattern: Mean step count and mean acceleration per hour of each day; Minutes in sustained ambulation ≥4 step (1–5 min, 6–10 min, 10+); Minutes spent in different bout lengths ≥ 12 milli-g (1–5 min, 6–10 min, 10+) | NR | Participant feedback: Questionnaire—Acceptability of both devices was high overall, wrist-worn device (96%) was more acceptable to patients than the ankle-worn device (83%) |
McCullagh et al., 2016 [113] | Observational/Cross sectional/Prospective: Teaching Hospital/Ireland: (Medical patients) | n = 154 | 77.5 [7.4] | Stepwatch Activity Monitor (SAM) (Modus health, Washington, US) | Continuous: up to 7 days | Sampling frequency = 128 Hz; Manufacturers software—based on answers to direct questions relating to (height, gait pattern and gait cycle); Epochs of 15 s | Volume: average daily step-count | 68% recruitment (eligible to recruited), 97% retention (recruited to analysed) | n = 4 saved incorrectly/irretrievable |
| Moreno et al., 2019 [46] | Randomised controlled trial: University Hospital/Respiratory and Clinical Medicine/Brazil: (Emergency) | n = 68, Experimental = 33/Control = 35 | Experimental—69 [7]/Control—69 [7] | Actigraph GT3X LLC. Pensacola, FL, USA | Continuous: during admission up to 20 days | NR | Volume: Mean steps per day: Sedentary behaviour: Time sedentary (%). Intensity: % of time light, moderate, hard, and very hard intensity level (thresholds ≤ 1951, 1952–5724, 5725–9498, ≥9499 cnts·min-1, respectively | 72% recruitment (eligible to recruited), 97% retention (recruited to analysed) | n = 2 died, n = 2 device malfunction |
| Norheim et al., 2017 [106] | Observational/Prospective: Geriatric Ward/Denmark: (Rehab) | n = 16 (pre-test to post-test) | 84.8 [1.9] | ActivPAL™ (PAL Technologies Ltd.©, Glasgow, UK) | Continuous: ≥24 h for both pre-test and post-test (leg resistance exercise) | NR | Volume: Time spent standing, and walking. Sedentary behaviour: Time spent sedentary (lying or sitting), | 3% recruitment (screened to consented), 79% retention (recruited to analysed) | n = 3 transferred, n = 1 sensor lost |
| Ostir et al., 2013 [10] | Observational/Prospective: University Hospital/Acute Care for Elders (ACE) hospital unit/USA: (Emergency) | n = 224 | Age—65–74 median = 108, IQR = 48.2/Age -75–84 median = 86, IQR = 38.4/Age—80 median = 30, IQR = 13.4 | StepWatch Activity Monitor (SAM) (2 axis) (Modus health, Washington, DC, USA) | Continuous: 24 h at start of admission, 24 h before discharge | NR | Volume: Total steps; Total minutes active (number of 1 min intervals recorded in a 24-h period with a step count greater than 0) | 87% recruitment (eligible to recruited)), 79% retention (recruited to final sample) | n = 24 removed for medical procedures/tests |
| Pedersen et al., 2013 [4] | Observational/Prospective: University Hospital/Acute medical admission ward: (Emergency) | n = 48, Ambulatory = 42/Nonambulatory = 6 | Ambulatory median = 84.7, IQR (78.6–87.2)/Nonambulatory median = 82.8, IQR (79.9–88.0) | Augmentech, Inc.; Pittsburgh, PA, USA | Continuous: within 48 h of admission up to discharge, <10 days | NR | Volume: Hours per day spent standing and/or walking, Sedentary behaviour: Hours per day spent lying, sitting: To define postures—algorithm identification from two axes by Angle = tan − 1(AX/AZ) | 72% recruitment (eligible to recruited), Retention unclear [one excluded due to lack of data] | n = 1 episode of acute psychosis (3 day pause), n = 2 removed after 3 and 4 days, n = 1 lack of accelerometer data * (removals by patients or staff for examinations *) |
| Tasheva et al., 2020 [60] | Observational/Prospective: University hospital/Medicine ward/Switzerland: (Emergency) | n = 177, Male = 106/Women = 71 | Men—79.7 [8.1]/Women—83.5 [8.6] | GENEActiv Original, ActivInsights Ltd., UK | Continuous:—during admission ≥24 h | Sampling frequency = 50 Hz | Intensity: Time spent in light or moderate activity levels (min per day, % of daily time); Sedentary behaviour: <30 mg inactivity, (30–99 mg light, ≥100 for moderate PA) Pattern: Activity per hour (average) (Physically active—in the highest quartile of time spent in light and moderate PA or spending ≥20 min/day in moderate PA) | 56% recruitment (screened to recruited), 84% retention (recruited to analysed) | Very short (<24 h) recordings or had a high percentage of accelerometer nonwear time * |
| Theou et al., 2019 | Observational/Prospective: Tertiary care hospital/Medicine Unit and Geriatric Assessment Unit/Canada: (Emergency) | n = 111, bedridden = 32/person assistance = 44/independent = 35 | Overall—82.2 [8.0], bedridden—84.8 [8.5]/person assist—81.6 [6.5]/independent—80 [8.9] | ActivPAL3™ (PAL Technologies Ltd.©, Glasgow, UK) | Continuous: up to 2 weeks | NR | Volume: Upright time (min); Number of upright bouts (per day). Pattern: Upright time and upright bouts during awake (7 a.m.–10 p.m.), night-time (10 p.m.–7 a.m.), morning (7 a.m.–12 p.m.), afternoon (12 p.m.–5 p.m.), evening (5 p.m.–10 p.m.). (outcomes measured every 15 s) | 45% recruitment (eligible to recruited), 85% retention (recruited to analysed) | n = 11 no valid ActivPAL data, n = 5 no mobility assessment within 48 hr of hospital admission, n = 3 withdrew from study |
| Mixed Admission | |||||||||
| Chaboyer et al., 2015 [58] | Observational/Prospective: Tertiary Hospital/Acute Medical Wards/Australia: (Emergency) | n = 84, 66 to 74 years = 18/>75 years = 44 | Overall median = 77, IQR = [64.0–85.8], 66 to 74 years median = 70, IQR = [68.8–72]/>75 years median = 85, IQR = [81.0–87.0] | ActiGraph, model GT3X+, Manufacturing Technology Inc., Pensacola, FL, USA | Continuous: 24 h | Butterworth low-pass filter with a cut-off frequency of 0.1 Hz; | Volume: Number of postural changes (24 h) (Angle data for postural change); Sedentary behaviour: % time sedentary (<100 counts/min); Intensity: % time in light (100–760 counts/min), moderate or greater (>760 counts/min) | NR | NR |
| Fisher et al., 2016 [9] | Observational/Prospective: Teaching hospital/Acute Medical Ward/USA: (Emergency—cardiovascular, pulmonary, infection, gastrointestinal, or endocrine) | n = 164 | 76.2 [7.0] | StepWatch Activity Monitor (2 axis) Orthocare Innovations | Continuous: Up to discharge | NR | Volume: Steps per day (mean) (recorded in 1-min intervals per 24-h day, off-axis accelerations are not registered i.e., lying down) | 82% retention (recruited to analysed) | n = 35 either discharged <48 h, withdrew, removed sensor nor resecured. |
| Sallis et al., 2015 [112] | Observational/Prospective: Community hospital/Medical-surgical units/USA: (ambulatory medical and surgical adult patients) | n = 777, Age 18–40 years = 111/41–65 years = 325/65–75 years = 187/>75 years = 151 | Overall—60 [17] (not provided for the subgroup 65 or over) | Tractivity® activity monitor (Tractivity®, Vancouver, BC, Canada) | Continuous: during admission | NR | Volume: Median step count per 24 h. Pattern: Distribution of step counts by percentage of accrued steps each hour over 24 h | NR | n = 10 device failure, 44% of sensors were lost mainly as nursing staff failed to remove on discharge, potential non wear by patient * |
| Oncology | |||||||||
| § Fernandes et al., 2006 [109] | Observational/Prospective/Two group comparative study: Tertiary hospital/UK: (women with cancer and matched median age group) | n = 50, patients = 25/volunteers = 25 | Subject median = 67, range = [46–90]/volunteers’ median = 63, range = [54–79] | Actimeters (Ambulatory Monitoring Inc., New York, NY, USA) | Continuous: 72 h | Epochs of 60 s | Volume: Mean activity score (counts/minute) up interval—during the time awake. Sleep: % Sleep—up interval and down interval; Sleep efficiency (% time asleep when attempting to sleep); Sleep latency (minutes trying to sleep until first 20 min block of sleep); Wake after sleep onset (min); (Sleep diary used to define up and down interval) Circadian Rhythm: 24-h autocorrelation coefficient (R24) (activity data during each 1-min ‘‘epoch’’ of a 24-h period with the activity levels during subsequent epochs) | 100% recruitment rate | NR |
| Jonker et al., 2020 [91] | Observational/Prospective: Tertiary academic hospital/Surgical Unit/Netherlands: (Elective—Cancer patients scheduled for surgery) | n = 37/Recovery = 15/Not recovered = 22 | Recovery—71.7 [4.8]/Not recovered—73.6 [5.2] | Fitbit Charge 2 (Fitbit Inc., San Francisco, CA, USA) | Continuous: Time 1—pre-op 7 days, Time 2—during admission—2 days, Time 3—3 month later 7 days. | NR | Volume: Daily step count. Intensity: Time engaged in moderate-vigorous physical activity (MVPA) (minutes per day spent on activities with an intensity of 3 Metabolic Equivalent of Tasks) | 49% recruitment (eligible to recruited), 80% retention (recruited to analysed) | n = 1 died, n = 1 dermatitis, n = 1 too stressful, n = 3 surgery cancelled, n = 2 withdrew due to complications, n = 1 too time consuming, n = 1 no data available |
| Porserud et al., 2019 [47] | Non-randomised controlled trial: University hospital/Sweden: Elective—abdominal surgery—colo-rectal, urinary, ovarian cancer) | n = 133, Activity board = 67/Standard treatment = 66 | Overall—68.1 [12.3], Activity board—69.3 [11.4]/Standard treatment—66 [67.0] | activPAL3 micro (PAL Technologies Ltd., Glasgow, UK) | Continuous: up to 5 days | NR | Volume: Time spent standing, stepping, sitting (min/day); Number of step counts; Sit to stand transitions. Sedentary behaviour: lying in bed/sitting, min/day (valid day—12 h wear time) | 89% retention (recruited to analysed) | Less than 12 h of wear time on any of the three days, early discharge * |
| Morikawa et al., 2018 [90] | Observational/Prospective: Hospital/Oncology Unit/Japan: (Elective—Non small cell lung cancer (Advanced) Elderly—scheduled to initiate first line chemotherapy | n = 18, cachexia = 11/noncachexia = 7 | Overall median = 74.5, range [70–82], Cachexia median = 74, Range [70–82]/Noncachexia median = 76, Range = [70–81] | Lifecorder®, Suzuken Co., Ltd., Japan) | Discontinuous: daytime, before admission through to during admission, 1st , 2nd, and 3rd week after discharge | NR | Volume: Daily steps (mean). | 96% recruitment (screened to recruited), 72% retention rate (recruited to completion) | n = 1 failure of accelerometer, (less than 5 h a day then that day excluded * |
