Retinal Prostheses: Engineering and Clinical Perspectives for Vision Restoration
Abstract
1. Introduction
1.1. Overview of Retinal Prostheses
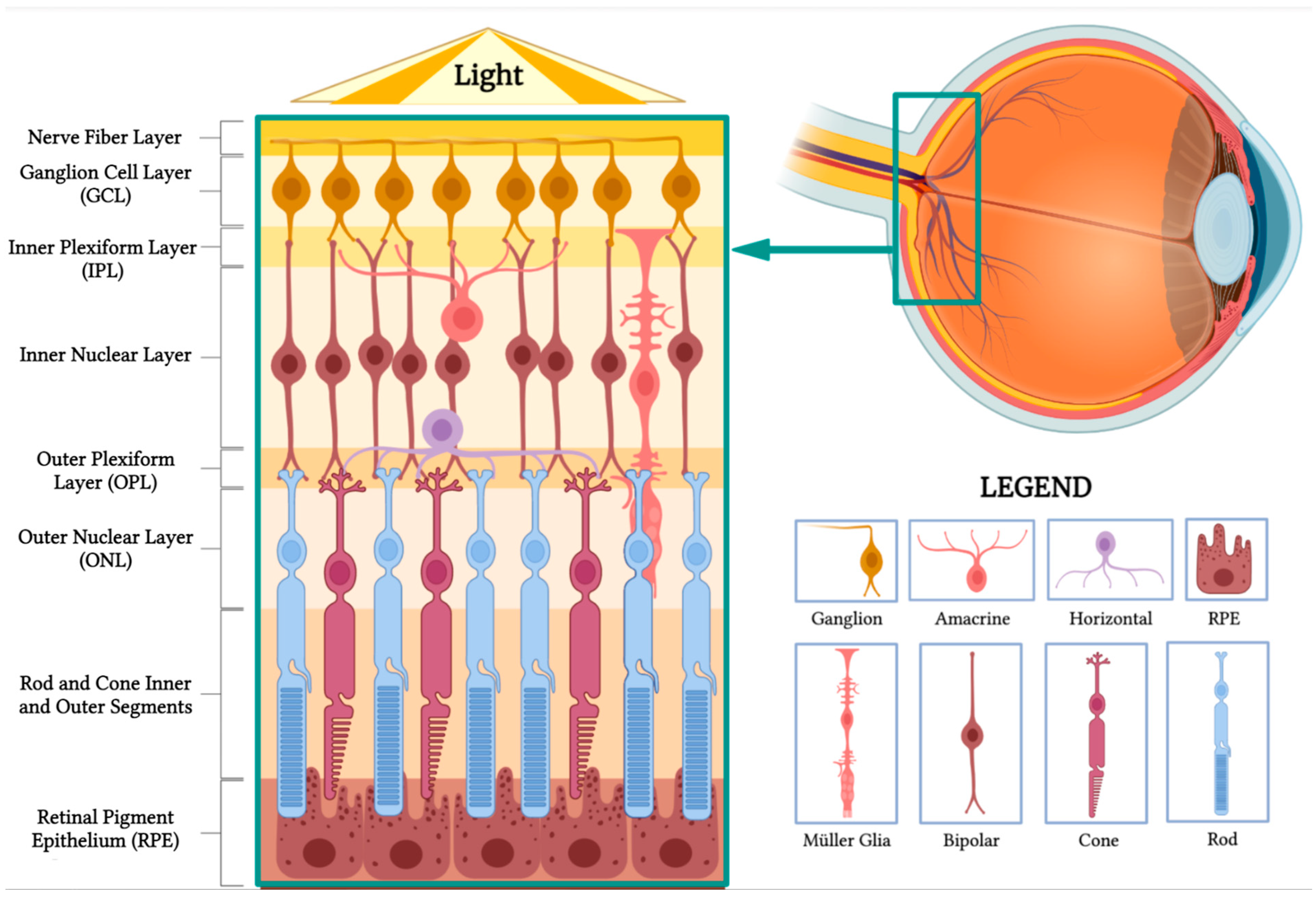
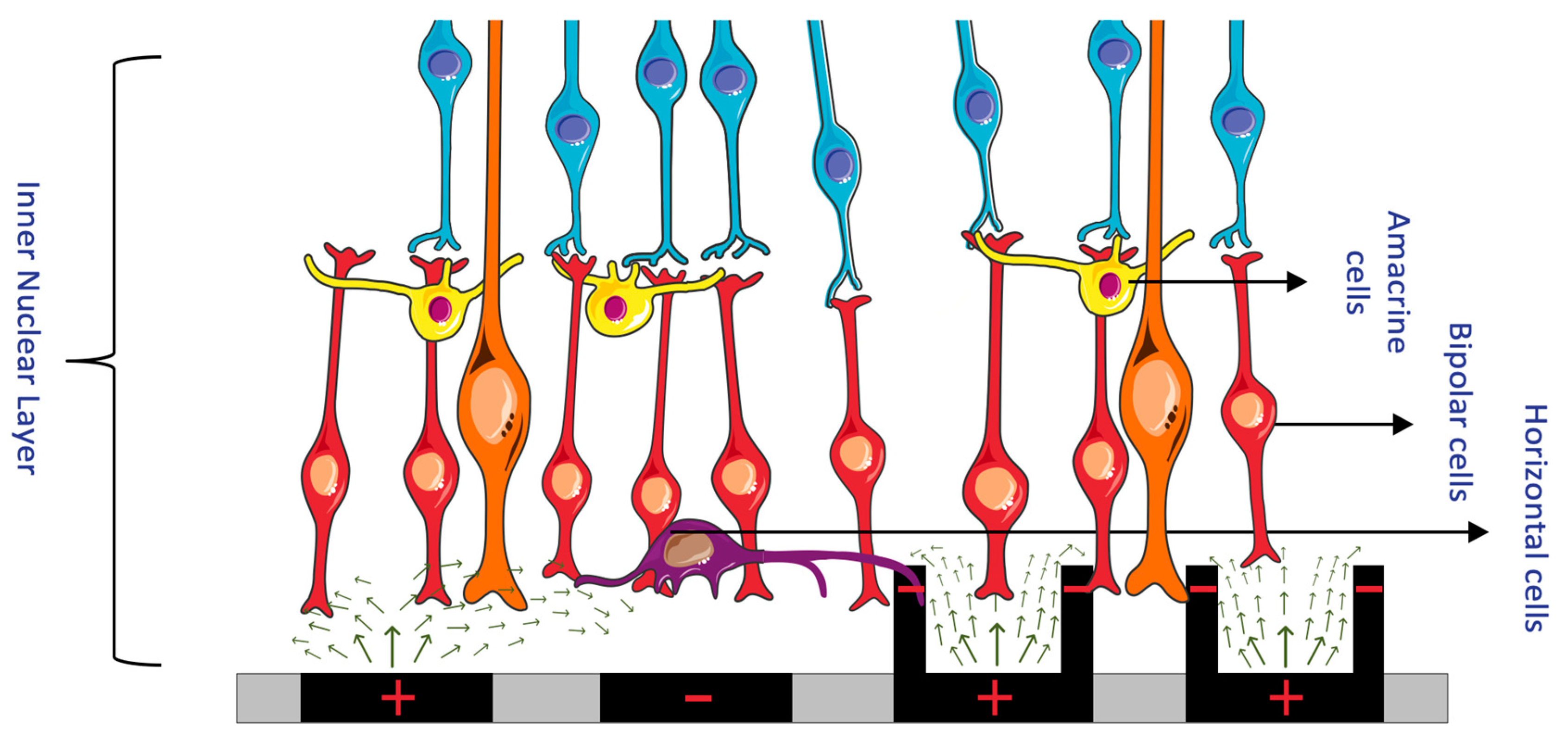
1.2. Overview of Retinal Structure and Function
- Nerve fiber layer: This layer contains the axons of retinal ganglion cells that coalesce to form the optic nerve.
- Ganglion cell layer: This layer is composed of the cell bodies of retinal ganglion cells, which transmit visual information to the brain.
- Inner plexiform layer: This layer consists of the synapses between bipolar and ganglion cells, facilitating signal processing and integration.
- Inner nuclear layer: This layer houses the cell bodies of bipolar, horizontal, and amacrine cells, which play essential roles in processing and transmitting visual information.
- Outer plexiform layer: This layer contains synapses between photoreceptors, bipolar cells, and horizontal cells, allowing for the initial processing of visual signals.
- Outer nuclear layer: This layer is made up of the cell bodies of rod and cone photoreceptors, which are responsible for capturing and converting light into neural signals.
- Rod and cone inner and outer segments: These segments are part of the photoreceptor cells, which include rods and cones. The inner segments contain vital cellular components, such as mitochondria, while the outer segments contain stacked discs rich in photopigments, which are essential for absorbing light and initiating the phototransduction cascade to produce neuronal signals.
