Fabrication of Carbon Nanofiber Incorporated with CuWO4 for Sensitive Electrochemical Detection of 4-Nitrotoluene in Water Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of CuWO4/CNF Nanocomposite
2.2. Fabrication of GCE/CNF/CuWO4 Electrode
3. Results
3.1. XRD Studies
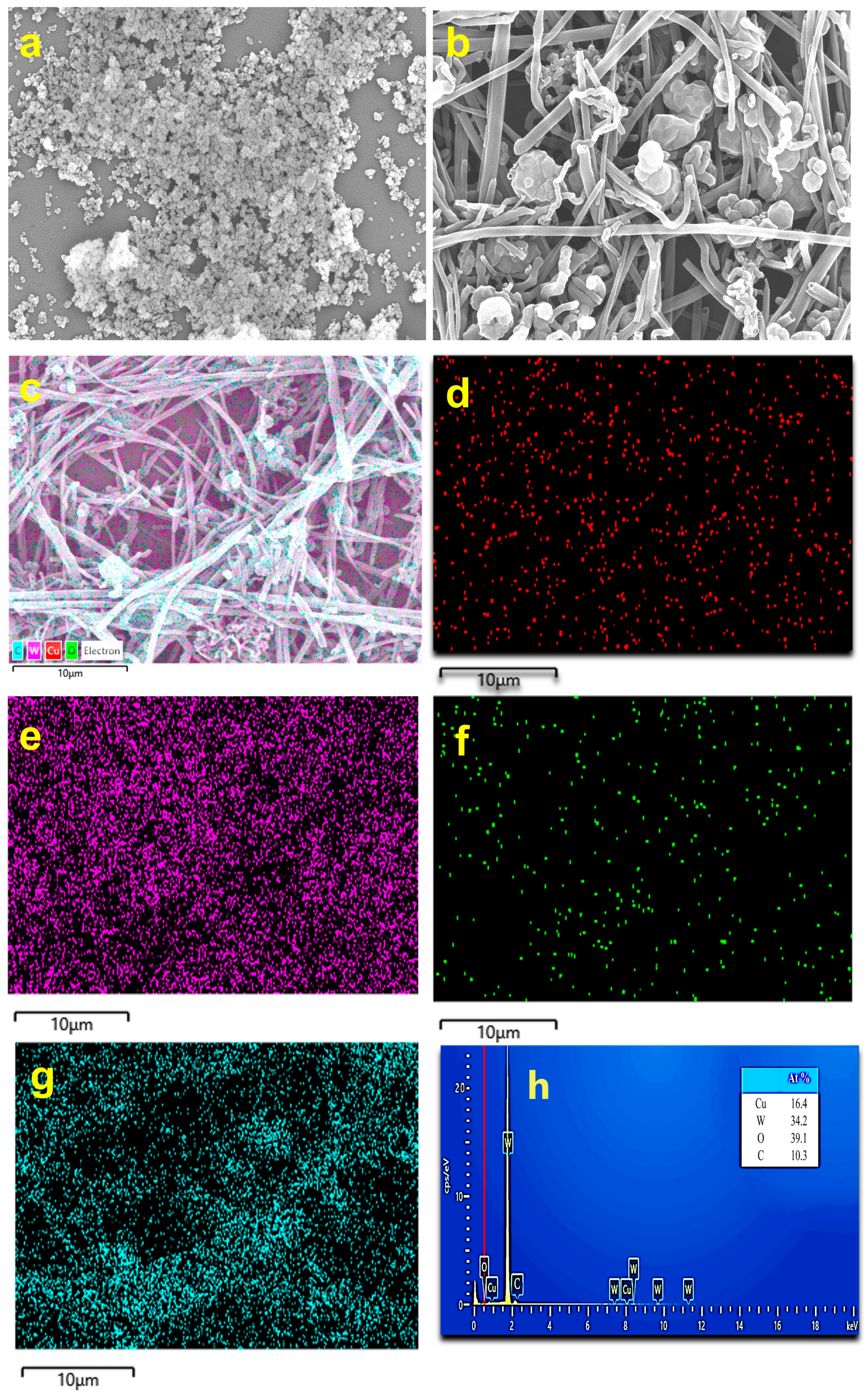
3.2. Structure and Morphology Analysis by FESEM and HRTEM Studies
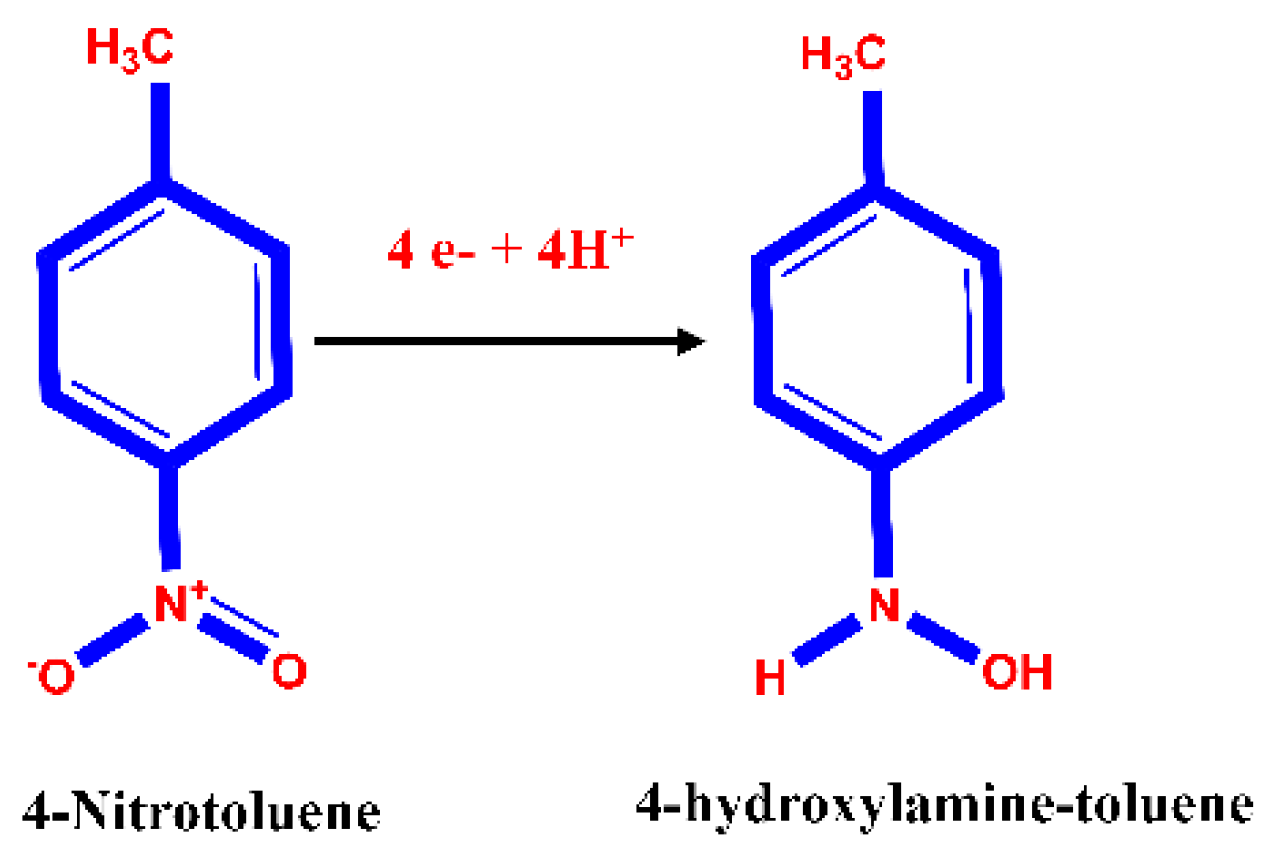
4. Electrochemical Reduction Studies of 4-NT on the GCE/CNF/CuWO4 Electrode
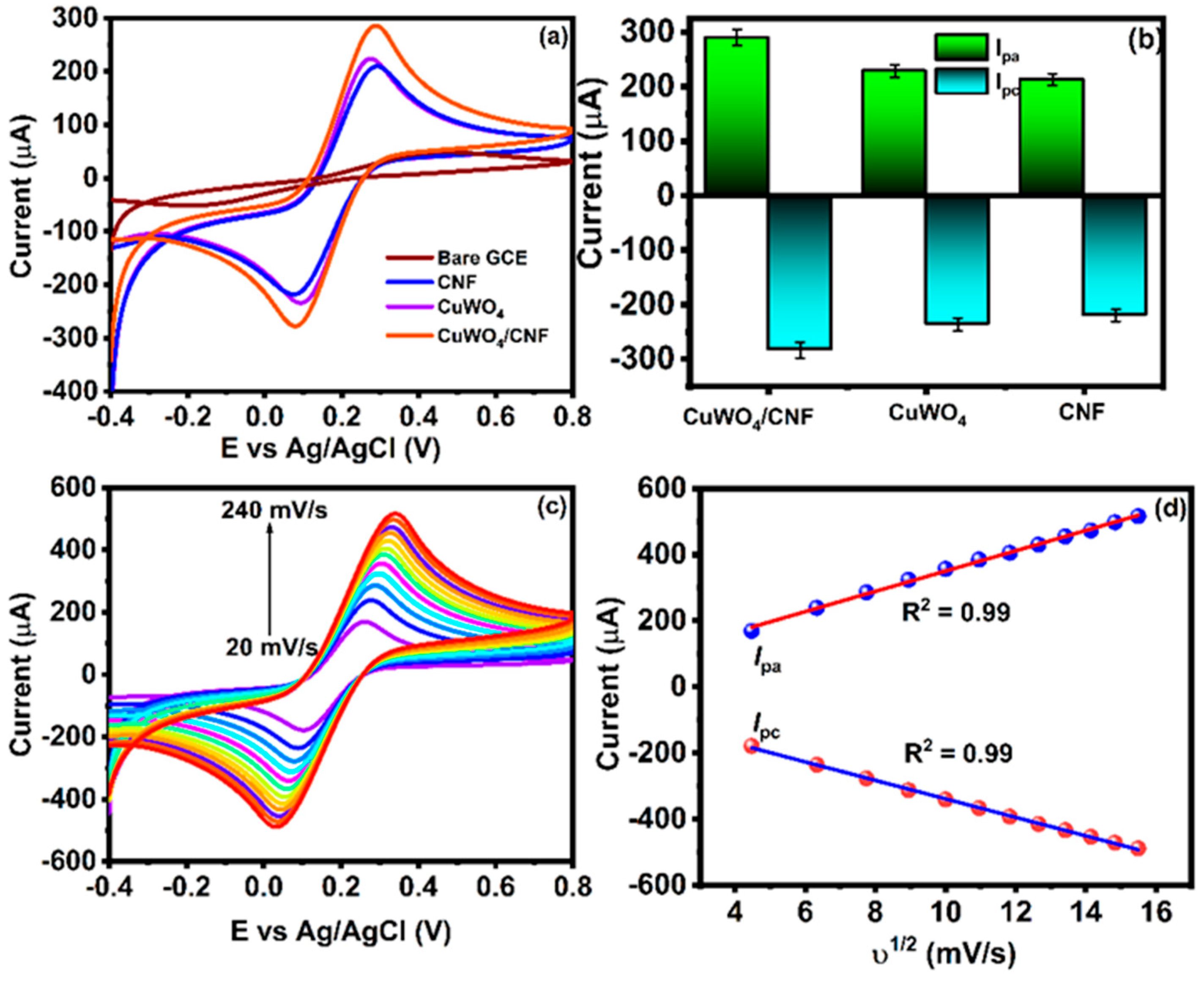
4.1. Electrochemical Behavior of Different Modified Electrodes
4.2. Different Films and Effects of Different Concentration Studies
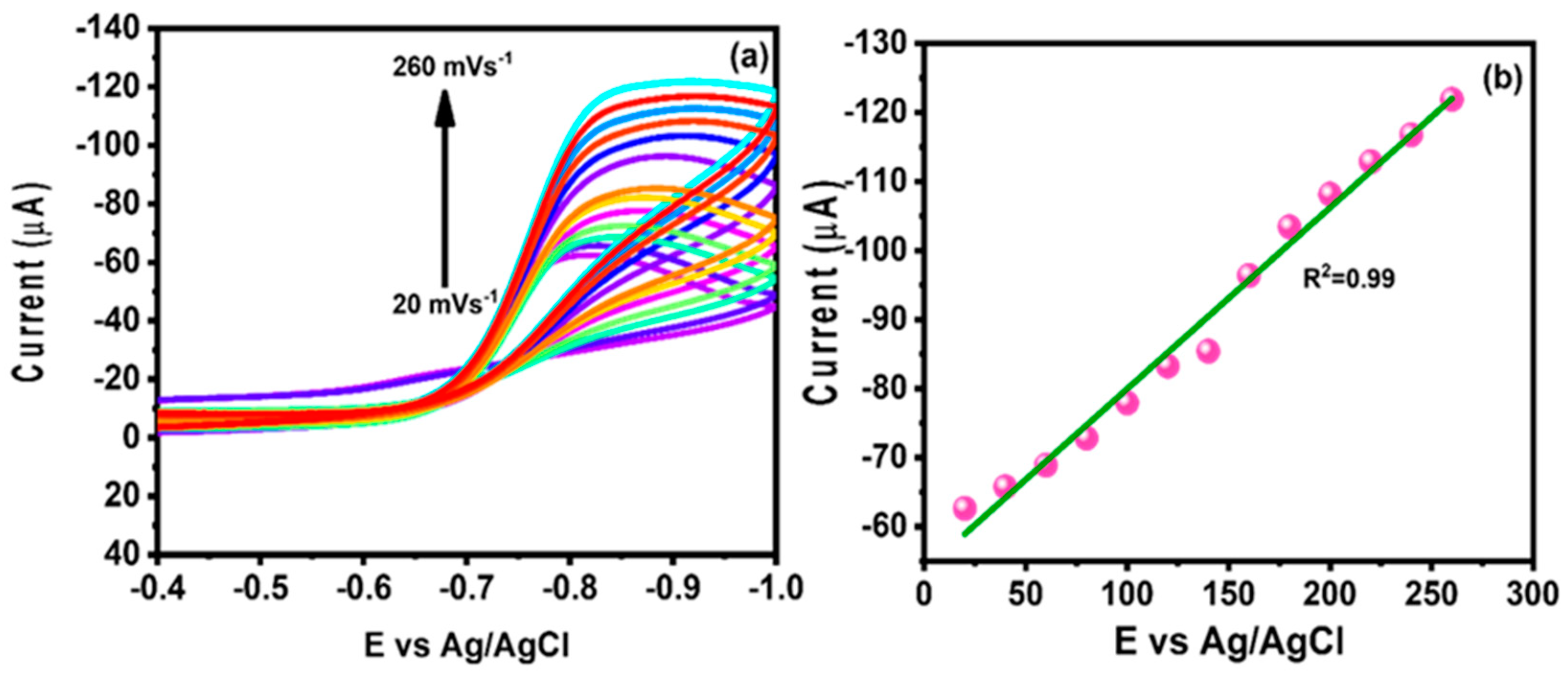
4.3. Effect of Different pH and Different Scan Rate Studies
4.4. Differential Pulse Voltammetry Studies
4.5. Interference Studies
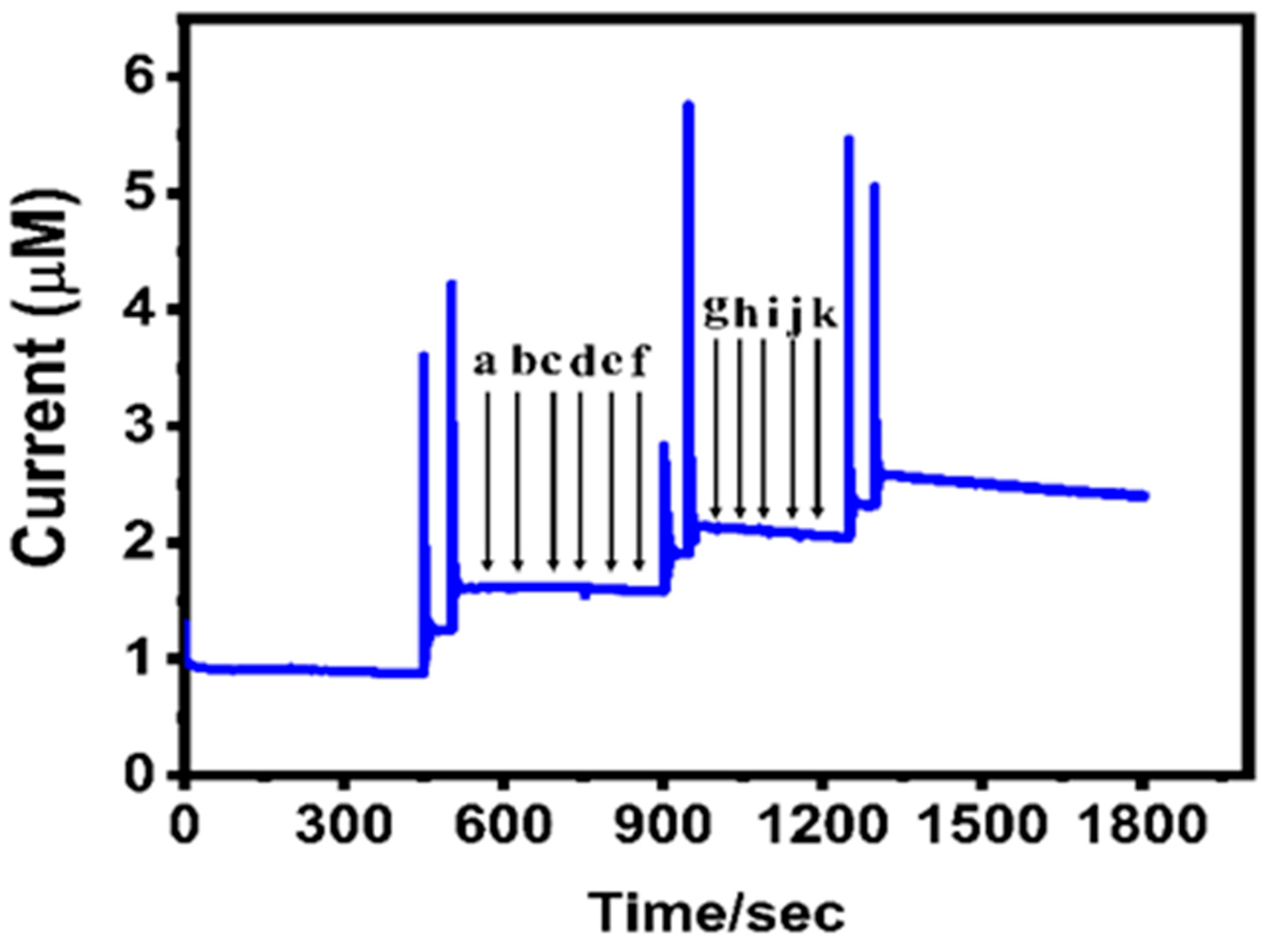
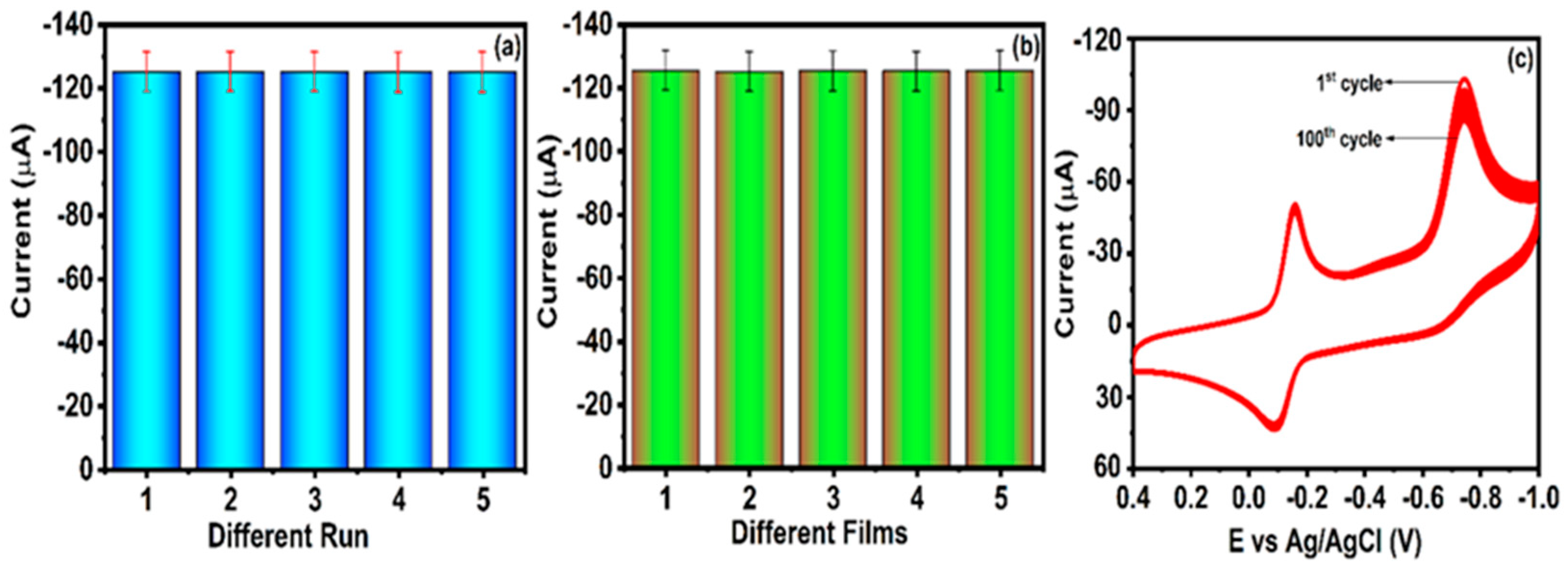
4.6. Repeatability, Reproducibility, and Stability Performance of GCE/CuWO4/CNF Electrode
4.7. Real Sample Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gou, Z.; Zhang, X.; Zuo, Y.; Tian, M.; Dong, B.; Lin, W. Pyrenyl-Functionalized Polysiloxane Based on Synergistic Effect for Highly Selective and Highly Sensitive Detection of 4-Nitrotoluene. ACS Appl. Mater. Interfaces 2019, 11, 30218–30227. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, U.; Garg, P.; Bhanjana, G.; Kaur, G.; Kaushik, A.; Chaudhary, G.R. Spherical Silver Oxide Nanoparticles for Fabrication of Electrochemical Sensor for Efficient 4-Nitrotoluene Detection and Assessment of their Antimicrobial Activity. Sci. Total Environ. 2022, 808, 152179. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, U.; Kaur, I.; Chauhan, A.; Kaur, N.; Kaur, G.; Chaudhary, G.R. Zinc oxide-Copper Sulfide Semiconductor Nano-heterostructure for Low-Level Electrochemical Detection of 4-Nitrotoluene. Electrochim. Acta 2023, 447, 142160. [Google Scholar] [CrossRef]