| Parkinsons Disease | |||||||||
| Ito et al., 2020 [89] | Observational/Prospective/Pilot study: Medical Centre/Neurology Department/Japan: (Elective—admitted for adjustment of medication or deep brain stimulation) | n = 11 | 67.1 [7.7] | Active Style Pro HJA-350IT, Omron Healthcare, Kyoto, Japan | Discontinuous: 10 h per days 07:00/07:30 am—05:30/07:00 p.m., during admission | Sampling frequency = 32 Hz; Range = 23.0 g; Epochs of 10 s | Volume: Time spent in physical activity (active/inactive); Sedentary behaviour: Time spent sedentary and inactive; Intensity: Physical activity level calculated by Total Energy Expenditure derived from a manufactured regression equation using METs assessed by the triaxial accelerometer. Pattern: METs variation over a day | ||
| Study | Study Setting (Admission Type) | Sample Size | Age: mean [Standard Deviation] | Sensor Technology | Monitoring Duration | Sensor Characteristics/Signal Processing | Reported Measures | Recruitment and Retention | Sensor Removal/Missing Data (Participant Feedback) |
|---|---|---|---|---|---|---|---|---|---|
| Stroke | |||||||||
| Bakken et al., 2012) [85] | Observational/Prospective [2 sites]: Hospital Trust, University Hospital/Medical Wards/Norway: (Emergency—First time stroke) | n = 90 | 68.4 [13.3] | Motion logger (Ambulatory Monitoring Inc.; Ardsley, NY, USA) | Continuous: 3 nights/two days | Epochs of 60 s: (Activity counts analysed in Hertz with the Cole–Kripke algorithm ActionW software (Ambulatory Monitoring) | Sleep: Total sleep time at night (mean minutes); Wake after sleep onset %; Number of awakenings; Daytime nap (mean minutes of sleep between 09:00–20:59). (Sleep period between 21:00–08:59) | 65% recruitment (screened to recruited) 95% retention (Time 1) | n = 3 deaths, n = 3 transfers, n = 3 missing data *, n = 13 not completed protocol/assessments. |
| Cardiac Medical/Surgical | |||||||||
| Amofah et al., 2016 [104] | Observational/Prospective: University Hospital/Cardiothoracic Surgical Centre/Norway: (Emergency and Elective -Octogenarians undergoing SAVR and TAVI | n = 143, SAVR = 78/TAVI = 65 | Overall—83 [2.7], SAVR—82 [2.0]/TAVI—85 [2.8] | Actiwatch 2 (Respironics, Philips Health Care, Best, The Netherlands | Continuous: 5 days post op | NR | Sleep: Total sleep time at night; Sleep efficiency; Wake time at night; Sleep time during day (between 07:00 and 23:00). (Sleep period between 23:00 and 07:00) | 89% recruitment (eligible to recruited) Unclear retention (7 nonresponsive/died *) | n = 7 non-responsive due to sedation, or died within five days after surgery |
| Gimenez et al., [50] | Observational/Prospective/Interventional: University Hospital/Cardiology Ward/Netherlands | n = 196, Intervention (dynamic light/dark cycle) = 100/Control = 96 | Overall—66.5 [13.1], Intervention—68.1 [12.2]/Control—64.9 [13.9] | Actigraph [no model provided] | Continuous: during admission | NR | Sleep: Total sleep duration (min); Sleep-onset latency (Measurement for time to bed/time in bed not clear) | 34% recruitment, 47% retention | n = 27 withdrew, n = 20 transferred, n = 5 moved to other room, n = 22 early discharge (<2 days, n = 2 unknown, n = 1 still hospitalised, n = 1 migraine, n = 1 skin irritation, |
| Redeker and Wykpisz, 1999 [118] | Observational/Prospective: University-affiliated Coronary Care Centre/Acute care/USA: (post coronary artery bypass surgery) | n = 22, middle age = 8, Older adults = 14 | Middle age—57.12 [6.62]/Older adults—72.36 [4.14] | The Mini Motion Logger (Ambulatory Monitoring Inc., Ardsley, NY, USA) | Continuous: during admission | Epochs of 60 s. Programmed for zero crossing mode. Proprietary software | Volume: Total activity counts. Pattern: Activity counts during 12-h intervals (day 0700–1900 h, night 1900–0700 h Circadian Rhythm: Acrophase (crest time of the fitted rhythmic function, or time of peak activity); Amplitude (half difference between peak and trough of the rhythm, or half maximum height of the oscillation); Mesor (rhythm adjusted mean); Percent rhythm (% variance in activity) | NR | NR |
| Takaesu et al., 2015 [74] | Observational/Prospective: Medical University Hospital/Coronary Care Unit/Japan: (Emergency) | n = 42, Subjects = 23/Control = 19 | NR | Actigraph [no model provided] | Continuous: 24 h, 6 a.m.—6 a.m. | NR | Sleep: Total sleep time at night (min); Total sleep time in the day (min); Sleep latency (min); Wake time after sleep onset (min); Sleep efficiency (%) (diurnal period 9 p.m.–6 a.m., nocturnal period 9 p.m.–6 am) | 100% retention | NR |
| Orthopedic Surgery/Fractures | |||||||||
| § Krenk et al., 2013 [98] | Observational/Prospective: Denmark: Elective—fast track THA and TKA) | n = 20 | Overall—70.5, range (61–89) | Actiwatch spectrum ambulatory activity device (Philips Respironics, Murrysville, PA, USA) | Continuous: Time 1, 3 days prior to surgery; Time 2, 7 days postoperatively. | (Proprietary—Respironics) | Volume: Maximum activity count per day; Mean activity count per minute; Total activity count (24 h—6 a.m. to 6 a.m.). Sleep: Mean day-time sleep (min); Mean night-time sleep (min); (Measurement for night-time taken from patients recorded lights-off and lights-on) | 83% recruitment (approached to recruited), 95% retention (recruited to analysed)1 excluded | Sensor never removed for more than 20 min |
| Miller et al., 2015 [97] | Observational/Prospective: Tertiary Hospital/Surgical Ward/USA: (Elective—Post-op—THA and TKA) | n = 50 | 65 [10.8] | Actiwatch 2 (Philips Respironics, Andover, MA, USA) | Continuous: first 2 nights post surgery | Epochs of 15 s: (Further processing: Actiwatch Respironics software ‘Philips Respironics’) | Sleep: Total sleep time (night-time); Sleep efficiency (Sleep diary used); Awake index (number of awakenings divided by the time difference between the initiation of sleep and the offset of sleep) | 100% retention rate | NR |
| Mixed Admissions (Delirium and Dementia) | |||||||||
| § Davoudi et al. 2019 [68] | Observational/Prospective: University Hospital/ICU/USA: (Emergency—post surgery delirium) | n = 17/Delirious = 4/Non-delirious = 8 | Overall Median = 69, IQR = (54.0–73.0)/Delirious Median = 72.5, IQR = (64.5 -74.5)/Non-delirious Median = 62.5, IQR = (37.7–73.0) | Actigraph GT3X (GT3X) devices (ActiGraph, LLC. Pensacola, FL, USA) | Continuous: Up to 7 days | Sampling frequency = 100 Hz; Analysed as 1-min activity counts | Volume: Activity counts (mean/SD); Root Mean Square of Sequential Differences: Root Mean Square of Sequential Differences/Standard Deviation. PATTERN: Activity counts (daytime 7 a.m.–7 p.m.—night-time 7 p.m.–7 a.m.) Mean/SD Sleep: Number of immobile minutes (day and night). Circadian Rhythm: M10—Activity intensity of 10-h window with highest sum of activity intensity; L5—Activity intensity 5-h window with lowest sum of activity; Relative amplitude—Difference between M10 and L5. | 55% retention (recruited to analysed) | n = 1 or 2 removed at patient’s request, during bathing, or during clinical routines Moved to different ward * |
| Jaiswal et al., 2020 [119] | Retrospective/Observational (secondary analysis of a Randomised Controlled Trial): Teaching Hospital/Medicine Wards/USA: (Inpatient delirium) | n = 70, Delirium = 17/no delirium = 53 | 81.5 [7.4] | Actiwatch Spectrum Plus (Philips Respironics, Murrysville, PA, USA) | Continuous: during admission | Epochs of 15 s: Further processing—Actiwatch Respironics software (Philips Respironics) | Sleep: Total sleep time Average 24-h total sleep time; Night-time sleep duration; Number of sleep bouts at night-time; Duration of sleep bout; Wake after sleep onset; Sleep efficiency; Fragmentation Index (using average length of sleep bout during night sleep); (Rest interval—manual based on decreased activity and light) | NR [secondary analysis on the complete sensor data] | Secondary analysis of those wearing sensors. |
| Leung et al., 2015 [95] | Observational/Prospective: University Medical Centre/USA: (Elective-delirium in non-cardiac surgical patients) | n = 50, No delirium = 43/Delirium = 7 | Overall—66 [11], range [43–91], No delirium—66.5 [11.1]/Delirium—70.1 [8.3] | Mini Motionlogger Actigraph (Ambulatory Monitoring, Inc., Ardsley, NY, USA | Continuous: Time1—home, 72 h; Time 2—hospital post-surgery, 72 h | Epochs of 60 s; Further processing—Action4 software—Cole–Kripke algorithm program (Ambulatory Monitoring, Inc., Ardsley, NY, USA) | Sleep: Total sleep time (night-time); Sleep onset latency (time event marker pressed to onset of sleep); Number of awakenings; Wake after sleep onset (%). (Sleep diary used) (Bedtime and final wake times determined by the diary entry matched with a 50% change in movement during the same 10-min block of time on actigraphy) | 83% retention [60 recruited, 50 analysed] | n = 6 surgery cancelled, no hospital data, n = 4 equipment failure |
| § Osse et al., 2009 [38] | Observational/Prospective: Medical centre/Cardiothoracic surgery dept/Holland: (Elective—Postcardiotomy delirium) | n = 79, ‘Non-cr-Del’(non-clinically relevant delirium) = 46, ‘Short-Del’ = 16, ’Sustained-Del’ = 17 | ‘Non-cr-Del—72.9 [4.9]/Short-Del—75.2 [4.1]/Sustained Del—75.2 [4.5] | Actiwatch (Cambridge Neurotechnology Ltd., Cambridge, UK) | Continuous: post-surgery for up to 6 days | Epochs of 60 s | Volume: Activity per minute (mean); Sedentary behaviour: Number of minutes immobile (based on the total number of minutes with a score of zero). Pattern:, Sedentary behaviour: number of minutes Immobile (night-time 23.00–06.00 h and daytime 06.00 h–23.00 h) Circadian Rhythm: Restlessness Index (addition of the percentage of time spent moving and the percentage immobility phases of 1 min); Activity Amplitude (difference between the least active 5 h period and the most active 10 h period within each 24-h period) | 90% recruitment (eligible to recruited), 91% retention (recruited to analysed) | n = 7 technical problems |
