A Ratiometric Fluorescence Amplification Using Copper Nanoclusters with o-Phenylenediamine Sensor for Determination of Mercury (II) in Natural Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of L-Cysteine-Capped Copper Nanocluster
2.3. Sensing Detection of Mercury (II)
2.4. Detection of Mercury (II) in Real Water Samples
2.5. Validation
3. Results and Discussion
3.1. Synthesized and Characterization of L-Cysteine-Capped Copper Nanoclusters
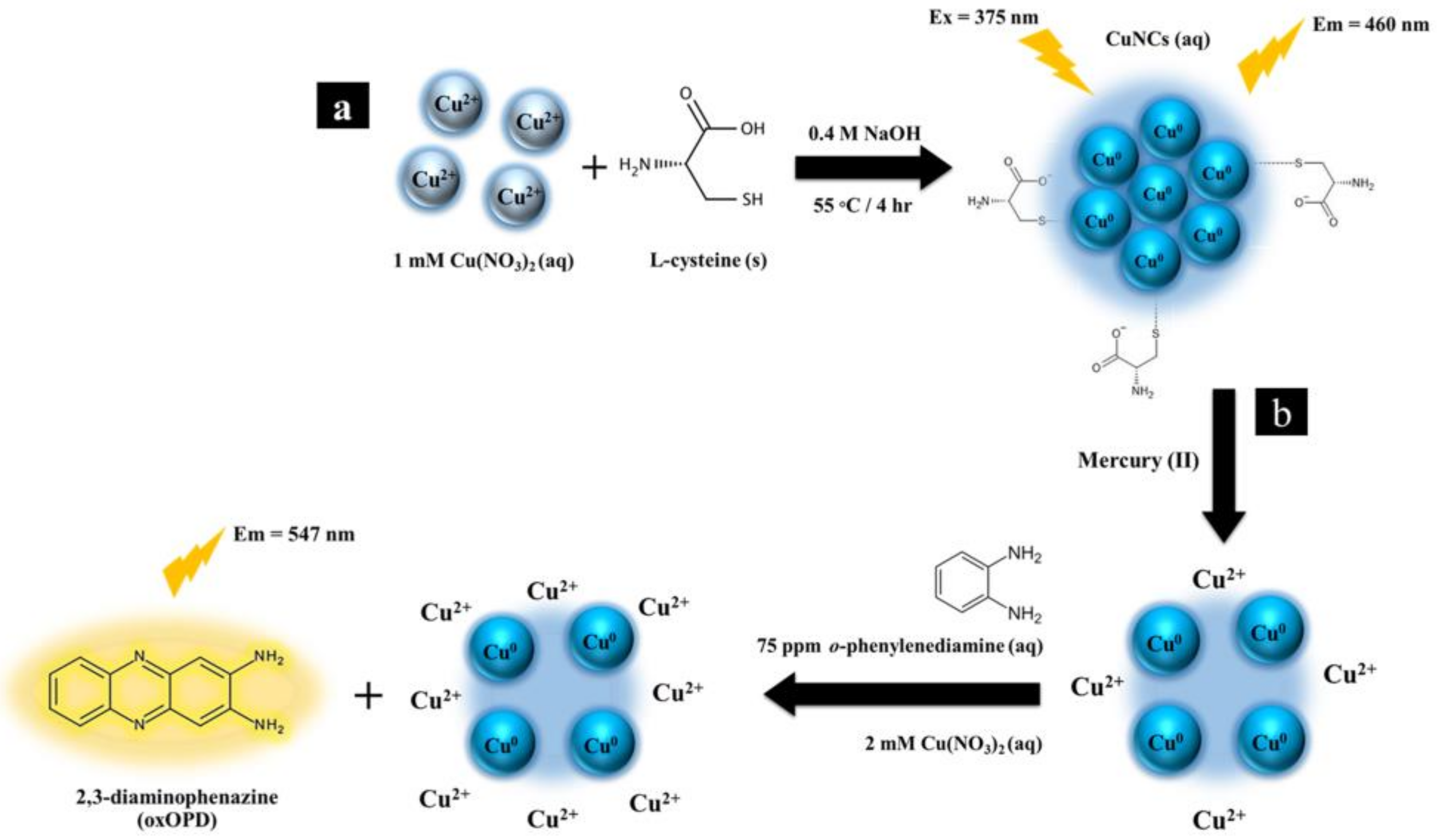
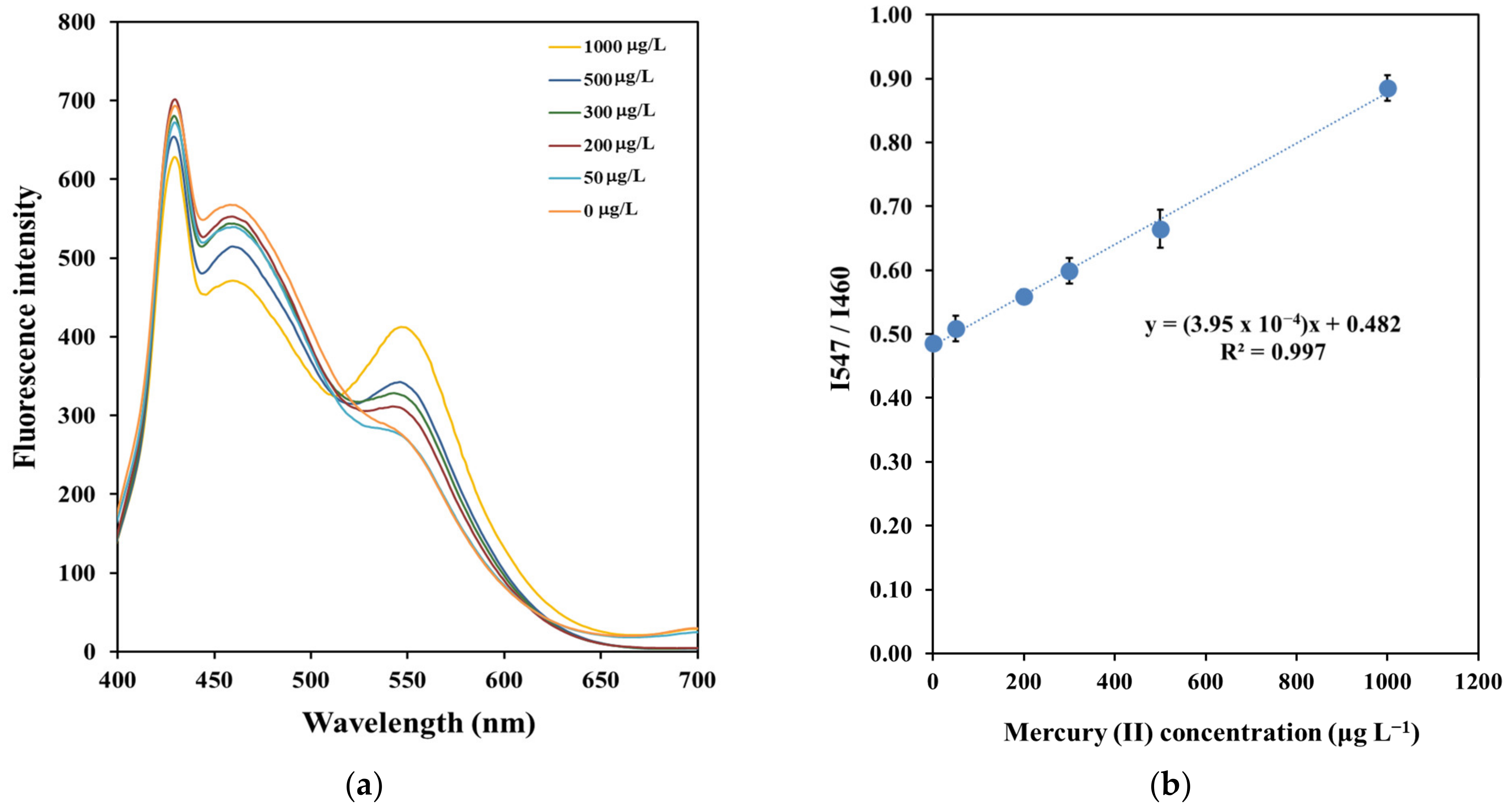
3.2. Sensing Strategy of Copper Nanoclusters with o-Phenylenediamine Sensors for the Determination of Mercury (II)
3.3. Optimization of the Experimental Conditions
3.3.1. Effect of o-Phenylenediamine Concentrations
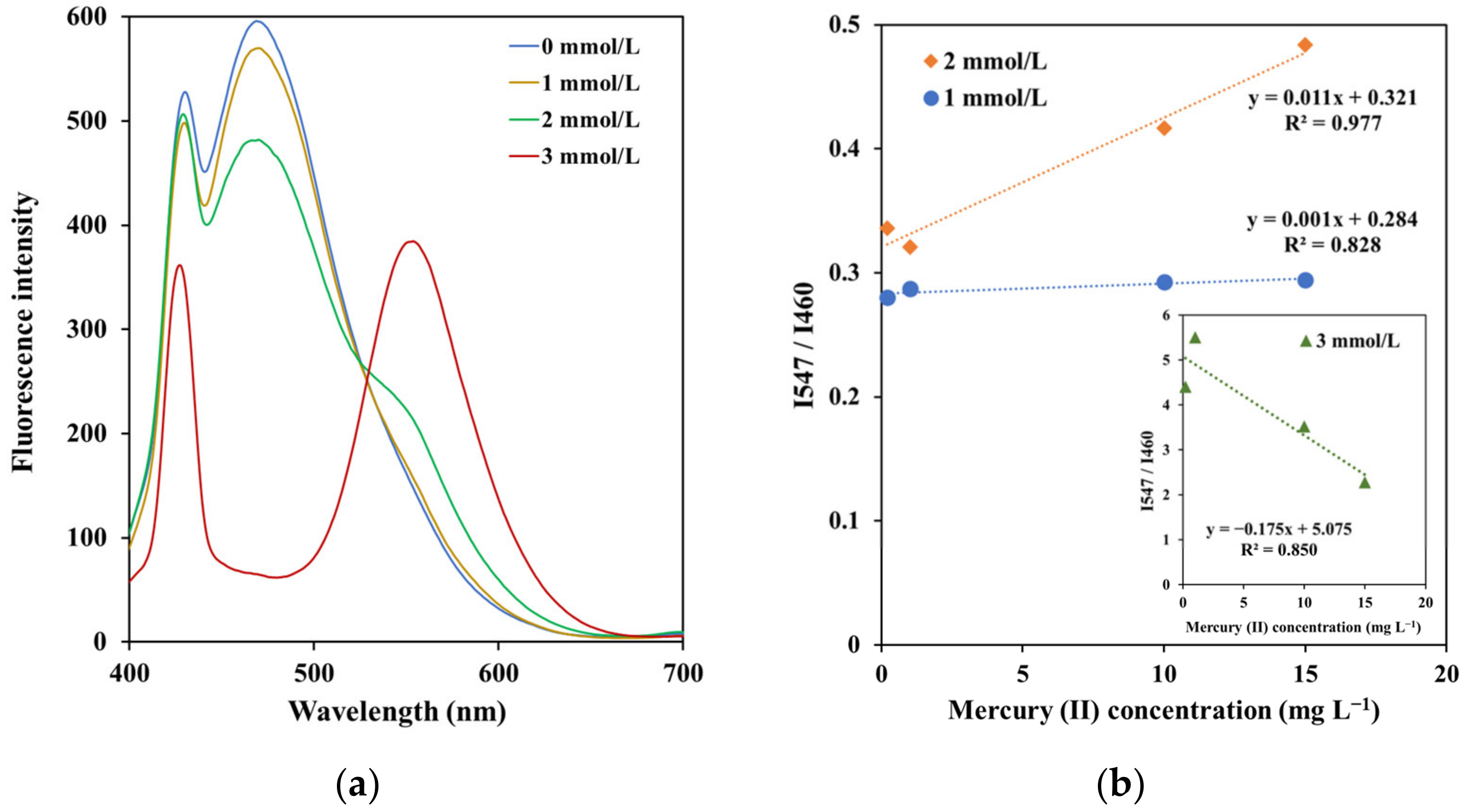
3.3.2. Effect of Copper (II) Nitrate Concentration
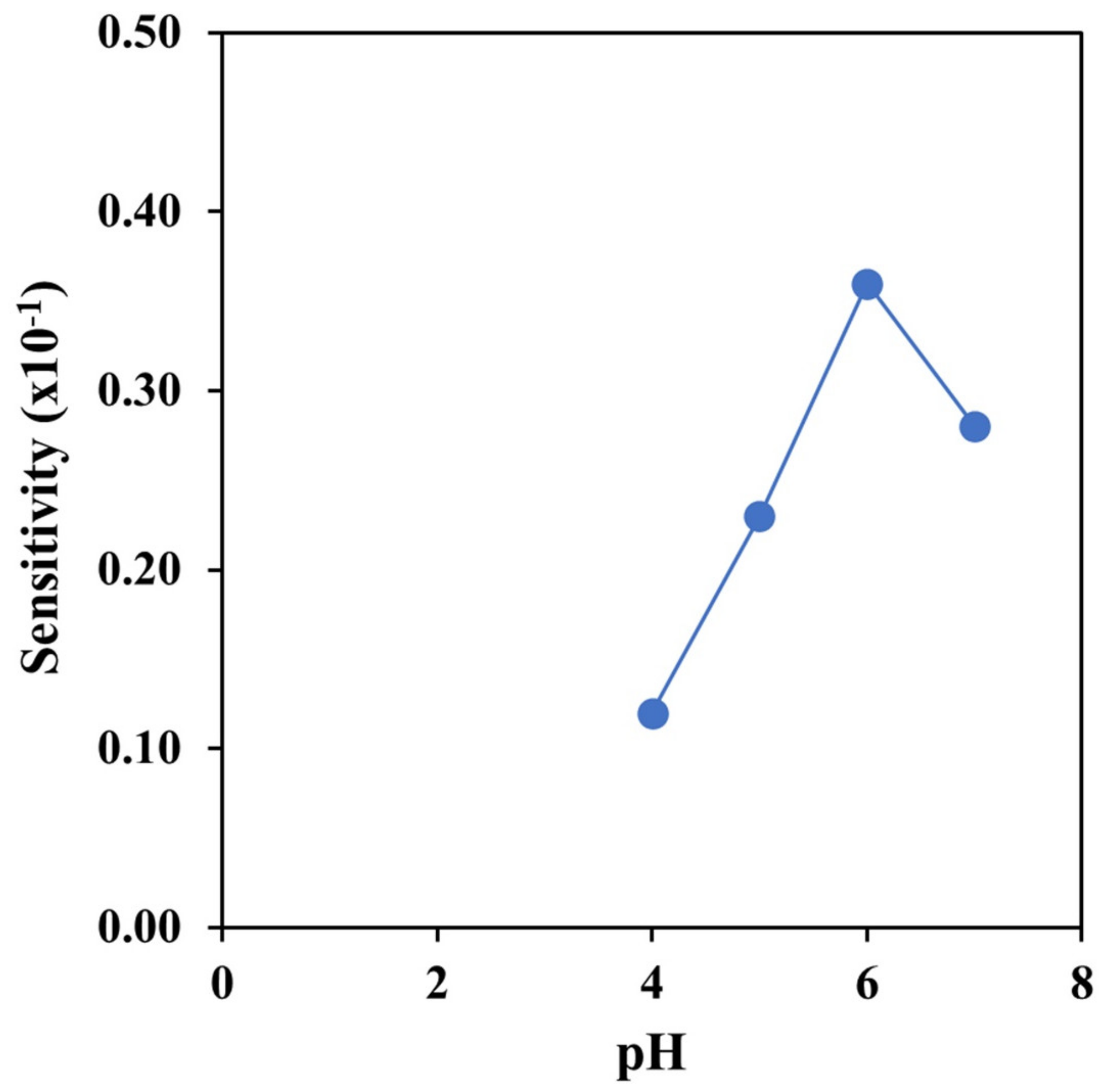
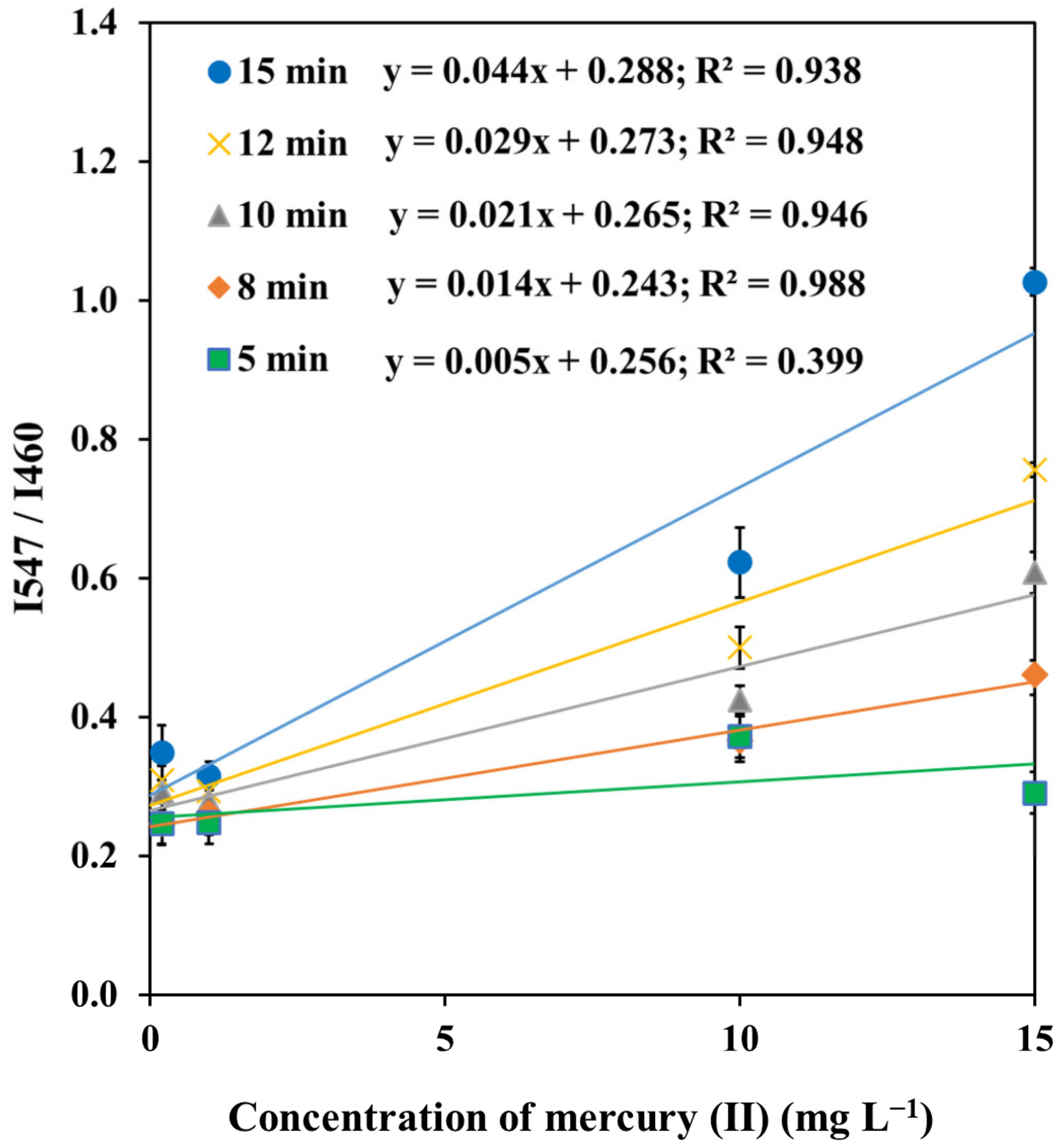
3.3.3. Effect of pH and Reaction Time
3.4. Method Validation and Fantastic Application
3.5. Selectivity of the Mercury (II) Detection System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bachmann, L.; Zezell, D.M.; Ribeiro, A.d.C.; Gomes, L.; Ito, A.S. Fluorescence Spectroscopy of Biological Tissues—A Review. Appl. Spectrosc. Rev. 2006, 41, 575–590. [Google Scholar] [CrossRef]
