Effect of One Step Solid State Reaction Route on the Semiconductor Behavior of the Spinel (NI, Co, and Mn)O4 to Be Used as Temperature Sensor
Abstract
:1. Introduction
2. Experimental Procedure
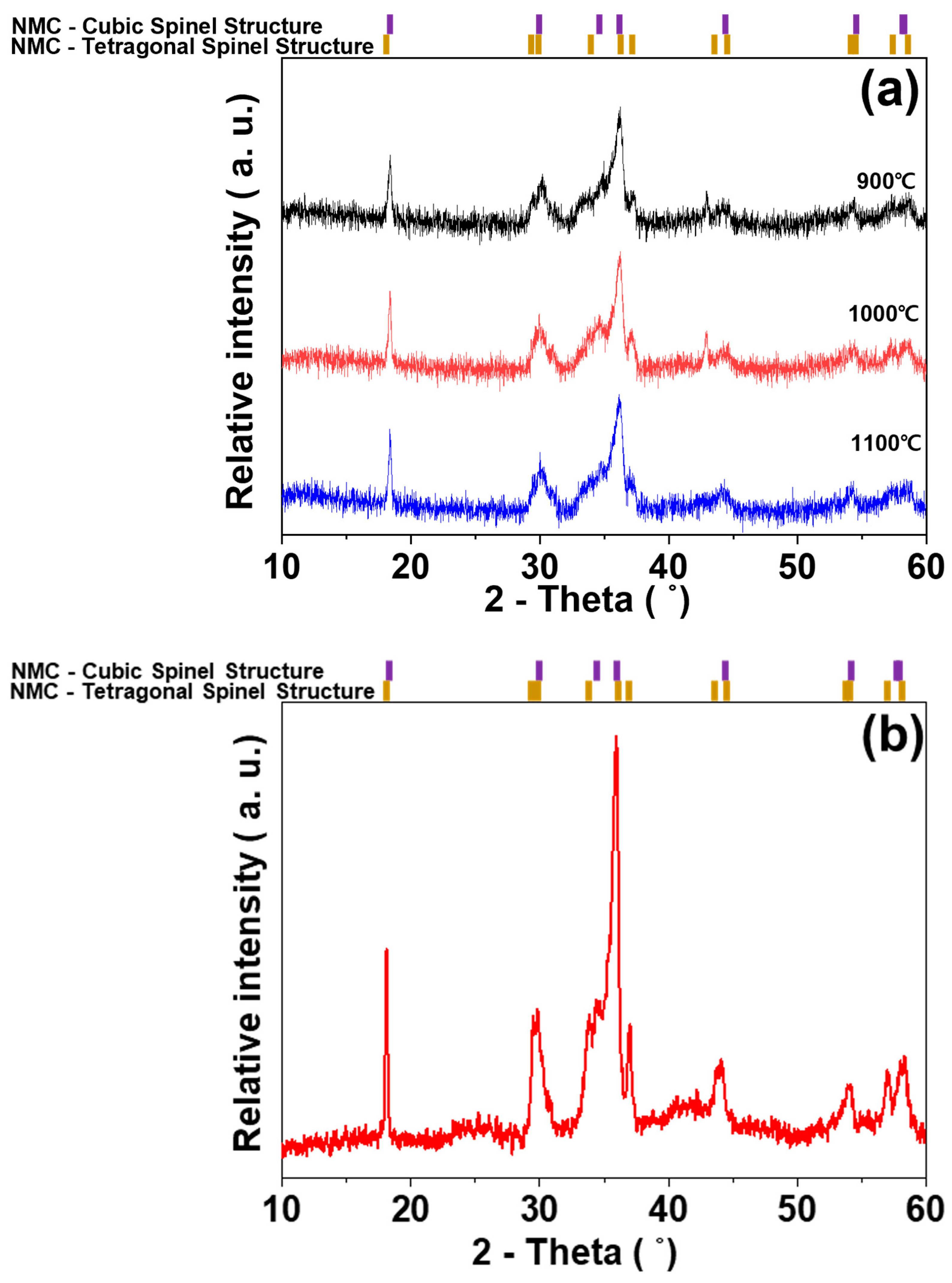
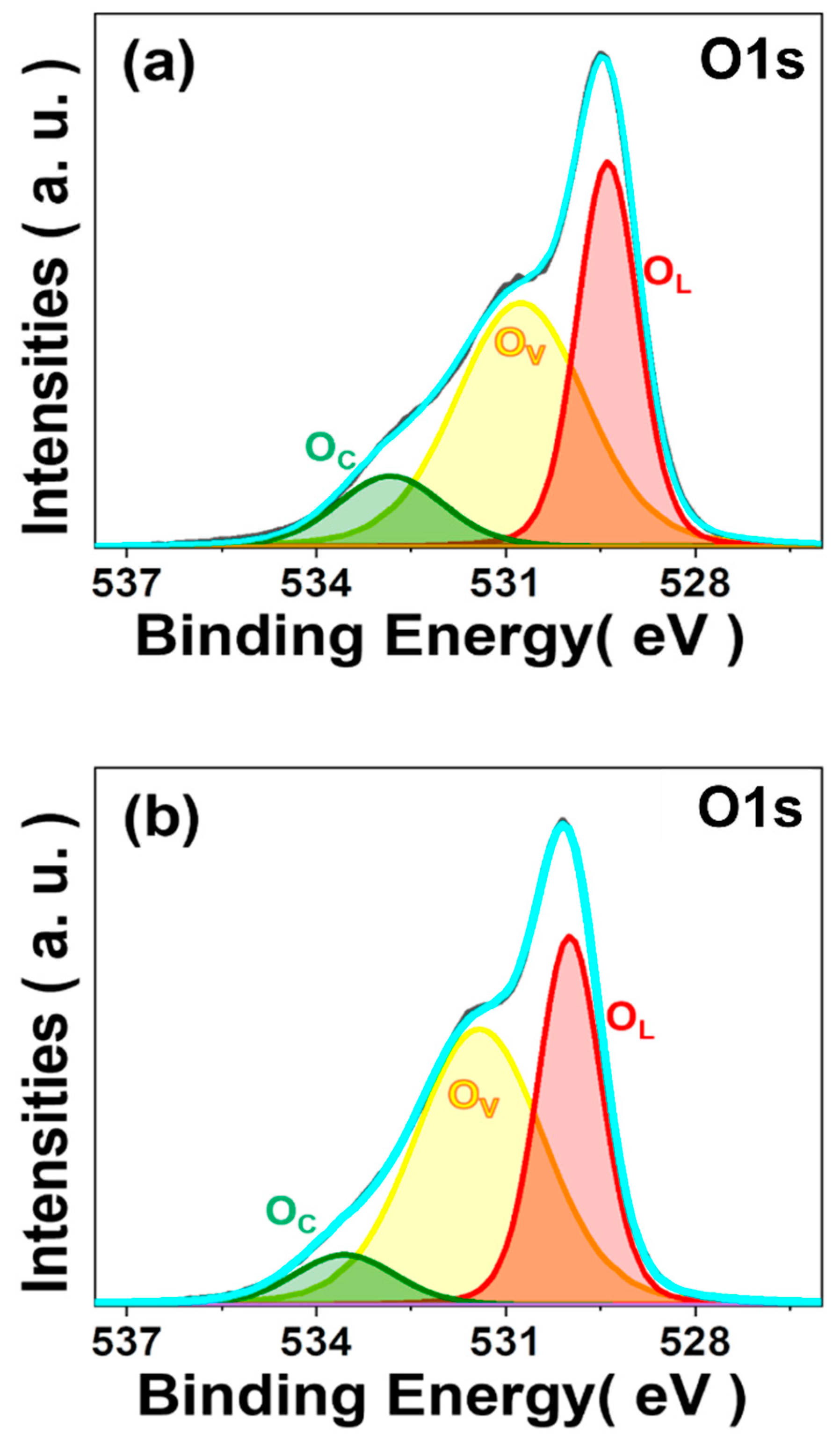
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mhin, S.; Han, H.; Kim, D.; Yeo, S.; Lee, J.-I.; Ryu, J.H. Phase evolution of (Ni, Co, Mn)O4 during heat treatment with high temperature in situ X-ray diffraction. Ceram. Int. 2015, 42, 5412–5417. [Google Scholar] [CrossRef]
- Zahid, T.; Li, W. A Comparative Study Based on the Least Square Parameter Identification Method for State of Charge Estimation of a LiFePO4 Battery Pack Using Three Model-Based Algorithms for Electric Vehicles. Energies 2016, 9, 720. [Google Scholar] [CrossRef] [Green Version]
- Han, H.; Park, K.R.; Hong, Y.-R.; Shim, K.; Mhin, S. Effect of Fe incorporation on cation distributions and hopping conductions in Ni-Mn-Co-O spinel oxides. J. Alloys Compd. 2018, 732, 486–490. [Google Scholar] [CrossRef]
- Jeon, J.-E.; Park, K.R.; Kim, K.M.; Ahn, C.; Lee, J.; Yu, D.-Y.; Bang, J.; Oh, N.; Han, H.; Mhin, S. Effect of Cu/Fe addition on the microstructures and electrical performances of Ni–Co–Mn oxides. J. Alloys Compd. 2020, 859, 157769. [Google Scholar] [CrossRef]
- Bhargava, A.; Eppstein, R.; Sun, J.; Smeaton, M.A.; Paik, H.; Kourkoutis, L.F.; Schlom, D.G.; Toroker, M.C.; Robinson, R.D. Breakdown of the Small-Polaron Hopping Model in Higher-Order Spinels. Adv. Mater. 2020, 32, e2004490. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Lee, J.S.; Ryu, J.H.; Kim, K.M.; Jones, J.L.; Lim, J.; Guillemet-Fritsch, S.; Lee, H.C.; Mhin, S. Effect of High Cobalt Concentration on Hopping Motion in Cobalt Manganese Spinel Oxide (CoxMn3–xO4, x ≥ 2.3). J. Phys. Chem. C 2016, 120, 13667–13674. [Google Scholar] [CrossRef] [Green Version]