| Tanev et al., 2017 [65] | Observational/Prospective: University Hospital/Psychiatry Unit/USA: (Emergency—dementia) | n = 28 | 82 [9] | Actigraph (Ambulatory Monitoring, Ardsley, NY, USA) | Discontinuous: 9 p.m.–7 a.m., during admission | Epochs of 60 s | Sleep: Mean sleep minutes (night-time); The deviation of each night’s sleep minutes from that mean (Time in bed defined as 9:00 p.m. and out of bed 7:30 a.m.) | NO | NR |
| Todd et al., 2017 [93] | Observational/Prospective: Teaching Hospital/Orthopedic Dept/Germany: (Elective -post-op delirium) | n = 101, Post op delirium = 27/No post op delirium = 74 | Overall—76.0 [6.0], Post op delirium—75.4 [5.7]/No post op delirium—77.6 [6.7] | Actiwatch, Cambridge Neurotechnology Co., Cambridge, UK | Continuous; 1 day before op, up to 7 days after | Epochs of 60 s. Actiwatch software version 5.17 (CamNtech, Cambridge, UK) | Sleep: Total Sleep Time (night-time); Wake after sleep onset in min; Wake after sleep onset percentage (Sleep diary used) (Sleep was defined as an actigraphy count of less than 80 per half-minute interval) | 77% recruitment [136 screened, 105 recruited], 85% retention [89 sensor data available] | n = 2 refused post op, n = 2 op cancelled, n = 1 died, n = 4 early discharge (n = 12 refused actigraphy, did not tolerate, or misplaced the watch during the night) |
| § Valembois et al., 2015 [33] | Observational/Prospective/Cross sectional: Intermediate care unit/Geriatric ward/France: (Emergency—Dementia and apathy or aberrant motor behaviour) | n = 183 | 84.9 [6.8] | Vivago (Vivago®, Vivago Oy, Espoo, Finland) | Continuous: 10 days | Proprietary software (Vista, Vivago, Finland) | Pattern: Mean motor activity (grouped by pre-defined 3-h periods during the day 0:00 to 2:59, 3.00 to 05.59, 06.00 to 8.59, 9.00 to 11.59, 12.00 to 14.59; 15.00 to 17.59; 18.00 to 20.59 and 21.00 to 23.59) Sleep: Total sleep time (min); Number night awakenings (n); (Proprietary software) | 100% retention (obtained analysable actigraphy data for all the patients included) | impaired sensor contact with skin/long period of time out of the detection zone* |
| Patients In Intensive Care | |||||||||
| Arttawejkul et al., 2020 [48] | Randomised Controlled Trial: Memorial hospital/Medical ICU Unit/Thailand | n = 17, Control = 9/Intervention (ear plugs, eye mask) = 8 | Control median = 76, IQR = 32/Intervention median = 67, IQR = 25 | Actiwatch® 2 (Respironics) | Continuous: during ICU stay | Epoch of 30 s (Further processing—by Actiware 6.0 software based on threshold of activity counts within the epoch and 2 min before and after that epoch) | Sleep: Total sleep time (night-time); Sleep efficiency; Sleep latency; Wake after sleep onset (Alongside Polysomnography Bedtime based on habitual bedtime at home, until 07:00) | 85% retention [20 recruited, 17 analysed] | n = 1, ICU discharge, n = 2 poor polysomnographic quality |
| Beecroft et al., 2008 [54] | Observational/Prospective (Validation study): General Hospital/Medical Surgical ICU/Canada: (Mechanically ventilated) | n = 12 | 68 [13] | Actiwatch—Model AW-64 (Philips Respironics, 1010 Murry Ridge Lane, Murrysville, PA 15668, USA) | Discontinuous: 8–12 h overnight | Sampling acceleration = 0.01 g, sampling frequency 32 Hz, epoch of 30 s: (Further processing by Actiware-Sleep v. 3.4, software—based on threshold of activity counts) | Sleep: Total sleep time (night-time); Sleep efficiency; Frequency of awakenings. (Alongside Polysomnography. Frequency of awakenings—calculated at each threshold for each patient) | NR | NR |
| Chen et a;., 2012 [49] | Randomised Clinical Trial: Medical Centre/Taiwan | n = 85, Experimental (valerian acupressure) = 41/Control = 44 | Experimental mean—72.1 [18.2]/Control—69.1 [15.1] | ActiGraph GT1M (ActiGraph, LLC. Pensacola, FL, USA) | Discontinuous: 10 p.m.–6 a.m. (2 nights) | Processing by proprietary ActiWeb software | Sleep: Total sleep time hours (night-time); Waking minutes; Waking frequency (Sleep based on ActiWeb software and sleep observation) | NR | NR |
| Other Surgery | |||||||||
| Ida et al., 2019 [92] | Observational/Prospective: Tertiary Hospital/Surgical Ward/Japan: (Elective—surgical lobectomy for lung cancer) | n = 24 | Overall median = 69, IQR = 4.3 | WGT3X-BT (ActiGraph, LLC. Pensacola, FL, USA) | Continuous: 1 day before surgery to 6 days after | NR | Sleep: Total sleep time (night-time); Sleep efficiency (Time tried to sleep recorded in sleep diaries, preoperative sleep efficiency < 85% was defined as acute sleep disruption) | 71% retention [24 recruited, 17 analysed] | n = 6 early discharge (within 4 days of surgery), n = 1 no sleep data for the third postoperative day |
| Older Adults | |||||||||
| § Beveridge et al., 2015 [116] | Observational/Prospective: Medical Centre/Medical Ward/USA | n = 120 (Subgroup with sleep data) (Overall = 300) | Subgroup activity (with sleep data)—65.5 [11.1] | Actiwatch2, Respironics, Inc., Murrysville, PA, USA | Continuous: during admission | Proprietary software (Actiware 5 ‘respironics inc’—summed over 15 and 60 s intervals | Volume: Average and maximum Activity Counts (min); Total Activity Counts. (Calculated from reported wake up on morning to bed that night) Sleep: Total time spent asleep (night-time); Sleep efficiency. (Assumed sleep period based on reported bedtimes and morning wake times) | 42% recruitment (consented to larger study to recruited), 71% retention rate [recruited to analysed] | n = 104 removed sensor at night or sensor failure, n = 49 removed during the day, |
| Dzierzewski et al., 2014 [115] | Observational/Prospective/Longitudinal: Veteran Affairs (VA) Medical centre/USA | n = 192, Study 1 = 85/Study 2 = 107 (intervention = 53, control = 54] | Overall—73.8 [9.4], Study 1—75 [8.5]/Study 2 intervention—73.3 [10.7]/Control—72.6 [9.5] | Octagonal Sleep Watch-L (Ambulatory Monitoring Inc.; Ardsley, New York, NY, USA) | Continuous: 7 nights/days | Epochs of 60 s. Further processing—commercially available software (ACT software, AMI)] | Sleep: Total night-time wake minutes; Daytime minutes asleep (time in bed individually reported) | NR for hospital data | NR for baseline data |
| Mixed Admission | |||||||||
| Alessi et al., 2008 [105] | Observational/Prospective [2 sites]: Veterans Administration Medical Centre/Community nursing home/USA: (Rehab) | n = 245, Facility A = 158/Facility B = 87 | Overall—80.6 [7.2], Facility A—82.0 [7.1]/Facility B—78.1 [6.7] | Octagonal Sleep Watch-L (Ambulatory Monitoring, Inc., AMI, Ardsley, NY, USA | Continuous: 7 days | Epochs of 60 s: Further processing—commercially available software (ACT software, AMI) using time above threshold (default algorithms) | Sleep: Total night-time sleep (hours); Mean night-time % sleep (hours asleep/hours between bedtime and wake-up time); Number of night-time awakenings. Daytime hours of sleep; Daytime percent sleep. (Night-time defined as the period between reported bedtime and reported wake-up time) | 25% recruitment, (admitted to recruited) | NR |
| Enomoto et al., 2010 [57] | Observational/Prospective [44 sites]: General Hospital/Mixed Acute Wards (not psychiatric or tuberculosis wards)/Japan: (Emergency) | n = 557 (analysed = 421) | Overall—72.8 [12.8], (analysed—72.5 [12.6]) | Lifecorder PLUS (LC) (Suzuken, Nagoya, Japan) | Continuous: 2 days and nights | NR | Sleep: Total sleep time (night-time); Total wake time; Sleep efficiency (Time in bed—lights out to wake approx. 9 pm–6 a.m., confirmed by nursing staff) | 76% retention (557 randomly selected to analysed) | Sudden change in physical condition/fever/severe dementia* missing data due to the amount of physical activity * |
| Macfarlane et al., 2019 [56] | Observational/Prospective: Hospital/Medical Assessment Ward/Australia: (Emergency) | n = 54 | 70.5 [17] | Sensewear actigraphy armband (Bodymedia Inc., Pittsburgh, PA, USA) | Discontinuous: 17 h—~4 p.m.–9 a.m.] | NR | Sleep: Total sleep duration (night-time mean); Sleep efficiency (Time in bed based on lights out 10 p.m. and lights on 06:45 a.m.) | 100% retention | NR |
| Missildine et al., 2010 [55] | Observational/Prospective/Pilot/Multicentre [2 sites]: Acute Care/Medical Unit/USA: (Emergency) | n = 48 | 79 | MicroMini Motionlogger (Ambulatory Monitoring, Ardsley, NY, USA) | Continuous: 24 h | Epochs of 60 s; Action-W, Version 2 software. | Sleep: Nocturnal sleep efficiency, Total sleep minutes/24 h, Total sleep minutes—night, Duration longest sleep episode—night (minutes), Number of wake episodes at night, Total wake minutes/24 h, Total wake minutes at night, Longest wake episode at night(minutes) (Daytime sleep—scored during the hours of 7 a.m.–11 p.m.) | Unclear | NR |
| Shear et al., 2014 [111] | Observational/Prospective: Academic Medical Centre/General Medicine Ward/USA | n = 424 | 65 [12] | Actiwatch 2 (Respironics Inc, Murrysville, PA, USA) | Continuous: up to discharge | NR | Sleep: Average sleep duration (night-time min); Sleep efficiency (%) (rest interval, sleep onset—taken from sleep log) | 53% recruitment (eligible to recruited), 76% retention (recruited to analysed) | n = 103 removal at night or failure |
| Vinzio et al., 2003 [22] | Observational/Prospective: University Hospital/Acute Care Unit/France: (Emergency—cardiac, respiratory or renal acute disease) | n = 10 | 81 [14] | Actiwatch-L (Cambridge Neurotechnology Ltd., Cambridge, UK) | Continuous: during admission | Epochs of 60 s | Circadian Rhythm: Relative amplitude (RA)—difference between M10 and L5, M10 and L5 onset time; Interdaily stability (IS) (perfect stability = 1, gaussian noise = 0); Intradaily variability (IV) (perfect sine wave = 0, gaussian noise = 2). | 80% retention (recruited to analysed) | n = 2 transferred to ICU/admitted for only 7 days, n = 2 died |
| Oncology | |||||||||
