Fully 3D-Printed Dry EEG Electrodes
Abstract
:1. Introduction
2. Materials and Methods
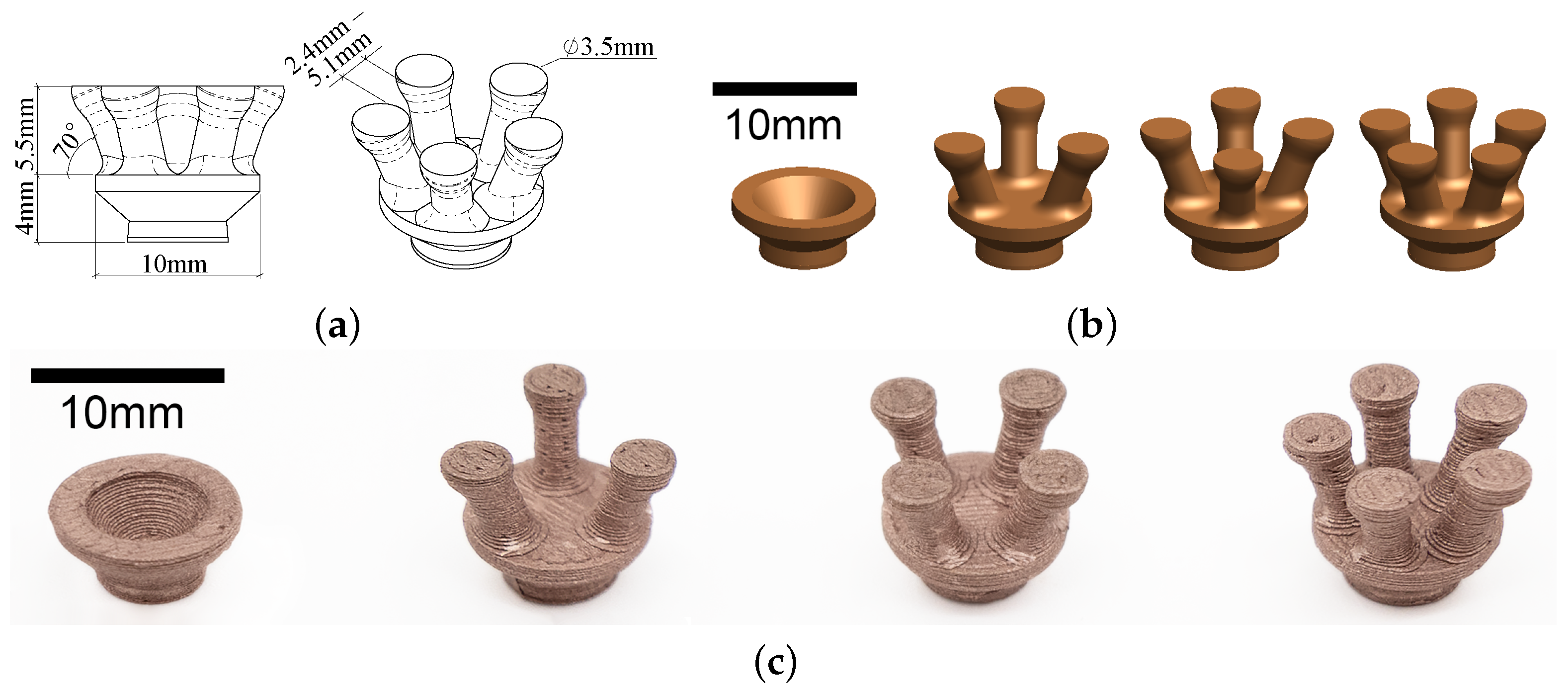
2.1. Electrode Design
2.2. Prototyping of Electrodes
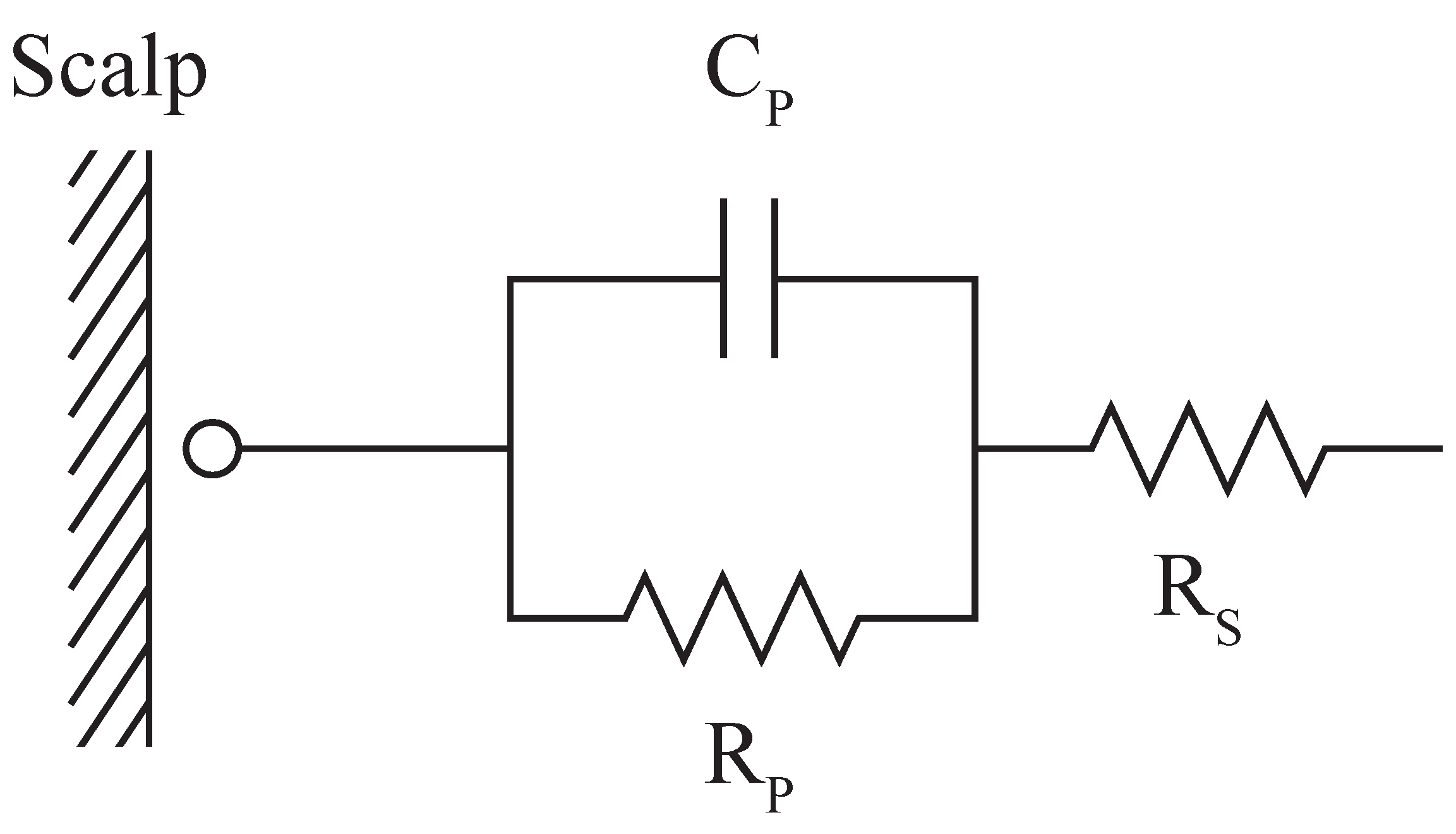
2.3. Contact Impedance Testing
2.4. Functional Testing
3. Results
3.1. Contact Impedance Measurement
3.1.1. Results for 0-Pin-Flat Electrode
3.1.2. Results for Different Numbers of Electrode Pins
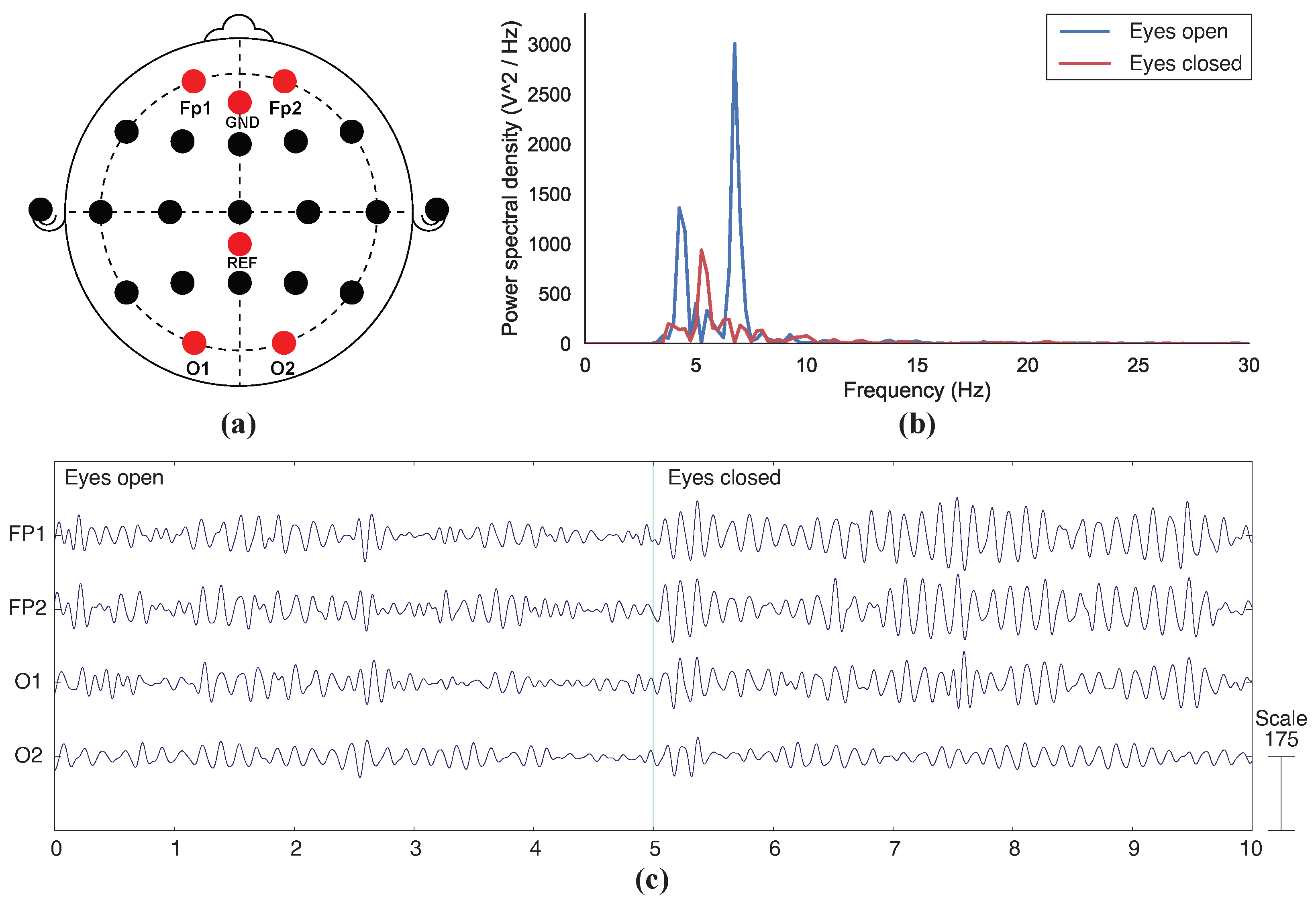
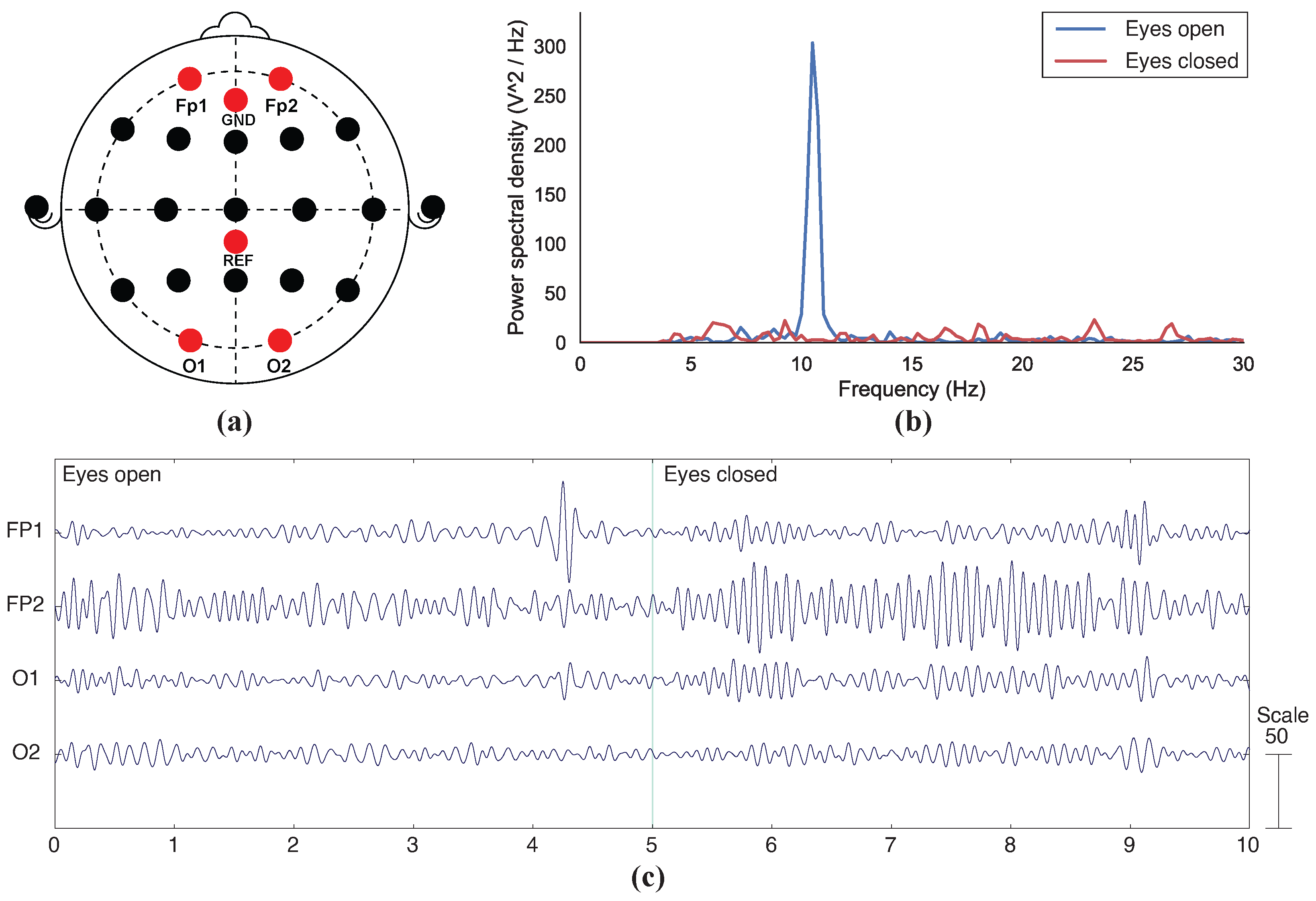
3.2. EEG Recording
4. Discussion