- Easwaramoorthy, D.; Yu, Y.C.; Huang, H.J. Chemiluminescence Detection of Paracetamol by a Luminol-Permanganate Based Reaction. Anal. Chim. Acta 2001, 439, 95–100. [Google Scholar] [CrossRef]
- Sazinas, R.; Andersen, S.Z.; Li, K.; Saccoccio, M.; Krempl, K.; Pedersen, J.B.; Chorkendorff, I. Towards Understanding of Electrolyte Degradation in Lithium-Mediated Non-aqueous Electrochemical Ammonia Synthesis with Gas chromatography-Mass Spectrometry. RSC Adv. 2021, 11, 31487–31498. [Google Scholar] [CrossRef]
- Liu, Y.; Mills, R.C.; Boncella, J.M.; Schanze, K.S. Fluorescent Polyacetylene Thin Film Sensor for Nitroaromatics. Langmuir 2001, 17, 7452–7455. [Google Scholar] [CrossRef]
- Selvan, P.S.; Gopinath, R.; Saravanan, V.S.; Gopal, N.; Kumar, A.S.; Periyasamy, K. Simultaneous Estimation of Paracetamol and Aceclofenac in Combined Dosage Forms by RP-HPLC Method. Asian J. Chem. 2007, 19, 1004. [Google Scholar]
- Hira, S.A.; Nallal, M.; Park, K.H. Fabrication of PdAg Nanoparticles Infused Metal-Organic Framework for Electrochemical and Solution-Chemical Reduction and Detection of Toxic 4-Nitrophenol. Sens. Actuators B Chem. 2019, 298, 126861. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, L.; Zhang, Y.; Tang, H. Electrochemical Sensoring of 2,4-Dinitrophenol by Using Composites of Graphene oxide with Surface Molecular Imprinted Polymer. Sens. Actuators B Chem. 2012, 171, 1151–1158. [Google Scholar] [CrossRef]
- Chakraborty, U.; Bhanjana, G.; Adam, J.; Mishra, Y.K.; Kaur, G.; Chaudhary, G.R.; Kaushik, A. A Flower-like ZnO–Ag2O Nanocomposite for Label and Mediator Free Direct Sensing of Dinitrotoluene. RSC Adv. 2020, 10, 27764–27774. [Google Scholar] [CrossRef]
