Review and Analysis of Tumour Detection and Image Quality Analysis in Experimental Breast Microwave Sensing
Abstract
:1. Introduction
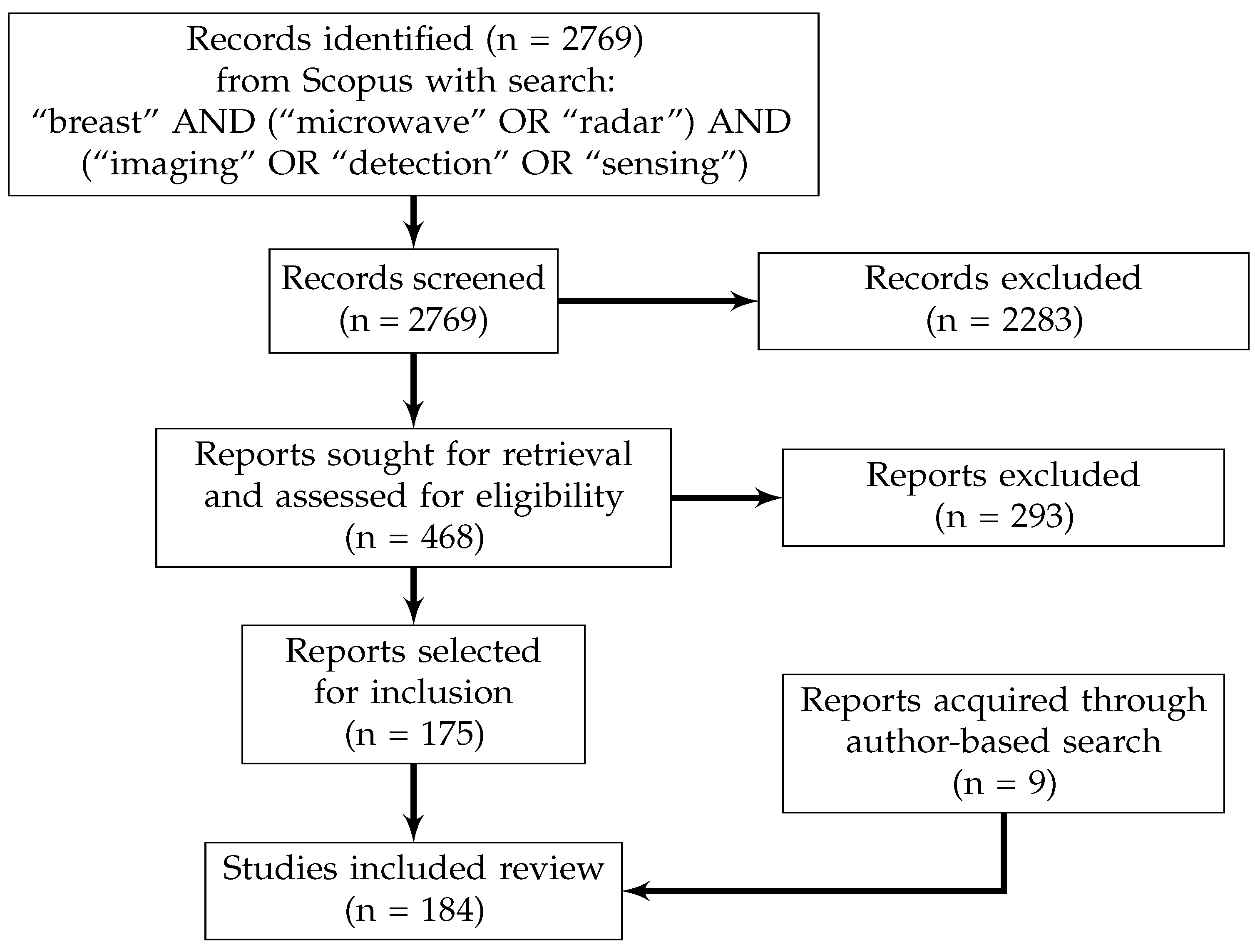
2. Review Methodology
- Used non-physical phantom materials (e.g., metal as a tumour analog);
- Examined contrast-enhanced breast microwave sensing;
- Examined multimodality imaging (e.g., using MRI-based prior information for microwave image reconstruction).
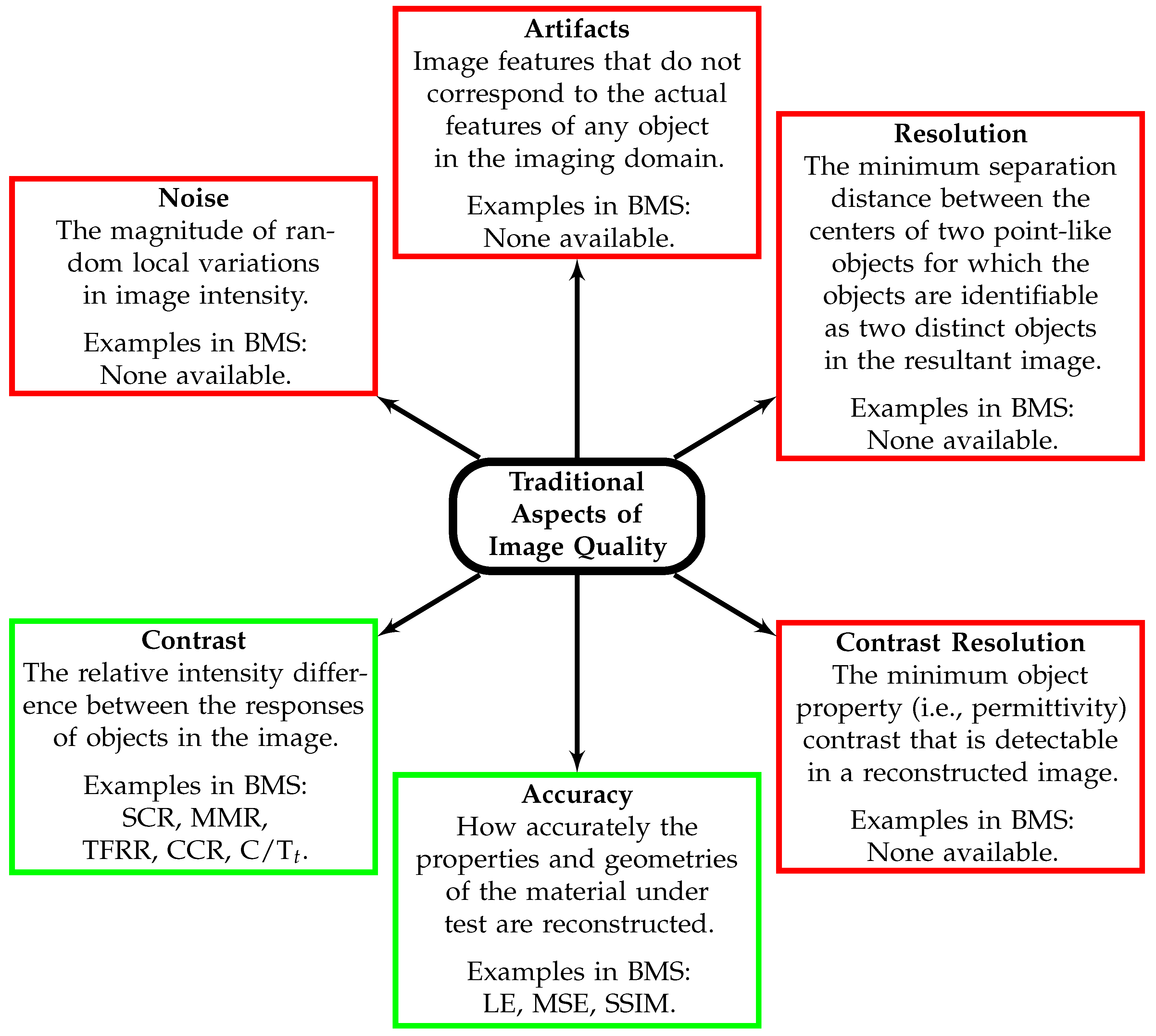
3. Image Quality Analysis
- The signal-to-clutter ratio (SCR);
- The signal-to-mean ratio (SMR);
- The mean-to-mean ratio (MMR);
- The tumour-to-fibroglandular response ratio (TFRR);
- The contrast-to-clutter ratio (CCR);
- The clutter-to-tumour ratio at threshold t (C/T);
- The localization error (LE);
- The mean squared error (and the associated family of error metrics) (MSE);
- The full-width at half-maximum area (FWHM);
- The and f metrics presented in [70];
- The structural similarity index measure (SSIM).
