Study of Cationic Surfactants Raw Materials for COVID-19 Disinfecting Formulations by Potentiometric Surfactant Sensor
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Measuring Setup
2.3. Potentiometric Titrations Procedure
3. Results
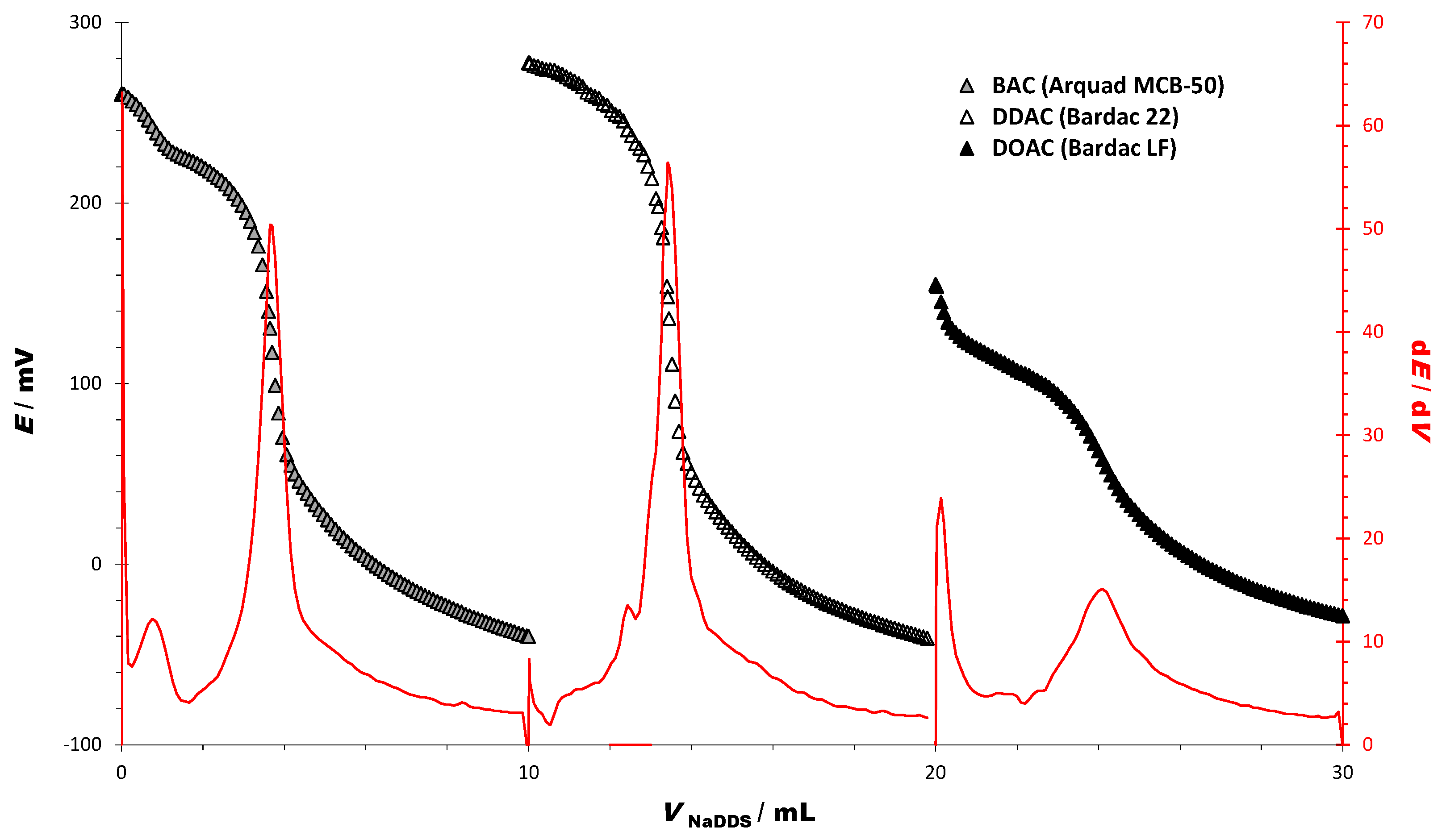
3.1. Titrations of Technical-Grade Cationic Surfactants
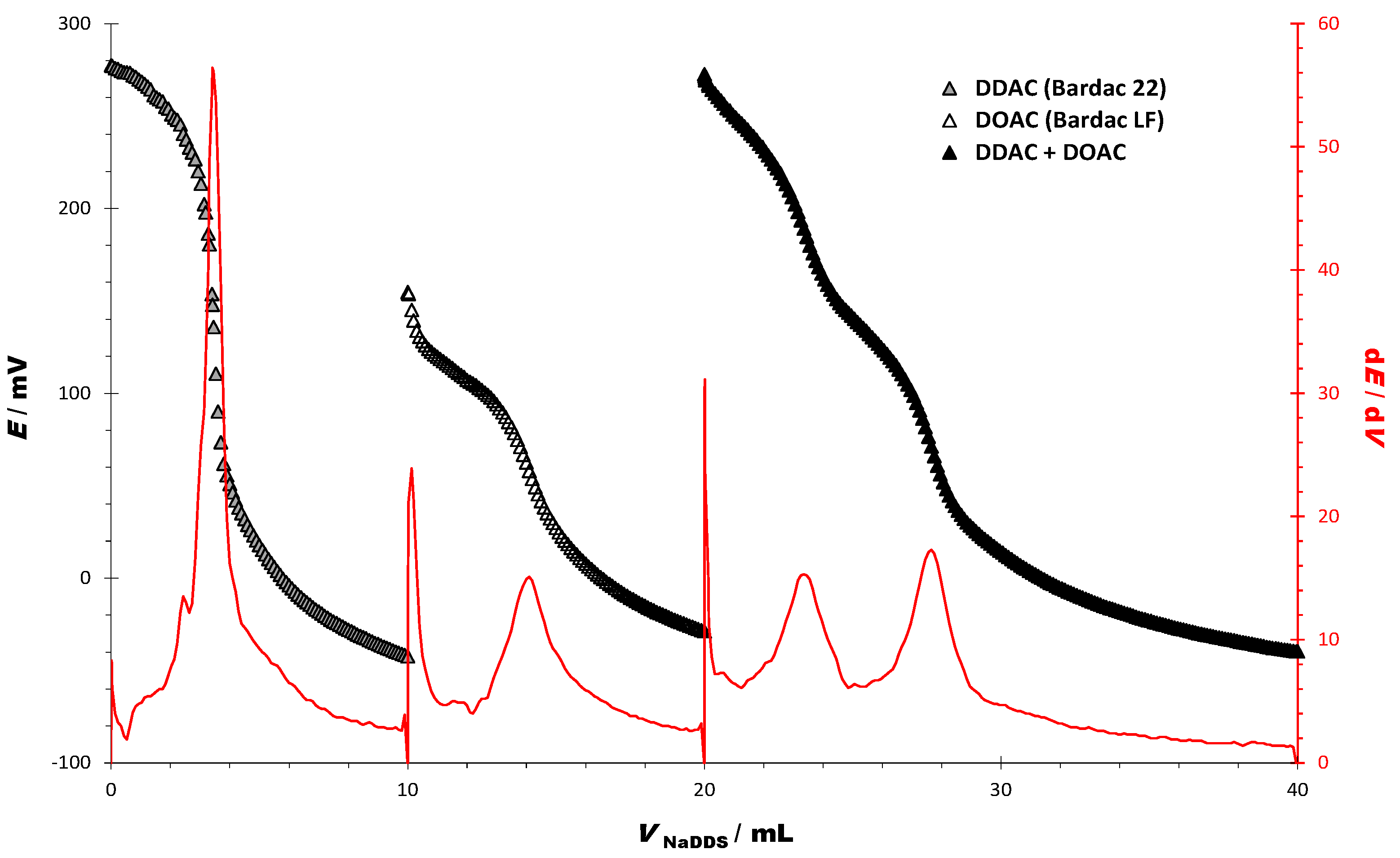
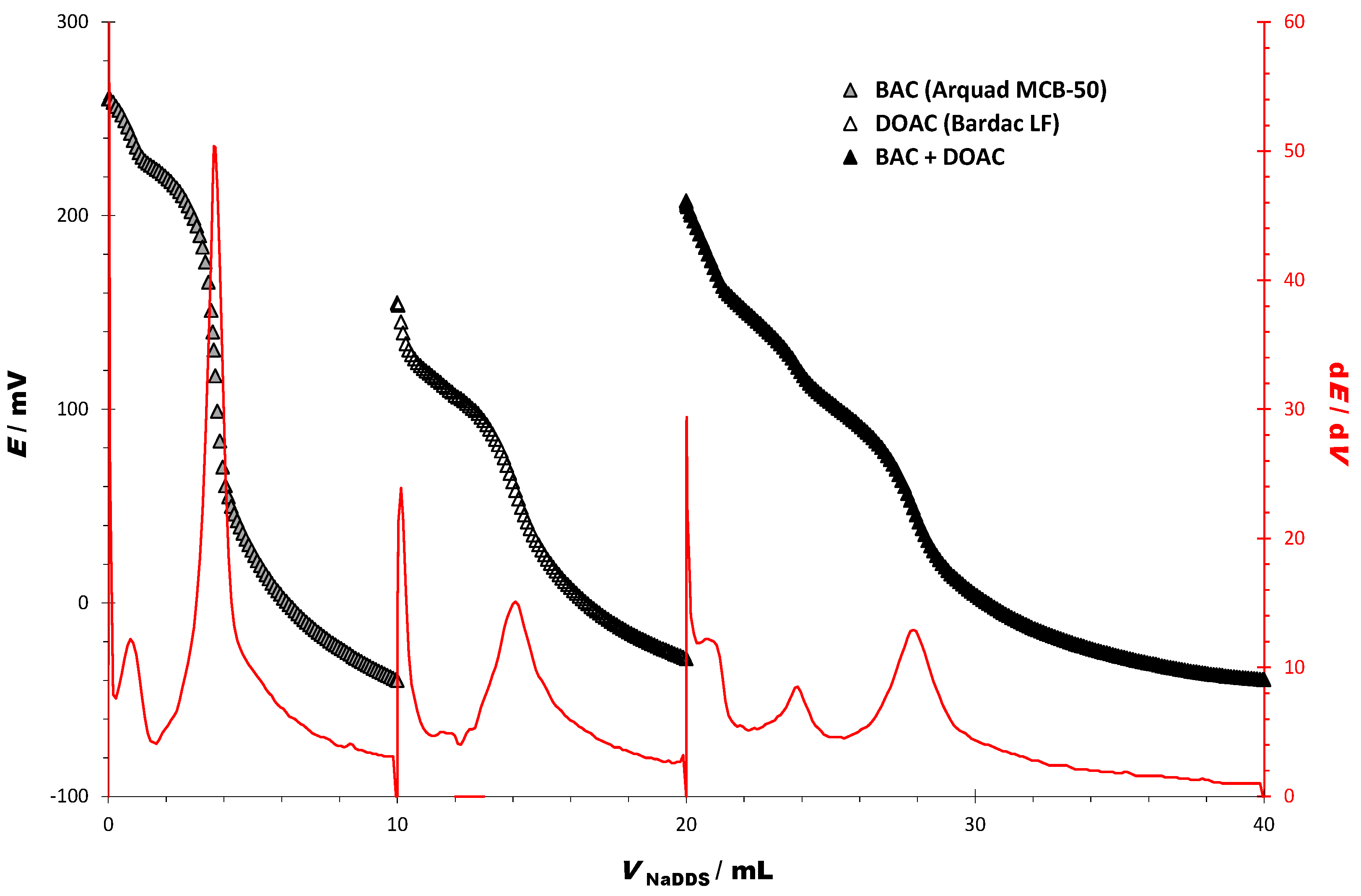
3.2. Titrations of Cationic Surfactants in Two-Component Model Mixtures
3.3. Influence of Non-Ionic Surfactants on the Titration of Cationic Surfactants
3.4. Potentiometric Determinations of Cationic Surfactants in Commercial Samples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hora, P.I.; Pati, S.G.; McNamara, P.J.; Arnold, W.A. Increased Use of Quaternary Ammonium Compounds during the SARS-CoV-2 Pandemic and Beyond: Consideration of Environmental Implications. Environ. Sci. Technol. Lett. 2020, 7, 622–631. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Y. Structure–activity relationship of cationic surfactants as antimicrobial agents. Curr. Opin. Colloid Interface Sci. 2020, 45, 28–43. [Google Scholar] [CrossRef]
- Falk, N.A. Surfactants as Antimicrobials: A Brief Overview of Microbial Interfacial Chemistry and Surfactant Antimicrobial Activity. J. Surfactants Deterg. 2019, 22, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Zakharova; Pashirova; Doktorovova; Fernandes; Sanchez-Lopez; Silva; Souto; Souto Cationic Surfactants: Self-Assembly, Structure-Activity Correlation and Their Biological Applications. Int. J. Mol. Sci. 2019, 20, 5534. [CrossRef] [PubMed]