4.1. Contact Impedance
4.2. Functional Testing
4.3. Limitations
4.3.1. Customisation Challenges
4.3.2. Fabrication Challenges
4.3.3. Material Properties
4.3.4. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nayak, C.S.; Anilkumar, A.C. EEG Normal Waveforms. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Usakli, A.B. Improvement of EEG Signal Acquisition: An Electrical Aspect for State of the Art of Front End. Comput. Intell. Neurosci. 2010, 2010, 630649. [Google Scholar] [CrossRef]
- Moffett, S.X.; O’Malley, S.M.; Man, S.; Hong, D.; Martin, J.V. Dynamics of high frequency brain activity. Sci. Rep. 2017, 7, 15758. [Google Scholar] [CrossRef] [PubMed]
- Niedermeyer, E. Ultrafast Frequencies and Full-Band EEG, Ultrafast EEG Activities and Their Significance. Clin. EEG Neurosci. 2005, 36, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Casson, A.J. Wearable EEG and beyond. Biomed. Eng. Lett. 2019, 9, 53–71. [Google Scholar] [CrossRef] [PubMed]
- Velcescu, A.; Lindley, A.; Cursio, C.; Krachunov, S.; Beach, C.; Brown, C.; Jones, A.; Casson, A. Flexible 3D-Printed EEG Electrodes. Sensors 2019, 19, 1650. [Google Scholar] [CrossRef]
- Krachunov, S.; Casson, A. 3D Printed Dry EEG Electrodes. Sensors 2016, 16, 1635. [Google Scholar] [CrossRef]
- Lee, M.S.; Paul, A.; Xu, Y.; Hairston, W.D.; Cauwenberghs, G. Characterization of Ag/AgCl Dry Electrodes for Wearable Electrophysiological Sensing. Front. Electron. 2022, 2, 700363. [Google Scholar] [CrossRef]
- Electrifi Conductive Filament. Available online: https://www.multi3dllc.com/product/electrifi/ (accessed on 17 April 2023).