4. Estimates of the Diagnostic Potential of Image-based Tumour Detection
5. Machine Learning-Based Tumour Detection
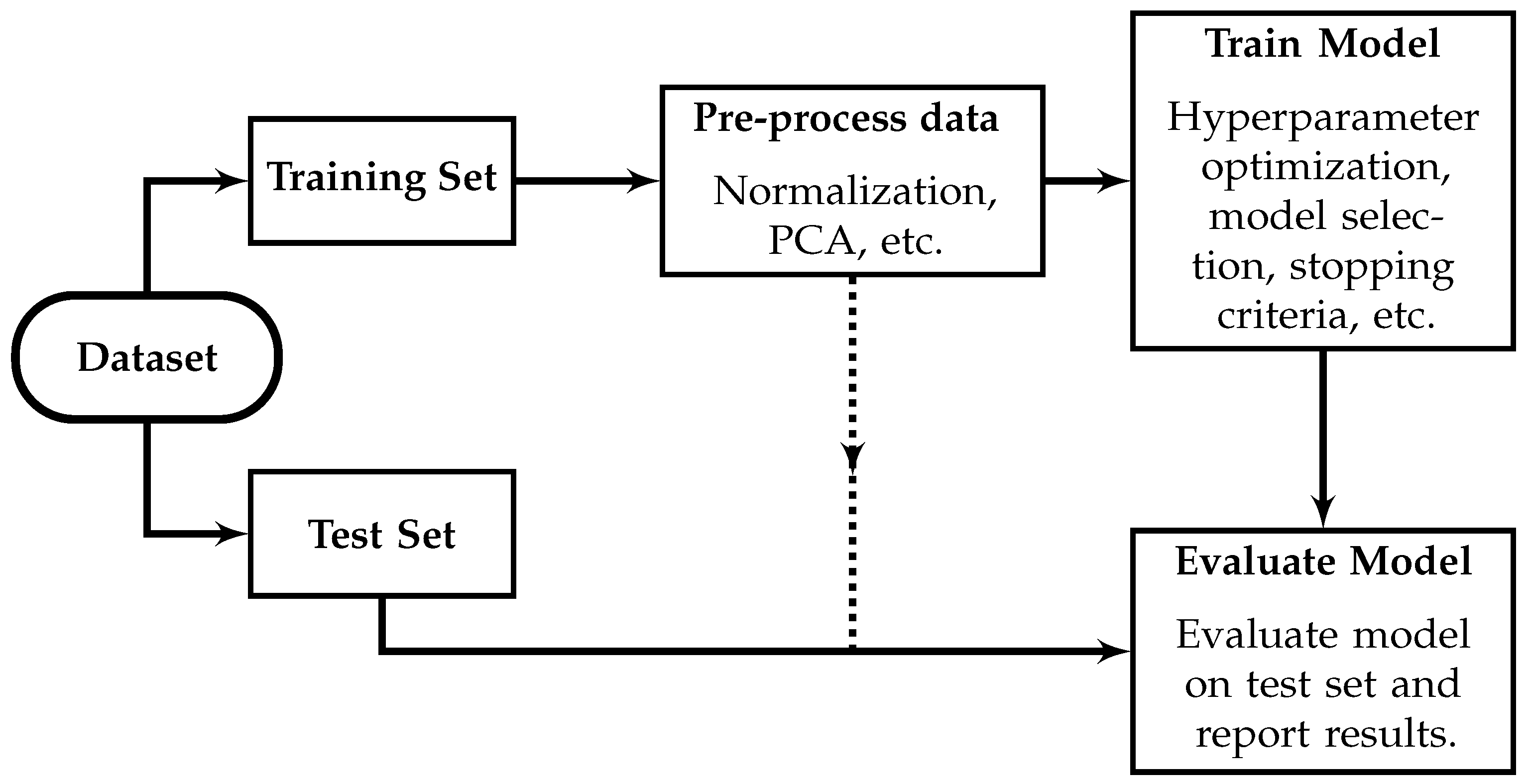
5.1. Appropriate Machine Learning Methodology
5.2. Analysis of Existing Estimates of the Diagnostic Performance of Machine Learning in Breast Microwave Sensing
6. Achievements, Challenges, and Recommendations
6.1. Achievements in Breast Microwave Sensing
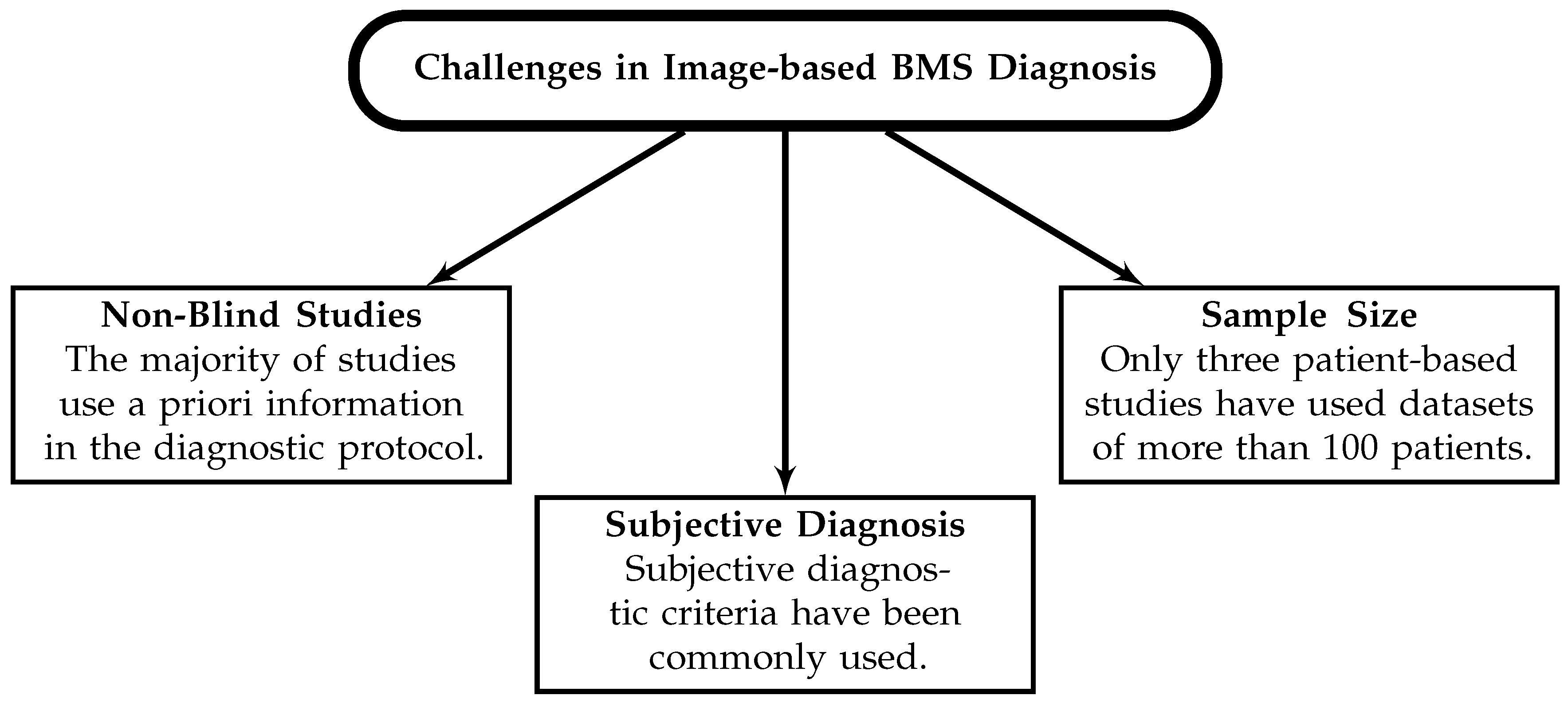
6.2. Challenges in Breast Microwave Sensing
6.3. Recommendations
- 1.
- Develop more robust image quality metrics that describe image contrast, resolution, noise, accuracy, and artifacts. Metrics that utilize distributions of intensities within an image may be more robust than current metrics, which use single-pixel values.
- 2.
- Coherence of image quality analysis within the literature should be considered when performing research. Multiple definitions for a common term, or multiple terms having the same definition, only obfuscate the academic literature surrounding breast microwave sensing. We recommend that given their relative prevalence in the literature (as the most commonly used definitions) the following definitions for the SCR, SMR, and localization error, should be used.
- 3.
- Compare reconstructed images of tumour-free and tumour-containing phantoms. The use of more robust image quality metrics will assist these comparisons, but even qualitative comparisons should be made.
- 4.
- Estimate the specificity of the technique using controlled phantom studies. Despite several patient-based investigations into the sensitivity of the modality, only two estimates of the specificity have been presented. Before patient or volunteer trials are conducted, the specificity must be estimated using controlled phantom studies.
- 5.
- Develop objective and robust tumour-detection criteria and utilize these in blind studies to estimate the modality’s diagnostic potential.
- 6.
- Published results should be reproducible, and methods should be transparent. Several articles have been missing important information that precludes result reproduction, including information regarding training and evaluation procedures of machine-learning methods, propagation speed estimation methodology (in radar-based image reconstruction), phantom information (dielectric property and geometry information), and reconstruction method. Open-source analysis, as in [53,116,118,119], and open-access datasets, as in [118,119,120], are the best methods for ensuring transparency and reproducibility and should be used when appropriate.
- 7.
- Machine-learning applications should be evaluated using diverse datasets and appropriate ML methodologies that are fully described and reproducible. Valid estimates of the diagnostic performance of ML-based diagnosis require adherence to appropriate ML standards, the use of a valid test set, and sufficient dataset diversity. The results from [118,119] should inform future work—the outer tissue geometries were a primary determinant of dataset diversity, and multiple measurements from the same phantom should be constrained to only the training or testing sets, and should not be used in both.
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMS | Breast Microwave Sensing |
| MRI | Magnetic Resonance Imaging |
| DAS | Delay-And-Sum |
| DMAS | Delay-Multiply-And-Sum |
| IDAS | Improved Delay-And-Sum |
| SCR | Signal-to-Clutter Ratio |
| SMR | Signal-to-Mean Ratio |
| TFRR | Tumour-to-Fibroglandular Response Ratio |
| CCR | Contrast-to-Clutter Ratio |
| LE | Localization Error |
| MSE | Mean Squared Error |
| FWHM | Full-Width at Half-Maximum |
| SSIM | Structural Similarity Index Measure |
| ROI | Region of Interest |
| ROC AUC | Area Under the Curve of the Receiver Operating Characteristic curve |
| ORR | Optimization-based Radar Reconstruction algorithm |
| FNR | False Negative Rate |
| TPR | True Positive Rate |
| ML | Machine Learning |
| SVM | Support Vector Machine |
| LDA | Linear Discriminant Analysis |
| QDA | Quadratic Discriminant Analysis |
| MLP | MultiLayer Perceptron |
| CNN | Convolutional Neural Network |
| DNN | Dense Neural Network |
| kNN | k-Nearest Neighbour |
| PCA | Principal Component Analysis |
Appendix A
| Index | Claim | References |
|---|---|---|
| (i) | Papers presenting images without quantitative analysis of image quality | [12,17,27,29,30,32,33,34,35,37,38,40,41,42,43,47,49,50,51,54,55,57,59,64,66,67,73,74,75,77,81,84,87,88,92,95,96,97,102,103,105,107,108,109,110,121,122,123,126,127,128,129,134,136,140,141,144,146,148,150,152,155,156,157,158,160,161,163,166,167,168,169,170,171,172,174,175,177,178,179,180,184,185,187,191,192,194,195,197] |
| (ii) | Papers presenting image-based analyses with healthy patients or phantoms | [12,25,26,30,33,40,41,42,43,45,49,50,56,63,67,69,72,84,85,87,88,90,91,100,107,108,109,110,120,122,129,131,132,133,134,135,140,142,148,158,162,165,166,169,173,175,184,186,188,190,195,198] |
| (iii) | Papers that performed quantitative analysis of healthy and unhealthy images | [25,26,45,56,61,63,69,70,72,85,90,91,100,101,120,131,132,133,142,162,165,173,186,190,198] |
| (iv) | Papers that have reported estimates of the diagnostic performance of image-based BMS | [47,94,100,111,120,132,133,134,141,143,170,201] |
| (v) | Papers that have estimated image-based diagnostic performance using patient datasets | [47,94,111,132,133,134,141,143,170,201] |
| (vi) | Papers that have estimated diagnostic performance using machine learning | [48,49,53,76,79,82,93,104,112,118,119,135,138,188,196,202] |
| (vii) | Papers that have estimated diagnostic performance using machine learning with patient datasets | [48,49,93,112,135,188,196,202] |
| (viii) | Papers that have estimated diagnostic performance using machine learning with phantom datasets | [53,76,79,82,104,118,119,138] |
| (ix) | Papers in which data leakage may have occurred | [48,82,135,138,196] |
| (x) | Papers in which data leakage explicitly occurred | [53,112,113,188,202] |
| (xi) | Papers that applied ML methods with datasets consisting of multiple measurements of the same phantom | [53,76,79,82,104,118,119,138] |
| (xii) | Papers that have reported sub-centimeter lesion detection | [13,17,25,26,32,34,36,40,43,45,47,48,51,52,54,57,58,60,64,69,71,83,99,100,114,124,129,148,154,158,163,164,167,168,170,171,172,173,174,175,177,182,187,199] |
References
- Gøtzsche, P.C.; Jørgensen, K.J. Screening for breast cancer with mammography. Cochrane Database Syst. Rev. 2013, 22, CD001877. [Google Scholar] [CrossRef] [PubMed]
- Moloney, B.M.; O’Loughlin, D.; Abd Elwahab, S.; Kerin, M.J. Breast cancer detection—A synopsis of conventional modalities and the potential role of microwave imaging. Diagnostics 2020, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Iskander, M.; Durney, C. Electromagnetic techniques for medical diagnosis: A review. Proc. IEEE 1980, 68, 126–132. [Google Scholar] [CrossRef]
- Sugitani, T.; Kubota, S.i.; Kuroki, S.i.; Sogo, K.; Arihiro, K.; Okada, M.; Kadoya, T.; Hide, M.; Oda, M.; Kikkawa, T. Complex permittivities of breast tumor tissues obtained from cancer surgeries. Appl. Phys. Lett. 2014, 104, 253702. [Google Scholar] [CrossRef]
- Lazebnik, M.; Popovic, D.; McCartney, L.; Watkins, C.B.; Lindstrom, M.J.; Harter, J.; Sewall, S.; Ogilvie, T.; Magliocco, A.; Breslin, T.M.; et al. A large-scale study of the ultrawideband microwave dielectric properties of normal, benign and malignant breast tissues obtained from cancer surgeries. Phys. Med. Biol. 2007, 52, 6093–6115. [Google Scholar] [CrossRef]
- Lazebnik, M.; McCartney, L.; Popovic, D.; Watkins, C.B.; Lindstrom, M.J.; Harter, J.; Sewall, S.; Magliocco, A.; Booske, J.H.; Okoniewski, M.; et al. A large-scale study of the ultrawideband microwave dielectric properties of normal breast tissue obtained from reduction surgeries. Phys. Med. Biol. 2007, 52, 2637. [Google Scholar] [CrossRef]
- Benny, R.; Anjit, T.A.; Mythili, P. An overview of microwave imaging for breast tumor detection. Prog. Electromagn. Res. B 2020, 87, 61–91. [Google Scholar] [CrossRef]
- Aldhaeebi, M.A.; Alzoubi, K.; Almoneef, T.S.; Bamatraf, S.M.; Attia, H.; Ramahi, O.M. Review of microwaves techniques for breast cancer detection. Sensors 2020, 20, 2390. [Google Scholar] [CrossRef]
- Kwon, S.; Lee, S. Recent advances in microwave imaging for breast cancer detection. Int. J. Biomed. Imaging 2016, 2016, 5054912. [Google Scholar] [CrossRef]
- Chandra, R.; Zhou, H.; Balasingham, I.; Narayanan, R.M. On the opportunities and challenges in microwave medical sensing and imaging. IEEE Trans. Biomed. Eng. 2015, 62, 1667–1682. [Google Scholar] [CrossRef]
- Fear, E.C.; Bourqui, J.; Curtis, C.; Mew, D.; Docktor, B.; Romano, C. Microwave breast imaging with a monostatic radar-based system: A study of application to patients. IEEE Trans. Microw. Theory Tech. 2013, 61, 2119–2128. [Google Scholar] [CrossRef]
- Fasoula, A.; Duchesne, L.; Moloney, B.; Gil Cano, J.; Chenot, C.; Oliveira, B.; Bernard, J.G.; Abd Elwahab, S.; Kerin, M. Pilot patient study with the Wavelia microwave breast imaging system for breast cancer detection: Clinical feasibility and identified technical challenges. In Proceedings of the 2020 14th European Conference on Antennas and Propagation (EuCAP), Copenhagen, Denmark, 15–20 March 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 1–5. [Google Scholar] [CrossRef]
- Klemm, M.; Craddock, I.J.; Leendertz, J.A.; Preece, A.; Benjamin, R. Radar-based breast cancer detection using a hemispherical antenna array – Experimental results. IEEE Trans. Antennas Propag. 2009, 57, 1692–1704. [Google Scholar] [CrossRef]
- Solis-Nepote, M.; Reimer, T.; Pistorius, S. An air-operated bistatic system for breast microwave radar imaging: Pre-clinical validation. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 1859–1862. [Google Scholar] [CrossRef]