- Mukherjee, S.; Vincent, C.K.; Jayasekera, H.W.; Yekhe, A.S. Antiviral efficacy of personal care formulations against Severe Acute Respiratory Syndrome Coronavirus 2. Infect. Dis. Health 2021, 26, 63–66. [Google Scholar] [CrossRef]
- Karamov, E.V.; Larichev, V.F.; Kornilaeva, G.V.; Fedyakina, I.T.; Turgiev, A.S.; Shibaev, A.V.; Molchanov, V.S.; Philippova, O.E.; Khokhlov, A.R. Cationic Surfactants as Disinfectants against SARS-CoV-2. Int. J. Mol. Sci. 2022, 23, 6645. [Google Scholar] [CrossRef] [PubMed]
- Rutala, W.; Weber, D. Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008; CDC: Chapel Hill, NC, USA, 2019. [Google Scholar]
- Simon, M.; Veit, M.; Osterrieder, K.; Gradzielski, M. Surfactants—Compounds for inactivation of SARS-CoV-2 and other enveloped viruses. Curr. Opin. Colloid Interface Sci. 2021, 55, 101479. [Google Scholar] [CrossRef]
- Rahaman, S.M.; Chowdhury, B.; Acharjee, A.; Singh, B.; Saha, B. Surfactant-based therapy against COVID-19: A review. Tenside Surfactants Deterg. 2021, 58, 410–415. [Google Scholar] [CrossRef]
- Sakač, N.; Madunić-Čačić, D.; Karnaš, M.; Ðurin, B.; Kovač, I.; Jozanović, M. The influence of plasticizers on the response characteristics of the surfactant sensor for cationic surfactant determination in disinfectants and antiseptics. Sensors 2021, 21, 3535. [Google Scholar] [CrossRef]
- Alygizakis, N.; Galani, A.; Rousis, N.I.; Aalizadeh, R.; Dimopoulos, M.A.; Thomaidis, N.S. Change in the chemical content of untreated wastewater of Athens, Greece under COVID-19 pandemic. Sci. Total Environ. 2021, 799, 149230. [Google Scholar] [CrossRef]
- Kaczerewska, O.; Martins, R.; Figueiredo, J.; Loureiro, S.; Tedim, J. Environmental behaviour and ecotoxicity of cationic surfactants towards marine organisms. J. Hazard. Mater. 2020, 392, 122299. [Google Scholar] [CrossRef]