- Kimura, M.; Nakatani, S.; Nishida, S.I.; Taketoshi, D.; Araki, N. 3D Printable Dry EEG Electrodes with Coiled-Spring Prongs. Sensors 2020, 20, 4733. [Google Scholar] [CrossRef]
- Damalerio, R.; Cheng, M.Y. Development of Dry EEG Electrodes and Dry EEG Cap for Neuromonitoring. In Proceedings of the 2020 IEEE 70th Electronic Components and Technology Conference (ECTC), Orlando, FL, USA, 3–30 June 2020; pp. 841–846. [Google Scholar] [CrossRef]
- Symeonidou, E.R.; Nordin, A.; Hairston, W.; Ferris, D. Effects of Cable Sway, Electrode Surface Area, and Electrode Mass on Electroencephalography Signal Quality during Motion. Sensors 2018, 18, 1073. [Google Scholar] [CrossRef]
- Cardenas, J.A.; Tsang, H.; Tong, H.; Abuzaid, H.; Price, K.; Cruz, M.A.; Wiley, B.J.; Franklin, A.D.; Lazarus, N. Flash ablation metallization of conductive thermoplastics. Addit. Manuf. 2020, 36, 101409. [Google Scholar] [CrossRef]
- Kim, M.J.; Cruz, M.A.; Ye, S.; Gray, A.L.; Smith, G.L.; Lazarus, N.; Walker, C.J.; Sigmarsson, H.H.; Wiley, B.J. One-step electrodeposition of copper on conductive 3D printed objects. Addit. Manuf. 2019, 27, 318–326. [Google Scholar] [CrossRef]
- Helena, D.; Ramos, A.; Varum, T.; Matos, J.N. The Use of 3D Printing Technology for Manufacturing Metal Antennas in the 5G/IoT Context. Sensors 2021, 21, 3321. [Google Scholar] [CrossRef] [PubMed]
- Mitra, D.; Roy, S.; Striker, R.; Burczek, E.; Aqueeb, A.; Wolf, H.; Kabir, K.S.; Ye, S.; Braaten, B.D. Conductive Electrifi and Nonconductive NinjaFlex Filaments based Flexible Microstrip Antenna for Changing Conformal Surface Applications. Electronics 2021, 10, 821. [Google Scholar] [CrossRef]
- Flowers, P.F.; Reyes, C.; Ye, S.; Kim, M.J.; Wiley, B.J. 3D printing electronic components and circuits with conductive thermoplastic filament. Addit. Manuf. 2017, 18, 156–163. [Google Scholar] [CrossRef]
- Aloqalaa, Z. Electrically Conductive Fused Deposition Modeling Filaments: Current Status and Medical Applications. Crystals 2022, 12, 1055. [Google Scholar] [CrossRef]
- Saharan, L.; Agbesoyin, T. Design and 3D printing of a capacitive sensor for biomedical applications. In Proceedings of the SPIE Smart Structures + Nondestructive Evaluation, Long Beach, CA, USA, 18 April 2022; Volume 12046, p. 1204612. [Google Scholar] [CrossRef]
- Kappenman, E.S.; Luck, S.J. The effects of electrode impedance on data quality and statistical significance in ERP recordings. Psychophysiology 2010, 47, 888–904. [Google Scholar] [CrossRef]
- Mascia, A.; Collu, R.; Spanu, A.; Fraschini, M.; Barbaro, M.; Cosseddu, P. Wearable System Based on Ultra-Thin Parylene C Tattoo Electrodes for EEG Recording. Sensors 2023, 23, 766. [Google Scholar] [CrossRef]
- Babiloni, C.; Barry, R.J.; Başar, E.; Blinowska, K.J.; Cichocki, A.; Drinkenburg, W.H.; Klimesch, W.; Knight, R.T.; Lopes da Silva, F.; Nunez, P.; et al. International Federation of Clinical Neurophysiology (IFCN)—EEG research workgroup: Recommendations on frequency and topographic analysis of resting state EEG rhythms. Part 1: Applications in clinical research studies. Clin. Neurophysiol. 2020, 131, 285–307. [Google Scholar] [CrossRef]
- Kawana, T.; Yoshida, Y.; Kudo, Y.; Iwatani, C.; Miki, N. Design and Characterization of an EEG-Hat for Reliable EEG Measurements. Micromachines 2020, 11, 635. [Google Scholar] [CrossRef]