- Lim, H.J.; Chua, B.; Son, A. Detection of Bisphenol a Using Palm-size Nano Aptamer Analyzer. Biosens. Bioelectron. 2017, 94, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Sangamithirai, D.; Ramanathan, S. Electrochemical Sensing Platform for the Detection of Nitroaromatics Using g-C3N4/V2O5 Nanocomposites Modified Glassy Carbon Electrode. Electrochim. Acta 2022, 434, 141308. [Google Scholar] [CrossRef]
- Alam, M.W.; Al, Q.H.S.; Souayeh, B.; Ahmed, W.; Albalawi, H.; Farhan, M.; Naeem, S. Novel Copper-Zinc-Manganese Ternary Metal Oxide Nanocomposite as Heterogeneous Catalyst for Glucose Sensor and Antibacterial Activity. Antioxidants 2022, 11, 1064. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.M.; Jiang, M.; Tang, D.; Qiao, L.; Xiao, H.Y.; Oropeza, F.E.; Zhang, K.H.L. Elucidating the Electronic structure of CuWO4 thin films for enhanced photoelectrochemical water splitting. J. Mater. Chem. A 2019, 7, 11895–11907. [Google Scholar] [CrossRef]
- Wang, C.; Fu, M.; Cao, J.; Wu, X.; Hu, X.; Dong, F. BaWO4/g-C3N4 heterostructure with excellent bifunctional photocatalytic performance. J. Chem. Eng. 2020, 385, 123833. [Google Scholar] [CrossRef]
- Sriram, B.; Shajahan, S.; Hsu, Y.F.; Wang, S.F.; Haija, M.A. Electrochemical Behavior of a Copper Tungstate-Fabricated Disposable Strip for the Rapid and Real-Time Detection. ACS Appl. Nano Mater. 2023, 6, 1–9. [Google Scholar] [CrossRef]
- Ahmed, J.; Ahamad, T.; Ubaidullah, M.; Al-Enizi, A.M.; Alhabarah, A.N.; Alhokbany, N.; Alshehri, S.M. RGO Supported NiWO4 Nanocomposites for Hydrogen Evolution Reactions. Mater. Lett. 2019, 240, 51–54. [Google Scholar] [CrossRef]
- Xu, X.; Pei, L.; Yang, Y.; Shen, J.; Ye, M. Facile Synthesis of NiWO4/Reduced Graphene Oxide Nanocomposite with Excellent Capacitive Performance for Supercapacitors. J. Alloys Compd. 2016, 654, 23–31. [Google Scholar] [CrossRef]