- Goh, C.F.; Ming, L.C.; Wong, L.C. Dermatologic reactions to disinfectant use during the COVID-19 pandemic. Clin. Dermatol. 2021, 39, 314–322. [Google Scholar] [CrossRef]
- Boyce, J.M.; Kelliher, S.; Vallande, N. Skin Irritation and Dryness Associated With Two Hand-Hygiene Regimens: Soap-and-Water Hand Washing Versus Hand Antisepsis With an Alcoholic Hand Gel. Infect. Control Hosp. Epidemiol. 2000, 21, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Maillard, J.-Y. Antimicrobial biocides in the healthcare environment: Efficacy, usage, policies, and perceived problems. Ther. Clin. Risk Manag. 2005, 1, 307–320. [Google Scholar] [PubMed]
- Kursunlu, A.N.; Koc, Z.E.; Obalı, A.Y.; Güler, E. A symmetric and selective fluorescent Cu (II) sensor based on bodipy and s-triazine. J. Lumin. 2014, 149, 215–220. [Google Scholar] [CrossRef]
- Kursunlu, A.N.; Acikbas, Y.; Ozmen, M.; Erdogan, M.; Capan, R. Haloalkanes and aromatic hydrocarbons sensing using Langmuir–Blodgett thin film of pillar[5]arene-biphenylcarboxylic acid. Colloids Surf. A Physicochem. Eng. Asp. 2019, 565, 108–117. [Google Scholar] [CrossRef]
- Paut, A.; Prkić, A.; Mitar, I.; Guć, L.; Marciuš, M.; Vrankić, M.; Krehula, S.; Tomaško, L. The New Ion-Selective Electrodes Developed for Ferric Cations Determination, Modified with Synthesized Al and Fe−Based Nanoparticles. Sensors 2021, 22, 297. [Google Scholar] [CrossRef] [PubMed]
- Ogino, K.; Abe, M. (Eds.) Mixed Surfactant Systems; Marcel Dekker, Inc.: New York, NY, USA, 1993. [Google Scholar]