- Gopal, K.V. Neurotoxic effects of mercury on auditory cortex networks growing on microelectrode arrays: A preliminary analysis. Neurotoxicol. Teratol. 2003, 25, 69–76. [Google Scholar] [CrossRef]
- Zhang, D.H.; Sun, L.Y. Determination of mercury by cold vapour atomic absorption spectrometry with derivative signal processing. Anal. Chim. Acta 1999, 395, 173–178. [Google Scholar] [CrossRef]
- Zhang, Y.; Adeloju, S.B. A novel sequential injection—Cold vapour atomic absorption spectrometric system for rapid and reliable determination of mercury. Talanta 2008, 74, 951–957. [Google Scholar] [CrossRef]
- de Jesus, A.; Zmozinski, A.V.; Vieira, M.A.; Ribeiro, A.S.; da Silva, M.M. Determination of mercury in naphtha and petroleum condensate by photochemical vapor generation atomic absorption spectrometry. Microchem. J. 2013, 110, 227–232. [Google Scholar] [CrossRef]
- Souza, J.P.; Cerveira, C.; Miceli, T.M.; Moraes, D.P.; Mesko, M.F.; Pereira, J.S.F. Evaluation of sample preparation methods for cereal digestion for subsequent As, Cd, Hg and Pb determination by AAS-based techniques. Food Chem. 2020, 321, 126715. [Google Scholar] [CrossRef]