- Retinal pigment epithelium (RPE): This is the outermost layer of the retina, located just beneath the photoreceptor cells. The RPE has several crucial functions in visual processing. Its cells absorb stray light, preventing light scatter and enhancing visual acuity. They also play a vital role in recycling photopigments and shuttling nutrients to the photoreceptors. Additionally, they facilitate the transport of metabolic waste products from the photoreceptors to the choroidal blood supply, thereby helping to maintain the health of the photoreceptor cells.
1.3. Overview of Retinal Physiology and Pathology
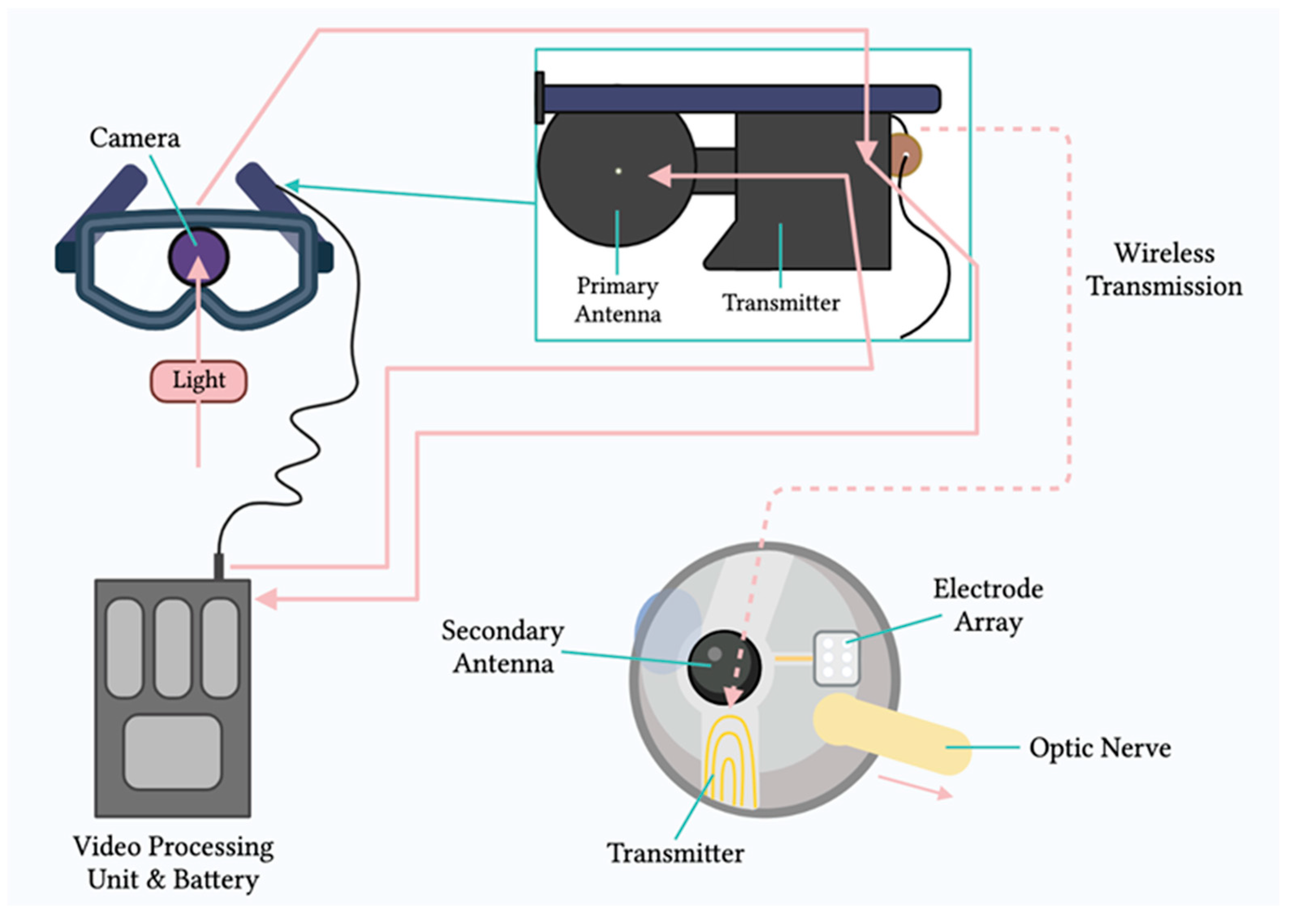
2. Principles of Electronic Retinal Prostheses
3. Engineering of Retinal Prostheses
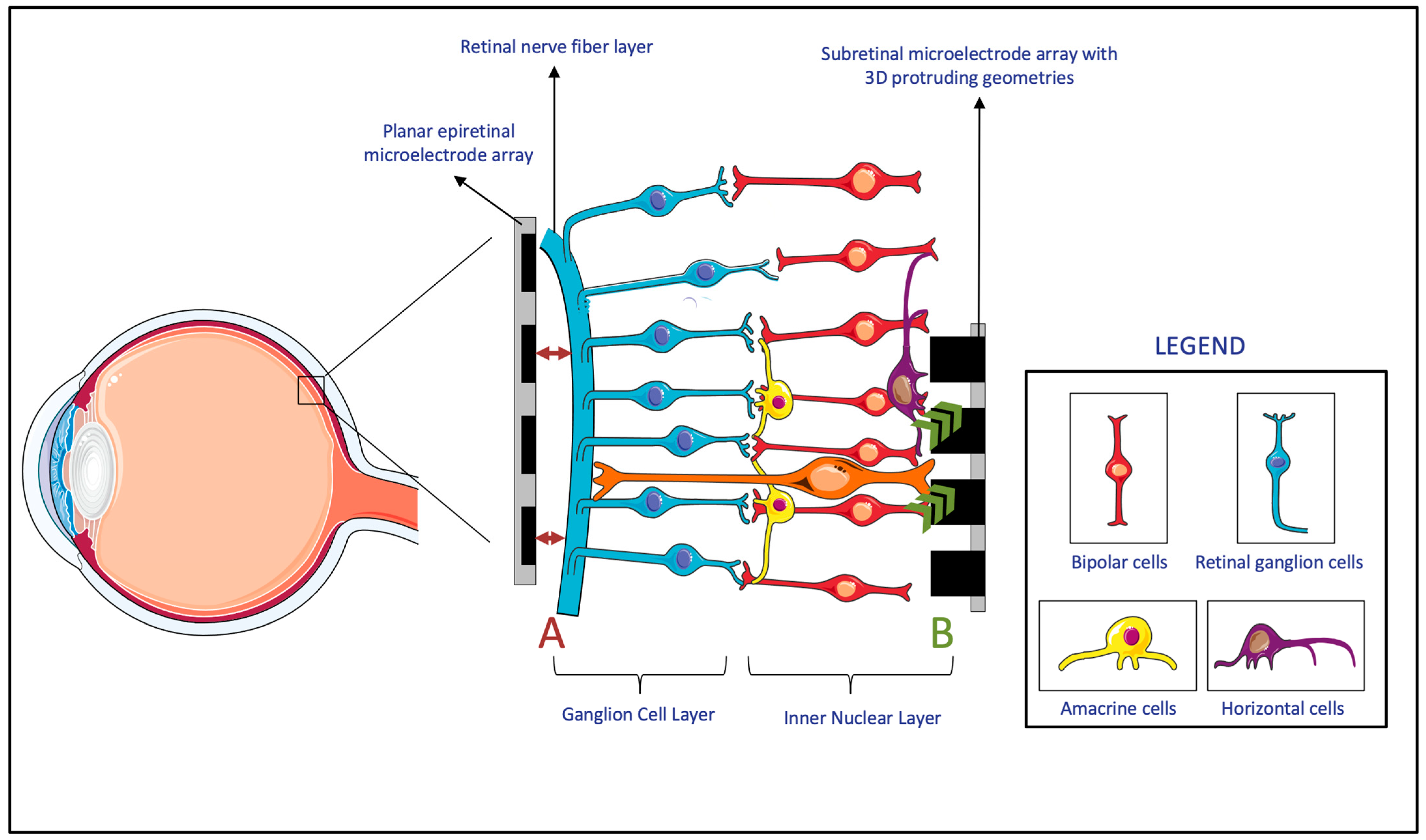
3.1. Electrode–Retina (ER) Topographical Alignment
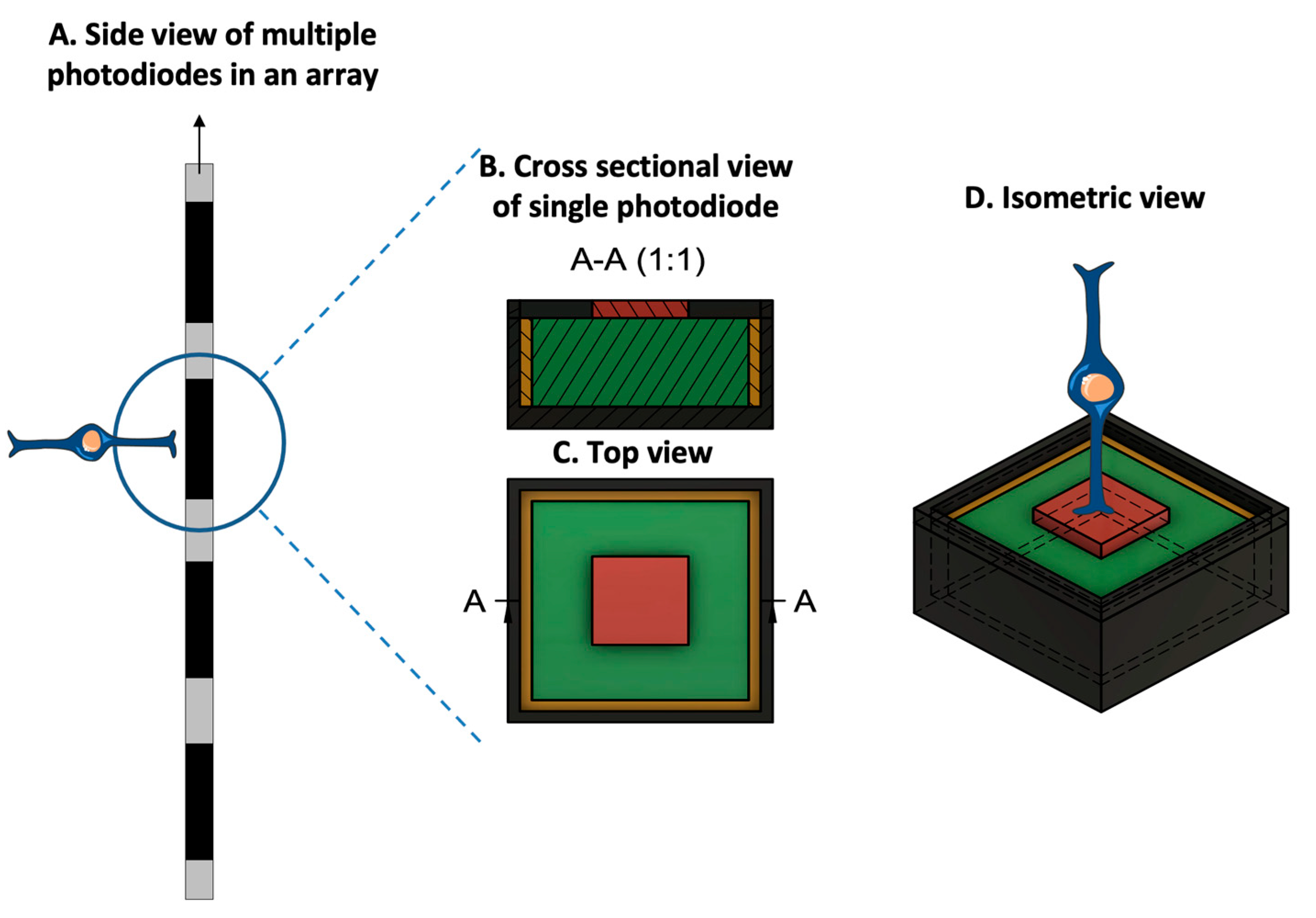
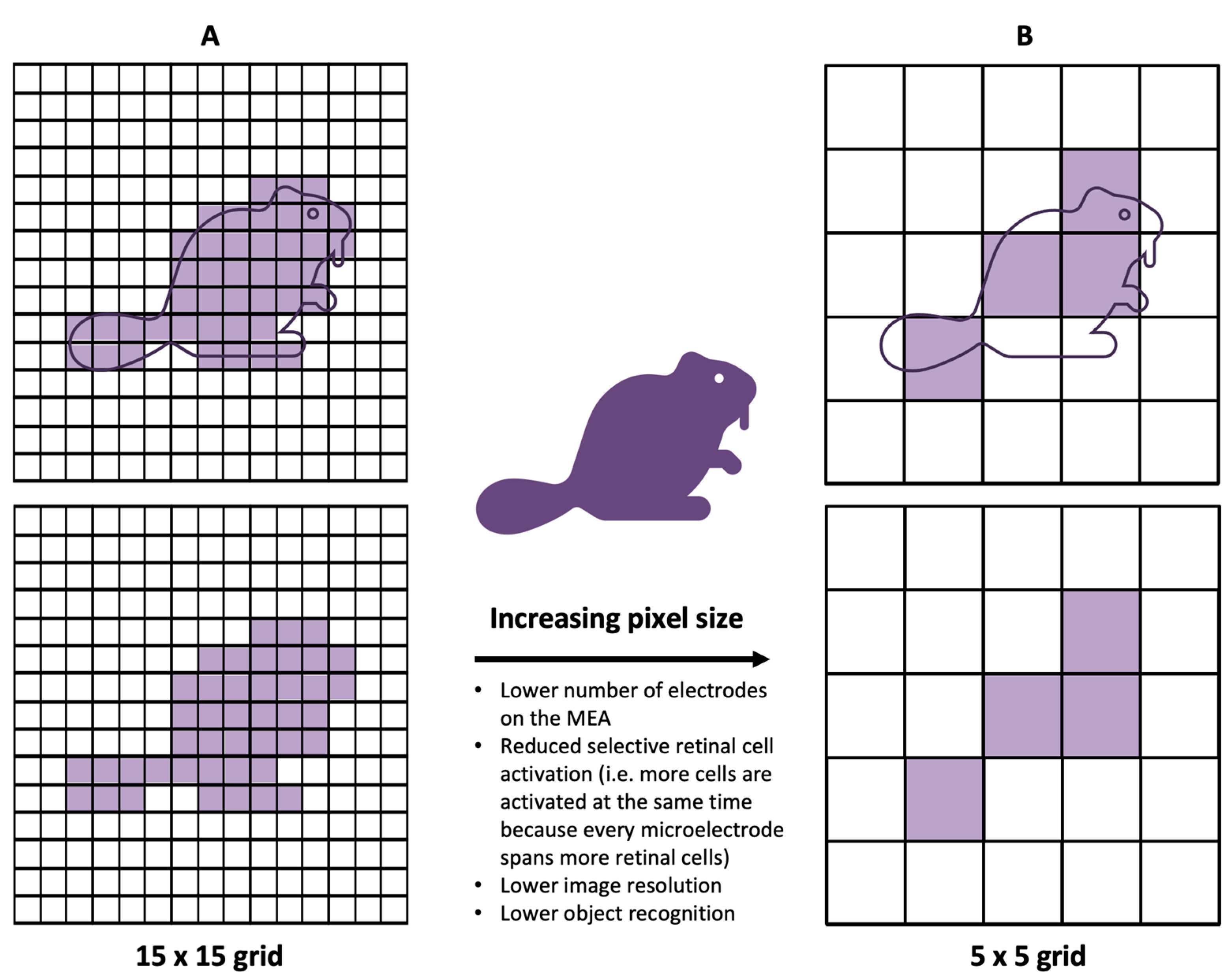
3.2. Electrode Size and Material, Charge Density, and Resolution Limit
3.3. Spatial Selectivity
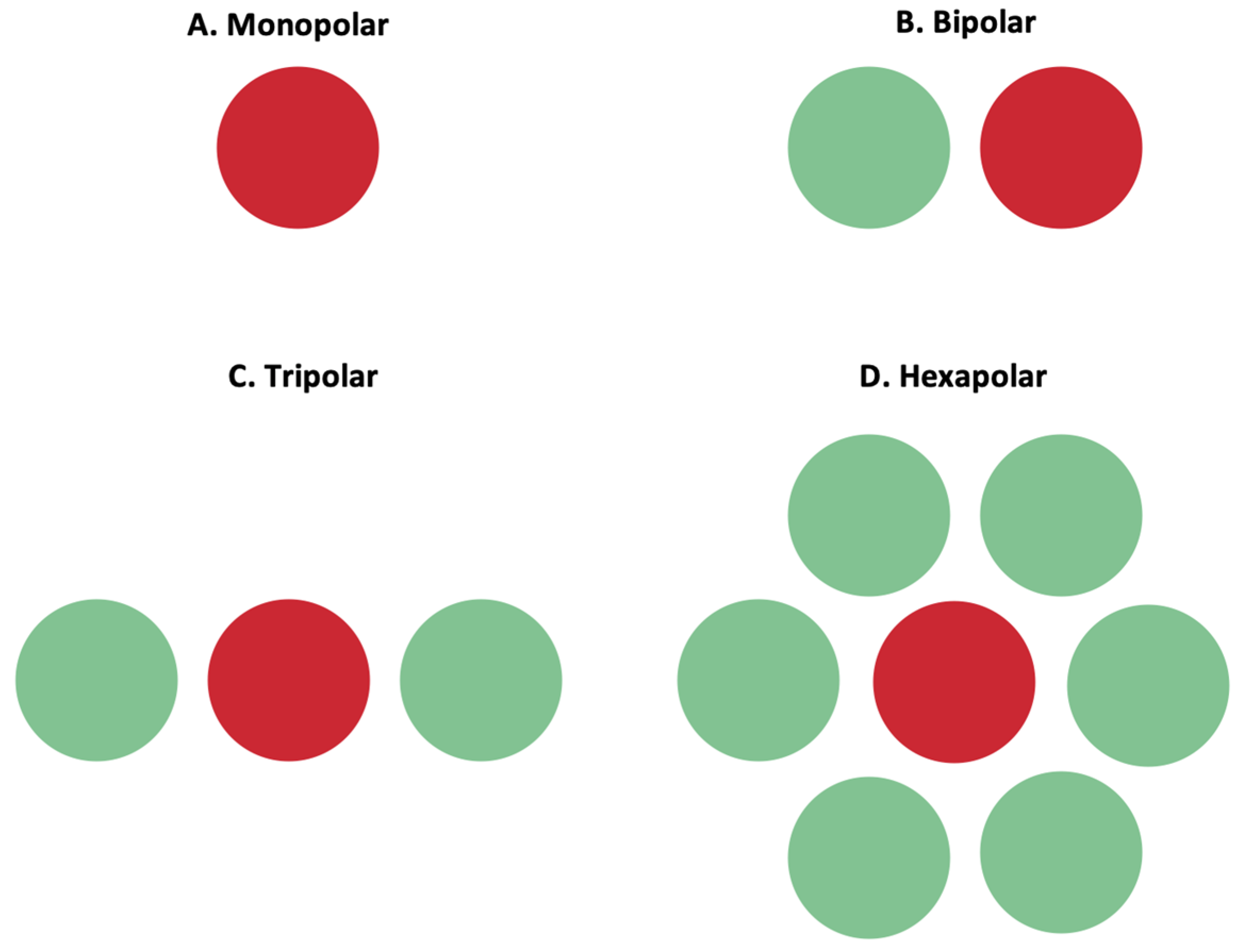
3.3.1. Return Electrodes for Electric Field Localization and Current Steering
3.3.2. Electric Stimulation Parameters for Selective Cell Activation and Chromatic Vision
3.4. Bidirectional/Closed-Loop Retinal Prostheses
4. Clinical Considerations for Retinal Prostheses
4.1. Importance of Patient Selection and Screening
- Aged 25 years or older;
- A prior history of useful vision;
- Profound visual loss resulting from the loss of photoreceptors (e.g., retinitis pigmentosa) that limits visual acuity to hand motion or bare light or no light perception in both eyes;
- A patient with no light perception must demonstrate retinal ability to respond to electrical stimulation, which can be confirmed through a dark-adapted flash test and visual evoked potential (VEP) testing;
- A patient must be in pseudophakic or aphakic status or have phakic status requiring cataract surgery or lensectomy prior to retinal prosthesis implantation;
- A patient must be able to attend post-implant clinical follow-up, device fitting, and visual rehabilitation.
- Vision better than counting fingers in one of the eyes;
- Ocular conditions that prevent adequate visualization of internal structures, such as corneal opacification;