- Fizer, O.; Fizer, M.; Sidey, V.; Studenyak, Y. Predicting the end point potential break values: A case of potentiometric titration of lipophilic anions with cetylpyridinium chloride. Microchem. J. 2021, 160, 105758. [Google Scholar] [CrossRef]
- Sakač, N.; Jozanović, M.; Karnaš, M.; Sak-Bosnar, M. A New Sensor for Determination of Anionic Surfactants in Detergent Products with Carbon Nanotubes as Solid Contact. J. Surfactants Deterg. 2017, 20, 881–889. [Google Scholar] [CrossRef]
- Hajduković, M.; Samardžić, M.; Galović, O.; Széchenyi, A.; Sak-Bosnar, M. A functionalized nanomaterial based, new, solid state cationic-surfactant-selective sensor with fast response and low noise. Sens. Actuators B Chem. 2017, 251, 795–803. [Google Scholar] [CrossRef]
- Karnaš, M.; Sakač, N.; Jozanović, M.; Tsakiri, M.; Kopriva, M.; Andrić, E.K.; Sak-Bosnar, M. The influence of plasticisers on response characteristics of anionic surfactant potentiometric sensor. Int. J. Electrochem. Sci. 2017, 12, 5921–5933. [Google Scholar] [CrossRef]
- Fizer, M.; Fizer, O.; Sidey, V.; Mariychuk, R.; Studenyak, Y. Experimental and theoretical study on cetylpyridinium dipicrylamide—A promising ion-exchanger for cetylpyridinium selective electrodes. J. Mol. Struct. 2019, 1187, 77–85. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Frolov, N.A.; Egorova, K.S.; Seitkalieva, M.M.; Ananikov, V.P. Quaternary Ammonium Compounds (QACs) and Ionic Liquids (ILs) as Biocides: From Simple Antiseptics to Tunable Antimicrobials. Int. J. Mol. Sci. 2021, 22, 6793. [Google Scholar] [CrossRef] [PubMed]
- Bureš, F. Quaternary Ammonium Compounds: Simple in Structure, Complex in Application. Top. Curr. Chem. 2019, 377, 14. [Google Scholar] [CrossRef]