| Chang et al., 2018 [110] | Observational/Prospective: University Hospital/Taiwan: Lung cancer -newly diagnosed) | n = 84, 65 or above = 42/Below 65 = 42 | Overall—65.45 [10.77], 65 or above—74.31 [6.07]/below 65–56.60 [6.12] | MicroMini Motionlogger (Ambulatory Monitoring Inc., New York, NY, USA) | Continuous: 3 days | NR | Circadian Rhythm: I < O, a percentage of activity counts less than the median activity during the rest span (rest span is defined according to sleep–wake logbook; median of I < O (88.04%) cut-off point above this—robust rest activity rhythm, below—disrupted) | 91% retention (recruited to analysed) | n = 8 deterioration of health |
| § Fernandes et al., 2006 [109] | Observational/Prospective/Two group comparative study: Tertiary hospital/UK: (women with cancer and matched median age group) | n = 50, patients = 25/volunteers = 25 | Subject median = 67, range = [46–90]/volunteers’ median = 63, range = [54–79] | Actimeters (Ambulatory Monitoring Inc., New York, NY, USA) | Continuous: 72 h | Epochs of 60 s | Volume: Mean activity score (counts/minute) up interval—during the time awake. Sleep: % Sleep—up interval and down interval; Sleep efficiency (% time asleep when attempting to sleep); Sleep latency (minutes trying to sleep until first 20 min block of sleep); Wake after sleep onset (min); (Sleep diary used to define up and down interval) Circadian Rhythm: 24-h autocorrelation coefficient (R24) (activity data during each 1-min ‘‘epoch’’ of a 24-h period with the activity levels during subsequent epochs) | 100% recruitment rate | NR |
| Jakobsen et al., 2020 [37] | Observational/Prospective: University Hospital/Palliative Medicine Dept/Norway: (Elective—advanced/metastatic cancer) | n = 40 | Overall Median = 70, range (41–91) | Actiwatch 2 (Philips Respironics, Inc., Murrysville, PA, USA) | Discontinuous: 4 p.m.–9 a.m., 17 h | Epoch of 30 s. Manufacturers’ software—medium sensitivity (40 activity counts) per epoch to score sleep, 10-min inactivity threshold for sleep onset | Sleep: Total sleep time (night-time); Sleep onset latency; Number of awakenings during the night; Wake after sleep onset; Sleep efficiency; (Sleep diary to establish time in bed and rest interval (lights off, lights on) | 32% recruitment (Screened to recruited), 98% retention (recruited to analysed) | n = 1 died, n = 2 not possible to generate sleep parameters from the Actiware software |
References
- Fisher, S.R.; Graham, J.E.; Brown, C.J.; Galloway, R.V.; Ottenbacher, K.J.; Allman, R.M.; Ostir, G.V. Factors that differentiate level of ambulation in hospitalised older adults. Age Ageing 2012, 41, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Villumsen, M.; Jorgensen, M.G.; Andreasen, J.; Rathleff, M.S.; Molgaard, C.M. Very Low Levels of Physical Activity in Older Patients During Hospitalization at an Acute Geriatric Ward: A Prospective Cohort Study. J. Aging Phys. Act. 2015, 23, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.J.; Redden, D.T.; Flood, K.L.; Allman, R.M. The underrecognized epidemic of low mobility during hospitalization of older adults. J. Am. Geriatr. Soc. 2009, 57, 1660–1665. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.M.; Bodilsen, A.C.; Petersen, J.; Beyer, N.; Andersen, O.; Lawson-Smith, L.; Kehlet, H.; Bandholm, T. Twenty-four-hour mobility during acute hospitalization in older medical patients. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Boyd, C.M.; Landefeld, C.S.; Counsell, S.R.; Palmer, R.M.; Fortinsky, R.H.; Kresevic, D.; Burant, C.; Covinsky, K.E. Recovery of Activities of Daily Living in Older Adults After Hospitalization for Acute Medical Illness. J. Am. Geriatr. Soc. 2008, 56, 2171–2179. [Google Scholar] [CrossRef] [PubMed]
- Covinsky, K.E.; Pierluissi, E.; Johnston, C.B. Hospitalization-associated disability: “She was probably able to ambulate, but I’m not sure”. Jama 2011, 306, 1782–1793. [Google Scholar] [CrossRef]
- Lafont, C.; Gérard, S.; Voisin, T.; Pahor, M.; Vellas, B.; The Members of IAGG/AMPA Task Force. Reducing “iatrogenic disability” in the hospitalized frail elderly. J. Nutr. Health Aging 2011, 15, 645–660. [Google Scholar] [CrossRef]
- Zisberg, A.; Shadmi, E.; Sinoff, G.; Gur-Yaish, N.; Srulovici, E.; Admi, H. Low mobility during hospitalization and functional decline in older adults. J. Am. Geriatr. Soc. 2011, 59, 266–273. [Google Scholar] [CrossRef]
- Fisher, S.R.; Graham, J.E.; Ottenbacher, K.J.; Deer, R.; Ostir, G.V. Inpatient Walking Activity to Predict Readmission in Older Adults. Arch. Phys. Med. Rehabil. 2016, 97, S226–S231. [Google Scholar] [CrossRef]
- Ostir, G.V.; Berges, I.M.; Kuo, Y.F.; Goodwin, J.S.; Fisher, S.R.; Guralnik, J.M. Mobility activity and its value as a prognostic indicator of survival in hospitalized older adults. J. Am. Geriatr. Soc. 2013, 61, 551–557. [Google Scholar] [CrossRef]
- Roepke, S.K.; Ancoli-Israel, S. Sleep disorders in the elderly. Indian J. Med. Res. 2010, 131, 302–310. [Google Scholar] [PubMed]
- Lee, C.Y.; Low, L.P.; Twinn, S. Understanding the sleep needs of older hospitalized patients: A review of the literature. Contemp. Nurse 2005, 20, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Missildine, K. Sleep and the sleep environment of older adults in acute care settings. J. Gerontol. Nurs. 2008, 34, 15–21. [Google Scholar] [CrossRef]
- Yoder, J.C.; Staisiunas, P.G.; Meltzer, D.O.; Knutson, K.L.; Arora, V.M. Noise and sleep among adult medical inpatients: Far from a quiet night. Arch. Intern. Med. 2012, 172, 68–70. [Google Scholar] [CrossRef]
- Stewart, N.H.; Arora, V.M. Sleep in Hospitalized Older Adults. Sleep Med. Clin. 2018, 13, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Delaney, L.J.; Currie, M.J.; Huang, H.C.; Lopez, V.; Van Haren, F. “They can rest at home”: An observational study of patients’ quality of sleep in an Australian hospital. BMC Health Serv. Res. 2018, 18, 524. [Google Scholar] [CrossRef]
- Arora, V.M.; Chang, K.L.; Fazal, A.Z.; Staisiunas, P.G.; Meltzer, D.O.; Zee, P.C.; Knutson, K.L.; Van Cauter, E. Objective Sleep Duration and Quality in Hospitalized Older Adults: Associations with Blood Pressure and Mood. J. Am. Geriatr. Soc. 2011, 59, 2185–2186. [Google Scholar] [CrossRef]
- Inouye, S.K.; Bogardus, S.T., Jr.; Charpentier, P.A.; Leo-Summers, L.; Acampora, D.; Holford, T.R.; Cooney, L.M., Jr. A multicomponent intervention to prevent delirium in hospitalized older patients. N. Engl. J. Med. 1999, 340, 669–676. [Google Scholar] [CrossRef]
- Prather, A.A.; Hall, M.; Fury, J.M.; Ross, D.C.; Muldoon, M.F.; Cohen, S.; Marsland, A.L. Sleep and antibody response to hepatitis B vaccination. Sleep 2012, 35, 1063–1069. [Google Scholar] [CrossRef]
- Martin, J.L.; Fiorentino, L.; Jouldjian, S.; Mitchell, M.; Josephson, K.R.; Alessi, C.A. Poor self-reported sleep quality predicts mortality within one year of inpatient post-acute rehabilitation among older adults. Sleep 2011, 34, 1715–1721. [Google Scholar] [CrossRef]
- Wu, L.; Sun, D. Sleep duration and falls: A systemic review and meta-analysis of observational studies. J. Sleep Res. 2017, 26, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Vinzio, S.; Ruellan, A.; Perrin, A.E.; Schlienger, J.L.; Goichot, B. Actigraphic assessment of the circadian rest-activity rhythm in elderly patients hospitalized in an acute care unit. Psychiatry Clin. Neurosci. 2003, 57, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Sallis, J.F.; Saelens, B.E. Assessment of physical activity by self-report: Status, limitations, and future directions. Res. Q. Exerc. Sport 2000, 71 (Suppl. 2), 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hauer, K.; Lord, S.R.; Lindemann, U.; Lamb, S.E.; Aminian, K.; Schwenk, M. Assessment of Physical Activity in Older People with and without Cognitive Impairment. J. Aging Phys. Act. 2011, 19, 347–372. [Google Scholar] [CrossRef] [PubMed]
- Hoey, L.M.; Fulbrook, P.; Douglas, J.A. Sleep assessment of hospitalised patients: A literature review. Int. J. Nurs. Stud. 2014, 51, 1281–1288. [Google Scholar] [CrossRef]
- Chen, K.Y.; Bassett, D.R., Jr. The technology of accelerometry-based activity monitors: Current and future. Med. Sci. Sport. Exerc. 2005, 37, S490–S500. [Google Scholar] [CrossRef]
- Migueles, J.H.; Cadenas-Sanchez, C.; Ekelund, U.; Delisle Nyström, C.; Mora-Gonzalez, J.; Löf, M.; Labayen, I.; Ruiz, J.R.; Ortega, F.B. Accelerometer Data Collection and Processing Criteria to Assess Physical Activity and Other Outcomes: A Systematic Review and Practical Considerations. Sport. Med. 2017, 47, 1821–1845. [Google Scholar] [CrossRef]
- Doherty, A.; Jackson, D.; Hammerla, N.; Plötz, T.; Olivier, P.; Granat, M.H.; Wareham, N.J. Large scale population assessment of physical activity using wrist worn accelerometers: The UK biobank study. PLoS ONE 2017, 12, e0169649. [Google Scholar] [CrossRef]
- Menai, M.; van Hees, V.T.; Elbaz, A.; Kivimaki, M.; Singh-Manoux, A.; Sabia, S. Accelerometer assessed moderate-to-vigorous physical activity and successful ageing: Results from the Whitehall II study. Sci. Rep. 2017, 8, 45772. [Google Scholar] [CrossRef]
- Bernaldo de Quirós, M.; Douma, E.H.; van den Akker-Scheek, I.; Lamoth, C.J.C.; Maurits, N.M. Quantification of Movement in Stroke Patients under Free Living Conditions Using Wearable Sensors: A Systematic Review. Sensors 2022, 22, 1050. [Google Scholar] [CrossRef]
- Ancoli-Israel, S.; Clopton, P.; Klauber, M.R.; Fell, R.; Mason, W. Use of wrist activity for monitoring sleep/wake in demented nursing-home patients. Sleep 1997, 20, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Mc Ardle, R.; Sverdrup, K.; Del Din, S.; Lord, S.; Kerse, N.; Rochester, L.; Taylor, L. Quantifying physical activity in aged residential care facilities: A structured review. Ageing Res. Rev. 2021, 67, 101298. [Google Scholar] [CrossRef] [PubMed]
- Valembois, L.; Oasi, C.; Pariel, S.; Jarzebowski, W.; Lafuente-Lafuente, C.; Belmin, J. Wrist Actigraphy: A Simple Way to Record Motor Activity in Elderly Patients with Dementia and Apathy or Aberrant Motor Behavior. J. Nutr. Health Aging 2015, 19, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health. Quality Assessment Tool for Observational Cohort and CrossSectional Studies. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 10 May 2021).