- Hagness, S.C.; Taflove, A.; Bridges, J. Two-dimensional FDTD analysis of a pulsed microwave confocal system for breast cancer detection: Fixed-focus and antenna-array sensors. IEEE Trans. Biomed. Eng. 1998, 45, 1470–1479. [Google Scholar] [CrossRef]
- Lim, H.B.; Nhung, N.T.T.; Li, E.P.; Thang, N.D. Confocal microwave imaging for breast cancer detection: Delay-multiply-and-sum image reconstruction algorithm. IEEE Trans. Biomed. Eng. 2008, 55, 1697–1704. [Google Scholar] [CrossRef] [PubMed]
- Klemm, M.; Craddock, I.J.; Leendertz, J.A.; Preece, A.; Benjamin, R. Improved delay-and-sum beamforming algorithm for breast cancer detection. Int. J. Antennas Propag. 2008, 2008, 761402. [Google Scholar] [CrossRef]
- Porter, E.; O’Loughlin, D. Pathway to demonstrating clinical efficacy of microwave breast imaging: Qualitative and quantitative performance assessment. IEEE J. Electromagn. RF Microwaves Med. Biol. 2022, 6, 439–448. [Google Scholar] [CrossRef]
- Prince, J.L. Medical Imaging Signals and Systems, 2nd ed.; Pearson: Boston, MA, USA, 2015. [Google Scholar]
- O’Loughlin, D.; O’Halloran, M.; Moloney, B.M.; Glavin, M.; Jones, E.; Elahi, M.A. Microwave breast imaging: Clinical advances and remaining challenges. IEEE Trans. Biomed. Eng. 2018, 65, 2580–2590. [Google Scholar] [CrossRef]
- Misilmani, H.M.E.; Naous, T.; Khatib, S.K.A.; Kabalan, K.Y. A survey on antenna designs for breast cancer detection using microwave imaging. IEEE Access 2020, 8, 102570–102594. [Google Scholar] [CrossRef]
- Modiri, A.; Goudreau, S.; Rahimi, A.; Kiasaleh, K. Review of breast screening: Toward clinical realization of microwave imaging. Med. Phys. 2017, 44, e446–e458. [Google Scholar] [CrossRef]
- Semenov, S. Microwave tomography: Review of the progress towards clinical applications. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2009, 367, 3021–3042. [Google Scholar] [CrossRef]
- Felicio, J.M.; Bioucas-Dias, J.M.; Costa, J.R.; Fernandes, C.A. Microwave breast imaging using a dry setup. IEEE Trans. Comput. Imaging 2020, 6, 167–180. [Google Scholar] [CrossRef]
- Flores-Tapia, D.; Pistorius, S. Real time breast microwave radar image reconstruction using circular holography: A study of experimental feasibility. Med. Phys. 2011, 38, 5420–5431. [Google Scholar] [CrossRef] [PubMed]
- Flores-Tapia, D.; Rodriguez, D.; Solis, M.; Kopotun, N.; Latif, S.; Maizlish, O.; Fu, L.; Gui, Y.; Hu, C.M.; Pistorius, S. Experimental feasibility of multistatic holography for breast microwave radar image reconstruction. Med. Phys. 2016, 43, 4674–4686. [Google Scholar] [CrossRef]
- Fogel, H.C.; Hughson, M.; Asefi, M.; Jeffrey, I.; LoVetri, J. An integrated microwave-ultrasound breast imaging system: Initial phantom results. In Proceedings of the 2022 16th European Conference on Antennas and Propagation (EuCAP), Madrid, Spain, 27 March–1 April 2022; IEEE: Piscataway, NJ, USA, 2022; pp. 1–5. [Google Scholar] [CrossRef]
- Ghavami, N.; Probert Smith, P.; Tiberi, G.; Edwards, D.; Craddock, I. Non-iterative beamforming based on Huygens principle for multistatic ultrawide band radar: Application to breast imaging. IET Microwaves Antennas Propag. 2015, 9, 1233–1240. [Google Scholar] [CrossRef]
- Golnabi, A.H.; Geimer, S.D.; Meaney, P.M.; Paulsen, K.D. Comparison of no-prior and soft-prior regularization in biomedical microwave imaging. J. Med. Phys. 2011, 36, 159–170. [Google Scholar] [CrossRef]
- Golnabi, A.H.; Meaney, P.M.; Epstein, N.R.; Paulsen, K.D. Microwave imaging for breast cancer detection: Advances in three dimensional image reconstruction. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; IEEE: Piscataway, NJ, USA, 2011; pp. 5730–5733. [Google Scholar] [CrossRef]
- Golnabi, A.H.; Meaney, P.M.; Paulsen, K.D. 3D microwave tomography of the breast using prior anatomical information. Med. Phys. 2016, 43, 1933–1944. [Google Scholar] [CrossRef]
- Grzegorczyk, T.M.; Meaney, P.M.; Jeon, S.I.; Geimer, S.D.; Paulsen, K.D. Importance of phase unwrapping for the reconstruction of microwave tomographic images. Biomed. Opt. Express 2011, 2, 315–330. [Google Scholar] [CrossRef]
- Grzegorczyk, T.M.; Meaney, P.M.; Kaufman, P.A.; di Florio-Alexander, R.M.; Paulsen, K.D. Fast 3-D tomographic microwave imaging for breast cancer detection. IEEE Trans. Med. Imaging 2012, 31, 1584–1592. [Google Scholar] [CrossRef]
- Guruswamy, S.; Chinniah, R.; Thangavelu, K. Design and implementation of compact ultra-wideband vivaldi antenna with directors for microwave-based imaging of breast cancer. Analog. Integr. Circuits Signal Process. 2021, 108, 45–57. [Google Scholar] [CrossRef]
- Halter, R.J.; Zhou, T.; Meaney, P.M.; Hartov, A.; Barth, R.J.; Rosenkranz, K.M.; Wells, W.A.; Kogel, C.A.; Borsic, A.; Rizzo, E.J.; et al. The correlation of in vivo and ex vivo tissue dielectric properties to validate electromagnetic breast imaging: Initial clinical experience. Physiol. Meas. 2009, 30, S121–S136. [Google Scholar] [CrossRef]
- Hathal, M.S.; Salih, S.S.; Hasan, A.H. Ultra-wideband featuring enhanced delay and sum algorithm and oriented for detecting early stage breast cancer. Prog. Electromagn. Res. M 2021, 100, 141–150. [Google Scholar] [CrossRef]
- Helbig, M.; Dahlke, K.; Hilger, I.; Kmec, M.; Sachs, J. Design and test of an imaging system for UWB breast cancer detection. Frequenz 2012, 66, 387–394. [Google Scholar] [CrossRef]
- Helbig, M.; Faenger, B.; Ley, S.; Hilger, I. Multistatic M-sequence UWB radar system for microwave breast imaging. In Proceedings of the 2021 IEEE Conference on Antenna Measurements & Applications (CAMA), Antibes Juan-les-Pins, France, 15–17 November 2021; IEEE: Piscataway, NJ, USA, 2021; pp. 540–545. [Google Scholar] [CrossRef]
- Henriksson, T.; Joachimowicz, N.; Conessa, C.; Bolomey, J.C. Quantitative microwave imaging for breast cancer detection using a planar 2.45 GHz system. IEEE Trans. Instrum. Meas. 2010, 59, 2691–2699. [Google Scholar] [CrossRef]
- Hossain, A.; Islam, M.T.; Islam, M.T.; Chowdhury, M.E.H.; Rmili, H.; Samsuzzaman, M. A planar ultrawideband patch antenna array for microwave breast tumor detection. Materials 2020, 13, 4918. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Samsuzzaman, M.; Islam, M.T.; Kibria, S.; Singh, M.J. A homogeneous breast phantom measurement system with an improved modified microwave imaging antenna sensor. Sensors 2018, 18, 2962. [Google Scholar] [CrossRef]
- Islam, M.; Samsuzzaman, M.; Islam, M.; Kibria, S. Experimental breast phantom imaging with metamaterial-inspired nine-antenna sensor array. Sensors 2018, 18, 4427. [Google Scholar] [CrossRef]
- Islam, M.T.; Samsuzzaman, M.; Kibria, S.; Islam, M.T. Experimental breast phantoms for estimation of breast tumor using microwave imaging systems. IEEE Access 2018, 6, 78587–78597. [Google Scholar] [CrossRef]
- Islam, M.T.; Mahmud, M.Z.; Islam, M.T.; Kibria, S.; Samsuzzaman, M. A low cost and portable microwave imaging system for breast tumor detection using UWB directional antenna array. Sci. Rep. 2019, 9, 15491. [Google Scholar] [CrossRef]
- Islam, M.T.; Islam, M.T.; Samsuzzaman, M.; Kibria, S.; Chowdhury, M.E.H. Microwave breast imaging using compressed sensing approach of iteratively corrected delay multiply and sum beamforming. Diagnostics 2021, 11, 470. [Google Scholar] [CrossRef]
- Jalilvand, M.; Li, X.; Zwirello, L.; Zwick, T. Ultra wideband compact near-field imaging system for breast cancer detection. IET Microwaves Antennas Propag. 2015, 9, 1009–1014. [Google Scholar] [CrossRef]
- Janjic, A.; Cayoren, M.; Akduman, I.; Yilmaz, T.; Onemli, E.; Bugdayci, O.; Aribal, M.E. SAFE: A novel microwave imaging system design for breast cancer screening and early detection—Clinical evaluation. Diagnostics 2021, 11, 533. [Google Scholar] [CrossRef] [PubMed]
- Janjic, A.; Akduman, I.; Cayoren, M.; Bugdayci, O.; Aribal, M.E. Microwave breast lesion classification—Results from clinical investigation of the SAFE microwave breast cancer system. Acad. Radiol. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Janjic, A.; Akduman, I.; Cayoren, M.; Bugdayci, O.; Aribal, M.E. Gradient-boosting algorithm for microwave breast lesion classification—SAFE clinical investigation. Diagnostics 2022, 12, 3151. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.I.; Kim, B.R.; Son, S.H. Clinical trial of microwave tomography imaging. In Proceedings of the 2016 URSI Asia-Pacific Radio Science Conference (URSI AP-RASC), Seoul, Republic of Korea, 21–25 August 2016; IEEE: Piscataway, NJ, USA, 2016; pp. 1–2. [Google Scholar] [CrossRef]
- Kaur, G.; Kaur, A. Monostatic radar-based microwave imaging of breast tumor detection using a compact cubical dielectric resonator antenna. Microw. Opt. Technol. Lett. 2021, 63, 196–204. [Google Scholar] [CrossRef]
- Kaur, G.; Kaur, A. “C” shaped dual polarized dielectric resonator antenna for the microwave imaging of breast tumor using beam-forming algorithms. Int. J. Microw. Comput.-Aided Eng. 2022, 32, e23178. [Google Scholar] [CrossRef]
- Al Khatib, S.K.; Naous, T.; Shubair, R.M.; el Misilmani, H.M. A deep learning framework for breast tumor detection and localization from microwave imaging data. In Proceedings of the 2021 28th IEEE International Conference on Electronics, Circuits, and Systems (ICECS), Dubai, United Arab Emirates, 28 November–1 December 2021; IEEE: Piscataway, NJ, USA, 2021; pp. 1–4. [Google Scholar] [CrossRef]
- Khor, W.C.; Abu Bakar, A.; Bialkowski, M.E. Investigations into breast cancer detection using ultra wide band microwave radar technique. In Proceedings of the 2009 Asia Pacific Microwave Conference, Singapore, 10–13 December 2019; IEEE: Piscataway, NJ, USA, 2009; pp. 712–715. [Google Scholar] [CrossRef]