- Mohapatra, S.; Yutao, L.; Goh, S.G.; Ng, C.; Luhua, Y.; Tran, N.H.; Gin, K.Y.-H. Quaternary ammonium compounds of emerging concern: Classification, occurrence, fate, toxicity and antimicrobial resistance. J. Hazard. Mater. 2023, 445, 130393. [Google Scholar] [CrossRef]
- McDonnell, G.; Russell, A.D. Antiseptics and Disinfectants: Activity, Action, and Resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef]
- Romanowski, E.G.; Yates, K.A.; Shanks, R.M.Q.; Kowalski, R.P. Benzalkonium Chloride Demonstrates Concentration-Dependent Antiviral Activity Against Adenovirus In Vitro. J. Ocul. Pharmacol. Ther. 2019, 35, 311–314. [Google Scholar] [CrossRef]
- Tsujimura, K.; Murase, H.; Bannai, H.; Nemoto, M.; Yamanaka, T.; Kondo, T. Efficacy of five commercial disinfectants and one anionic surfactant against equine herpesvirus type 1. J. Vet. Med. Sci. 2015, 77, 1545–1548. [Google Scholar] [CrossRef]
- Lukasik, J.; Bradley, M.L.; Scott, T.M.; Dea, M.; Koo, A.; Hsu, W.-Y.; Bartz, J.A.; Farrah, S.R. Reduction of Poliovirus 1, Bacteriophages, Salmonella Montevideo, and Escherichia coli O157:H7 on Strawberries by Physical and Disinfectant Washes. J. Food Prot. 2003, 66, 188–193. [Google Scholar] [CrossRef]
- Baker, N.; Williams, A.J.; Tropsha, A.; Ekins, S. Repurposing Quaternary Ammonium Compounds as Potential Treatments for COVID-19. Pharm. Res. 2020, 37, 104. [Google Scholar] [CrossRef]
- Jantafong, T.; Ruenphet, S.; Punyadarsaniya, D.; Takehara, K. The study of effect of didecyl dimethyl ammonium bromide on bacterial and viral decontamination for biosecurity in the animal farm. Vet. World 2018, 11, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Rutala, W.A.; Weber, D.J. Disinfection, sterilization, and antisepsis: An overview. Am. J. Infect. Control 2016, 44, e1–e6. [Google Scholar] [CrossRef]