- Santos, E.J.d.; Herrmann, A.B.; Frescura, V.L.A.; Curtius, A.J. Evaluation of slurry preparation procedures for the simultaneous determination of Hg and Se in biological samples by axial view ICP OES using on-line chemical vapor generation. Anal. Chim. Acta 2005, 548, 166–173. [Google Scholar] [CrossRef]
- García-Mesa, J.C.; Montoro Leal, P.; López Guerrero, M.M.; Vereda Alonso, E.I. Simultaneous determination of noble metals, Sb and Hg by magnetic solid phase extraction on line ICP OES based on a new functionalized magnetic graphene oxide. Microchem. J. 2019, 150, 104141. [Google Scholar] [CrossRef]
- Liu, Z.; Xing, Z.; Li, Z.; Zhu, Z.; Ke, Y.; Jin, L.; Hu, S. The online coupling of high performance liquid chromatography with atomic fluorescence spectrometry based on dielectric barrier discharge induced chemical vapor generation for the speciation of mercury. J. Anal. At. Spectrom. 2017, 32, 678–685. [Google Scholar] [CrossRef]
- Lima, A.F.; Da Costa, M.C.; Ferreira, D.C.; Richter, E.M.; Munoz, R.A.A. Fast ultrasound-assisted treatment of inorganic fertilizers for mercury determination by atomic absorption spectrometry and microwave-induced plasma spectrometry with the aid of the cold-vapor technique. Microchem. J. 2015, 118, 40–44. [Google Scholar] [CrossRef]