- Han, H.; Davis, C.; Nino, J.C. Variable Range Hopping Conduction in BaTiO3 Ceramics Exhibiting Colossal Permittivity. J. Phys. Chem. C 2014, 118, 9137–9142. [Google Scholar] [CrossRef]
- Khopkar, P.; Kulkarni, J.; Darshane, V. Structural, transport and thermoanalytical studies of some coprecipitated spinels. Thermochim. Acta 1985, 93, 481–484. [Google Scholar] [CrossRef]
- Yokoyama, T.; Abe, Y.; Meguro, T.; Komeya, K.; Kondo, K.; Sasamoto, S.K. Preparation and Electrical Properties of Sintered Bodies Composed of Monophase Spinel Mn(2-X)Co2XNi(1-X)O4 (0 \LeqX \Leq 1) Derived from Rock-Salt-Type Oxides. Jpn. J. Appl. Phys. 1996, 35, 5775. [Google Scholar] [CrossRef]
- Sazelee, N.; Din, M.F.M.; Ismail, M.; Rather, S.-U.; Bamufleh, H.S.; Alhumade, H.; Taimoor, A.A.; Saeed, U. Effect of LaCoO3 Synthesized via Solid-State Method on the Hydrogen Storage Properties of MgH2. Materials 2023, 16, 2449. [Google Scholar] [CrossRef]
- Wang, J.; Dreyer, S.L.; Wang, K.; Ding, Z.; Diemant, T.; Karkera, G.; Ma, Y.; Sarkar, A.; Zhou, B.; Gorbunov, M.V.; et al. P2-type layered high-entropy oxides as sodium-ion cathode materials. Mater. Futures 2022, 1, 035104. [Google Scholar] [CrossRef]
- Shchelkanova, M.; Shekhtman, G.; Pershina, S.; Vovkotrub, E. Physico-Chemical Properties of NaV3O8 Prepared by Solid-State Reaction. Materials 2021, 14, 6976. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, S.-D. Acceleration of applied voltage on metallic ion migration of wires in NTC thermistor temperature sensors. Eng. Fail. Anal. 2013, 28, 252–263. [Google Scholar] [CrossRef]
- Wiendartun; Syarif, D.G. The Effect of MnO2 Content and Sintering Atmosphere on The Electrical Properties of Iron Titanium Oxide NTC Thermistors using Yarosite. J. Physics Conf. Ser. 2017, 812, 012120. [Google Scholar] [CrossRef]
- Cheng, F.; Wang, J.; Zhang, H.; Chang, A.; Kong, W.; Zhang, B.; Chen, L. Phase transition and electrical properties of Ni1−x Zn x Mn2O4 (0 ≤ x ≤ 1.0) NTC ceramics. J. Mater. Sci. Mater. Electron. 2014, 26, 1374–1380. [Google Scholar] [CrossRef]