- Osimitz, T.G.; Droege, W. Quaternary ammonium compounds: Perspectives on benefits, hazards, and risk. Toxicol. Res. Appl. 2021, 5, 239784732110490. [Google Scholar] [CrossRef]
- Dellanno, C.; Vega, Q.; Boesenberg, D. The antiviral action of common household disinfectants and antiseptics against murine hepatitis virus, a potential surrogate for SARS coronavirus. Am. J. Infect. Control 2009, 37, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Sakač, N.; Madunić-Čačić, D.; Marković, D.; Ventura, B.D.; Velotta, R.; Ptiček Siročić, A.; Matasović, B.; Sermek, N.; Đurin, B.; Šarkanj, B.; et al. The 1,3-Dioctadecyl-1H-imidazol-3-ium Based Potentiometric Surfactant Sensor for Detecting Cationic Surfactants in Commercial Products. Sensors 2022, 22, 9141. [Google Scholar] [CrossRef]
- Sakač, N.; Madunić-Čačić, D.; Marković, D.; Hok, L.; Vianello, R.; Vrček, V.; Šarkanj, B.; Đurin, B.; Della Ventura, B.; Velotta, R.; et al. Potentiometric Surfactant Sensor for Anionic Surfactants Based on 1,3-dioctadecyl-1H-imidazol-3-ium tetraphenylborate. Chemosensors 2022, 10, 523. [Google Scholar] [CrossRef]
- Madunić-Čačić, D.; Sak-Bosnar, M.; Galović, O.; Sakač, N.; Matešić-Puač, R. Determination of cationic surfactants in pharmaceutical disinfectants using a new sensitive potentiometric sensor. Talanta 2008, 76, 259–264. [Google Scholar] [CrossRef]
- ISO2871-2:2010; Surface Active Agents—Detergents—Determination of Cationic-Active Matter Content—Part 2: Cationic-Active Matter of Low Molecular Mass (between 200 and 500). Management Centre: Brussels, Belgium, 2010.