- Ocular conditions that affect device functionality, such as optic neuropathy, central retinal artery occlusion (CRAO), central retinal vein occlusion (CRVO), retinal detachment, severe strabismus, and amblyopia;
- Systemic conditions that contraindicate general anesthesia;
- Presence of metallic or other implantable devices in the head (e.g., cochlear implants);
- Hearing impairments that can interfere with a patient’s interaction with the Argus II device;
- Inability to comply with post-operative follow-up and rehabilitation due to cognitive decline or other conditions such as dementia or developmental disability.
- Light perception without projection or no light perception in hereditary retinal diseases (retinitis pigmentosa, choroideremia, and Usher syndrome);
- Primary rod cone or cone rod dystrophies in their end-stage diseases;
- A prior history of normal visual function for >12 years.;
- A prior history of pseudophakia or aphakic status prior to retinal prosthesis implantation;
- A fluorescein angiography showing retinal vascular perfusion in all four quadrants of the macula;
- Evidence of inner retinal function (ganglion cells and optic nerve function) observed by the ability to elicit phosphene thresholds;
- Ability to give written informed consent and to attend follow-up and visual rehabilitation.
- Ophthalmic conditions with relevant effects upon visual function (glaucoma, diabetic neuropathy, retinal detachment, optic neuropathies, heavy clumped pigmentation at posterior lobe, and cystoid macular edema);
- Retina < 100 μm or no layering of the inner retina shown by OCT;
- Scar tissue (epiretinal, intraretinal, subretinal, and macular pucker);
- Occipital stroke;
- Congenital blindness and severe amblyopia;
- Substantial corneal opacity or any opacification of ocular structures that prevent clear image transmission;
- Active inflammation (uveitis);
- Systemic conditions that could pose significant risks during general anesthesia (cardiovascular/pulmonary/severe metabolic conditions such as diabetes);
- Life expectancy < 1 year;
- Inability to comply with post-operative follow-up and rehabilitation due to psychiatric/neurological diseases (Parkinson’s, dementia, MS, epilepsy, and severe depression and anxiety).
4.1.1. Pre-Operative Assessment, Examination, and Imaging