- Mikhailik, V.B.; Kraus, H.; Miller, G.; Mykhaylyk, M.S.; Wahl, D. Luminescence of CaWO4, CaMoO4, and ZnWO4 Scintillating Crystals Under Different Excitations. J. Appl. Phys. 2005, 97, 083523. [Google Scholar] [CrossRef] [Green Version]
- Habibi, M.M.; Mousavi, M.; Shadman, Z.; Ghasemi, J.B. Preparation of a Nonenzymatic Electrochemical Sensor Based on a g-C3N4/MWO4 (M: Cu, Mn, Co, Ni) Composite for the Determination of H2O2. New J. Chem. 2022, 46, 3766–3776. [Google Scholar] [CrossRef]
- Gaillard, N.; Chang, Y.; DeAngelis, A.; Higgins, S.; Braun, A. A Nanocomposite Photoelectrode Made of 2.2 eV Band gap Copper Tungstate (CuWO4) and Multi-wall carbon nanotubes for Solar-Assisted Water Splitting. Int. J. Hydrogen Energy 2013, 38, 3166–3176. [Google Scholar] [CrossRef]
- Davi, M.; Mann, M.; Ma, Z.; Schrader, F.; Drichel, A.; Budnyk, S.; Slabon, A. An MnNCN-Derived Electrocatalyst for CuWO4 Photoanodes. Langmuir 2018, 34, 3845–3852. [Google Scholar] [CrossRef]
- Shad, N.A.; Bajwa, S.Z.; Amin, N.; Taj, A.; Hameed, S.; Khan, Y.; Dai, Z.; Cao, C.; Khan, W.S. Solution Growth of 1D Zinc Tungstate (ZnWO4) Nanowires; Design, Morphology, and Electrochemical Sensor Fabrication for Selective Detection of Chloramphenicol. J. Hazard. Mater. 2019, 367, 205–214. [Google Scholar] [CrossRef]
- Dinh, P.H.; Pham, T.D.; Thuan, D.V.; Cam, N.T.D.; Hanh, N.T.; Van Ha, H.; Tri, N.L.M. CuWO4 Decorated by Polypyrrole (PPy) Protector/Sensitizer for Novel Photocatalytic and Stable Water Splitting for Hydrogen Generation. Int. J. Hydrogen Energy 2020, 45, 21442–21449. [Google Scholar] [CrossRef]
- Hosseinpour, M.S.M.; Sobhani, N.A. Simple Synthesis and Characterization of Copper Tungstate Nanoparticles: Investigation of Surfactant Effect and Its Photocatalyst Application. J. Mater. Sci. Mater. Electron. 2016, 27, 7548–7553. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Weier, M.L.; Duong, L.V.; Frost, R.L. Microwave-Assisted Synthesis and Characterisation of Divalent Metal Tungstate Nanocrystalline Minerals: Ferberite, Hubnerite, Sanmartinite, Scheelite and Stolzite. Mater. Chem. Phys. 2004, 88, 438–443. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.-H.; Liu, B.; Mo, M.-S.; Huang, J.-H.; Liu, X.-M.; Qian, Y.-T. General Synthesis of Single-Crystal Tungstate Nanorods/Nanowires: A Facile, Low-Temperature Solution Approach. Adv. Funct. Mater. 2003, 13, 639–647. [Google Scholar] [CrossRef]
- Bagwade, P.P.; Malavekar, D.B.; Ubale, S.B.; Bulakhe, R.N.; In, I.; Patil, U.M.; Lokhande, C.D. Synthesis, Characterization and Supercapacitive Application of Nanocauliflower-like Cobalt Tungstate Thin Films by Successive Ionic Layer Adsorption and Reaction (SILAR) Method. Electrochim. Acta 2022, 408, 139933. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E.; Rahighi, R. Toward Single-DNA Electrochemical Biosensing by Graphene Nanowalls. ACS Nano 2012, 6, 2904–2916. [Google Scholar] [CrossRef]
- Bounegru, A.V.; Apetrei, C. Carbonaceous Nanomaterials Employed in the Development of Electrochemical Sensors Based on Screen-Printing Technique-A Review. Catalysts 2020, 10, 680. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, F.; Wang, H.; Gao, L.; Wang, Z. A Sensor Based on Au Nanoparticles/Carbon Nitride/Graphene Composites for the Detection of Chloramphenicol and Ciprofloxacin. ECS J. Solid State Sci. Technol. 2018, 7, M201. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, S.; Wang, J.; Yu, A.; Wei, G. Carbon Nanofiber-Based Functional Nanomaterials for Sensor Applications. J. Nanomater. 2019, 9, 1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, G.; Zhao, F. Electrochemical Sensor for Chloramphenicol Based on Novel Multiwalled Carbon Nanotubes@ Molecularly Imprinted Polymer. Biosens. Bioelectron. 