- Khoshdel, V.; Asefi, M.; Ashraf, A.; LoVetri, J. Full 3D microwave breast imaging using a deep-learning technique. J. Imaging 2020, 6, 80. [Google Scholar] [CrossRef]
- Kibria, S.; Samsuzzaman, M.; Islam, M.T.; Mahmud, M.Z.; Misran, N.; Islam, M.T. Breast phantom imaging using iteratively corrected coherence factor delay and sum. IEEE Access 2019, 7, 40822–40832. [Google Scholar] [CrossRef]
- Klemm, M.; Craddock, I.; Leendertz, J.; Preece, A.; Benjamin, R. Experimental and clinical results of breast cancer detection using UWB microwave radar. In Proceedings of the 2008 IEEE Antennas and Propagation Society International Symposium, San Diego, CA, USA, 5–11 July 2008; IEEE: Piscataway, NJ, USA, 2008; pp. 1–4. [Google Scholar] [CrossRef]
- Klemm, M.; Leendertz, J.; Gibbins, D.; Craddock, I.; Preece, A.; Benjamin, R. Microwave radar-based breast cancer detection: Imaging in inhomogeneous breast phantoms. IEEE Antennas Wirel. Propag. Lett. 2009, 8, 1349–1352. [Google Scholar] [CrossRef]
- Klemm, M.; Craddock, I.J.; Leendertz, J.A.; Preece, A.; Gibbins, D.R.; Shere, M.; Benjamin, R. Clinical trials of a UWB imaging radar for breast cancer. In Proceedings of the Fourth European Conference on Antennas and Propagation, Barcelona, Spain, 12–16 April 2010; IEEE: Piscataway, NJ, USA, 2010; pp. 1–4. [Google Scholar]
- Klemm, M.; Leendertz, J.A.; Gibbins, D.; Craddock, I.J.; Preece, A.; Benjamin, R. Microwave radar-based differential breast cancer imaging: Imaging in homogeneous breast phantoms and low contrast scenarios. IEEE Trans. Antennas Propag. 2010, 58, 2337–2344. [Google Scholar] [CrossRef]
- Kranold, L.; Taherzadeh, M.; Nabki, F.; Coates, M.; Popovic, M. Microwave breast screening prototype: System miniaturization with IC pulse radio. IEEE J. Electromagn. RF Microwaves Med. Biol. 2021, 5, 168–178. [Google Scholar] [CrossRef]
- Kranold, L.; Ozmen, M.; Coates, M.; Popovic, M. Microwave radar for breast health monitoring: System performance protocol. In Proceedings of the 2020 IEEE MTT-S International Microwave Biomedical Conference (IMBioC), Toulouse, France, 14–17 December 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 1–3. [Google Scholar] [CrossRef]
- Kranold, L.; Popovic, M. RF radar nreast health monitoring: System evaluation with post-biopsy marker. IEEE J. Electromagn. RF Microwaves Med. Biol. 2021, 5, 148–154. [Google Scholar] [CrossRef]
- Kumari, V.; Ahmed, A.; Kanumuri, T.; Shakher, C.; Sheoran, G. Early detection of cancerous tissues in human breast utilizing near field microwave holography. Int. J. Imaging Syst. Technol. 2020, 30, 391–400. [Google Scholar] [CrossRef]
- Kurrant, D.J.; Fear, E.C.; Westwick, D.T. Tumor response estimation in radar-based microwave breast cancer detection. IEEE Trans. Biomed. Eng. 2008, 55, 2801–2811. [Google Scholar] [CrossRef] [PubMed]
- Kurrant, D.; Bourqui, J.; Fear, E. Surface estimation for microwave imaging. Sensors 2017, 17, 1658. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, Y.; Miura, S.; Nishina, Y.; Mukumoto, K.; Ogura, H.; Sakahara, H. Clinical test of microwave mammography. In Proceedings of the 2013 IEEE Antennas and Propagation Society International Symposium (APSURSI), 7–13 July 2013; IEEE: Piscataway, NJ, USA, 2013; pp. 2028–2029. [Google Scholar] [CrossRef]
- Lai, J.C.Y.; Soh, C.B.; Gunawan, E.; Low, K.S. UWB microwave imaging for breast cancer detection—Experiments with heterogeneous breast phantoms. Prog. Electromagn. Res. M 2011, 16, 19–29. [Google Scholar] [CrossRef]
- Latif, S.I.; Flores Tapia, D.; Rodriguez Herrera, D.; Solis Nepote, M.; Pistorius, S.; Shafai, L. A directional antenna in a matching liquid for microwave radar imaging. Int. J. Antennas Propag. 2015, 2015, 751739. [Google Scholar] [CrossRef]
- Lavoie, B.R.; Okoniewski, M.; Fear, E.C. Estimating the effective permittivity for reconstructing accurate microwave-radar images. PLoS ONE 2016, 11, e0160849. [Google Scholar] [CrossRef]
- Lazaro, A.; Girbau, D.; Villarino, R. Simulated and experimental investigation of microwave imaging using UWB. Prog. Electromagn. Res. 2009, 94, 263–280. [Google Scholar] [CrossRef]
- Li, D.; Meaney, P.M.; Tosteson, T.D.; Jiang, S.; Kerner, T.E.; McBride, T.O.; Pogue, B.W.; Hartov, A.; Paulsen, K.D. Comparisons of three alternative breast modalities in a common phantom imaging experiment. Med. Phys. 2003, 30, 2194–2205. [Google Scholar] [CrossRef]
- Dun, L.; Meaney, P.; Paulsen, K. Conformal microwave imaging for breast cancer detection. IEEE Trans. Microw. Theory Tech. 2003, 51, 1179–1186. [Google Scholar] [CrossRef]
- Li, D.; Meaney, P.M.; Raynolds, T.; Pendergrass, S.A.; Fanning, M.W.; Paulsen, K.D. Parallel-detection microwave spectroscopy system for breast imaging. Rev. Sci. Instrum. 2004, 75, 2305–2313. [Google Scholar] [CrossRef]
- Li, Q.; Xiao, X.; Wang, L.; Song, H.; Kono, H.; Liu, P.; Lu, H.; Kikkawa, T. Direct extraction of tumor response based on ensemble empirical mode decomposition for image reconstruction of early breast cancer detection by UWB. IEEE Trans. Biomed. Circuits Syst. 2015, 9, 710–724. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Porter, E.; Santorelli, A.; Popovic, M.; Coates, M. Microwave breast cancer detection via cost-sensitive ensemble classifiers: Phantom and patient investigation. Biomed. Signal Process. Control 2017, 31, 366–376. [Google Scholar] [CrossRef]
- Li, H.; Zhang, H.; Kong, Y.; Zhou, C. Flexible dual-polarized UWB antenna sensors for breast tumor detection. IEEE Sens. J. 2022, 22, 13648–13658. [Google Scholar] [CrossRef]
- Liu, G.; Xiao, X.; Song, H.; Lu, M.; Kikkawa, T. An adaptive window-based hybrid artifact removal method for ultra-wide band imaging enhancement of early breast cancer detection. Biomed. Signal Process. Control 2021, 70, 102980. [Google Scholar] [CrossRef]
- Lu, M.; Xiao, X.; Pang, Y.; Liu, G.; Lu, H. Detection and localization of breast cancer using UWB microwave technology and CNN-LSTM framework. IEEE Trans. Microw. Theory Tech. 2022, 70, 5085–5094. [Google Scholar] [CrossRef]
- Lu, M.; Xiao, X.; Liu, G.; Lu, H.; Pang, Y.; Kikkawa, T. Breast tumor detection by 1D-convolutional neural network based on ultra-wide-band microwave technology. Meas. Sci. Technol. 2023, 34, 025702. [Google Scholar] [CrossRef]
- Ma, H.; Sasada, S.; Okada, M.; Kikkawa, T.; Kidera, S. Clinical test of surface rejection method for microwave breast cancer imaging. In Proceedings of the 2021 IEEE USNC-URSI Radio Science Meeting (Joint with AP-S Symposium), Singapore, 4–10 December 2021; IEEE: Piscataway, NJ, USA, 2021; pp. 43–44. [Google Scholar] [CrossRef]
- Martins, R.A.; Felicio, J.M.; Costa, J.R.; Fernandes, C.A. Comparison of slot-based and Vivaldi antennas for breast tumor detection using machine learning and microwave imaging algorithms. In Proceedings of the 2021 15th European Conference on Antennas and Propagation (EuCAP), Dusseldorf, Germany, 22–26 March 2021; IEEE: Piscataway, NJ, USA, 2021; pp. 1–5. [Google Scholar] [CrossRef]
- Martins, R.A.; Felicio, J.M.; Costa, J.R.; Fernandes, C.A. Systematic analysis of microwave breast imaging detection of different-sized malignant and benign tumors. In Proceedings of the 2022 16th European Conference on Antennas and Propagation (EuCAP), Madrid, Spain, 27 March–1 April 2022; IEEE: Piscataway, NJ, USA, 2022; pp. 1–4. [Google Scholar] [CrossRef]
- Meaney, P.; Fanning, M.; Li, D.; Poplack, S.; Paulsen, K. A clinical prototype for active microwave imaging of the breast. IEEE Trans. Microw. Theory Tech. 2000, 48, 1841–1853. [Google Scholar] [CrossRef]
- Meaney, P.M.; Demidenko, E.; Yagnamurthy, N.K.; Li, D.; Fanning, M.W.; Paulsen, K.D. A two-stage microwave image reconstruction procedure for improved internal feature extraction. Med. Phys. 2001, 28, 2358–2369. [Google Scholar] [CrossRef]
- Meaney, P.M.; Yagnamurthy, N.K.; Paulsen, K.D. Pre-scaled two-parameter Gauss-Newton image reconstruction to reduce property recovery imbalance. Phys. Med. Biol. 2002, 47, 1101–1119. [Google Scholar] [CrossRef]
- Meaney, P.M.; Pendergrass, S.A.; Fanning, M.W.; Paulsen, K.D. Importance of using a reduced contrast coupling medium in 2D microwave breast imaging. J. Electromagn. Waves Appl. 2003, 17, 333–355. [Google Scholar] [CrossRef]
- Meaney, P.M.; Fanning, M.W.; Raynolds, T.; Fox, C.J.; Fang, Q.; Kogel, C.A.; Poplack, S.P.; Paulsen, K.D. Initial clinical experience with microwave breast imaging in women with normal mammography. Acad. Radiol. 2007, 14, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Meaney, P.M.; Fang, Q.; Rubaek, T.; Demidenko, E.; Paulsen, K.D. Log transformation benefits parameter estimation in microwave tomographic imaging. Med. Phys. 2007, 34, 2014–2023. [Google Scholar] [CrossRef] [PubMed]
- Meaney, P.M.; Kaufman, P.A.; Muffly, L.S.; Click, M.; Poplack, S.P.; Wells, W.A.; Schwartz, G.N.; di Florio-Alexander, R.M.; Tosteson, T.D.; Li, Z.; et al. Microwave imaging for neoadjuvant chemotherapy monitoring: Initial clinical experience. Breast Cancer Res. 2013, 15, R35. [Google Scholar] [CrossRef]
- Meaney, P.M.; Geimer, S.D.; Paulsen, K.D. Two-step inversion with a logarithmic transformation for microwave breast imaging. Med. Phys. 2017, 44, 4239–4251. [Google Scholar] [CrossRef]
- Medina, Y.; Augusto, M.; Paz, A.V. Microwave imaging for breast cancer detection: Experimental comparison of confocal and holography algorithms. In Proceedings of the 2016 IEEE ANDESCON, Arequipa, Peru, 19–21 October 2016; IEEE: Piscataway, NJ, USA, 2016; pp. 1–4. [Google Scholar] [CrossRef]
- Moloney, B.M.; McAnena, P.F.; Elwahab, S.M.; Fasoula, A.; Duchesne, L.; Gil Cano, J.D.; Glynn, C.; O’Connell, A.; Ennis, R.; Lowery, A.J.; et al. The Wavelia microwave breast imaging system–tumour discriminating features and their clinical usefulness. Br. J. Radiol. 2021, 94, 20210907. [Google Scholar] [CrossRef]