4.1.2. Post-Operative Rehabilitation
4.2. Safety and Adverse Events
4.2.1. Epiretinal Prostheses
4.2.2. Subretinal Prostheses
4.2.3. Suprachoroidal Prostheses
4.3. Visual Function and Outcomes
4.3.1. Epiretinal Prostheses
4.3.2. Subretinal Prostheses
4.3.3. Suprachorodial Prostheses
4.4. Rehabilitative Programs
5. Comparison of Alternative Emerging Therapies to Retinal Prostheses
5.1. Cell-Based Therapies
5.2. Gene-Based Therapies
5.3. Optogenetics
5.4. Verdict
6. Recent Advancements in Retinal Prosthesis Technology
6.1. Advances in Engineering of Prostheses
6.1.1. Material Science
6.1.2. Visual Field Size
6.1.3. Artificial Intelligence
6.1.4. Preserving Residual Visual Field
6.2. Outlook on Retinal Prostheses
- –
- Limited effectiveness: While retinal prostheses can provide some degree of visual perception, the quality and resolution of the restored vision are still limited; current prosthetic devices cannot fully replicate the complexity and functionality of the natural retina;
- –
- Surgical complexity: Implanting retinal prostheses requires delicate and technically challenging surgical procedures with inherent risks;
- –
- Patient eligibility: Selection criteria are crucial to ensure that candidates have specific visual and anatomical characteristics that can benefit from the device; factors such as residual vision, retinal health, and overall eye condition need to be carefully evaluated, leading to a limited pool of eligible candidates;
- –
- Long-term durability: The longevity of retinal prostheses poses significant challenges; over time, the implant may encounter issues such as mechanical failure, degradation, or tissue response that can affect its performance, which are still unknown for now; we still do not know if a prostheses already implanted in a patient’s body can be repaired or replaced by a newer more developed version in the future;
- –
- Cost and accessibility: Retinal prostheses are currently expensive due to the advanced technology involved and the complexity of the surgical procedure; the high costs can restrict access to these treatments for many patients, limiting their availability and adoption in clinical practice; reducing costs and increasing accessibility are important considerations for the broader application of retinal prostheses.