2015, 64, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Huan, K.; Li, Y.; Deng, D.; Wang, H.; Wang, D.; Li, M.; Luo, L. Composite-controlled electrospinning of CuSn bimetallic nanoparticles/carbon nanofibers for electrochemical glucose sensor. Appl. Surf. Sci. 2022, 573, 151528. [Google Scholar] [CrossRef]
- Gorska, A.; Zambrzycki, M.; Bator, B.P.; Piech, R. New Electrochemical Sensor Based on Electrospun Carbon Nanofibers Modified with Cobalt Nanoparticles and Its Application for Atorvastatin Determination. J. Electrochem. Soc. 2022, 169, 096503. [Google Scholar] [CrossRef]
- Sakthinathan, S.; Rajakumaran, R.; Keyan, A.K.; Yu, C.L.; Wu, C.F.; Vinothini, S.; Chen, S.M.; Chiu, T.W. Novel Construction of Carbon Nanofiber/CuCrO2 Composite for Selective Determination of 4-Nitrophenol in Environmental Samples and for Supercapacitor Application. RSC Adv. 2021, 11, 15856–15870. [Google Scholar] [CrossRef]
- Anupriya, J.; Rajakumaran, R.; Chen, S.M.; Karthik, R.; Kumar, J.V.; Shim, J.J.; Lee, J.W. Raspberry-Like CuWO4 Hollow Spheres Anchored on Sulfur-Doped g-C3N4 Composite: An Efficient Electrocatalyst for Selective Electrochemical Detection of Antibiotic Drug Nitrofurazone. Chemosphere 2022, 296, 133997. [Google Scholar] [CrossRef]
- Pavitra, V.; Praveen, B.M.; Nagaraju, G. Exploring the Spondias Mombin (Hog plum) Mediated ZnWO4–CuWO4 Nanocomposite for Photocatalysis and Electrochemical Nitrite Sensing. Mater. Chem. Phys. 2023, 293, 126882. [Google Scholar] [CrossRef]
- Karthika, A.; Karuppasamy, P.; Selvarajan, S.; Suganthi, A.; Rajarajan, M. Electrochemical Sensing of Nicotine Using CuWO4 Decorated Reduced Graphene oxide Immobilized Glassy Carbon Electrode. Ultrason. Sonochem. 2019, 55, 196–206. [Google Scholar] [CrossRef]