- Moloney, B.M.; McAnena, P.F.; Abd Elwahab, S.M.; Fasoula, A.; Duchesne, L.; Gil Cano, J.D.; Glynn, C.; O’Connell, A.; Ennis, R.; Lowery, A.J.; et al. Microwave imaging in breast cancer – Results from the first-in-human clinical investigation of the Wavelia system. Acad. Radiol. 2022, 29, S211–S222. [Google Scholar] [CrossRef]
- Nemez, K.; Baran, A.; Asefi, M.; LoVetri, J. Modeling error and calibration techniques for a faceted metallic chamber for magnetic field microwave imaging. IEEE Trans. Microw. Theory Tech. 2017, 65, 4347–4356. [Google Scholar] [CrossRef]
- Norouzzadeh, E.; Chamaani, S.; Moll, J.; Kexel, C.; Nguyen, D.H.; Hübner, F.; Bazrafshan, B.; Vogl, T.J.; Krozer, V. Numerical and experimental analysis of a transmission-based breast imaging system: A study of application to patients. Int. J. Microw. Wirel. Technol. 2020, 12, 469–476. [Google Scholar] [CrossRef]
- Oliveira, B.L.; O’Loughlin, D.; O’Halloran, M.; Porter, E.; Glavin, M.; Jones, E. Microwave breast imaging: Experimental tumour phantoms for the evaluation of new breast cancer diagnosis systems. Biomed. Phys. Eng. Express 2018, 4, 025036. [Google Scholar] [CrossRef]
- O’Loughlin, D.; Oliveira, B.; Elahi, M.; Glavin, M.; Jones, E.; Popovic, M.; O’Halloran, M. Parameter search algorithms for microwave radar-based breast imaging: Focal quality metrics as fitness functions. Sensors 2017, 17, 2823. [Google Scholar] [CrossRef]
- O’Loughlin, D.; Glavin, M.; Jones, E.; O’Halloran, M. Evaluation of experimental microwave radar-based images: Evaluation criteria. In Proceedings of the 2018 IEEE International Symposium on Antennas and Propagation & USNC/URSI National Radio Science Meeting, Boston, MA, USA, 8–13 July 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 895–896. [Google Scholar] [CrossRef]
- O’Loughlin, D.; Oliveira, B.L.; Santorelli, A.; Porter, E.; Glavin, M.; Jones, E.; Popovic, M.; O’Halloran, M. Sensitivity and specificity estimation using patient-specific microwave imaging in diverse experimental breast phantoms. IEEE Trans. Med. Imaging 2019, 38, 303–311. [Google Scholar] [CrossRef] [PubMed]
- O’Loughlin, D.; Elahi, M.A.; Lavoie, B.R.; Fear, E.C.; O’Halloran, M. Assessing patient-specific microwave breast imaging in clinical case studies. Sensors 2021, 21, 8048. [Google Scholar] [CrossRef] [PubMed]
- Oloumi, D.; Winter, R.S.C.; Kordzadeh, A.; Boulanger, P.; Rambabu, K. Microwave imaging of breast tumor using time-domain UWB circular-SAR technique. IEEE Trans. Med. Imaging 2020, 39, 934–943. [Google Scholar] [CrossRef]
- Ozmen, H.; Kurt, M.B. Radar-based microwave breast cancer detection system with a high-performance ultrawide band antipodal Vivaldi antenna. Turk. J. Electr. Eng. Comput. Sci. 2021, 29, 2326–2345. [Google Scholar] [CrossRef]
- Patel, P.; Raina, A. Comparison of machine learning algorithms for tumor detection in breast microwave imaging. In Proceedings of the 2021 11th International Conference on Cloud Computing, Data Science & Engineering (Confluence), Noida, India, 28–29 January 2021; IEEE: Piscataway, NJ, USA, 2021; pp. 882–886. [Google Scholar] [CrossRef]
- Porter, E.; Kirshin, E.; Santorelli, A.; Popovic, M. Microwave breast screening in the time-domain: Identification and compensation of measurement-induced uncertainties. Prog. Electromagn. Res. B 2013, 55, 115–130. [Google Scholar] [CrossRef]
- Porter, E.; Kirshin, E.; Santorelli, A.; Coates, M.; Popovic, M. Time-domain multistatic radar system for microwave breast screening. IEEE Antennas Wirel. Propag. Lett. 2013, 12, 229–232. [Google Scholar] [CrossRef]
- Porter, E.; Santorelli, A.; Popovic, M. Time-domain microwave radar applied to breast imaging: Measurement reliability in a clinical setting. Prog. Electromagn. Res. 2014, 149, 119–132. [Google Scholar] [CrossRef]
- Porter, E.; Santorelli, A.; Kazemi, R.; Popovic, M. Microwave time-domain radar: Healthy tissue variations over the menstrual cycle. IEEE Antennas Wirel. Propag. Lett. 2015, 14, 1310–1313. [Google Scholar] [CrossRef]
- Porter, E.; Coates, M.; Popovic, M. An early clinical study of time-domain microwave radar for breast health monitoring. IEEE Trans. Biomed. Eng. 2016, 63, 530–539. [Google Scholar] [CrossRef]
- Porter, E.; Bahrami, H.; Santorelli, A.; Gosselin, B.; Rusch, L.A.; Popovic, M. A wearable microwave antenna array for time-domain breast tumor screening. IEEE Trans. Med. Imaging 2016, 35, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Preece, A.W.; Craddock, I.; Shere, M.; Jones, L.; Winton, H.L. MARIA M4: Clinical evaluation of a prototype ultrawideband radar scanner for breast cancer detection. J. Med. Imaging 2016, 3, 033502. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.P.; Dey, M.; Tiberi, G.; Sani, L.; Vispa, A.; Raspa, G.; Duranti, M.; Ghavami, M.; Dudley, S. Machine learning approaches for automated lesion detection in microwave breast imaging clinical data. Sci. Rep. 2019, 9, 10510. [Google Scholar] [CrossRef]
- Rana, S.P.; Dey, M.; Loretoni, R.; Duranti, M.; Sani, L.; Vispa, A.; Ghavami, M.; Dudley, S.; Tiberi, G. Radial basis function for breast lesion detection from MammoWave clinical data. Diagnostics 2021, 11, 1930. [Google Scholar] [CrossRef] [PubMed]
- Rasappan, R.; Anwar, N.S.N.; Zanoon, T.F.; Sun, T.S.; Ain, M.F.; Abdullah, M.Z. Microwave 3D imaging system featuring the phase coherence factor for improved beamforming. Curr. Med. Imaging Former. Curr. Med. Imaging Rev. 2022, 18, 939–951. [Google Scholar] [CrossRef]
- Reimer, T.; Nepote, M.S.; Pistorius, S. Initial results using an MLEM-based reconstruction algorithm for breast microwave radar imaging. In Proceedings of the 2018 18th International Symposium on Antenna Technology and Applied Electromagnetics (ANTEM), Waterloo, ON, Canada, 19–22 August 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 1–2. [Google Scholar] [CrossRef]
- Reimer, T.; Solis-Nepote, M.; Pistorius, S. The application of an iterative structure to the delay-and-sum and the delay-multiply-and-sum beamformers in breast microwave imaging. Diagnostics 2020, 10, 411. [Google Scholar] [CrossRef]
- Reimer, T.; Solis-Nepote, M.; Pistorius, S. The impact of the inverse chirp z-transform on breast microwave radar image reconstruction. Int. J. Microw. Wirel. Technol. 2020, 12, 848–854. [Google Scholar] [CrossRef]
- Reimer, T.; Krenkevich, J.; Pistorius, S. An open-access experimental dataset for breast microwave imaging. In Proceedings of the 2020 14th European Conference on Antennas and Propagation (EuCAP), Copenhagen, Denmark, 15–20 March 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 1–5. [Google Scholar] [CrossRef]
- Reimer, T.; Pistorius, S. The diagnostic performance of machine learning in breast microwave sensing on an experimental dataset. IEEE J. Electromagn. RF Microwaves Med. Biol. 2022, 6, 139–145. [Google Scholar] [CrossRef]
- Reimer, T.; Pistorius, S. An optimization-based approach to radar image reconstruction in breast microwave sensing. Sensors 2021, 21, 8172. [Google Scholar] [CrossRef]
- Herrera, D.R.; Reimer, T.; Nepote, M.S.; Pistorius, S. Manufacture and testing of anthropomorphic 3D-printed breast phantoms using a microwave radar algorithm optimized for propagation speed. In Proceedings of the 2017 11th European Conference on Antennas and Propagation (EUCAP), Paris, France, 19–24 March 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 3480–3484. [Google Scholar] [CrossRef]
- Rubaek, T.; Meaney, P.M.; Meincke, P.; Paulsen, K.D. Nonlinear microwave imaging for breast-cancer screening using Gauss-Newton’s method and the CGLS inversion algorithm. IEEE Trans. Antennas Propag. 2007, 55, 2320–2331. [Google Scholar] [CrossRef]
- Rubæk, T.; Meaney, P.M.; Paulsen, K.D. A contrast source inversion algorithm formulated using the log-phase formulation. Int. J. Antennas Propag. 2011, 2011, 849894. [Google Scholar] [CrossRef]
- Ruvio, G.; Solimene, R.; Cuccaro, A.; Gaetano, D.; Browne, J.E.; Ammann, M.J. Breast cancer detection using interferometric MUSIC: Experimental and numerical assessment. Med. Phys. 2014, 41, 103101. [Google Scholar] [CrossRef] [PubMed]
- Rydholm, T.; Fhager, A.; Persson, M.; Meaney, P.M. A first evaluation of the realistic Supelec-breast phantom. IEEE J. Electromagn. RF Microwaves Med. Biol. 2017, 1, 59–65. [Google Scholar] [CrossRef]
- Sagheer, M.; Sami, H.; Riaz, K.; Qasim Mehmood, M.; Zubair, M. Performance omparison of image reconstruction algorithms in microwave imaging for breast cancer screening. In Proceedings of the 2021 1st International Conference on Microwave, Antennas & Circuits (ICMAC), Islamabad, Pakistan, 21–22 December 2021; IEEE: Piscataway, NJ, USA, 2021; pp. 1–2. [Google Scholar] [CrossRef]
- Saied, I.; Arslan, T.; Ullah, R.; Liu, C.; Wang, F. Hardware accelerator for wearable and portable radar-based microwave breast imaging systems. In Proceedings of the 2021 IEEE International Symposium on Circuits and Systems (ISCAS), Daegu, Republic of Korea, 22–28 May 2021; IEEE: Piscataway, NJ, USA, 2021; pp. 1–5. [Google Scholar] [CrossRef]
- Sakamoto, T.; Song, H.; Kikkawa, T. Radar imaging of breast cancer using Kirchhoff migration and singular value decomposition. In Proceedings of the 2017 IEEE Conference on Antenna Measurements & Applications (CAMA), Tsukuba, Japan, 4–6 December 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 268–271. [Google Scholar] [CrossRef]
- Salvador, S.M.; Vecchi, G. Experimental tests of microwave breast cancer detection on phantoms. IEEE Trans. Antennas Propag. 2009, 57, 1705–1712. [Google Scholar] [CrossRef]
- Sami, H.; Sagheer, M.; Riaz, K.; Mehmood, M.Q.; Zubair, M. Machine learning-based approaches for breast cancer detection in microwave imaging. In Proceedings of the 2021 IEEE USNC-URSI Radio Science Meeting (Joint with AP-S Symposium), Singapore, 4–10 December 2021; IEEE: Piscataway, NJ, USA, 2021; pp. 72–73. [Google Scholar] [CrossRef]