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahabadi, N.; Al Khalili, Y. Neuroanatomy, Retina. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Kolb, H.; Fernandez, E.; Nelson, R. Anatomy and Physiology of the Retina; University of Utah Health Sciences Center: Salt Lake City, UT, USA, 1995. [Google Scholar]
- Abbasi, B.; Rizzo, J.F. Advances in Neuroscience, Not Devices, Will Determine the Effectiveness of Visual Prostheses. Semin. Ophthalmol. 2021, 36, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Mirochnik, R.M.; Pezaris, J.S. Contemporary Approaches to Visual Prostheses. Mil. Med. Res. 2019, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, D. Translation of a Photovoltaic Retinal Prosthesis. Nat. Biomed. Eng. 2020, 4, 137–138. [Google Scholar] [CrossRef]
- Cehajic-Kapetanovic, J.; Singh, M.S.; Zrenner, E.; MacLaren, R.E. Bioengineering Strategies for Restoring Vision. Nat. Biomed. Eng. 2022, 7, 387–404. [Google Scholar] [CrossRef] [PubMed]
- Zrenner, E.; Bartz-Schmidt, K.U.; Benav, H.; Besch, D.; Bruckmann, A.; Gabel, V.-P.; Gekeler, F.; Greppmaier, U.; Harscher, A.; Kibbel, S.; et al. Subretinal Electronic Chips Allow Blind Patients to Read Letters and Combine Them to Words. Proc. Biol. Sci. 2011, 278, 1489–1497. [Google Scholar] [CrossRef]
- Mathieson, K.; Loudin, J.; Goetz, G.; Huie, P.; Wang, L.; Kamins, T.I.; Galambos, L.; Smith, R.; Harris, J.S.; Sher, A.; et al. Photovoltaic Retinal Prosthesis with High Pixel Density. Nat. Photonics 2012, 6, 391–397. [Google Scholar] [CrossRef]
- Huang, T.W.; Kamins, T.I.; Chen, Z.C.; Wang, B.-Y.; Bhuckory, M.; Galambos, L.; Ho, E.; Ling, T.; Afshar, S.; Shin, A.; et al. Vertical-Junction Photodiodes for Smaller Pixels in Retinal Prostheses. J. Neural Eng. 2021, 18, 036015. [Google Scholar] [CrossRef]
- Watterson, W.J.; Montgomery, R.D.; Taylor, R.P. Modeling the Improved Visual Acuity Using Photodiode Based Retinal Implants Featuring Fractal Electrodes. Front. Neurosci. 2018, 12, 277. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.; Stamp, M.; Apollo, N.V.; Ganesan, K.; Meffin, H.; Prawer, S.; Garrett, D.J.; Ibbotson, M.R. Improved Visual Acuity Using a Retinal Implant and an Optimized Stimulation Strategy. J. Neural Eng. 2019, 17, 016018. [Google Scholar] [CrossRef]
- Xu, Y.; Pang, S. Microelectrode Array With Integrated Pneumatic Channels for Dynamic Control of Electrode Position in Retinal Implants. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 2292–2298. [Google Scholar] [CrossRef] [PubMed]
- Avraham, D.; Yitzhaky, Y. Simulating the Perceptual Effects of Electrode–Retina Distance in Prosthetic Vision. J. Neural Eng. 2022, 19, 035001. [Google Scholar] [CrossRef] [PubMed]
- Flores, T.; Huang, T.; Bhuckory, M.; Ho, E.; Chen, Z.; Dalal, R.; Galambos, L.; Kamins, T.; Mathieson, K.; Palanker, D. Honeycomb-Shaped Electro-Neural Interface Enables Cellular-Scale Pixels in Subretinal Prosthesis. Sci. Rep. 2019, 9, 10657. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.W.; Kim, N.; Ahn, J.; Cha, S.; Goo, Y.S.; Kim, S. A 3D Flexible Microelectrode Array for Subretinal Stimulation. J. Neural Eng. 2019, 16, 056016. [Google Scholar] [CrossRef]
- Vu, Q.A.; Seo, H.W.; Choi, K.-E.; Kim, N.; Kang, Y.N.; Lee, J.; Park, S.-H.; Kim, J.T.; Kim, S.; Kim, S.-W. Structural Changes in the Retina after Implantation of Subretinal Three-Dimensional Implants in Mini Pigs. Front. Neurosci. 2022, 16, 1010445. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.W.; Kim, N.; Kim, S. Fabrication of Subretinal 3D Microelectrodes with Hexagonal Arrangement. Micromachines 2020, 11, 467. [Google Scholar] [CrossRef]
- Flores, T.; Lei, X.; Huang, T.; Lorach, H.; Dalal, R.; Galambos, L.; Kamins, T.; Mathieson, K.; Palanker, D. Optimization of Pillar Electrodes in Subretinal Prosthesis for Enhanced Proximity to Target Neurons. J. Neural Eng. 2018, 15, 036011. [Google Scholar] [CrossRef]
- Abbott, C.J.; Baglin, E.K.; Kolic, M.; McGuinness, M.B.; Titchener, S.A.; Young, K.A.; Yeoh, J.; Luu, C.D.; Ayton, L.N.; Petoe, M.A.; et al. Interobserver Agreement of Electrode to Retina Distance Measurements in a Second-Generation (44-Channel) Suprachoroidal Retinal Prosthesis. Transl. Vis. Sci. Technol. 2022, 11, 4. [Google Scholar] [CrossRef]
- Sharafkhani, N.; Kouzani, A.Z.; Adams, S.D.; Long, J.M.; Lissorgues, G.; Rousseau, L.; Orwa, J.O. Neural Tissue-Microelectrode Interaction: Brain Micromotion, Electrical Impedance, and Flexible Microelectrode Insertion. J. Neurosci. Methods 2022, 365, 109388. [Google Scholar] [CrossRef]
- Zhou, M.; Kang, D.H.; Kim, J.; Weiland, J.D. Shape Morphable Hydrogel/Elastomer Bilayer for Implanted Retinal Electronics. Micromachines 2020, 11, 392. [Google Scholar] [CrossRef]
- Ferlauto, L.; Airaghi Leccardi, M.J.I.; Chenais, N.A.L.; Gilliéron, S.C.A.; Vagni, P.; Bevilacqua, M.; Wolfensberger, T.J.; Sivula, K.; Ghezzi, D. Design and Validation of a Foldable and Photovoltaic Wide-Field Epiretinal Prosthesis. Nat. Commun. 2018, 9, 992. [Google Scholar] [CrossRef]
- Vagni, P.; Airaghi Leccardi, M.J.I.; Vila, C.-H.; Zollinger, E.G.; Sherafatipour, G.; Wolfensberger, T.J.; Ghezzi, D. POLYRETINA Restores Light Responses in Vivo in Blind Göttingen Minipigs. Nat. Commun. 2022, 13, 3678. [Google Scholar] [CrossRef] [PubMed]
- Rincón Montes, V.; Gehlen, J.; Ingebrandt, S.; Mokwa, W.; Walter, P.; Müller, F.; Offenhäusser, A. Development and in Vitro Validation of Flexible Intraretinal Probes. Sci. Rep. 2020, 10, 19836. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.; Meffin, H.; Garrett, D.J.; Ibbotson, M.R. Stimulation Strategies for Improving the Resolution of Retinal Prostheses. Front. Neurosci. 2020, 14, 262. [Google Scholar] [CrossRef] [PubMed]
- Palanker, D.; Le Mer, Y.; Mohand-Said, S.; Sahel, J.A. Simultaneous Perception of Prosthetic and Natural Vision in AMD Patients. Nat. Commun. 2022, 13, 513. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.C.; Wang, B.-Y.; Goldstein, A.K.; Butt, E.; Mathieson, K.; Palanker, D. Photovoltaic Implant Simulator Reveals Resolution Limits in Subretinal Prosthesis. J. Neural Eng. 2022, 19, 055008. [Google Scholar] [CrossRef]
- Palanker, D.; Le Mer, Y.; Mohand-Said, S.; Muqit, M.; Sahel, J.A. Photovoltaic Restoration of Central Vision in Atrophic Age-Related Macular Degeneration. Ophthalmology 2020, 127, 1097–1104. [Google Scholar] [CrossRef]
- Liu, Z.; Kurokawa, K.; Zhang, F.; Lee, J.J.; Miller, D.T. Imaging and Quantifying Ganglion Cells and Other Transparent Neurons in the Living Human Retina. Proc. Natl. Acad. Sci. USA 2017, 114, 12803–12808. [Google Scholar] [CrossRef]
- Shim, S.; Eom, K.; Jeong, J.; Kim, S.J. Retinal Prosthetic Approaches to Enhance Visual Perception for Blind Patients. Micromachines 2020, 11, 535. [Google Scholar] [CrossRef]
- Zheng, X.S.; Yang, Q.; Vazquez, A.L.; Tracy Cui, X. Imaging the Efficiency of Poly(3,4-Ethylenedioxythiophene) Doped with Acid-Functionalized Carbon Nanotube and Iridium Oxide Electrode Coatings for Microstimulation. Adv. NanoBiomed Res. 2021, 1, 2000092. [Google Scholar] [CrossRef]