- Abe, Y.; Meguro, T.; Oyamatsu, S.; Yokoyama, T.; Komeya, K. Formation region of monophase with cubic spinel-type oxides in Mn–Co–Ni ternary system. J. Mater. Sci. 1999, 34, 4639–4644. [Google Scholar] [CrossRef]
- Park, K.R.; Han, H.; Hou, D.; Jones, J.L.; Oh, N.; Ahn, C.; Lee, J.; Lee, S.H.; Mhin, S. Crystal structures and electrical properties of cobalt manganese spinel oxides. Mater. Today Commun. 2020, 25, 101298. [Google Scholar] [CrossRef]
- Gaál, F.; Szöllösy, I.; Arnold, M.; Paulik, F. Determination of the organic matter, metal carbonate and mobile water in soils simultaneous TG, DTG, DTA and EGA techniques. J. Therm. Anal. Calorim. 1994, 42, 1007–1016. [Google Scholar] [CrossRef]
- Mhin, S.; Han, H.; Kim, K.M.; Lim, J.; Kim, D.; Lee, J.-I.; Ryu, J.H. Synthesis of (Ni, Mn, Co)O4 nanopowder with single cubic spinel phase via combustion method. Ceram. Int. 2016, 42, 13654–13658. [Google Scholar] [CrossRef]
- de Vidales, J.M.; Rojas, R.; Vila, E.; García-Martínez, O. Low temperature synthesis of tetragonal nickel manganite spinels: Thermal behaviour and reactivity. Mater. Res. Bull. 1994, 29, 1163–1173. [Google Scholar] [CrossRef]
- Wang, W.; Kuai, L.; Cao, W.; Huttula, M.; Ollikkala, S.; Ahopelto, T.; Honkanen, A.-P.; Huotari, S.; Yu, M.; Geng, B. Mass-Production of Mesoporous MnCo2O4 Spinels with Manganese(IV)- and Cobalt(II)-Rich Surfaces for Superior Bifunctional Oxygen Electrocatalysis. Angew. Chem. Int. Ed. 2017, 56, 14977–14981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preda, I.; Gutiérrez, A.; Abbate, M.; Yubero, F.; Méndez, J.; Alvarez, L.; Soriano, L. Interface effects in theNi2px-ray photoelectron spectra of NiO thin films grown on oxide substrates. Phys. Rev. B 2008, 77, 075411. [Google Scholar] [CrossRef] [Green Version]