- Jakobsen, G.; Engstrom, M.; Thronaes, M.; Lohre, E.T.; Kaasa, S.; Fayers, P.; Hjermstad, M.J.; Klepstad, P. Sleep quality in hospitalized patients with advanced cancer: An observational study using self-reports of sleep and actigraphy. Support. Care Cancer 2020, 28, 2015–2023. [Google Scholar] [CrossRef]
- Osse, R.J.; Tulen, J.H.M.; Bogers, A.J.J.C.; Hengeveld, M.W. Disturbed circadian motor activity patterns in postcardiotomy delirium. Psychiatry Clin. Neurosci. 2009, 63, 56–64. [Google Scholar] [CrossRef]
- Cook, D.J.; Thompson, J.E.; Prinsen, S.K.; Dearani, J.A.; Deschamps, C. Functional recovery in the elderly after major surgery: Assessment of mobility recovery using wireless technology. Ann. Thorac. Surg. 2013, 96, 1057–1061. [Google Scholar] [CrossRef]
- Dall, C.H.; Andersen, H.; Povlsen, T.M.; Henriksen, M. Evaluation of a technology assisted physical activity intervention among hospitalised patients: A randomised study. Eur. J. Intern. Med. 2019, 69, 50–56. [Google Scholar] [CrossRef]
- van Dijk-Huisman, H.C.; Weemaes, A.T.; Boymans, T.A.; Lenssen, A.F.; de Bie, R.A. Smartphone App with an Accelerometer Enhances Patients’ Physical Activity Following Elective Orthopedic Surgery: A Pilot Study. Sensors 2020, 20, 4317. [Google Scholar] [CrossRef]
- Mahlberg, R.; Walther, S.; Eichmann, U.; Tracik, F.; Kunz, D. Effects of rivastigmine on actigraphically monitored motor activity in severe agitation related to Alzheimer’s disease: A placebo-controlled pilot study. Arch. Gerontol. Geriatr. 2007, 45, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, B.; Cooper-Evans, M.S.; Durran, M.; Montenegro, J.M. An exploration of walking activity among older psychiatric inpatients. Int. J. Ther. Rehabil. 2007, 14, 532–537. [Google Scholar] [CrossRef]
- Jonsson, M.; Hurtig-Wennlöf, A.; Ahlsson, A.; Vidlund, M.; Cao, Y.; Westerdahl, E. In-hospital physiotherapy improves physical activity level after lung cancer surgery: A randomized controlled trial. Physiotherapy 2019, 105, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Cohen, Y.; Zisberg, A.; Chayat, Y.; Gur-Yaish, N.; Gil, E.; Levin, C.; Rand, D.; Agmon, M. Walking for Better Outcomes and Recovery: The Effect of WALK-FOR in Preventing Hospital-Associated Functional Decline Among Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 1664–1670. [Google Scholar] [CrossRef]
- Moreno, N.A.; de Aquino, B.G.; Garcia, I.F.; Tavares, L.S.; Costa, L.F.; Giacomassi, I.W.S.; Lunardi, A.C. Physiotherapist advice to older inpatients about the importance of staying physically active during hospitalisation reduces sedentary time, increases daily steps and preserves mobility: A randomised trial. J. Physiother. 2019, 65, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Porserud, A.; Aly, M.; Nygren-Bonnier, M.; Hagströmer, M. Objectively measured mobilisation is enhanced by a new behaviour support tool in patients undergoing abdominal cancer surgery. Eur. J. Surg. Oncol. 2019, 45, 1847–1853. [Google Scholar] [CrossRef] [PubMed]
- Arttawejkul, P.; Reutrakul, S.; Muntham, D.; Chirakalwasan, N. Effect of Nighttime Earplugs and Eye Masks on Sleep Quality in Intensive Care Unit Patients. Indian J. Crit. Care Med. 2020, 24, 6–10. [Google Scholar]
- Chen, J.H.; Chao, Y.H.; Lu, S.F.; Shiung, T.F.; Chao, Y.F. The effectiveness of valerian acupressure on the sleep of ICU patients: A randomized clinical trial. Int. J. Nurs. Stud. 2012, 49, 913–920. [Google Scholar] [CrossRef]
- Gimenez, M.C.; Geerdinck, L.M.; Versteylen, M.; Leffers, P.; Meekes, G.J.; Herremans, H.; de Ruyter, B.; Bikker, J.W.; Kuijpers, P.M.; Schlangen, L.J. Patient room lighting influences on sleep, appraisal and mood in hospitalized people. J. Sleep Res. 2017, 26, 236–246. [Google Scholar] [CrossRef]
- Donaire-Gonzalez, D.; Gimeno-Santos, E.; Serra, I.; Roca, J.; Balcells, E.; Rodríguez, E.; Farrero, E.; Antó, J.M.; Garcia-Aymerich, J.; PAC-COPD Study Group. Validation of the Yale Physical Activity Survey in Chronic Obstructive Pulmonary Disease Patients. [Spanish]. Arch. De Bronconeumol. 2011, 47, 552–560. [Google Scholar] [CrossRef]
- Denkinger, M.D.; Flick, S.E.; Nikolaus, T.; Becker, C.; Aminian, K.; Lindemann, U. Assessing physical activity in inpatient rehabilitation—Sensor-based validation of the PAIR. Eur. Rev. Aging Phys. Act. 2014, 11, 133–139. [Google Scholar] [CrossRef]
- Thorup, C.B.; Andreasen, J.J.; Sorensen, E.E.; Gronkjaer, M.; Dinesen, B.I.; Hansen, J. Accuracy of a step counter during treadmill and daily life walking by healthy adults and patients with cardiac disease. BMJ. Open 2017, 7, e011742. [Google Scholar] [CrossRef] [PubMed]
- Beecroft, J.M.; Ward, M.; Younes, M.; Crombach, S.; Smith, O.; Hanly, P.J. Sleep monitoring in the intensive care unit: Comparison of nurse assessment, actigraphy and polysomnography. Intensive Care Med. 2008, 34, 2076–2083. [Google Scholar] [CrossRef] [PubMed]
- Missildine, K.; Bergstrom, N.; Meininger, J.; Richards, K.; Foreman, M.D. Sleep in hospitalized elders: A pilot study. Geriatr. Nurs. 2010, 31, 263–271. [Google Scholar] [CrossRef]
- Macfarlane, M.; Rajapakse, S.; Loughran, S. What prevents patients sleeping on an acute medical ward? An actigraphy and qualitative sleep study. Sleep Health 2019, 5, 666–669. [Google Scholar] [CrossRef]
- Enomoto, M.; Tsutsui, T.; Higashino, S.; Otaga, M.; Higuchi, S.; Aritake, S.; Hida, A.; Tamura, M.; Matsuura, M.; Kaneita, Y.; et al. Sleep-related problems and use of hypnotics in inpatients of acute hospital wards. Gen. Hosp. Psychiatry 2010, 32, 276–283. [Google Scholar] [CrossRef]
- Chaboyer, W.; Mills, P.M.; Roberts, S.; Latimer, S. Physical activity levels and torso orientations of hospitalized patients at risk of developing a pressure injury: An observational study. Int. J. Nurs. Pract. 2015, 21, 11–17. [Google Scholar] [CrossRef]
- Theou, O.; Kehler, D.S.; Godin, J.; Mallery, K.; MacLean, M.A.; Rockwood, K. Upright time during hospitalization for older inpatients: A prospective cohort study. Exp. Gerontol. 2019, 126, 110681. [Google Scholar] [CrossRef]
- Tasheva, P.; Kraege, V.; Vollenweider, P.; Roulet, G.; Méan, M.; Marques-Vidal, P. Accelerometry assessed physical activity of older adults hospitalized with acute medical illness—An observational study. BMC Geriatr. 2020, 20, 382. [Google Scholar] [CrossRef]
- Lim, S.E.; Dodds, R.; Bacon, D.; Sayer, A.A.; Roberts, H.C. Physical activity among hospitalised older people: Insights from upper and lower limb accelerometry. Aging Clin. Exp. Res. 2018, 30, 1363–1369. [Google Scholar] [CrossRef]
- Kolk, D.; Aarden, J.J.; MacNeil-Vroomen, J.L.; Reichardt, L.A.; van Seben, R.; van der Schaaf, M.; Wold, J. Factors Associated with Step Numbers in Acutely Hospitalized Older Adults: The Hospital-Activities of Daily Living Study. J. Am. Med. Dir. Assoc. 2021, 22, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Fisher, S.R.; Goodwin, J.S.; Protas, E.J.; Kuo, Y.F.; Graham, J.E.; Ottenbacher, K.J.; Ostir, G.V. Ambulatory activity of older adults hospitalized with acute medical illness. J. Am. Geriatr. Soc. 2011, 59, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Estrup, S.; Kjer, C.K.W.; Vilhelmsen, F.; Poulsen, L.M.; Gogenur, I.; Mathiesen, O. Physical function and actigraphy in intensive care survivors-A prospective 3-month follow-up cohort study. Acta Anaesthesiol. Scand 2019, 63, 647–652. [Google Scholar] [CrossRef]
- Tanev, K.S.; Winokur, A.; Pitman, R.K. Sleep Patterns and Neuropsychiatric Symptoms in Hospitalized Patients with Dementia. J. Neuropsychiatry Clin. Neurosci. 2017, 29, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Fleiner, T.; Gersie, M.; Ghosh, S.; Mellone, S.; Zijlstra, W.; Haussermann, P. Prominent physical inactivity in acute dementia care: Psychopathology seems to be more important than the dose of sedative medication. Int. J. Geriatr. Psychiatry 2019, 34, 308–314. [Google Scholar] [CrossRef]
- Evensen, S.; Bourke, A.K.; Lydersen, S.; Sletvold, O.; Saltvedt, I.; Wyller, T.B.; Taraldsen, K. Motor activity across delirium motor subtypes in geriatric patients assessed using body-worn sensors: A Norwegian cross-sectional study. BMJ Open 2019, 9, e026401. [Google Scholar] [CrossRef]
- Davoudi, A.; Malhotra, K.R.; Shickel, B.; Siegel, S.; Williams, S.; Ruppert, M.; Rashidi, P. Intelligent ICU for Autonomous Patient Monitoring Using Pervasive Sensing and Deep Learning. Sci. Rep. 2019, 9, 8020. [Google Scholar] [CrossRef]
- Schmal, H.; Holsgaard-Larsen, A.; Izadpanah, K.; Brønd, J.C.; Madsen, C.F.; Lauritsen, J. Validation of Activity Tracking Procedures in Elderly Patients after Operative Treatment of Proximal Femur Fractures. Rehabil. Res. Pract. 2018, 2018, 3521271. [Google Scholar] [CrossRef]
- Marsault, L.V.; Ryg, J.; Madsen, C.F.; Holsgaard-Larsen, A.; Lauritsen, J.; Schmal, H. Objectively Measured Physical Activity and Its Association with Functional Independence, Quality of Life and In-Hospital Course of Recovery in Elderly Patients with Proximal Femur Fractures: A Prospective Cohort Study. Rehabil. Res. Pract. 2020, 2020, 5907652. [Google Scholar] [CrossRef]
- Kronborg, L.; Bandholm, T.; Palm, H.; Kehlet, H.; Kristensen, M.T. Physical Activity in the Acute Ward Following Hip Fracture Surgery is Associated with Less Fear of Falling. J. Aging Phys. Act. 2016, 24, 525–532. [Google Scholar] [CrossRef]
- Keppler, A.M.; Holzschuh, J.; Pfeufer, D.; Neuerburg, C.; Kammerlander, C.; Böcker, W.; Fürmetz, J. Postoperative physical activity in orthogeriatric patients—New insights with continuous monitoring. Injury 2020, 51, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Davenport, S.J.; Arnold, M.; Hua, C.; Schenck, A.; Batten, S.; Taylor, N.F. Physical Activity Levels During Acute Inpatient Admission After Hip Fracture are Very Low. Physiother Res. Int. 2015, 20, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Takaesu, Y.; Futenma, K.; Kobayashi, M.; Komada, Y.; Tanaka, N.; Yamashina, A.; Inoue, Y. A preliminary study on the relationships between diurnal melatonin secretion profile and sleep variables in patients emergently admitted to the coronary care unit. Chronobiol. Int. 2015, 32, 875–879. [Google Scholar] [CrossRef]
- Floegel, T.A.; Allen, K.D.; Buman, M.P. A pilot study examining activity monitor use in older adults with heart failure during and after hospitalization. Geriatr. Nurs. 2019, 40, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Tsai, L.L.; Alison, J.A.; McKenzie, D.K.; McKeough, Z.J. Physical activity levels improve following discharge in people admitted to hospital with an acute exacerbation of chronic obstructive pulmonary disease. Chron. Respir Dis. 2016, 13, 23–32. [Google Scholar] [CrossRef]