- Sani, L.; Paoli, M.; Raspa, G.; Ghavami, N.; Sacchetti, F.; Saracini, A.; Ercolani, S.; Vannini, E.; Duranti, M. Initial clinical validation of a novel microwave apparatus for testing breast integrity. In Proceedings of the 2016 IEEE International Conference on Imaging Systems and Techniques (IST), Chania, Greece, 4–6 October 2016; IEEE: Piscataway, NJ, USA, 2016; pp. 278–282. [Google Scholar] [CrossRef]
- Sani, L.; Paoli, M.; Raspa, G.; Vispa, A.; Ghavami, N.; Tiberi, G.; Saracini, A.; Ercolani, S.; Vannini, E.; Duranti, M. Microwave apparatus for testing breast integrity based on Huygens principle: Clinical validation on 16 subjects. In Proceedings of the Loughborough Antennas & Propagation Conference (LAPC 2017), Loughborough, UK, 12–13 November 2018; Institution of Engineering and Technology: Loughborough, UK, 2017. [Google Scholar] [CrossRef]
- Sani, L.; Ghavami, N.; Vispa, A.; Paoli, M.; Raspa, G.; Ghavami, M.; Sacchetti, F.; Vannini, E.; Ercolani, S.; Saracini, A.; et al. Novel microwave apparatus for breast lesions detection: Preliminary clinical results. Biomed. Signal Process. Control 2019, 52, 257–263. [Google Scholar] [CrossRef]
- Sani, L.; Vispa, A.; Loretoni, R.; Duranti, M.; Ghavami, N.; Alvarez Sánchez-Bayuela, D.; Caschera, S.; Paoli, M.; Bigotti, A.; Badia, M.; et al. Breast lesion detection through MammoWave device: Empirical detection capability assessment of microwave images’ parameters. PLoS ONE 2021, 16, e0250005. [Google Scholar] [CrossRef]
- Sani, L.; Vispa, A.; Ghavami, N.; Sanchez-Bayuela, D.A.; Badia, M.; Bigotti, A.; Raspa, G.; Castellano, C.R.; Ghavami, M.; Tiberi, G. MammoWave breast imaging device: A procedure for device’s characterization via phantom measurements and subsequent clinical trials’ preliminary results. In Proceedings of the 2021 IEEE Conference on Antenna Measurements & Applications (CAMA), Antibes Juan-les-Pins, France, 15–17 November 2021; IEEE: Piscataway, NJ, USA, 2021; pp. 483–486. [Google Scholar] [CrossRef]
- Santorelli, A.; Chudzik, M.; Kirshin, E.; Porter, E.; Lujambio, A.; Arnedo, I.; Popovic, M.; Schwartz, J.D. Experimental demonstration of pulse shaping for time-domain microwave breast imaging. Prog. Electromagn. Res. 2013, 133, 309–329. [Google Scholar] [CrossRef]
- Santorelli, A.; Kirshin, E.; Porter, E.; Popovic, M.; Schwartz, J. Improved calibration for an experimental time-domain microwave imaging system. In Proceedings of the 2013 7th European Conference on Antennas and Propagation (EuCAP), Gothenburg, Sweden, 8–12 April 2013; IEEE: Piscataway, NJ, USA, 2013; pp. 801–805. [Google Scholar]
- Santorelli, A.; Porter, E.; Kirshin, E.; Liu, Y.J.; Popovic, M. Investigation of classifiers for tumor detection with an experimental time-domain breast screening system. Prog. Electromagn. Res. 2014, 144, 45–57. [Google Scholar] [CrossRef]
- Santorelli, A.; Porter, E.; Kang, E.; Piske, T.; Popovic, M.; Schwartz, J.D. A time-domain microwave system for breast cancer detection using a flexible circuit board. IEEE Trans. Instrum. Meas. 2015, 64, 2986–2994. [Google Scholar] [CrossRef]
- Santorelli, A.; Laforest, O.; Porter, E.; Popovic, M. Image classification for a time-domain microwave radar system: Experiments with stable modular breast phantoms. In Proceedings of the 2015 9th European Conference on Antennas and Propagation (EuCAP), Lisbon, Portugal, 13–17 April 2015; IEEE: Piscataway, NJ, USA, 2015; pp. 1–5. [Google Scholar]
- Sasada, S.; Masumoto, N.; Song, H.; Kajitani, K.; Emi, A.; Kadoya, T.; Arihiro, K.; Kikkawa, T.; Okada, M. Portable impulse-radar detector for breast cancer: A pilot study. J. Med. Imaging 2018, 5, 1. [Google Scholar] [CrossRef]
- Shah Karam, S.A.; O’Loughlin, D.; Asl, B.M. A novel sophisticated form of DMAS beamformer: Application to breast cancer detection. Biomed. Signal Process. Control 2022, 74, 103516. [Google Scholar] [CrossRef]
- Shere, M.; Lyburn, I.; Sidebottom, R.; Massey, H.; Gillett, C.; Jones, L. MARIA©M5: A multicentre clinical study to evaluate the ability of the Micrima radio-wave radar breast imaging system (MARIA©) to detect lesions in the symptomatic breast. Eur. J. Radiol. 2019, 116, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Shipilov, S.; Eremeev, A.; Yakubov, V.; Fedyanin, I.; Satarov, R.; Zavyalova, K.; Shipilova, S.; Balzovsky, E. Use of multi-angle ultra-wide band microwave sounding for high resolution breast imaging. Med. Phys. 2020, 47, 5147–5157. [Google Scholar] [CrossRef] [PubMed]
- Sill, J.; Fear, E. Tissue sensing adaptive radar for breast cancer detection–Experimental investigation of simple tumor models. IEEE Trans. Microw. Theory Tech. 2005, 53, 3312–3319. [Google Scholar] [CrossRef]
- Solimene, R.; Basile, B.; Browne, J.; Cuccaro, A.; Dell’Aversano, A.; Ruvio, G. An incoherent radar imaging system for medical applications. In Proceedings of the 2021 IEEE Conference on Antenna Measurements & Applications (CAMA), Antibes Juan-les-Pins, France, 15–17 November 2021; IEEE: Piscataway, NJ, USA, 2021; pp. 493–498. [Google Scholar] [CrossRef]
- Solis Nepote, M.; Herrera, D.R.; Tapia, D.F.; Latif, S.; Pistorius, S. A comparison study between horn and Vivaldi antennas for 1.5–6 GHz breast microwave radar imaging. In Proceedings of the 8th European Conference on Antennas and Propagation (EuCAP 2014), The Hague, The Netherlands, 6–11 April 2014; IEEE: Piscataway, NJ, USA, 2014; pp. 59–62. [Google Scholar] [CrossRef]
- Son, S.H. Preclinical prototype development of a microwave tomography system for breast cancer detection. ETRI J. 2010, 32, 901–910. [Google Scholar] [CrossRef]
- Song, H.; Kono, H.; Seo, Y.; Azhari, A.; Somei, J.; Suematsu, E.; Watarai, Y.; Ota, T.; Watanabe, H.; Hiramatsu, Y.; et al. A radar-based breast cancer detection system using CMOS integrated circuits. IEEE Access 2015, 3, 2111–2121. [Google Scholar] [CrossRef]
- Song, H.; Sasada, S.; Kadoya, T.; Okada, M.; Arihiro, K.; Xiao, X.; Kikkawa, T. Detectability of breast tumor by a hand-held impulse-radar detector: Performance evaluation and pilot clinical study. Sci. Rep. 2017, 7, 16353. [Google Scholar] [CrossRef]
- Song, H.; Sasada, S.; Masumoto, N.; Kadoya, T.; Shiroma, N.; Orita, M.; Arihiro, K.; Okada, M.; Kikkawa, T. Detectability of breast tumors in excised breast tissues of total mastectomy by IR-UWB-radar-based breast cancer detector. IEEE Trans. Biomed. Eng. 2019, 66, 2296–2305. [Google Scholar] [CrossRef]
- Song, H.; Watanabe, H.; Xiao, X.; Kikkawa, T. Influence of air-gaps between antennas and breast on impulse-radar-based breast cancer detection. In Proceedings of the 2019 13th European Conference on Antennas and Propagation (EuCAP), Krakow, Poland, 31 March–5 April 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 1–2. [Google Scholar]
- Song, H.; Sasada, S.; Masumoto, N.; Kadoya, T.; Okada, M.; Arihiro, K.; Xiao, X.; Kikkawa, T. A two-stage rotational surface clutter suppression method for microwave breast imaging with multistatic impulse-radar detector. IEEE Trans. Instrum. Meas. 2020, 69, 9586–9598. [Google Scholar] [CrossRef]
- Sugitani, T.; Kubota, S.; Toya, A.; Xiao, X.; Kikkawa, T. A compact 4 × 4 planar UWB antenna array for 3D breast cancer detection. IEEE Antennas Wirel. Propag. Lett. 2013, 12, 733–736. [Google Scholar] [CrossRef]
- Tajik, D.; Foroutan, F.; Shumakov, D.S.; Pitcher, A.D.; Nikolova, N.K. Real-time microwave imaging of a compressed breast phantom with planar scanning. IEEE J. Electromagn. RF Microwaves Med. Biol. 2018, 2, 154–162. [Google Scholar] [CrossRef]
- Tajik, D.; Nikolova, N.K.; Noseworthy, M.D. Improving quantitative microwave holography through simultaneous use of the Born and Rytov approximations. In Proceedings of the 2019 16th European Radar Conference (EuRAD), Paris, France, 2–4 October 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 281–284. [Google Scholar]
- Tajik, D.; Trac, J.; Nikolova, N.K. Spatial esolution evaluation of a microwave system for breast cancer screening. In Proceedings of the 2019 13th European Conference on Antennas and Propagation (EuCAP), Krakow, Poland, 31 March–5 April 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 1–5. [Google Scholar]
- Tangwachirapan, S.; Sawangsri, P.; Pinitkiatisakul, P.; Thongdit, P.; Thaiwirot, W. Breast cancer detection based microwave imaging using single antipodal Vivaldi antenna. In Proceedings of the 2022 Research, Invention, and Innovation Congress: Innovative Electricals and Electronics (RI2C), Bangkok, Thailand, 4–5 August 2022; IEEE: Piscataway, NJ, USA, 2022; pp. 97–100. [Google Scholar] [CrossRef]
- Tiberi, G.; Ghavami, N.; Sanchez-Bayuela, D.A.; Sani, L.; Vispa, A.; Bigotti, A.; Badia, M.; Papini, L.; Raspa, G.; Castellano, C.R.; et al. MammoWave breast imaging device: Path to clinical validation, results and implications in future population-based breast screening programs. In Proceedings of the 2022 16th European Conference on Antennas and Propagation (EuCAP), Madrid, Spain, 27 March–1 April 2022; IEEE: Piscataway, NJ, USA, 2022; pp. 1–4. [Google Scholar] [CrossRef]
- Torres-Quispe, H.A.; Patino-Escarcina, R.E. Improving UWB image reconstruction for breast cancer diagnosis by doing an iterative analysis of radar signals. In Pattern Recognition and Artificial Intelligence; Springer International Publishing: Cham, Switzerland, 2022; Volume 13363, pp. 435–446, Series Title: Lecture Notes in Computer Science. [Google Scholar] [CrossRef]