- Xu, Z.; Lu, Y.; Qin, S.; Wu, T.; Qin, B. Electrical Stimulation Scheme Optimization for Retinal Prosthesis: Considerations from Biological Perspective. Ann. Eye Sci. 2020, 5, 13. [Google Scholar] [CrossRef]
- Weiland, J.D.; Fink, W.; Humayun, M.; Liu, W.; Rodger, D.C.; Tai, Y.-C.; Tarbell, M. Progress Towards A High-Resolution Retinal Prosthesis. In Proceedings of the 2005 IEEE Engineering in Medicine and Biology 27th Annual Conference, Shanghai, China, 17–18 January 2005; pp. 7373–7375. [Google Scholar]
- Cui, H.; Xie, X.; Xu, S.; Chan, L.L.H.; Hu, Y. Electrochemical Characteristics of Microelectrode Designed for Electrical Stimulation. BioMed. Eng. OnLine 2019, 18, 86. [Google Scholar] [CrossRef]
- Wang, B.-Y.; Chen, Z.C.; Bhuckory, M.; Kochnev Goldstein, A.; Palanker, D. Pixel Size Limit of the PRIMA Implants: From Humans to Rodents and Back. J. Neural Eng. 2022, 19, 055003. [Google Scholar] [CrossRef]
- Zeng, Q.; Yu, S.; Fan, Z.; Huang, Y.; Song, B.; Zhou, T. Nanocone-Array-Based Platinum-Iridium Oxide Neural Microelectrodes: Structure, Electrochemistry, Durability and Biocompatibility Study. Nanomaterials 2022, 12, 3445. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Valet, M.; Dégardin, J.; Boucherit, L.; Illa, X.; de la Cruz, J.; Del Corro, E.; Bousquet, J.; Garrido, J.A.; Hébert, C.; et al. Novel Graphene Electrode for Retinal Implants: An in Vivo Biocompatibility Study. Front. Neurosci. 2021, 15, 615256. [Google Scholar] [CrossRef] [PubMed]
- Vafaiee, M.; Mohammadpour, R.; Vossoughi, M.; Asadian, E.; Janahmadi, M.; Sasanpour, P. Carbon Nanotube Modified Microelectrode Array for Neural Interface. Front. Bioeng. Biotechnol. 2020, 8, 582713. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Qin, N.; Chong, Y.; Diao, Y.; Yiliguma; Wang, Z.; Xue, T.; Jiang, M.; Zhang, J.; Zheng, G. Nanowire Arrays Restore Vision in Blind Mice. Nat. Commun. 2018, 9, 786. [Google Scholar] [CrossRef]
- Fan, V.H.; Grosberg, L.E.; Madugula, S.S.; Hottowy, P.; Dabrowski, W.; Sher, A.; Litke, A.M.; Chichilnisky, E.J. Epiretinal Stimulation with Local Returns Enhances Selectivity at Cellular Resolution. J. Neural Eng. 2019, 16, 025001. [Google Scholar] [CrossRef]
- Erickson-Davis, C.; Korzybska, H. What Do Blind People “See” with Retinal Prostheses? Observations and Qualitative Reports of Epiretinal Implant Users. PLoS ONE 2021, 16, e0229189. [Google Scholar] [CrossRef]
- Tandon, P.; Bhaskhar, N.; Shah, N.; Madugula, S.; Grosberg, L.; Fan, V.H.; Hottowy, P.; Sher, A.; Litke, A.M.; Chichilnisky, E.J.; et al. Automatic Identification of Axon Bundle Activation for Epiretinal Prosthesis. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 2496–2502. [Google Scholar] [CrossRef]
- Grosberg, L.E.; Ganesan, K.; Goetz, G.A.; Madugula, S.S.; Bhaskhar, N.; Fan, V.; Li, P.; Hottowy, P.; Dabrowski, W.; Sher, A.; et al. Activation of Ganglion Cells and Axon Bundles Using Epiretinal Electrical Stimulation. J. Neurophysiol. 2017, 118, 1457–1471. [Google Scholar] [CrossRef]
- Weitz, A.C.; Nanduri, D.; Behrend, M.R.; Gonzalez-Calle, A.; Greenberg, R.J.; Humayun, M.S.; Chow, R.H.; Weiland, J.D. Improving the Spatial Resolution of Epiretinal Implants by Increasing Stimulus Pulse Duration. Sci. Transl. Med. 2015, 7, 318ra203. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, D.H.; Chang, Y.-C.; Mirzakhalili, E.; Weiland, J.D. Closed-Loop Optimization of Retinal Ganglion Cell Responses to Epiretinal Stimulation: A Computational Study. In Proceedings of the 2021 10th International IEEE/EMBS Conference on Neural Engineering (NER), Virtual Event, Italy, 4–6 May 2021; pp. 597–600. [Google Scholar]
- Wang, B.-Y.; Chen, Z.C.; Bhuckory, M.; Huang, T.; Shin, A.; Zuckerman, V.; Ho, E.; Rosenfeld, E.; Galambos, L.; Kamins, T.; et al. Electronic Photoreceptors Enable Prosthetic Visual Acuity Matching the Natural Resolution in Rats. Nat. Commun. 2022, 13, 6627. [Google Scholar] [CrossRef] [PubMed]
- Madugula, S.S.; Gogliettino, A.R.; Zaidi, M.; Aggarwal, G.; Kling, A.; Shah, N.P.; Vilkhu, R.; Hays, M.R.; Nguyen, H.; Fan, V.; et al. Focal Electrical Stimulation of Human Retinal Ganglion Cells for Vision Restoration. J. Neural Eng. 2022, 19, 066040. [Google Scholar] [CrossRef] [PubMed]
- Yunzab, M.; Soto-Breceda, A.; Maturana, M.; Kirkby, S.; Slattery, M.; Newgreen, A.; Meffin, H.; Kameneva, T.; Burkitt, A.N.; Ibbotson, M.; et al. Preferential Modulation of Individual Retinal Ganglion Cells by Electrical Stimulation. J. Neural Eng. 2022, 19, 045003. [Google Scholar] [CrossRef]
- Borda, E.; Gaillet, V.; Airaghi Leccardi, M.J.I.; Zollinger, E.G.; Moreira, R.C.; Ghezzi, D. Three-Dimensional Multilayer Concentric Bipolar Electrodes Restrict Spatial Activation in Optic Nerve Stimulation. J. Neural Eng. 2022, 19, 036016. [Google Scholar] [CrossRef]
- Goetz, J.; Jessen, Z.F.; Jacobi, A.; Mani, A.; Cooler, S.; Greer, D.; Kadri, S.; Segal, J.; Shekhar, K.; Sanes, J.R.; et al. Unified Classification of Mouse Retinal Ganglion Cells Using Function, Morphology, and Gene Expression. Cell Rep. 2022, 40, 111040. [Google Scholar] [CrossRef]
- Roh, H.; Otgondemberel, Y.; Eom, J.; Kim, D.; Im, M. Electrically-Evoked Responses for Retinal Prostheses Are Differentially Altered Depending on Ganglion Cell Types in Outer Retinal Neurodegeneration Caused by Crb1 Gene Mutation. Front. Cell. Neurosci. 2023, 17, 1115703. [Google Scholar] [CrossRef]
- Yue, L.; Castillo, J.; Gonzalez, A.C.; Neitz, J.; Humayun, M.S. Restoring Color Perception to the Blind: An Electrical Stimulation Strategy of Retina in Patients with End-Stage Retinitis Pigmentosa. Ophthalmology 2021, 128, 453–462. [Google Scholar] [CrossRef]
- Stanga, P.E.; Hafezi, F.; Sahel, J.A.; daCruz, L.; Merlini, F.; Coley, B.; Greenberg, R.J.; Argus II Study Group. Patients Blinded By Outer Retinal Dystrophies Are Able To Perceive Color Using The ArgusTm II Retinal Prosthesis System. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4949. [Google Scholar]
- Stanga, P.E.; Sahel, J.A., Jr.; daCruz, L.; Hafezi, F.; Merlini, F.; Coley, B.; Greenberg, R.J.; Argus II Study Group. Patients Blinded by Outer Retinal Dystrophies Are Able to Perceive Simultaneous Colors Using the Argus® II Retinal Prosthesis System. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6952. [Google Scholar]
- Towle, V.L.; Pham, T.; McCaffrey, M.; Allen, D.; Troyk, P.R. Toward the Development of a Color Visual Prosthesis. J. Neural Eng. 2021, 18, 023001. [Google Scholar] [CrossRef]
- Paknahad, J.; Loizos, K.; Yue, L.; Humayun, M.S.; Lazzi, G. Color and Cellular Selectivity of Retinal Ganglion Cell Subtypes through Frequency Modulation of Electrical Stimulation. Sci. Rep. 2021, 11, 5177. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Ghaffari, D.H.; Chow, R.H.; Weiland, J.D. Stimulation Strategies for Selective Activation of Retinal Ganglion Cell Soma and Threshold Reduction. J. Neural Eng. 2019, 16, 026017. [Google Scholar] [CrossRef]