- Nong, C.; Yang, B.; Li, X.; Feng, S.; Cui, H. An Ultrasensitive Electrochemical Immunosensor Based on In-situ Growth of CuWO4 Nanoparticles on MoS2 and Chitosan-Gold Nanoparticles for Cortisol Detection. Microchem. J. 2022, 179, 107434. [Google Scholar] [CrossRef]
- Ruiz-Fuertes, J.; Errandonea, D.; Segura, A.; Manjón, F.J.; Zhu, Z.; Tu, C.Y. Growth, Characterization, and High-Pressure Optical Studies of CuWO4. High Press. Res. 2008, 28, 565–570. [Google Scholar] [CrossRef]
- Lin, J.; Mei, Q.; Duan, Y.; Yu, C.; Ding, Y.; Li, L. A Highly Sensitive Electrochemical Sensor based on Nanoflower-like MoS2-Ag-CNF Nanocomposites for the Detection of VB2. J. Nanopart. Res. 2020, 22, 1–11. [Google Scholar] [CrossRef]
- Mohammad, A.; Ahmad, K.; Qureshi, A.; Tauqeer, M.; Mobin, S.M. Zinc Oxide-Graphitic Carbon Nitride Nanohybrid as an Efficient Electrochemical Sensor and Photocatalyst. Sens. Actuators B Chem. 2018, 277, 467–476. [Google Scholar] [CrossRef]
- Xiao, Q.; Lu, S.; Huang, C.; Su, W.; Zhou, S.; Sheng, J.; Huang, S. An Electrochemical Chiral Sensor Based on Amino-Functionalized Graphene Quantum Dots/β-Cyclodextrin Modified Glassy Carbon Electrode for Enantioselective Detection of Tryptophan Isomers. J. Iran. Chem. Soc. 2017, 14, 1957–1970. [Google Scholar] [CrossRef]
- Rani, S.; Dilbaghi, N.; Kumar, S.; Varma, R.S.; Malhotra, R. Rapid Redox Sensing of P-Nitrotoluene in Real Water Samples Using Silver Nanoparticles. Inorg. Chem. Commun. 2020, 120, 108157. [Google Scholar] [CrossRef]
- Yuan, S.; Bo, X.; Guo, L. In-Situ Growth of Iron-Based Metal-Organic Framework Crystal on Ordered Mesoporous Carbon for Efficient Electrocatalysis of p-Nitrotoluene and Hydrazine. Anal. Chim. Acta 2018, 1024, 73–83. [Google Scholar] [CrossRef]
- Ahmad, K.; Mohammad, A.; Mobin, S.M. Hydrothermally Grown α-MnO2 Nanorods as Highly Efficient Low-Cost Counter-Electrode Material for Dye-Sensitized Solar Cells and Electrochemical Sensing Applications. Electrochim. Acta 2017, 252, 549–557. [Google Scholar] [CrossRef]