- Orme, M.W.; Harvey-Dunstan, T.C.; Boral, I.; Chaplin, E.J.; Hussain, S.F.; Morgan, M.D.; Steiner, M.C.; Singh, S.J.; Greening, N.J. Changes in physical activity during hospital admission for chronic respiratory disease. Respirology 2019, 24, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Borges, R.C.; Carvalho, C.R.F. Physical activity in daily life in Brazilian COPD patients during and after exacerbation. COPD 2012, 9, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Strømmen, A.M.; Christensen, T.; Jensen, K. Quantitative measurement of physical activity in acute ischemic stroke and transient ischemic attack. Stroke 2014, 45, 3649–3655. [Google Scholar] [CrossRef]
- Sheedy, R.; Kramer, S.F.; Johnson, L.; Shields, N.; Churilov, L.; Cadilhac, D.A.; Bernhardt, J. Acute Hospital Admission for Stroke Is Characterised by Inactivity. Stroke Res. Treat. 2020, 2020, 5879295. [Google Scholar] [CrossRef]
- Norvang, O.P.; Hokstad, A.; Taraldsen, K.; Tan, X.; Lydersen, S.; Indredavik, B.; Askim, T. Time spent lying, sitting, and upright during hospitalization after stroke: A prospective observation study. BMC Neurol. 2018, 18, 138. [Google Scholar] [CrossRef]
- Kerr, A.; Rowe, P.; Esson, D.; Barber, M. Changes in the physical activity of acute stroke survivors between inpatient and community living with early supported discharge: An observational cohort study. Physiotherapy 2016, 102, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Iacovelli, C.; Caliandro, P.; Rabuffetti, M.; Padua, L.; Simbolotti, C.; Reale, G.; Ferrarin, M.; Rossini, P.M. Actigraphic measurement of the upper limbs movements in acute stroke patients. J. Neuroeng. Rehabil. 2019, 16, 153. [Google Scholar] [CrossRef] [PubMed]
- Gebruers, N.; Truijen, S.; Engelborghs, S.; De Deyn, P.P. Predictive value of upper-limb accelerometry in acute stroke with hemiparesis. J. Rehabil. Res. Dev. 2013, 50, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Bakken, L.N.; Kim, H.S.; Finset, A.; Lerdal, A. Stroke patients’ functions in personal activities of daily living in relation to sleep and socio-demographic and clinical variables in the acute phase after first-time stroke and at six months of follow-up. J. Clin. Nurs. 2012, 21, 1886–1895. [Google Scholar] [CrossRef] [PubMed]
- Askim, T.; Bernhardt, J.; Churilov, L.; Fredriksen, K.R.; Indredavik, B. Changes in physical activity and related functional and disability levels in the first six months after stroke: A longitudinal follow-up study. J. Rehabil. Med. 2013, 45, 423–428. [Google Scholar] [CrossRef]
- Kunkel, D.; Burnett, M.; Ashburn, A. Physical inactivity post-stroke: A 3-year longitudinal study. Disabil. Rehabil. 2015, 37, 304–310. [Google Scholar] [CrossRef]
- Pitta, F.; Troosters, T.; Probst, V.S.; Spruit, M.A.; Decramer, M.; Gosselink, R. Physical activity and hospitalization for exacerbation of COPD. Chest 2006, 129, 536–544. [Google Scholar] [CrossRef]
- Ito, H.; Yokoi, D.; Kobayashi, R.; Okada, H.; Kajita, Y.; Okuda, S. The relationships between three-axis accelerometer measures of physical activity and motor symptoms in patients with Parkinson’s disease: A single-center pilot study. BMC Neurol. 2020, 20, 340. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, A.; Naito, T.; Sugiyama, M.; Okayama, T.; Aoyama, T.; Tanuma, A.; Omae, K.; Takahashi, T. Impact of Cancer Cachexia on Hospitalization-associated Physical Inactivity in Elderly Patients with Advanced Non-small-cell Lung Cancer. Asia Pac. J. Oncol. Nurs. 2018, 5, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Jonker, L.T.; Hendriks, S.; Lahr, M.M.; van Munster, B.C.; de Bock, G.H.; van Leeuwen, B.L. Postoperative recovery of accelerometer-based physical activity in older cancer patients. Eur. J. Surg. Oncol. 2020, 46, 2083–2090. [Google Scholar] [CrossRef] [PubMed]
- Ida, M.; Onodera, H.; Yamauchi, M.; Kawaguchi, M. Preoperative sleep disruption and postoperative functional disability in lung surgery patients: A prospective observational study. J. Anesth. 2019, 33, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Todd, O.M.; Gelrich, L.; MacLullich, A.M.; Driessen, M.; Thomas, C.; Kreisel, S.H. Sleep Disruption at Home As an Independent Risk Factor for Postoperative Delirium. J. Am. Geriatr. Soc. 2017, 65, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Maybrier, H.R.; King, C.R.; Crawford, A.E.; Mickle, A.M.; Emmert, D.A.; Wildes, T.S.; ENGAGES Study Investigators. Early Postoperative Actigraphy Poorly Predicts Hypoactive Delirium. J. Clin. Sleep Med. 2019, 15, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.M.; Sands, L.P.; Newman, S.; Meckler, G.; Xie, Y.; Gay, C.; Lee, K. Preoperative Sleep Disruption and Postoperative Delirium. J. Clin. Sleep Med. 2015, 11, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Twiggs, J.; Salmon, L.; Kolos, E.; Bogue, E.; Miles, B.; Roe, J. Measurement of physical activity in the pre- and early post-operative period after total knee arthroplasty for Osteoarthritis using a Fitbit Flex device. Med. Eng. Phys. 2018, 51, 31–40. [Google Scholar] [CrossRef]
- Miller, A.; Roth, T.; Roehrs, T.; Yaremchuk, K. Correlation between sleep disruption on postoperative pain. Otolaryngol.—Head Neck Surg. 2015, 152, 964–968. [Google Scholar] [CrossRef]
- Krenk, L.; Jennum, P.; Kehlet, H. Activity, sleep and cognition after fast-track hip or knee arthroplasty. J. Arthroplast. 2013, 28, 1265–1269. [Google Scholar] [CrossRef]
- Hayashi, K.; Kako, M.; Suzuki, K.; Takagi, Y.; Terai, C.; Yasuda, S.; Nishida, Y. Impact of variation in physical activity after total joint replacement. J. Pain Res. 2018, 11, 2399–2406. [Google Scholar] [CrossRef]
- Mungovan, S.F.; Singh, P.; Gass, G.C.; Smart, N.A.; Hirschhorn, A.D. Effect of physical activity in the first five days after cardiac surgery. J. Rehabil. Med. 2017, 49, 71–77. [Google Scholar] [CrossRef]
- Evensen, S.; Sletvold, O.; Lydersen, S.; Taraldsen, K. Physical activity among hospitalized older adults—An observational study. BMC Geriatr. 2017, 17, 110. [Google Scholar] [CrossRef]
- Peiris, C.L.; Taylor, N.F.; Shields, N. Patients receiving inpatient rehabilitation for lower limb orthopaedic conditions do much less physical activity than recommended in guidelines for healthy older adults: An observational study. J. Physiother. 2013, 59, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Izawa, K.P.; Watanabe, S.; Hirano, Y.; Matsushima, S.; Suzuki, T.; Oka, K.; Akashi, Y.J. Gender-related differences in maximum gait speed and daily physical activity in elderly hospitalized cardiac inpatients: A preliminary study. Medicine 2015, 94, e623. [Google Scholar] [CrossRef] [PubMed]
- Amofah, H.A.; Brostrom, A.; Fridlund, B.; Bjorvatn, B.; Haaverstad, R.; Hufthammer, K.O.; Kuiper, K.K.; Ranhoff, A.H.; Norekval, T.M.; Investigators, C. Sleep in octogenarians during the postoperative phase after transcatheter or surgical aortic valve replacement. Eur. J. Cardiovasc. Nurs. 2016, 15, 168–177. [Google Scholar] [CrossRef]
- Alessi, C.A.; Martin, J.L.; Webber, A.P.; Alam, T.; Littner, M.R.; Harker, J.O.; Josephson, K.R. More daytime sleeping predicts less functional recovery among older people undergoing inpatient post-acute rehabilitation. Sleep 2008, 31, 1291–1300. [Google Scholar] [PubMed]
- Norheim, K.L.; Cullum, C.K.; Andersen, J.L.; Kjaer, M.; Karlsen, A. Inflammation Relates to Resistance Training-induced Hypertrophy in Elderly Patients. Med. Sci. Sport. Exerc. 2017, 49, 1079–1085. [Google Scholar] [CrossRef]
- Klenk, J.; Wekenmann, S.; Schwickert, L.; Lindemann, U.; Becker, C.; Rapp, K. Change of Objectively-Measured Physical Activity during Geriatric Rehabilitation. Sensors 2019, 19, 5451. [Google Scholar] [CrossRef]
- Matsuo, T.; Sakaguchi, T.; Ishida, A.; Yuguchi, S.; Saito, K.; Nakajima, M.; Ujikawa, T.; Morisawa, T.; Chikazawa, G.; Takahashi, T. Effect of in-hospital physical activity on cardiovascular prognosis in lower extremity bypass for claudication. J. Phys. Sci. 2015, 27, 1855–1859. [Google Scholar] [CrossRef]
- Fernandes, R.; Stone, P.; Andrews, P.; Morgan, R.; Sharma, S. Comparison between fatigue, sleep disturbance, and circadian rhythm in cancer inpatients and healthy volunteers: Evaluation of diagnostic criteria for cancer-related fatigue. J. Pain Symptom Manag. 2006, 32, 245–254. [Google Scholar] [CrossRef]
- Chang, W.P.; Smith, R.; Lin, C.C. Age and rest-activity rhythm as predictors of survival in patients with newly diagnosed lung cancer. Chronobiol. Int. 2018, 35, 188–197. [Google Scholar] [CrossRef]
- Shear, T.C.; Balachandran, J.S.; Mokhlesi, B.; Spampinato, L.M.; Knutson, K.L.; Meltzer, D.O.; Arora, V.M. Risk of sleep apnea in hospitalized older patients. J. Clin. Sleep Med. 2014, 10, 1061–1066. [Google Scholar] [CrossRef]
- Sallis, R.; Roddy-Sturm, Y.; Chijioke, E.; Litman, K.; Kanter, M.H.; Huang, B.Z.; Shen, E.; Nguyen, H.Q. Stepping toward discharge: Level of ambulation in hospitalized patients. J. Hosp. Med. 2015, 10, 384–389. [Google Scholar] [CrossRef] [PubMed]
- McCullagh, R.; Dillon, C.; Dahly, D.; Horgan, N.F.; Timmons, S. Walking in hospital is associated with a shorter length of stay in older medical inpatients. Physiol. Meas. 2016, 37, 1872–1884. [Google Scholar] [CrossRef] [PubMed]
- Hartley, P.; Keevil, V.L.; Westgate, K.; White, T.; Brage, S.; Romero-Ortuno, R.; Deaton, C. Using Accelerometers to Measure Physical Activity in Older Patients Admitted to Hospital. Curr. Gerontol. Geriatr. Res. 2018, 2018, 3280240. [Google Scholar] [CrossRef] [PubMed]
- Dzierzewski, J.M.; Fung, C.H.; Jouldjian, S.; Alessi, C.A.; Irwin, M.R.; Martin, J.L. Decrease in daytime sleeping is associated with improvement in cognition after hospital discharge in older adults. J. Am. Geriatr. Soc. 2014, 62, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Beveridge, C.; Knutson, K.; Spampinato, L.; Flores, A.; Meltzer, D.O.; Van Cauter, E.; Arora, V.M. Daytime Physical Activity and Sleep in Hospitalized Older Adults: Association with Demographic Characteristics and Disease Severity. J. Am. Geriatr. Soc. 2015, 63, 1391–1400. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Kumamaru, M.; Jenkins, S.; Saitoh, M.; Morisawa, T.; Matsuda, H. In-patient step count predicts re-hospitalization after cardiac surgery. J. Cardiol. 2015, 66, 286–291. [Google Scholar] [CrossRef]
- Redeker, N.S.; Wykpisz, E. Effects of age on activity patterns after coronary artery bypass surgery. Heart Lung 1999, 28, 5–14. [Google Scholar] [CrossRef]
- Jaiswal, S.J.; Kang, D.Y.; Wineinger, N.E.; Owens, R.L. Objectively measured sleep fragmentation is associated with incident delirium in older hospitalized patients: Analysis of data collected from an randomized controlled trial. J. Sleep Res. 2020, 30, e13205. [Google Scholar] [CrossRef]