- Hu, J.; Yu, X.; Zhang, X.; Jing, C.; Liu, T.; Hu, X.; Lu, S.; Uvdal, K.; Gao, H.W.; Hu, Z. Rapid detection of mercury (II) ions and water content by a new rhodamine B-based fluorescent chemosensor. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 241, 118657. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.M.; Koneswaran, M.; Narayanaswamy, R. A review on fluorescent inorganic nanoparticles for optical sensing applications. RSC Adv. 2016, 6, 21624–21661. [Google Scholar] [CrossRef]
- Li, Y.-L.; Leng, Y.-M.; Zhang, Y.-J.; Li, T.-H.; Shen, Z.-Y.; Wu, A.-G. A new simple and reliable Hg2+ detection system based on anti-aggregation of unmodified gold nanoparticles in the presence of O-phenylenediamine. Sens. Actuators B Chem. 2014, 200, 140–146. [Google Scholar] [CrossRef]
- Tolessa, T.; Tan, Z.Q.; Yin, Y.G.; Liu, J.F. Single-drop gold nanoparticles for headspace microextraction and colorimetric assay of mercury (II) in environmental waters. Talanta 2018, 176, 77–84. [Google Scholar] [CrossRef]
- Chen, Z.; Lou, T.; Wu, Q.; Li, K.; Tan, L.; Sun, J. A facile label-free colorimetric sensor for Hg2+ based on Hg-triangular silver nanoplates with amalgam-like structure. Sens. Actuators B Chem. 2015, 221, 365–369. [Google Scholar] [CrossRef]