| SURFACTANT INVESTIGATED | ||||
|---|---|---|---|---|
| Chemical Name | Abbreviation | Commercial Name | Mean Mr Declared | Manufacturer/ Country |
| Benzalkonium chloride | BAC | Arquad MCB-50 | 352.5 | AkzoNobel/ Netherlands |
| N,N-Didecyl-N,N-dimethylammonium chloride | DDAC | Bardac 22 | 361.0 | Lonza/ Swisserland |
| N,N-Dioctyl-N,N-dimethylammonium chloride | DOAC | Bardac LF | 312.0 | |
| CHARACTERISTICS OF THE TITRATION CURVES | BAC | DDAC | DOAC |
|---|---|---|---|
| Starting potential (E/mV) | 260.16 | 277.44 | 154.84 |
| Ending potential (E/mV) | −40.2 | −42.34 | −28.77 |
| ΔE/mV | −300.9 | −319.78 | −183.61 |
| End point | |||
| dE/dV (End point) | 50.4 | 56.4 | 15.1 |
| EP (E/mV) | 130.52 | 147.94 | 58.00 |
| Surfactant Used | SURFACTANT CONTENT * | |||||||
|---|---|---|---|---|---|---|---|---|
| DODI-TPB Sensor | DMI-TPB Sensor [39] | Two-Phase Titration [40] | ||||||
| Found (%) | RSD (%) | Rel. Error (%) | Found (%) | RSD (%) | Rel. Error (%) | Found (%) | RSD (%) | |
| BAC | 51.70 | 0.226 | 1.63 | 51.88 | 0.195 | 1.99 | 50.87 | 0.563 |
| DDAC | 50.06 | 0.174 | −0.64 | 51.77 | 0.207 | 2.76 | 50.38 | 0.608 |
| DOAC | 50.52 | 0.433 | −1.81 | 50.88 | 0.460 | −1.11 | 51.45 | 1.151 |
| CHARACTERISTICS OF THE TITRATION CURVES | DDAC | DOAC | DDAC + DOAC |
|---|---|---|---|
| Starting potential (E/mV) | 277.44 | 154.84 | 272.67 |
| Ending potential (E/mV) | −42.34 | −28.77 | −39.73 |
| ΔE/mV | −319.78 | −183.61 | −312.40 |
| End point (EP) | |||
| dE/dV (EP1) | 56.4 | 15.1 | 15.3 |
| dE/dV (EP2) | - | - | 17.3 |
| EP 1 (E/mV) | 147.94 | 58.00 | 189.04 |
| EP 2 (E/mV) | - | - | 71.18 |
| CHARACTERISTICS OF THE TITRATION CURVES | BAC | DOAC | BAC + DOAC |
|---|---|---|---|
| Starting potential (E/mV) | 260.16 | 154.84 | 247.22 |
| Ending potential (E/mV) | −40.2 | −28.77 | −39.67 |
| ΔE/mV | −300.9 | −183.61 | −247.22 |
| End point (EP) | |||
| dE/dV (EP1) | 50.4 | 15.1 | 8.5 |
| dE/dV (EP2) | - | - | 12.9 |
| EP 1 (E/mV) | 130.52 | 58.00 | 121.65 |
| EP 2 (E/mV) | - | - | 46.08 |
| Expected (%) | Obtained (%) * | Recovery (%) | |||
|---|---|---|---|---|---|
| Two-Component Mixture DDAC + DOAC | |||||
| DDAC | 50.06 | 48.18 | 96.24 | ||