- Middelkoop, H.A.; van Dam, E.M.; Smilde-van den Doel, D.A.; Van Dijk, G. 45-hour continuous quintuple-site actimetry: Relations between trunk and limb movements and effects of circadian sleep-wake rhythmicity. Psychophysiology 1997, 34, 199–203. [Google Scholar] [CrossRef]
- Migueles, J.H.; Rowlands, A.V.; Huber, F.; Sabia, S.; van Hees, V.T. GGIR: A Research Community–Driven Open Source R Package for Generating Physical Activity and Sleep Outcomes From Multi-Day Raw Accelerometer Data. J. Meas. Phys. Behav. 2019, 2, 188–196. [Google Scholar] [CrossRef]
- Rosenberger, M.E.; Haskell, W.L.; Albinali, F.; Mota, S.; Nawyn, J.; Intille, S. Estimating activity and sedentary behavior from an accelerometer on the hip or wrist. Med. Sci. Sport. Exerc. 2013, 45, 964–975. [Google Scholar] [CrossRef] [PubMed]
- Mannini, A.; Sabatini, A.M. On-line classification of human activity and estimation of walk-run speed from acceleration data using support vector machines. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2011, 2011, 3302–3305. [Google Scholar] [PubMed]
- Moore, C.C.; McCullough, A.K.; Aguiar, E.J.; Ducharme, S.W.; Tudor-Locke, C. Toward Harmonized Treadmill-Based Validation of Step-Counting Wearable Technologies: A Scoping Review. J. Phys. Act. Health 2020, 17, 840–852. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.E.R.; Ibrahim, K.; Sayer, A.A.; Roberts, H.C. Assessment of Physical Activity of Hospitalised Older Adults: A Systematic Review. J. Nutr. Health Aging 2018, 22, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Chernbumroong, S.; Cang, S.; Yu, H. Genetic algorithm-based classifiers fusion for multisensor activity recognition of elderly people. IEEE J. Biomed. Health Inf. 2015, 19, 282–289. [Google Scholar] [CrossRef]
- Tedesco, S.; Sica, M.; Ancillao, A.; Timmons, S.; Barton, J.; O’Flynn, B. Accuracy of consumer-level and research-grade activity trackers in ambulatory settings in older adults. PLoS ONE 2019, 14, e0216891. [Google Scholar] [CrossRef]
- Floegel, T.A.; Florez-Pregonero, A.; Hekler, E.B.; Buman, M.P. Validation of Consumer-Based Hip and Wrist Activity Monitors in Older Adults with Varied Ambulatory Abilities. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 229–236. [Google Scholar] [CrossRef]
- Ekelund, U.; Tarp, J.; Steene-Johannessen, J.; Hansen, B.H.; Jefferis, B.; Fagerland, M.W.; Whincup, P.; Diaz, K.M.; Hooker, S.P.; Chernofsky, A.; et al. Dose-response associations between accelerometry measured physical activity and sedentary time and all cause mortality: Systematic review and harmonised meta-analysis. BMJ. 2019, 366, l4570. [Google Scholar] [CrossRef]
- Owen, N.; Sparling, P.B.; Healy, G.N.; Dunstan, D.W.; Matthews, C.E. Sedentary behavior: Emerging evidence for a new health risk. Mayo. Clin. Proc. 2010, 85, 1138–1141. [Google Scholar] [CrossRef]
- Dohrn, I.-M.; Gardiner, P.A.; Winkler, E.; Welmer, A.-K. Device-measured sedentary behavior and physical activity in older adults differ by demographic and health-related factors. Eur. Rev. Aging Phys. Act. 2020, 17, 8. [Google Scholar] [CrossRef]
- Reed, D.L.; Sacco, W.P. Measuring Sleep Efficiency: What Should the Denominator Be? J. Clin. Sleep Med. 2016, 12, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.; Li, Y.; Rueschman, M.N.; Winkelman, J.W.; Ellenbogen, J.M.; Solet, J.M.; Dulin, H.; Berkman, L.F.; Buxton, O.M. Measuring sleep: Accuracy, sensitivity, and specificity of wrist actigraphy compared to polysomnography. Sleep 2013, 36, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
- Sivertsen, B.; Omvik, S.; Havik, O.E.; Pallesen, S.; Bjorvatn, B.; Nielsen, G.H.; Straume, S.; Nordhus, I.H. A Comparison of Actigraphy and Polysomnography in Older Adults Treated for Chronic Primary Insomnia. Sleep 2006, 29, 1353–1358. [Google Scholar] [CrossRef]
- Holfeld, B.; Ruthig, J.C. A longitudinal examination of sleep quality and physical activity in older adults. J. Appl. Gerontol. 2014, 33, 791–807. [Google Scholar] [CrossRef] [PubMed]
- Vanderlinden, J.; Boen, F.; van Uffelen, J.G.Z. Effects of physical activity programs on sleep outcomes in older adults: A systematic review. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 11. [Google Scholar] [CrossRef]
- Montoye, A.H.K.; Moore, R.W.; Bowles, H.R.; Korycinski, R.; Pfeiffer, K.A. Reporting accelerometer methods in physical activity intervention studies: A systematic review and recommendations for authors. Br. J. Sport. Med. 2018, 52, 1507. [Google Scholar] [CrossRef]
- Migueles, J.H.; Cadenas-Sanchez, C.; Alcantara, J.M.A.; Leal-Martín, J.; Mañas, A.; Ara, I.; Glynn, N.W.; Shiroma, E.J. Calibration and Cross-Validation of Accelerometer Cut-Points to Classify Sedentary Time and Physical Activity from Hip and Non-Dominant and Dominant Wrists in Older Adults. Sensors 2021, 21, 3326. [Google Scholar] [CrossRef]
- Watson, K.B.; Carlson, S.A.; Carroll, D.D.; Fulton, J.E. Comparison of accelerometer cut points to estimate physical activity in US adults. J. Sport. Sci. 2014, 32, 660–669. [Google Scholar] [CrossRef]
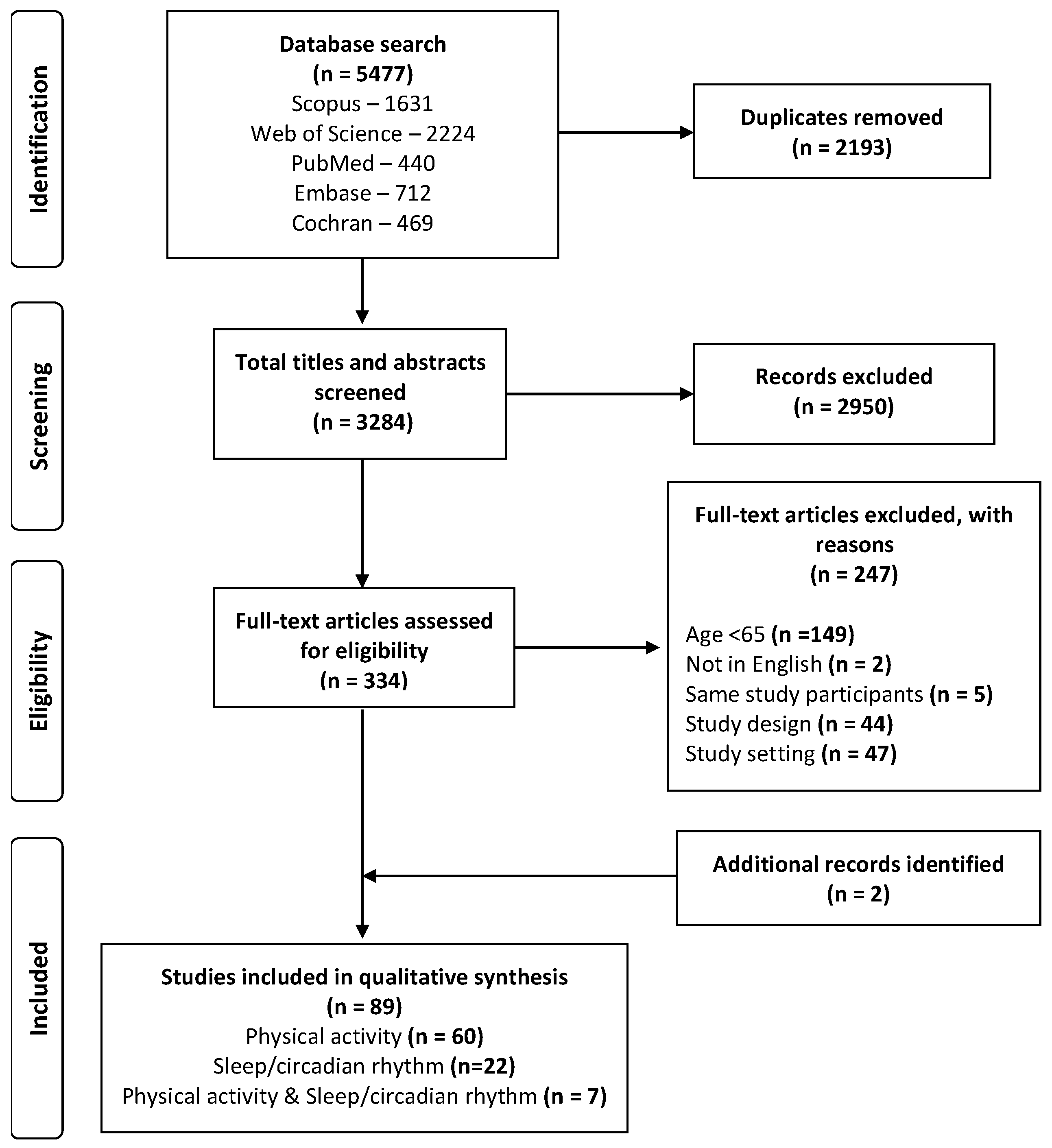

| Sensor Position | Physical Activity | SCR | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study Population Clinical Group | Author | Wrist | Thigh | Ankle | Waist | Upper Arm | Chest | Lower Back | Hip | Lower Leg | NR | General Mobility | Walking | Body Positions | Transitions | Arm Movement | Sedentary | Sleep | Circadian Rhythm |
| Stroke | (Askim et al., 2013) [86] |  |  |  |  | ||||||||||||||
| (Bakken et al., 2012) [85] |  |  | |||||||||||||||||
| (Gebruers et al., 2013) [84] | ❷ |  | |||||||||||||||||
| (Iacovelli et al., 2019) [83] | ❷ |  | |||||||||||||||||
| (Kerr et al., 2016) [82] |  |  |  |  | |||||||||||||||
| (Kunkel et al., 2015) [87] |  |  |  |  | |||||||||||||||
| (Norvang et al., 2018) [81] |  |  |  |  | |||||||||||||||
| (Sheedy et al., 2020) [80] |  |  |  |  | |||||||||||||||
| (Strommen et al., 2014) [79] | ❷ | ❷ |  |  |  | ||||||||||||||
| Respiratory | (Borges and Carvalho, 2012) [78] |  |  |  |  | ||||||||||||||
| (Dall et al., 2019) [40] |  |  |  |  |  | ||||||||||||||
| (Donaire-Gonzalez, 2011) [51] |  |  |  |  | |||||||||||||||
| (Orme et al., 2019) [77] |  |  | |||||||||||||||||
| (Pitta et al., 2006) [88] |  |  |  |  | |||||||||||||||
| (Tsai et al., 2016) [76] |  |  |  |  | |||||||||||||||
| Cardiac | (Amofah et al., 2016) [104] |  |  | ||||||||||||||||
| (Cook et al., 2013) [39] |  |  | |||||||||||||||||
| (Floegel et al., 2019) [75] |  |  |  |  |  |  | |||||||||||||
| (Gimenez et al., 2017) [50] |  |  | |||||||||||||||||
| (Izawa et al., 2015) [103] |  |  |  | ||||||||||||||||
| (Mungovan et al., 2017) [100] |  |  |  | ||||||||||||||||
| (Redeker and Wykpisz, 1999) [118] |  |  |  | ||||||||||||||||
| (Takaesu et al., 2015) [74] |  |  | |||||||||||||||||
| (Takahashi et al., 2015) [117] |  |  | |||||||||||||||||
| (Thorup et al., 2017) [53] |  |  |  | ||||||||||||||||
| Orthopedic | (Davenport et al., 2015) [73] |  |  |  |  | ||||||||||||||
| (Denkinger et al., 2014) [52] |  |  |  | ||||||||||||||||
| (Hayashi et al., 2018) [99] |  |  | |||||||||||||||||
| (Keppler et al., 2020) [72] |  |  | |||||||||||||||||
| (Krenk et al., 2013) [98] |  |  |  | ||||||||||||||||
| (Kronborg et al., 2016) [71] |  |  |  | ||||||||||||||||
| (Marsault et al., 2020) [70] |  |  |  | ||||||||||||||||
| (Miller et al., 2015) [97] |  |  | |||||||||||||||||
| (Peiris et al., 2013) [102] |  |  |  |  | |||||||||||||||
| (Schmal et al., 2018) [69] |  |  |  |  |  | ||||||||||||||
| (Twiggs et al., 2018) [96] |  |  | |||||||||||||||||
| (van Dijk-Huisman et al., 2020) [41] |  |  | |||||||||||||||||
| Mixed (Delirium, Dementia) | (Davoudi et al., 2019) [68] |  |  |  |  |  |  | ||||||||||||
| (Evensen et al., 2019) [67] |  |  |  |  |  |  | |||||||||||||
| (Fleiner et al., 2019) [66] |  |  |  |  | |||||||||||||||
| (Jaiswal et al., 2020) [119] |  |  | |||||||||||||||||
| (Leung et al., 2015) [95] |  |  | |||||||||||||||||
| (Mahlberg et al., 2007) [42] |  |  | |||||||||||||||||
| (Maybrier et al., 2019) [94] |  |  |  | ||||||||||||||||
| (Osse et al., 2009) [38] |  |  |  |  | |||||||||||||||
| (Stubbs, 2007) [43] |  |  | |||||||||||||||||
| (Tanev et al., 2017) [65] |  |  | |||||||||||||||||
| (Todd et al., 2017) [93] |  |  | |||||||||||||||||
| (Valembois et al., 2015) [33] |  |  |  | ||||||||||||||||
| Intensive Care | (Arttawejkul et al., 2020) [48] |  |  | ||||||||||||||||
| (Beecroft et al., 2008) [54] |  |  | |||||||||||||||||
| (Chen et al., 2012) [49] | * | * |  | ||||||||||||||||
| (Estrup et al., 2019) [64] |  |  | |||||||||||||||||
| Other Surgery | (Ida et al., 2019) [92] |  |  | ||||||||||||||||
| (Jonsson et al., 2019) [44] |  |  |  | ||||||||||||||||
| (Matsuo et al., 2015) [108] |  |  |  | ||||||||||||||||
| Older Adults | (Beveridge et al., 2015) [116] |  |  |  | |||||||||||||||
| (Brown et al., 2009) [3] |  |  |  |  | |||||||||||||||
| (Cohen et al., 2019) [45] |  |  | |||||||||||||||||
| (Dzierzewski et al., 2014) [115] |  |  | |||||||||||||||||
| (Evensen et al., 2017) [101] |  |  |  | ||||||||||||||||
| (Fisher, 2011) [63] |  |  | |||||||||||||||||
| (Hartley, 2018) [114] |  |  |  |  | |||||||||||||||
| (Klenk et al., 2019) [107] |  |  |  | ||||||||||||||||
| (Kolk et al., 2021) [62] |  |  | |||||||||||||||||
| (Lim et al., 2018) [61] |  |  |  |  | |||||||||||||||
| (McCullagh, 2016) [113] |  |  | |||||||||||||||||
| (Moreno et al., 2019) [46] |  |  |  |  | |||||||||||||||
| (Norheim et al., 2017) [106] |  |  |  |  | |||||||||||||||
| (Ostir et al., 2013) [10] |  |  | |||||||||||||||||
| (Pedersen et al., 2013) [4] |  |  |  |  |  | ||||||||||||||
| (Tasheva et al., 2020) [60] |  |  |  | ||||||||||||||||
| (Thorup et al., 2017) [53] |  |  | |||||||||||||||||
| Mixed admissions | (Alessi et al., 2008) [105] |  |  | ||||||||||||||||
| (Chaboyer et al., 2015) [58] |  |  |  |  | |||||||||||||||
| (Enomoto et al., 2010) [57] |  |  | |||||||||||||||||
| (Fisher et al., 2016) [9] |  |  | |||||||||||||||||
| (Macfarlane et al., 2019) [56] |  |  | |||||||||||||||||
| (Missildine et al., 2010) [55] |  |  | |||||||||||||||||
| (Sallis et al., 2015) [112] |  |  | |||||||||||||||||
| (Shear et al., 2014) [111] |  |  | |||||||||||||||||
| (Vinzio et al., 2003) [22] |  |  | |||||||||||||||||
| Oncology | (Chang et al., 2018) [110] |  |  | ||||||||||||||||
| (Fernandes et al., 2006) [109] |  |  |  |  | |||||||||||||||
| (Jakobsen et al., 2020) [37] |  |  | |||||||||||||||||
| (Jonker et al., 2020) [91] |  |  |  | ||||||||||||||||
| (Porserud et al., 2019) [47] |  |  |  |  |  |  | |||||||||||||
| Morikawa et al. (2018) [90] |  |  | |||||||||||||||||
| PAR | (Ito et al., 2020) [89] |  |  |  | |||||||||||||||
 = single sensor, ❷ = bilateral (left and right), * = optional location (either/or), row shaded = both PA and SCR outcomes. Abbreviations: PAR = Parkinson’s disease, PA = Physical activity, SCR = Sleep/circadian rhythm.