- Vasquez, J.T.; Vipiana, F.; Casu, M.; Vacca, M.; Sarwar, I.; Scapaticci, R.; Joachimowicz, N.; Duchene, B. Experimental assessment of qualitative microwave imaging using a 3-D realistic breast phantom. In Proceedings of the 2017 11th European Conference on Antennas and Propagation (EUCAP), Paris, France, 19–24 March 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 2728–2731. [Google Scholar] [CrossRef]
- Vispa, A.; Sani, L.; Paoli, M.; Bigotti, A.; Raspa, G.; Ghavami, N.; Caschera, S.; Ghavami, M.; Duranti, M.; Tiberi, G. UWB device for breast microwave imaging: Phantom and clinical validations. Measurement 2019, 146, 582–589. [Google Scholar] [CrossRef]
- Wang, L.; Simpkin, R.; Al-Jumaily, A.M. Holographic microwave imaging array: Experimental investigation of breast tumour detection. In Proceedings of the 2013 IEEE International Workshop on Electromagnetics, Applications and Student Innovation Competition, Hong Kong, China, 1–3 August 2013; IEEE: Piscataway, NJ, USA, 2013; pp. 61–64. [Google Scholar] [CrossRef]
- Wang, Y.; Abbosh, A.M.; Henin, B.; Nguyen, P.T. Synthetic bandwidth radar for ultra-wideband microwave imaging systems. IEEE Trans. Antennas Propag. 2014, 62, 698–705. [Google Scholar] [CrossRef]
- Xiao, X.; Liu, Y.; Song, H.; Kikkawa, T. Optimal microwave breast imaging using quality metrics and simulated annealing algorithm. Int. J. RF Microw. Comput.-Aided Eng. 2020, 30, e22364. [Google Scholar] [CrossRef]
- Yang, F.; Sun, L.; Hu, Z.; Wang, H.; Pan, D.; Wu, R.; Zhang, X.; Chen, Y.; Zhang, Q. A large-scale clinical trial of radar-based microwave breast imaging for Asian women: Phase I. In Proceedings of the 2017 IEEE International Symposium on Antennas and Propagation & USNC/URSI National Radio Science Meeting, San Diego, CA, USA, 9–14 July 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 781–783. [Google Scholar] [CrossRef]
- Zanoon, T.F.; Hathal, M.S.; Abdullah, M.Z. Comparing image reconstruction algorithms for microwave camera featuring ultra wide band sensor. In Proceedings of the 2011 IEEE International Conference on Imaging Systems and Techniques, Batu Ferringhi, Malaysia, 17–18 May 2011; IEEE: Piscataway, NJ, USA, 2011; pp. 112–117. [Google Scholar] [CrossRef]
- Zanoon, T.F.; Abdullah, M.Z. Early stage breast cancer detection by means of time-domain ultra-wide band sensing. Meas. Sci. Technol. 2011, 22, 114016. [Google Scholar] [CrossRef]
- Zerrad, F.E.; Taouzari, M.; Makroum, E.M.; Ahmad, S.; Alkurt, F.O.; Karaaslan, M.; Islam, M.T.; Hussein, M.I. Symmetrical and asymmetrical breast phantoms with 3D-printed anatomical structure for microwave imaging of breast cancer. IEEE Access 2022, 10, 96896–96908. [Google Scholar] [CrossRef]
- Adachi, M.; Nakagawa, T.; Fujioka, T.; Mori, M.; Kubota, K.; Oda, G.; Kikkawa, T. Feasibility of portable microwave imaging device for breast cancer detection. Diagnostics 2021, 12, 27. [Google Scholar] [CrossRef]
- Ahadi, M.; Nourinia, J.; Ghobadi, C. Square monopole antenna application in localization of tumors in three dimensions by confocal microwave imaging for breast cancer detection: Experimental measurement. Wirel. Pers. Commun. 2021, 116, 2391–2409. [Google Scholar] [CrossRef]
- Asok, A.O.; Gokul Nath, S.J.; Dey, S. Non-invasive breast tumor detection with antipodal Vivaldi antenna using monostatic approach. Int. J. RF Microw. Comput.-Aided Eng. 2022, 32, e23539. [Google Scholar] [CrossRef]
- Bakar, A.; Ireland, D.; Abbosh, A.; Wang, Y. Experimental assessment of microwave diagnostic tool for ultra-wideband breast cancer detection. Prog. Electromagn. Res. M 2012, 23, 109–121. [Google Scholar] [CrossRef]
- Bassi, M.; Caruso, M.; Khan, M.S.; Bevilacqua, A.; Capobianco, A.D.; Neviani, A. An integrated microwave imaging radar with planar antennas for breast cancer detection. IEEE Trans. Microw. Theory Tech. 2013, 61, 2108–2118. [Google Scholar] [CrossRef]
- Bialkowski, M.E. Ultra wideband microwave system with novel image reconstruction strategies for breast cancer detection. In Proceedings of the The 40th European Microwave Conference, Paris, France, 28–30 September 2010; IEEE: Piscataway, NJ, USA, 2010; pp. 537–540. [Google Scholar] [CrossRef]
- Bilgin, E.; ÇayOren, M.; Joof, S.; Cansiz, G.; Yilmaz, T.; Akduman, I. Single-slice microwave imaging of breast cancer by reverse time migration. Med. Phys. 2022, 49, 6599–6608. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Angulo, C.; Martinez-Lozano, A.; Gutierrez-Mazon, R.; Juan, C.G.; Garcia-Martinez, H.; Arias-Rodriguez, J.; Sabater-Navarro, J.M.; Avila-Navarro, E. Non-invasive microwave-based imaging system for early detection of breast tumours. Biosensors 2022, 12, 752. [Google Scholar] [CrossRef]
- Bourqui, J.; Sill, J.M.; Fear, E.C. A prototype system for measuring microwave frequency reflections from the breast. Int. J. Biomed. Imaging 2012, 2012, 851234. [Google Scholar] [CrossRef]
- Bourqui, J.; Kuhlmann, M.; Kurrant, D.; Lavoie, B.; Fear, E. Adaptive monostatic system for measuring microwave reflections from the breast. Sensors 2018, 18, 1340. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.G.; Geddert, N.; Asefi, M.; LoVetri, J.; Jeffrey, I. Hybridizable discontinuous Galerkin method contrast source inversion of 2-D and 3-D dielectric and magnetic targets. IEEE Trans. Microw. Theory Tech. 2019, 67, 1766–1777. [Google Scholar] [CrossRef]
- Byrne, D.; Craddock, I.J. Time-domain wideband adaptive beamforming for radar breast imaging. IEEE Trans. Antennas Propag. 2015, 63, 1725–1735. [Google Scholar] [CrossRef]
- Byrne, D.; Sarafianou, M.; Craddock, I.J. Compound radar approach for breast imaging. IEEE Trans. Biomed. Eng. 2017, 64, 40–51. [Google Scholar] [CrossRef]
- Casu, M.R.; Vacca, M.; Tobon, J.A.; Pulimeno, A.; Sarwar, I.; Solimene, R.; Vipiana, F. A COTS-based microwave imaging system for breast-cancer detection. IEEE Trans. Biomed. Circuits Syst. 2017, 11, 804–814. [Google Scholar] [CrossRef]
- Conceicao, R.C.; Byrne, D.; Ghavami, N.; Smith, P.P.; Craddock, I. Spectral filtering in phase delay beamforming for multistatic UWB breast cancer imaging. In Proceedings of the 2015 9th European Conference on Antennas and Propagation (EuCAP), Lisbon, Portugal, 13–17 April 2015; IEEE: Piscataway, NJ, USA, 2015; pp. 1–4. [Google Scholar]
- Cuccaro, A.; Dell’Aversano, A.; Ruvio, G.; Browne, J.; Solimene, R. Incoherent radar imaging for breast cancer detection and experimental validation against 3D multimodal breast phantoms. J. Imaging 2021, 7, 23. [Google Scholar] [CrossRef]
- De Jesus Aragão, A.; Carvalho, D.; Sanches, B.; Van Noije, W.A. Low-cost device for breast cancer screening: A dry setup IR-UWB proposal. Biomed. Signal Process. Control 2023, 79, 104078. [Google Scholar] [CrossRef]
- Deprez, J.F.; Klemm, M.; Probert Smith, P.; Craddock, I. Twin target correction for ultra-wideband radar imaging of breast tumours. In Proceedings of the 2010 IEEE International Symposium on Biomedical Imaging: From Nano to Macro, Rotterdam, The Netherlands, 14–17 April 2010; IEEE: Piscataway, NJ, USA, 2010; pp. 213–216. [Google Scholar] [CrossRef]
- Dey, M.; Rana, S.P.; Loretoni, R.; Duranti, M.; Sani, L.; Vispa, A.; Raspa, G.; Ghavami, M.; Dudley, S.; Tiberi, G. Automated breast lesion localisation in microwave imaging employing simplified pulse coupled neural network. PLoS ONE 2022, 17, e0271377. [Google Scholar] [CrossRef] [PubMed]
- Elahi, M.; Curtis, C.; Lavoie, B.; Glavin, M.; Jones, E.; Fear, E.; O’Halloran, M. Performance of leading artifact removal algorithms assessed across microwave breast imaging prototype scan configurations. Comput. Med. Imaging Graph. 2017, 58, 33–44. [Google Scholar] [CrossRef]
- Elahi, M.; O’Loughlin, D.; Lavoie, B.; Glavin, M.; Jones, E.; Fear, E.; O’Halloran, M. Evaluation of image reconstruction algorithms for confocal microwave imaging: Application to patient data. Sensors 2018, 18, 1678. [Google Scholar] [CrossRef] [PubMed]
- Epstein, N.R.; Meaney, P.M.; Paulsen, K.D. 3D parallel-detection microwave tomography for clinical breast imaging. Rev. Sci. Instrum. 2014, 85, 124704. [Google Scholar] [CrossRef]
- Eremeev, A.I.; Vasin, V.V.; Satarov, R.N.; Tseplyaev, I.S.; Shipilova, S.S. Application of the migration method for radiotomography of breast cancer. J. Phys. Conf. Ser. 2021, 2140, 012027. [Google Scholar] [CrossRef]
- Fang, Q.; Meaney, P.; Paulsen, K. Microwave image reconstruction of tissue property dispersion characteristics utilizing multiple-frequency information. IEEE Trans. Microw. Theory Tech. 2004, 52, 1866–1875. [Google Scholar] [CrossRef]
- Fang, Q.; Meaney, P.; Paulsen, K. The multidimensional phase unwrapping integral and applications to microwave tomographical image reconstruction. IEEE Trans. Image Process. 2006, 15, 3311–3324. [Google Scholar] [CrossRef]
- Fasoula, A.; Moloney, B.; Duchesne, L.; Cano, J.G.; Oliveira, B.; Bernard, J.G.; Kerin, M. Super-resolution radar imaging for breast cancer detection with microwaves: The integrated information selection criteria. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 1868–1874. [Google Scholar] [CrossRef]
- Fasoula, A.; Duchesne, L.; Gil Cano, J.D.; Moloney, B.M.; Abd Elwahab, S.M.; Kerin, M.J. Automated breast lesion detection and characterization with the Wavelia microwave breast imaging system: Methodological proof-of-concept on first-in-human patient data. Appl. Sci. 2021, 11, 9998. [Google Scholar] [CrossRef]
- Fasoula, A.; Duchesne, L.; Abdoush, Y.; Baracco, J. Frequency-dependent, configurable, sensor fidelity zone for microwave breast imaging: System dimensioning and image quality enhancement. In Proceedings of the 2021 IEEE Conference on Antenna Measurements & Applications (CAMA), Antibes Juan-les-Pins, France, 15–17 November 2021; IEEE: Piscataway, NJ, USA, 2021; pp. 487–492. [Google Scholar] [CrossRef]