- Mathaweesansurn, A.; Vittayakorn, N.; Detsri, E. Highly Sensitive and Selective Colorimetric Sensor of Mercury (II) based on Layer-by-Layer Deposition of Gold/Silver Bimetallic Nanoparticles. Molecules 2020, 25, 4443. [Google Scholar] [CrossRef]
- Yahyazadeh, E.; Shemirani, F. Easily synthesized carbon dots for determination of mercury(II) in water samples. Heliyon 2019, 5, e01596. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Xiao, X.; Xing, X.; Wang, Z.; Zou, T.; Wang, Z.; Zhao, R.; Wang, Y. Rhodamine B assisted graphene quantum dots flourescent sensor system for sensitive recognition of mercury ions. J. Lumin. 2019, 207, 273–281. [Google Scholar] [CrossRef]
- Wang, K.; Dong, E.-F.; Fang, M.; Chen, T.; Zhu, W.-J.; Li, C. Construction of ratio fluorescence sensor based on CdTe quantum dots and benzocoumarin-3-carboxylic acid for Hg2+ detection. Chin. J. Anal. Chem. 2022, 50, 100070. [Google Scholar] [CrossRef]
- Er Demirhan, B.; Şatana Kara, H.E.; Demirhan, B. One-step green aqueous synthesis of blue light emitting copper nanoclusters for quantitative determination of food color Ponceau 4R. J. Photochem. Photobiol. A Chem. 2021, 417, 113356. [Google Scholar] [CrossRef]