= single sensor, ❷ = bilateral (left and right), * = optional location (either/or), row shaded = both PA and SCR outcomes. Abbreviations: PAR = Parkinson’s disease, PA = Physical activity, SCR = Sleep/circadian rhythm.| Activity Domain | Description | Frequency of Papers Assessing Physical Activity Metrics % (n) |
|---|---|---|
| Volume Measures | Time spent in physical activity for a specified time frame | 97% (65) |
| Number of steps/step counts | Number of individual steps over a given time period. | 48% (32) |
| Time spent upright | Time spent standing and/or walking. | 37% (25) |
| Time spent lying and/or sitting | Time spent in a lying and/or sitting position | 24% (16) |
| Activity counts per day | An arbitrary term to describe acceleration integrated over a given time period (epoch) | 19% (13) |
| Number of postural transitions | The number of transitions from sitting to standing and/or standing to sitting. | 9% (6) |
| Number or duration of bouts | A bout is defined as the time between two activity events (e.g., an episode of stepping) | 7% (5) |
| Vector magnitude metrics | Derived from raw acceleration data in multiple axes as determined by each study: Euclidean norm minus one (ENMO)/Signal vector magnitude (SVM)/Root mean-squared activity (RMSactivity) (per time period) | 7% (5) |
| Other acceleration metrics | Epoch-related aggregated metric: Motor Activity index (MAe1:MAe2)/Asymmetry Rate Index(AR1_24h:AR2_24h) | 1% (1) |
| Intensity Measures | The rate of magnitude in which physical activities are performed, indicating metabolic demand of the activity | 24% (16) |
| Active minutes defined by various metrics | Acceleration thresholds (SVM)/counts (defined by cut points)/(can be categorised into subgroups (i.e., light/moderate) | 12% (7) |
| Moderate intensity (min per day) | Physical activity that burns between 3.0–6.0 (3.0–6.0 METs) times as much energy per minute as when resting | 3% (2) |
| Moderate or above intensity (min per day) | Physical activity that burns more than 3.0 (>3.0 METs) times as much energy per minute as when resting | 6% (4) |
| MET minutes active per day/week | A MET minute being the amount of energy expended during a minute while resting (various cut points used) | 4% (3) |
| Active/Physical energy expenditure (Calories per day) | The amount of energy required to carry out physical functions (breathing, exercising) when active | 4% (3) |
| Low/light/mild intensity (min per day) | Physical activity that burns between 1.5–3.0 (<3.0 METs) times as much energy per minute as when resting | 3% (2) |
| Total energy expenditure (Calories per day) | The amount of energy required to carry out physical functions (breathing, exercising) | 3% (2) |
| Movement intensity | Walking speed m/s2 | 3% (2) |
| Vigorous intensity (min per day) | Physical activity that burns between 6.0–9.0 (6.0–9.0 METs) times as much energy per minute as you do resting | 1% (1) |
| Very vigorous intensity (min per day) | Physical activity that burns between >9.0 (>9.0 METs) times as much energy per minute as you do resting | 1% (1) |
| Sedentary (min per day) | Physical activity that burns between 0–1.5 (0–1.5 METs) times as much energy per minute as you do resting | 1% (1) |
| Pattern Measures | The distribution of physical activity per day/week | 27% (18) |
| Night-time activity | Time spent in physical activity during night-time hours determined by each study | 13% (9) |
| Daytime activity | Time spent in physical activity during daytime hours determined by each study | 10% (7) |
| Daily variation in physical activity | Similarities/differences for time in physical activity over specified time period e.g., days | 7% (5) |
| Hour to hour variation in physical activity | Similarities/differences for time in physical activity over specified time period e.g., 24 h | 6% (4) |
| Evening activity | Time spent in physical activity during evening hours determined by each study | 6% (4) |
| Morning activity | Time spent in physical activity during morning hours determined by each study | 4% (3) |
| Afternoon activity | Time spent in physical activity during afternoon hours determined by each study | 4% (3) |
| Bout length variation | Similarities or differences in the episodes of activity (e.g., bout lengths) for a specified period of time | 1% (1) |
| Sleep and Circadian Rhythm Domain | Description | Frequency of Papers Assessing Sleep/Circadian Rhythm Metric % (n) |
|---|---|---|
| Sleep Measures | Variables used for study of sleep quality (metrics to define sleep depend on study criteria) | 86% (25) |
| Total sleep time at night | Time spent asleep over the night period. (Depending on the criteria and methodology applied to the study) | 76% (22) |
| Sleep efficiency | Percentage of time between sleep or bedtime and final awakening, which was spent asleep (Depending on the criteria and methodology applied to the study) | 48% (14) |
| Wake after sleep onset (minutes) (WASO) | Amount of time awake during the night after sleep onset to sleep offset (can be displayed as a %) | 28% (8) |
| Total sleep time during the day | Time spent asleep over the day period. (Depending on the criteria and methodology applied to the study) | 28% (8) |
| Number of awakenings | Number of wakeful events after sleep onset (Depending on the criteria and methodology applied to the study). | 28% (8) |
| Sleep-onset latency | Time from the intention to sleep to sleep onset (e.g., lights out/lying in bed, as defined by study) | 21% (6) |
| Total wake time (minutes) | Time spent awake over specified time period, e.g., at night/24 h. (Depending on the criteria and methodology applied to the study) | 17% (5) |
| Total sleep time (TST) | Time spent asleep over specified time period. (Depending on the criteria and methodology applied to the study). | 14% (4) |
| Awake index/Sleep fragmentation index | Number of awakenings divided by the time difference between the initiation of sleep and the offset of sleep | 7% (2) |
| Sleep bouts—number/duration | Sleep bout defined as between two waking events (Depending on the criteria and methodology applied to the study) | 3% (1) |
| Longest sleep episode at night (minutes) | The longest time between two waking events from the onset of sleep | 3% (1) |
| Longest wake episode at night (minutes) | The longest awake time from the onset of sleep | 3% (1) |
| Sleep pattern metrics | Standardised measure for each night’s sleep metric from a set value (usually mean) | 3% (1) |
| Circadian rhythm measures | Variables to study day night variation in rest or rest patterns that follow a 24-h cycle. | 21% (6) |
| Activity amplitude | The difference between the least active 5 h period and the most active 10 h period within each 24-h period | 10% (3) |
| Restlessness Index | Addition of percentage of time spent moving and the percentage immobility phases of 1 min | 3% (1) |
| Interdaily stability (IS) | 24-h rhythmic component in evaluating the ‘invariability’ between days | 3% (1) |
| Lowest mean activity during any stretch of 5 continuous hours (L5) | Mean activity of the 5 hr with the lowest activity within the 24 hr | 3% (1) |
| Highest mean activity during any stretch of 10 continuous hours (M10) | Mean activity of the 10 h with the highest activity within the 24 h | 3% (1) |
| Intradaily variability (IV) | Fragmentation of the rhythm (Representing the frequency and extent of transitions between rest and activity) | 3% (1) |
| I < O | A percentage of activity counts that are less than the median activity during the rest span. | 3% (1) |
| 24-h autocorrelation coefficient (R24) | Comparing activity data during each 1-min ‘‘epoch’’ of a 24-h period with the activity levels during subsequent epochs | 3% (1) |
| Cosinor parameter: acrophase | Crest time of the fitted rhythmic function, or time of peak activity | 3% (1) |
| Cosinor parameter: amplitude | Half of the difference between the peak and the trough of the rhythm, or half the maximum height of the oscillation | 3% (1) |
| Cosinor parameter: mesor | Rhythm adjusted mean | 3% (1) |
| Cosinor parameter: percent rhythm | Percent of the variance in activity accounted for by the cosine curve | 3% (1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bate, G.L.; Kirk, C.; Rehman, R.Z.U.; Guan, Y.; Yarnall, A.J.; Del Din, S.; Lawson, R.A. The Role of Wearable Sensors to Monitor Physical Activity and Sleep Patterns in Older Adult Inpatients: A Structured Review. Sensors 2023, 23, 4881. https://doi.org/10.3390/s23104881
Bate GL, Kirk C, Rehman RZU, Guan Y, Yarnall AJ, Del Din S, Lawson RA. The Role of Wearable Sensors to Monitor Physical Activity and Sleep Patterns in Older Adult Inpatients: A Structured Review. Sensors. 2023; 23(10):4881. https://doi.org/10.3390/s23104881
Chicago/Turabian StyleBate, Gemma L., Cameron Kirk, Rana Z. U. Rehman, Yu Guan, Alison J. Yarnall, Silvia Del Din, and Rachael A. Lawson. 2023. "The Role of Wearable Sensors to Monitor Physical Activity and Sleep Patterns in Older Adult Inpatients: A Structured Review" Sensors 23, no. 10: 4881. https://doi.org/10.3390/s23104881
APA StyleBate, G. L., Kirk, C., Rehman, R. Z. U., Guan, Y., Yarnall, A. J., Del Din, S., & Lawson, R. A. (2023). The Role of Wearable Sensors to Monitor Physical Activity and Sleep Patterns in Older Adult Inpatients: A Structured Review. Sensors, 23(10), 4881. https://doi.org/10.3390/s23104881