- Fear, E.; Low, A.; Sill, J.; Stuchly, M. Microwave system for breast tumor detection: Experimental concept evaluation. In Proceedings of the IEEE Antennas and Propagation Society International Symposium (IEEE Cat. No.02CH37313), Antonio, TX, USA, 16–21 June 2002; IEEE: Piscataway, NJ, USA, 2002; Volume 1, pp. 819–822. [Google Scholar] [CrossRef]
- Fear, E.; Sill, J.; Stuchly, M. Experimental feasibility study of confocal microwave imaging for breast tumor detection. IEEE Trans. Microw. Theory Tech. 2003, 51, 887–892. [Google Scholar] [CrossRef]
- Felicio, J.M.; Bioucas-Dias, J.M.; Costa, J.R.; Fernandes, C.A. Antenna design and near-field characterization for medical microwave imaging applications. IEEE Trans. Antennas Propag. 2019, 67, 4811–4824. [Google Scholar] [CrossRef]
- Poplack, S.P.; Tosteson, T.D.; Wells, W.A.; Pogue, B.W.; Meaney, P.M.; Hartov, A.; Kogel, C.A.; Soho, S.K.; Gibson, J.J.; Paulsen, K.D. Electromagnetic breast imaging: Results of a pilot study in women with abnormal mammograms. Radiology 2007, 243, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.P.; Dey, M.; Loretoni, R.; Duranti, M.; Ghavami, M.; Dudley, S.; Tiberi, G. Radiation-free microwave technology for breast lesion detection using supervised machine learning model. Tomography 2023, 9, 105–129. [Google Scholar] [CrossRef]
- Wang, Z.; Bovik, A.; Sheikh, H.; Simoncelli, E. Image quality assessment: From error visibility to structural similarity. IEEE Trans. Image Process. 2004, 13, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Reimer, T.; Sacristan, J.; Pistorius, S. Improving the diagnostic capability of microwave radar imaging systems using machine learning. In Proceedings of the 2019 13th European Conference on Antennas and Propagation (EuCAP), Krakow, Poland, 31 March–5 April 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 1–5. [Google Scholar]
- Conceicao, R.C.; O’Halloran, M.; Glavin, M.; Jones, E. Evaluation of features and classifiers for classification of early-stage breast cancer. J. Electromagn. Waves Appl. 2011, 25, 1–14. [Google Scholar] [CrossRef]
- Kaufman, S.; Rosset, S.; Perlich, C.; Stitelman, O. Leakage in data mining: Formulation, detection, and avoidance. ACM Trans. Knowl. Discov. Data 2012, 6. [Google Scholar] [CrossRef]
- Chiavegatto Filho, A.; Batista, A.F.D.M.; dos Santos, H.G. Data leakage in health outcomes prediction with machine learning. Comment on “Prediction of incident hypertension within the next year: Prospective study using statewide electronic health records and machine learning”. J. Med. Internet Research 2021, 23, e10969. [Google Scholar] [CrossRef]
- Conceicao, R.C.; Medeiros, H.; O’Halloran, M.; Rodriguez-Herrera, D.; Flores-Tapia, D.; Pistorius, S. Initial classification of breast tumour phantoms using a UWB radar prototype. In Proceedings of the 2013 International Conference on Electromagnetics in Advanced Applications (ICEAA), Turin, Italy, 9–13 September 2013; IEEE: Piscataway, NJ, USA, 2013; pp. 720–723. [Google Scholar] [CrossRef]
- Conceicao, R.C.; Medeiros, H.; Godinho, D.M.; O’Halloran, M.; Rodriguez-Herrera, D.; Flores-Tapia, D.; Pistorius, S. Classification of breast tumor models with a prototype microwave imaging system. Med. Phys. 2020, 47, 1860–1870. [Google Scholar] [CrossRef]
- Conceicao, R.; O’Halloran, M.; Glavin, M.; Jones, E. Support vector machines for the classification of early-stage breast cancer based on radar target signatures. Prog. Electromagn. Res. B 2010, 23, 311–327. [Google Scholar] [CrossRef]

| Signal to Clutter Ratio | Signal to Mean Ratio | Tumour to Fibroglandular Response Ratio | Contrast to Clutter Ratio | Mean-to-Mean Ratio | Localization Error | Mean Squared Error | Structural Similarity Index Measure | Full Width at Half Maximum | |
|---|---|---|---|---|---|---|---|---|---|
| Mathematical Definition | Various | ||||||||
| Publications | [13,36,61,62,63,65,100,106,116,117,124,139,140,153,165,189,199] | [61,62,63,116,117,124,189] | [26,69,147] | [26,69,147] | [14,99,164,173,178,190] | [14,62,63,65,98,116,117,124,137,139,153,200] | [31,39,72,85,86,89,91,125,180,193] | [176] | [101,114,189,190] |
| Measures | Image contrast | Image contrast | Image contrast | Ratio of contrast to noise | Image contrast | Accuracy of target localization | Image accuracy | Image accuracy | Image accuracy |
| Best use | Contrast maximum target to maximum non-target response | Contrast maximum target to mean non-target response | Contrast maximum target to maximum non-target response | Compare contrast to noise | Compare mean target response to mean non-target response | Describe target positioning error in image | Summary description of image accuracy | Summary description of image accuracy | Describe extent of the highest-intensity response |
| Challenges | Non-robust, requires subjective definition of target region and/or a priori knowledge of tissue geometry. | Requires subjective definition of target region and/or a priori knowledge of tissue geometry. | Requires a priori knowledge of tissue geometry, non-robust. | Requires a priori knowledge of tissue geometries and properties. Only applicable to quantitative reconstruction methods. Only applicable as a summary metric for image accuracy due to summation over the image space. | FWHM may not be limited to a tumour response. The FWHM from images of healthy and tumour-containing breasts may be similar, depending on the geometry of the fibroglandular breast tissues. | ||||
| Article | Image Reconstruction Method | Sensitivity Estimate | Specificity Estimate | ROC AUC Estimate | Dataset Information |
|---|---|---|---|---|---|
| Poplack et al. [201] | Tomographic | – | – | (80 ± 12)% | <130 patients (<80 with abnormal mammography, 50 with normal mammography) |
| Preece et al. [111] | Modified DAS | 74% | – | – | 66 patients (all abnormal mammography) |
| Shere et al. [143] | – | 76% | – | – | 225 patients (all with benign or malignant lesions) |
| Sani et al. [132] | Huygens Principle | 91% | – | – | 16 patients (8 healthy breasts, 12 non-healthy breasts) |
| Sani et al. [133] | Huygens Principle | 70% | 65% | – | 45 patients (22 healthy breasts, 29 non-healthy breasts) |
| Sani et al. [134] | Huygens Principle | 74% | 62% | – | 58 patients (103 breasts, 52 with no radiological findings, 51 with radiological findings) |
| Sasada et al. [141] | – | 100% | – | – | 5 patients (all with tumours larger than 1 cm) |
| Adachi et al. [170] | DAS | 100% | – | – | 9 patients with breast cancer |
| O’Loughlin et al. [100] | DAS | 80% | 20% | – | 115 phantoms (110 with tumours, 5 without tumours) |
| Janjic et al. [47] | Qualitative inverse scattering | 63% | – | – | 115 patients, all with known breast lesions |
| Reimer et al. [120] | DAS | ≤71% | ≤44% | – | 200 phantom scans (100 healthy; 100 tumour-containing) |
| DMAS | ≤77% | ≤40% | – | ||
| ORR | ≤82% | ≤56% | – | ||
| Moloney et al. [94] | TR-MUSIC | 87.5% | – | – | 24 patients (11 patients with biopsy-proven malignancy, 13 patients with either unaspirated cysts or biopsy-proven benign lesions) |
| Article | Classification Algorithm | Sensitivity Estimate | Specificity Estimate | Accuracy Estimate | ROC AUC Estimate | F1 Score | Dataset Information |
|---|---|---|---|---|---|---|---|
| Santorelli et al. [138] | SVM, LDA | 76.71% | 67.48% | – | – | – | 230 phantom scans |
| Li et al. [76] | SVM ensemble | 97% | 99% | – | – | – | 150 phantom scans |
| Rana et al. [112] | SVM, MLP, kNN | 97.7% | 99.7% | 98.9% | 93.7% | 98.6% | 18 patients (12 healthy breast scans and 11 non-healthy breast scans) |
| Sani et al. [135] | – | 88% | 59.3% | 80.4% | – | 86.8% | 102 breast scans (27 without radiological findings, 75 with radiological findings) |
| Rana et al. [202] | SVMs with quadratic and Gaussian kernels | 97.2% | 94.5% | 95.5% | – | 94.1% | 61 breast scans (36 with lesion, 25 without lesion) from 34 patients |
| Dey et al. [188] | PCNN | 81.82% | 98% | – | – | – | 61 breast scans (36 with lesion, 25 without lesion) from 34 patients |
| Reimer et al. [118] | Logistic regression | (95 ± 6)% | (80 ± 10)% | (85 ± 4)% | (94.4 ± 0.5)% | 80.85% | 249 phantom scans |
| Al Khatib et al. [53] | CNN | – | – | – | – | 92% | 1008 phantom scans |
| Reimer et al. [119] | Logistic regression, CNN, DNN | – | – | – | (90 ± 3)% | – | 1008 phantom scans |
| Martins et al. [82] | kNN, LDA, SVM | – | – | 85.00% | – | – | 5 phantom scans |
| Fasoula et al. [196] | QDA | 77.1% | 100.0% | – | – | – | 24 patients (11 patients with biopsy-proven malignancy, 13 patients with either unaspirated cysts or biopsy-proven benign lesions) |
| Moloney et al. [93] | QDA | 87.5% | – | – | – | – | 24 patients |
| Janjic et al. [48] | AdaBoost | 79% | 77% | 78% | 74% | 78% | 113 patients (43 with malignant lesions, 70 with benign lesions) |
| Janjic et al. [49] | Gradient Boosting Ensemble | 80% | 83% | 81% | 80% | 85% | 54 patients (25 with malignant lesions, 29 with benign lesions) |
| Lu et al. [79] | CNN LSTM | – | – | 89.5% | – | – | 1000 phantom scans |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reimer, T.; Pistorius, S. Review and Analysis of Tumour Detection and Image Quality Analysis in Experimental Breast Microwave Sensing. Sensors 2023, 23, 5123. https://doi.org/10.3390/s23115123
Reimer T, Pistorius S. Review and Analysis of Tumour Detection and Image Quality Analysis in Experimental Breast Microwave Sensing. Sensors. 2023; 23(11):5123. https://doi.org/10.3390/s23115123
Chicago/Turabian StyleReimer, Tyson, and Stephen Pistorius. 2023. "Review and Analysis of Tumour Detection and Image Quality Analysis in Experimental Breast Microwave Sensing" Sensors 23, no. 11: 5123. https://doi.org/10.3390/s23115123
APA StyleReimer, T., & Pistorius, S. (2023). Review and Analysis of Tumour Detection and Image Quality Analysis in Experimental Breast Microwave Sensing. Sensors, 23(11), 5123. https://doi.org/10.3390/s23115123