- Jepson, L.H.; Hottowy, P.; Mathieson, K.; Gunning, D.E.; Dąbrowski, W.; Litke, A.M.; Chichilnisky, E.J. Spatially Patterned Electrical Stimulation to Enhance Resolution of Retinal Prostheses. J. Neurosci. 2014, 34, 4871–4881. [Google Scholar] [CrossRef] [PubMed]
- Vėbraitė, I.; Hanein, Y. In the Eye of the Storm: Bi-Directional Electrophysiological Investigation of the Intact Retina. Front. Neurosci. 2022, 16, 829323. [Google Scholar] [CrossRef] [PubMed]
- Caravaca-Rodriguez, D.; Gaytan, S.P.; Suaning, G.J.; Barriga-Rivera, A. Implications of Neural Plasticity in Retinal Prosthesis. Investig. Ophthalmol. Vis. Sci. 2022, 63, 11. [Google Scholar] [CrossRef]
- Kang, H.; Abbasi, W.H.; Kim, S.-W.; Kim, J. Fully Integrated Light-Sensing Stimulator Design for Subretinal Implants. Sensors 2019, 19, 536. [Google Scholar] [CrossRef]
- Xu, A.; Beyeler, M. Retinal Ganglion Cells Undergo Cell Type-specific Functional Changes in a Biophysically Detailed Model of Retinal Degeneration. BioRxiv 2023. [Google Scholar] [CrossRef]
- Haji Ghaffari, D.; Akwaboah, A.D.; Mirzakhalili, E.; Weiland, J.D. Real-Time Optimization of Retinal Ganglion Cell Spatial Activity in Response to Epiretinal Stimulation. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 2733–2741. [Google Scholar] [CrossRef] [PubMed]
- Ly, K.; Guo, T.; Tsai, D.; Muralidharan, M.; Shivdasani, M.N.; Lovell, N.H.; Dokos, S. Simulating the Impact of Photoreceptor Loss and Inner Retinal Network Changes on Electrical Activity of the Retina. J. Neural Eng. 2022, 19, 065002. [Google Scholar] [CrossRef]
- Paknahad, J.; Kosta, P.; Bouteiller, J.-M.C.; Humayun, M.S.; Lazzi, G. Mechanisms Underlying Activation of Retinal Bipolar Cells through Targeted Electrical Stimulation: A Computational Study. J. Neural Eng. 2021, 18, 066034. [Google Scholar] [CrossRef]
- Iseri, E.; Kosta, P.; Paknahad, J.; Bouteiller, J.-M.C.; Lazzi, G. A Computational Model Simulates Light-Evoked Responses in the Retinal Cone Pathway. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Guadalajara, Mexico, 1–5 November 2021; pp. 4482–4486. [Google Scholar] [CrossRef]
- Italiano, M.L.; Guo, T.; Lovell, N.H.; Tsai, D. Improving the Spatial Resolution of Artificial Vision Using Midget Retinal Ganglion Cell Populations Modeled at the Human Fovea. J. Neural Eng. 2022, 19, 035002. [Google Scholar] [CrossRef]
- Relic, L.; Zhang, B.; Tuan, Y.-L.; Beyeler, M. Deep Learning-Based Perceptual Stimulus Encoder for Bionic Vision. In Proceedings of the Augmented Humans International Conference, Chiba, Japan, 13–15 March 2022. [Google Scholar]
- Machida, S. Clinical Applications of the Photopic Negative Response to Optic Nerve and Retinal Diseases. J. Ophthalmol. 2012, 2012, 397178. [Google Scholar] [CrossRef] [PubMed]
- Finn, A.P.; Grewal, D.S.; Vajzovic, L. Argus II Retinal Prosthesis System: A Review of Patient Selection Criteria, Surgical Considerations, and Post-Operative Outcomes. Clin. Ophthalmol. 2018, 12, 1089–1097. [Google Scholar] [CrossRef]
- Zrenner, E.; Bartz-Schmidt, K.; Besch, D.; Gekeler, F.; Koitschev, A.; Sachs, H.; Štingl, K. The Subretinal Implant ALPHA: Implantation and Functional Results; Springer: Cham, Switzerland, 2017; pp. 65–83. ISBN 978-3-319-41874-2. [Google Scholar]
- Stingl, K.; Schippert, R.; Bartz-Schmidt, K.U.; Besch, D.; Cottriall, C.L.; Edwards, T.L.; Gekeler, F.; Greppmaier, U.; Kiel, K.; Koitschev, A.; et al. Interim Results of a Multicenter Trial with the New Electronic Subretinal Implant Alpha AMS in 15 Patients Blind from Inherited Retinal Degenerations. Front. Neurosci. 2017, 11, 445. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.W.; Pfeiffer, R.L.; Ferrell, W.D.; Watt, C.B.; Marmor, M.; Marc, R.E. Retinal Remodeling in Human Retinitis Pigmentosa. Exp. Eye Res. 2016, 150, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Yoon, C.K.; Yu, H.G. Ganglion Cell-Inner Plexiform Layer and Retinal Nerve Fibre Layer Changes within the Macula in Retinitis Pigmentosa: A Spectral Domain Optical Coherence Tomography Study. Acta Ophthalmol. 2018, 96, e180–e188. [Google Scholar] [CrossRef]
- Geruschat, D.R.; Flax, M.; Tanna, N.; Bianchi, M.; Fisher, A.; Goldschmidt, M.; Fisher, L.; Dagnelie, G.; Deremeik, J.; Smith, A.; et al. FLORATM: Phase I Development of a Functional Vision Assessment for Prosthetic Vision Users. Clin. Exp. Optom. 2015, 98, 342–347. [Google Scholar] [CrossRef]
- Caspi, A.; Roy, A.; Wuyyuru, V.; Rosendall, P.E.; Harper, J.W.; Katyal, K.D.; Barry, M.P.; Dagnelie, G.; Greenberg, R.J. Eye Movement Control in the Argus II Retinal-Prosthesis Enables Reduced Head Movement and Better Localization Precision. Investig. Ophthalmol. Vis. Sci. 2018, 59, 792–802. [Google Scholar] [CrossRef]
- Stanga, P.E.; Tsamis, E.; Siso-Fuertes, I.; Dorn, J.D.; Merlini, F.; Fisher, A.; Crawford, F.I.; Kasbia, S.S.; Papayannis, A.; Baseler, H.A.; et al. Electronic Retinal Prosthesis for Severe Loss of Vision in Geographic Atrophy in Age-Related Macular Degeneration: First-in-Human Use. Eur. J. Ophthalmol. 2021, 31, 920–931. [Google Scholar] [CrossRef]
- Schaffrath, K.; Schellhase, H.; Walter, P.; Augustin, A.; Chizzolini, M.; Kirchhof, B.; Grisanti, S.; Wiedemann, P.; Szurman, P.; Richard, G.; et al. One-Year Safety and Performance Assessment of the Argus II Retinal Prosthesis: A Postapproval Study. JAMA Ophthalmol. 2019, 137, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Delyfer, M.-N.; Gaucher, D.; Mohand-Saïd, S.; Barale, P.-O.; Rezaigua-Studer, F.; Ayello-Scheer, S.; Dollfus, H.; Dorn, J.D.; Korobelnik, J.-F.; Sahel, J.-A. Improved Performance and Safety from Argus II Retinal Prosthesis Post-Approval Study in France. Acta Ophthalmol. 2021, 99, e1212–e1221. [Google Scholar] [CrossRef] [PubMed]
- Muqit, M.M.K.; Velikay-Parel, M.; Weber, M.; Dupeyron, G.; Audemard, D.; Corcostegui, B.; Sahel, J.; Le Mer, Y. Six-Month Safety and Efficacy of the Intelligent Retinal Implant System II Device in Retinitis Pigmentosa. Ophthalmology 2019, 126, 637–639. [Google Scholar] [CrossRef] [PubMed]
- Kurt, A.; Özbek, R.; Erbas, O. Systematic Review of Novel Advances in Epiretinal and Subretinal Prosthesis. J. Exp. Basic Med. Sci. 2022, 2, 326–335. [Google Scholar] [CrossRef]
- Bloch, E.; Luo, Y.; da Cruz, L. Advances in Retinal Prosthesis Systems. Ther. Adv. Ophthalmol. 2019, 11, 2515841418817501. [Google Scholar] [CrossRef]
- Stingl, K.; Bartz-Schmidt, K.U.; Besch, D.; Chee, C.K.; Cottriall, C.L.; Gekeler, F.; Groppe, M.; Jackson, T.L.; MacLaren, R.E.; Koitschev, A.; et al. Subretinal Visual Implant Alpha IMS—Clinical Trial Interim Report. Vis. Res. 2015, 111, 149–160. [Google Scholar] [CrossRef]
- Edwards, T.L.; Cottriall, C.L.; Xue, K.; Simunovic, M.P.; Ramsden, J.D.; Zrenner, E.; MacLaren, R.E. Assessment of the Electronic Retinal Implant Alpha AMS in Restoring Vision to Blind Patients with End-Stage Retinitis Pigmentosa. Ophthalmology 2018, 125, 432–443. [Google Scholar] [CrossRef]