| DOAC | 50.52 | 53.43 | 105.76 | ||
| Two-Component Mixture BAC + DOAC | |||||
| BAC | 51.70 | 53.69 | 103.86 | ||
| DOAC | 50.52 | 50.42 | 99.81 | ||
| CHARACTERISTICS OF THE TITRATION CURVES | DDAC: Triton X-100 (m/m) | ||||
|---|---|---|---|---|---|
| 1:0 | 1:1 | 1:3 | 1:5 | 1:10 | |
| ΔE/mV | 333.2 | 320.3 | 298.5 | 274.4 | 243.0 |
| dE/dV | 71.9 | 71.0 | 61.4 | 60.3 | 41.0 |
| DODI-TPB Sensor (%) | Two-Phase Titration (%) * | Recovery (%) | |||
|---|---|---|---|---|---|
| DDAC (EP1) | DOAC (∆EP) | Sum (EP2) | |||
| Sample 1 | 2.031 | 3.413 | 5.444 | 5.325 | 102.2 |
| Sample 2 | 1.526 | 3.521 | 5.047 | 5.073 | 99.4 |
| Sample 3 | 3.672 | 1.245 | 4.917 | 5.061 | 97.1 |
| Sample 4 | 2.677 | 1.673 | 4.350 | 4.275 | 101.7 |
| Sample 5 | 0.974 | 4.622 | 5.596 | 5.612 | 99.7 |
| Sample 6 | 2.856 | 1.956 | 4.812 | 4.728 | 101.7 |
| BAC (EP1) | DOAC (∆EP) | Sum (EP2) | |||
| Sample 7 | 2.367 | 1.264 | 3.631 | 3.602 | 100.8 |
| Sample 8 | 1.362 | 1.374 | 2.736 | 2.838 | 96.4 |
| Sample 9 | 0.934 | 1.427 | 2.361 | 2.381 | 99.1 |
| Sample 10 | 0.882 | 2.783 | 3.665 | 3.751 | 97.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakač, N.; Madunić-Čačić, D.; Marković, D.; Jozanović, M. Study of Cationic Surfactants Raw Materials for COVID-19 Disinfecting Formulations by Potentiometric Surfactant Sensor. Sensors 2023, 23, 2126. https://doi.org/10.3390/s23042126
Sakač N, Madunić-Čačić D, Marković D, Jozanović M. Study of Cationic Surfactants Raw Materials for COVID-19 Disinfecting Formulations by Potentiometric Surfactant Sensor. Sensors. 2023; 23(4):2126. https://doi.org/10.3390/s23042126
Chicago/Turabian StyleSakač, Nikola, Dubravka Madunić-Čačić, Dean Marković, and Marija Jozanović. 2023. "Study of Cationic Surfactants Raw Materials for COVID-19 Disinfecting Formulations by Potentiometric Surfactant Sensor" Sensors 23, no. 4: 2126. https://doi.org/10.3390/s23042126
APA StyleSakač, N., Madunić-Čačić, D., Marković, D., & Jozanović, M. (2023). Study of Cationic Surfactants Raw Materials for COVID-19 Disinfecting Formulations by Potentiometric Surfactant Sensor. Sensors, 23(4), 2126. https://doi.org/10.3390/s23042126