- Yang, R.; Wei, Y.; Yu, Y.; Gao, C.; Wang, L.; Liu, J.H.; Huang, X.J. Make It Different: The Plasma Treated Multi-Walled Carbon Nanotubes Improve Electrochemical Performances toward Nitroaromatic Compounds. Electrochim. Acta 2012, 76, 354–362. [Google Scholar] [CrossRef]







| Electrodes | Techniques | Linear Range | LOD (nM) | Reference |
|---|---|---|---|---|
| ZnO-CN/GCE | CV | - | 100.0 | [43] |
| Ag NPs/Au | Amperometry | 0.010–0.100 | 92.00 | [45] |
| NH2-Fe-MIL-88B@OMC-3 | DPV | 20.00–225.0 | 8000 | [46] |
| GCE/α-MnO2 | CV | 0.162–48.80 | 144.0 | [47] |
| Pn-MWCNTs/Po-MWCNTs | SWASV | 0.5–4, 0.2–1.2 | 442, 282.2 | [48] |
| CuWO4/CNF/GCE | DPV | 0.2–100 | 86.15 | This work |
| Samples | Added (µM) | Found (µM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| River water | 10 | 9.15 | 91.51 | 0.121 |
| 20 | 19.08 | 95.42 | 0.102 | |
| 30 | 29.08 | 96.95 | 0.070 | |
| Tap Water | 10 | 9.18 | 91.80 | 0.079 |
| 20 | 19.27 | 96.35 | 0.108 | |
| 30 | 29.13 | 97.10 | 0.089 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meenakshi, G.A.; Sakthinathan, S.; Chiu, T.-W. Fabrication of Carbon Nanofiber Incorporated with CuWO4 for Sensitive Electrochemical Detection of 4-Nitrotoluene in Water Samples. Sensors 2023, 23, 5668. https://doi.org/10.3390/s23125668
Meenakshi GA, Sakthinathan S, Chiu T-W. Fabrication of Carbon Nanofiber Incorporated with CuWO4 for Sensitive Electrochemical Detection of 4-Nitrotoluene in Water Samples. Sensors. 2023; 23(12):5668. https://doi.org/10.3390/s23125668
Chicago/Turabian StyleMeenakshi, Ganesh Abinaya, Subramanian Sakthinathan, and Te-Wei Chiu. 2023. "Fabrication of Carbon Nanofiber Incorporated with CuWO4 for Sensitive Electrochemical Detection of 4-Nitrotoluene in Water Samples" Sensors 23, no. 12: 5668. https://doi.org/10.3390/s23125668