- Shang, L.; Dong, S.; Nienhaus, G.U. Ultra-small fluorescent metal nanoclusters: Synthesis and biological applications. Nano Today 2011, 6, 401–418. [Google Scholar] [CrossRef]
- Sun, Z.; Li, J.; Yang, Y.; Tong, Y.; Li, H.; Wang, C.; Du, L.; Jiang, Y. Ratiometric Fluorescent Biosensor Based on Self-Assembled Fluorescent Gold Nanoparticles and Duplex-Specific Nuclease-Assisted Signal Amplification for Sensitive Detection of Exosomal miRNA. Bioconjug Chem. 2022, 33, 1698–1706. [Google Scholar] [CrossRef]
- Sivasankaran, U.; Radecki, J.; Radecka, H.; Girish Kumar, K. Copper nanoclusters: An efficient fluorescence sensing platform for quinoline yellow. Luminescence 2019, 34, 243–248. [Google Scholar] [CrossRef]
- Ma, Y.; Yu, Y.; Lin, B.; Zhang, L.; Cao, Y.; Guo, M. A novel signal amplification strategy based on the use of copper nanoclusters for ratiometric fluorimetric determination of o-phenylenediamine. Mikrochim. Acta 2019, 186, 206. [Google Scholar] [CrossRef]
- Luo, T.; Zhang, S.; Wang, Y.; Wang, M.; Liao, M.; Kou, X. Glutathione-stabilized Cu nanocluster-based fluorescent probe for sensitive and selective detection of Hg(2+) in water. Luminescence 2017, 32, 1092–1099. [Google Scholar] [CrossRef] [Green Version]



| OPD Concentration (mg L−1) | Equation | R2 |