- Töpfer, J.; Feltz, A.; Gräf, D.; Hackl, B.; Raupach, L.; Weissbrodt, P. Cation Valencies and Distribution in the Spinels NiMn2O4 and MzNiMn2−zO4 (M = Li, Cu) Studied by XPS. Phys. Status Solidi A 1992, 134, 405–415. [Google Scholar] [CrossRef]
- Zhao, M.; Deng, J.; Liu, J.; Li, Y.; Liu, J.; Duan, Z.; Xiong, J.; Zhao, Z.; Wei, Y.; Song, W.; et al. Roles of Surface-Active Oxygen Species on 3DOM Cobalt-Based Spinel Catalysts MxCo3–xO4 (M = Zn and Ni) for NOx-Assisted Soot Oxidation. ACS Catal. 2019, 9, 7548–7567. [Google Scholar] [CrossRef]
- Sahoo, S.; Parashar, S.K.S.; Ali, S.M. CaTiO3 nano ceramic for NTCR thermistor based sensor application. J. Adv. Ceram. 2014, 3, 117–124. [Google Scholar] [CrossRef] [Green Version]
- Wen, S.; Wang, S.; Chung, D. Carbon fiber structural composites as thermistors. Sens. Actuators A Phys. 1999, 78, 180–188. [Google Scholar] [CrossRef]
- Han, H.; Lee, H.; Lim, J.; Kim, K.M.; Hong, Y.-R.; Lee, J.; Forrester, J.; Ryu, J.H.; Mhin, S. Hopping conduction in (Ni,Co,Mn)O4 prepared by different synthetic routes: Conventional and spark plasma sintering. Ceram. Int. 2017, 43, 16070–16075. [Google Scholar] [CrossRef] [Green Version]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, D.; Mhin, S. Effect of One Step Solid State Reaction Route on the Semiconductor Behavior of the Spinel (NI, Co, and Mn)O4 to Be Used as Temperature Sensor. Sensors 2023, 23, 5380. https://doi.org/10.3390/s23125380
Ko D, Mhin S. Effect of One Step Solid State Reaction Route on the Semiconductor Behavior of the Spinel (NI, Co, and Mn)O4 to Be Used as Temperature Sensor. Sensors. 2023; 23(12):5380. https://doi.org/10.3390/s23125380
Chicago/Turabian StyleKo, Daehyeon, and Sungwook Mhin. 2023. "Effect of One Step Solid State Reaction Route on the Semiconductor Behavior of the Spinel (NI, Co, and Mn)O4 to Be Used as Temperature Sensor" Sensors 23, no. 12: 5380. https://doi.org/10.3390/s23125380