- Hairston, W.D.; Slipher, G.A.; Yu, A.B. Ballistic gelatin as a putative substrate for EEG phantom devices. arXiv 2016, arXiv:1609.07691. [Google Scholar] [CrossRef]
- Owda, A.Y.; Casson, A.J. Electrical properties, accuracy, and multi-day performance of gelatine phantoms for electrophysiology. BioRxiv 2020. [Google Scholar] [CrossRef]
- Rieiro, H.; Diaz-Piedra, C.; Morales, J.M.; Catena, A.; Romero, S.; Roca-Gonzalez, J.; Fuentes, L.J.; Di Stasi, L.L. Validation of Electroencephalographic Recordings Obtained with a Consumer-Grade, Single Dry Electrode, Low-Cost Device: A Comparative Study. Sensors 2019, 19, 2808. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Huang, H.; Schwab, N.; Tanner, J.; Rajan, A.; Lam, N.B.; Zaborszky, L.; Li, C.S.R.; Price, C.C.; Ding, M. From eyes-closed to eyes-open: Role of cholinergic projections in EC-to-EO alpha reactivity revealed by combining EEG and MRI. Hum. Brain Mapp. 2019, 40, 566–577. [Google Scholar] [CrossRef]
- Tasolamprou, A.C.; Mentzaki, D.; Viskadourakis, Z.; Economou, E.N.; Kafesaki, M.; Kenanakis, G. Flexible 3D Printed Conductive Metamaterial Units for Electromagnetic Applications in Microwaves. Materials 2020, 13, 3879. [Google Scholar] [CrossRef] [PubMed]
- Barši Palmić, T.; Slavič, J.; Boltežar, M. Process Parameters for FFF 3D-Printed Conductors for Applications in Sensors. Sensors 2020, 20, 4542. [Google Scholar] [CrossRef]




| Electrode Configuration | Contact Surface Area (mm2) | Volume (mm3) | Cost of Material (USD) |
|---|---|---|---|
| 0-pin-flat | 42.41 | 130.22 | 0.68 |
| 3-pin | 28.86 | 314.98 | 1.66 |
| 4-pin | 38.48 | 350.89 | 1.84 |
| 5-pin | 48.11 | 386.75 | 2.03 |
| Filament | Resistivity (cm) |
|---|---|
| Palmiga PI-ETPU | 30–700 |
| Black Magic 3D | 0.6 |
| Proto-pasta | 30 |
| Multi3D Electrifi | 0.006 |
| Printing Parameters | Value |
|---|---|
| Nozzle diameter | 0.35 mm |
| Print temperature | 140 C |
| Bed temperature | 25 C |
| Flow rate | 110% |
| Print speed | 15.0 mm/s |
| Primary layer height | 0.16 mm |
| Infill outline overlap | 50% |
| Minimum infill length | 0.00 mm |
| First layer height | 125% |
| First layer speed | 40% |
| Electrode Configuration | () | () | (F) |
|---|---|---|---|
| Neurospec dry flat | 11.67 | 16.61 | 58.18 |
| Neurospec dry 2 mm | 25.43 | 17.85 | 39.80 |
| 0-pin-flat | 94.35 | 90.28 | 15.67 |
| 3-pin | 316.47 | 178.95 | 10.15 |
| 4-pin | 415.93 | 154.57 | 6.51 |
| 5-pin | 295.47 | 212.31 | 8.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, A.; Perera, P.; Sarsenbayeva, Z.; McEwan, A.; De Silva, A.C.; Withana, A. Fully 3D-Printed Dry EEG Electrodes. Sensors 2023, 23, 5175. https://doi.org/10.3390/s23115175
Tong A, Perera P, Sarsenbayeva Z, McEwan A, De Silva AC, Withana A. Fully 3D-Printed Dry EEG Electrodes. Sensors. 2023; 23(11):5175. https://doi.org/10.3390/s23115175
Chicago/Turabian StyleTong, Adele, Praneeth Perera, Zhanna Sarsenbayeva, Alistair McEwan, Anjula C. De Silva, and Anusha Withana. 2023. "Fully 3D-Printed Dry EEG Electrodes" Sensors 23, no. 11: 5175. https://doi.org/10.3390/s23115175
APA StyleTong, A., Perera, P., Sarsenbayeva, Z., McEwan, A., De Silva, A. C., & Withana, A. (2023). Fully 3D-Printed Dry EEG Electrodes. Sensors, 23(11), 5175. https://doi.org/10.3390/s23115175