|---|---|---|
| 10 | y = 0.003x + 0.483 | 0.146 |
| 25 | y = 0.001x + 0.585 | 0.141 |
| 50 | y = 0.004x + 0.832 | 0.454 |
| 75 | y = 0.032x + 0.719 | 0.981 |
| 100 | y = 0.041x + 0.993 | 0.865 |
| Sample | Spiked Mercury (II) (μg L−1) | Mercury (II) Concentrations (μg L−1) (±SD) | Recovery a (%) | |
|---|---|---|---|---|
| This Work | ICP-OES | |||
| S-1 | 50 | 53.2 ± 0.5 | 50.7 ± 0.2 | 106.4 |
| 100 | 105.7 ± 2.4 | 98.4 ± 0.8 | 105.7 | |
| S-2 | 50 | 51.2 ± 0.3 | 50.9 ± 0.3 | 102.4 |
| 100 | 106.1 ± 4.1 | 97.8 ± 1.0 | 106.1 | |
| S-3 | 50 | 48.4 ± 1.7 | 52.5 ± 1.1 | 96.8 |
| 100 | 97.5 ± 2.9 | 102.4 ± 0.4 | 97.5 | |
| S-4 | 50 | 49.2 ± 2.8 | 51.3 ± 1.6 | 98.4 |
| 100 | 96.9 ± 3.3 | 103.1 ± 2.1 | 96.9 | |
| S-5 | 50 | 51.3 ± 1.4 | 52.8 ± 1.7 | 102.6 |
| 100 | 98.9 ± 5.1 | 103.7 ± 2.4 | 98.9 | |
| Techniques | Sample | Analysis Time (min) | Linear Range (μg L−1) | LOD (μg L−1) | %RSD | [Ref] |
|---|---|---|---|---|---|---|
| Sequential injection—CVAAS | Water | 0.33 | 0–60 | 0.2 | 1.8 | [4] |
| Photochemical vapor generation—AAS | Naphtha and condensate | 30 | 0–100 | 0.6 | 1.2–8.8 | [5] |
| Magnetic on-line solid phase extraction—ICP-OES | Water | 4.6 | 0.2–1000 | 0.05 | 1.6 | [8] |
| HPLC-AFS | Tuna fish (CRM) | 14 | 5–200 | 1.6 | 50 | [9] |
| Rhodamine B-banded fluorescent chemosensor | Water | 0.02 | 0–6 | 3.2 | 2.2–4.9 | [11] |
| AuNPs/UV–vis spectrophotometer | Water | 20 | 2–400 | 1.0 | - | [13] |
| CuNCs/fluorescence spectrophotometer | Water | 8 | 0–1000 | 18 | 2.2–5.8 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phoungsiri, A.; Lerdpiriyaskulkij, N.; Monvisade, P.; Detsri, E.; Mathaweesansurn, A. A Ratiometric Fluorescence Amplification Using Copper Nanoclusters with o-Phenylenediamine Sensor for Determination of Mercury (II) in Natural Water. Sensors 2023, 23, 5429. https://doi.org/10.3390/s23125429
Phoungsiri A, Lerdpiriyaskulkij N, Monvisade P, Detsri E, Mathaweesansurn A. A Ratiometric Fluorescence Amplification Using Copper Nanoclusters with o-Phenylenediamine Sensor for Determination of Mercury (II) in Natural Water. Sensors. 2023; 23(12):5429. https://doi.org/10.3390/s23125429
Chicago/Turabian StylePhoungsiri, Ampika, Natee Lerdpiriyaskulkij, Pathavuth Monvisade, Ekarat Detsri, and Arjnarong Mathaweesansurn. 2023. "A Ratiometric Fluorescence Amplification Using Copper Nanoclusters with o-Phenylenediamine Sensor for Determination of Mercury (II) in Natural Water" Sensors 23, no. 12: 5429. https://doi.org/10.3390/s23125429