- Ayton, L.N.; Blamey, P.J.; Guymer, R.H.; Luu, C.D.; Nayagam, D.A.X.; Sinclair, N.C.; Shivdasani, M.N.; Yeoh, J.; McCombe, M.F.; Briggs, R.J.; et al. First-in-Human Trial of a Novel Suprachoroidal Retinal Prosthesis. PLoS ONE 2014, 9, e115239. [Google Scholar] [CrossRef]
- Petoe, M.A.; Titchener, S.A.; Kolic, M.; Kentler, W.G.; Abbott, C.J.; Nayagam, D.A.X.; Baglin, E.K.; Kvansakul, J.; Barnes, N.; Walker, J.G.; et al. A Second-Generation (44-Channel) Suprachoroidal Retinal Prosthesis: Interim Clinical Trial Results. Transl. Vis. Sci. Technol. 2021, 10, 12. [Google Scholar] [CrossRef]
- da Cruz, L.; Dorn, J.D.; Humayun, M.S.; Dagnelie, G.; Handa, J.; Barale, P.-O.; Sahel, J.-A.; Stanga, P.E.; Hafezi, F.; Safran, A.B.; et al. Five-Year Safety and Performance Results from the Argus II Retinal Prosthesis System Clinical Trial. Ophthalmology 2016, 123, 2248–2254. [Google Scholar] [CrossRef]
- Karapanos, L.; Abbott, C.J.; Ayton, L.N.; Kolic, M.; McGuinness, M.B.; Baglin, E.K.; Titchener, S.A.; Kvansakul, J.; Johnson, D.; Kentler, W.G.; et al. Functional Vision in the Real-World Environment With a Second-Generation (44-Channel) Suprachoroidal Retinal Prosthesis. Transl. Vis. Sci. Technol. 2021, 10, 7. [Google Scholar] [CrossRef]
- Titchener, S.A.; Kvansakul, J.; Shivdasani, M.N.; Fallon, J.B.; Nayagam, D.A.X.; Epp, S.B.; Williams, C.E.; Barnes, N.; Kentler, W.G.; Kolic, M.; et al. Oculomotor Responses to Dynamic Stimuli in a 44-Channel Suprachoroidal Retinal Prosthesis. Transl. Vis. Sci. Technol. 2020, 9, 31. [Google Scholar] [CrossRef]
- Carignan, M.; Courchesne, M.; Cantin, S.; Moore, V.; Poncet, F. Visual Rehabilitation After Retinal Prosthesis Implantation: An 18-Month Case Report, From Candidate Selection to Follow-Up. J. Vis. Impair. Blind. 2022, 116, 167–182. [Google Scholar] [CrossRef]
- Avraham, D.; Jung, J.-H.; Yitzhaky, Y.; Peli, E. Retinal Prosthetic Vision Simulation: Temporal Aspects. J. Neural Eng. 2021, 18, 0460d9. [Google Scholar] [CrossRef]
- Thorn, J.T.; Chenais, N.A.L.; Hinrichs, S.; Chatelain, M.; Ghezzi, D. Virtual Reality Validation of Naturalistic Modulation Strategies to Counteract Fading in Retinal Stimulation. J. Neural Eng. 2022, 19, 026016. [Google Scholar] [CrossRef]
- Stiles, N.R.B.; Patel, V.R.; Weiland, J.D. Multisensory Perception in Argus II Retinal Prosthesis Patients: Leveraging Auditory-Visual Mappings to Enhance Prosthesis Outcomes. Vis. Res. 2021, 182, 58–68. [Google Scholar] [CrossRef]
- Rachitskaya, A.; Yuan, A.; Davidson, S.; Streicher, M.; DeBenedictis, M.; Rosenfeldt, A.B.; Alberts, J. Computer-Assisted Immersive Visual Rehabilitation in Argus II Retinal Prosthesis Recipients. Ophthalmol. Retin. 2020, 4, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Jindal, N.; Banik, A.; Prabhakar, S.; Vaiphie, K.; Anand, A. Alteration of Neurotrophic Factors After Transplantation of Bone Marrow Derived Lin-ve Stem Cell in NMDA-Induced Mouse Model of Retinal Degeneration. J. Cell. Biochem. 2017, 118, 1699–1711. [Google Scholar] [CrossRef] [PubMed]
- Mandai, M.; Fujii, M.; Hashiguchi, T.; Sunagawa, G.A.; Ito, S.; Sun, J.; Kaneko, J.; Sho, J.; Yamada, C.; Takahashi, M. IPSC-Derived Retina Transplants Improve Vision in Rd1 End-Stage Retinal-Degeneration Mice. Stem Cell Rep. 2017, 8, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Soleymaninejadian, E.; Pramanik, K.; Samadian, E. Immunomodulatory Properties of Mesenchymal Stem Cells: Cytokines and Factors. Am. J. Reprod. Immunol. 2012, 67, 1–8. [Google Scholar] [CrossRef]
- Bassi, Ê.J.; de Almeida, D.C.; Moraes-Vieira, P.M.M.; Câmara, N.O.S. Exploring the Role of Soluble Factors Associated with Immune Regulatory Properties of Mesenchymal Stem Cells. Stem Cell Rev. Rep. 2012, 8, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Kolomeyer, A.M.; Zarbin, M.A. Trophic Factors in the Pathogenesis and Therapy for Retinal Degenerative Diseases. Surv. Ophthalmol. 2014, 59, 134–165. [Google Scholar] [CrossRef]
- Sachdeva, M.M.; Eliott, D. Stem Cell-Based Therapy for Diseases of the Retinal Pigment Epithelium: From Bench to Bedside. Semin. Ophthalmol. 2016, 31, 25–29. [Google Scholar] [CrossRef]
- Pan, T.; Shen, H.; Yuan, S.; Lu, G.; Zhang, Y.; Wang, H.; Zhao, Y.; Sun, X.; Liu, Q. Combined Transplantation With Human Mesenchymal Stem Cells Improves Retinal Rescue Effect of Human Fetal RPE Cells in Retinal Degeneration Mouse Model. Investig. Ophthalmol. Vis. Sci. 2020, 61, 9. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.-K.; Tosi, J.; Kasanuki, J.M.; Chou, C.L.; Kong, J.; Parmalee, N.; Wert, K.J.; Allikmets, R.; Lai, C.-C.; Chien, C.-L.; et al. Transplantation of Reprogrammed Embryonic Stem Cells Improves Visual Function in a Mouse Model for Retinitis Pigmentosa. Transplantation 2010, 89, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Klassen, H.J.; Ng, T.F.; Kurimoto, Y.; Kirov, I.; Shatos, M.; Coffey, P.; Young, M.J. Multipotent Retinal Progenitors Express Developmental Markers, Differentiate into Retinal Neurons, and Preserve Light-Mediated Behavior. Investig. Ophthalmol. Vis. Sci. 2004, 45, 4167–4173. [Google Scholar] [CrossRef]
- Stern, J.H.; Tian, Y.; Funderburgh, J.; Pellegrini, G.; Zhang, K.; Goldberg, J.L.; Ali, R.R.; Young, M.; Xie, Y.; Temple, S. Regenerating Eye Tissues to Preserve and Restore Vision. Cell Stem Cell 2018, 22, 834–849. [Google Scholar] [CrossRef]
- Sharma, R.; Khristov, V.; Rising, A.; Jha, B.S.; Dejene, R.; Hotaling, N.; Li, Y.; Stoddard, J.; Stankewicz, C.; Wan, Q.; et al. Clinical-Grade Stem Cell–Derived Retinal Pigment Epithelium Patch Rescues Retinal Degeneration in Rodents and Pigs. Sci. Transl. Med. 2019, 11, eaat5580. [Google Scholar] [CrossRef]
- Kashani, A.H.; Lebkowski, J.S.; Rahhal, F.M.; Avery, R.L.; Salehi-Had, H.; Dang, W.; Lin, C.-M.; Mitra, D.; Zhu, D.; Thomas, B.B.; et al. A Bioengineered Retinal Pigment Epithelial Monolayer for Advanced, Dry Age-Related Macular Degeneration. Sci. Transl. Med. 2018, 10, eaao4097. [Google Scholar] [CrossRef]
- Kashani, A.H.; Uang, J.; Mert, M.; Rahhal, F.; Chan, C.; Avery, R.L.; Dugel, P.; Chen, S.; Lebkowski, J.; Clegg, D.O.; et al. Surgical Method for Implantation of a Biosynthetic Retinal Pigment Epithelium Monolayer for Geographic Atrophy: Experience from a Phase 1/2a Study. Ophthalmol. Retin. 2020, 4, 264–273. [Google Scholar] [CrossRef]
- Ong, T.; Pennesi, M.E.; Birch, D.G.; Lam, B.L.; Tsang, S.H. Adeno-Associated Viral Gene Therapy for Inherited Retinal Disease. Pharm. Res. 2019, 36, 34. [Google Scholar] [CrossRef]
- Trapani, I.; Puppo, A.; Auricchio, A. Vector Platforms for Gene Therapy of Inherited Retinopathies. Prog. Retin. Eye Res. 2014, 43, 108–128. [Google Scholar] [CrossRef]
- Trapani, I.; Auricchio, A. Has Retinal Gene Therapy Come of Age? From Bench to Bedside and Back to Bench. Hum. Mol. Genet. 2019, 28, R108–R118. [Google Scholar] [CrossRef]
- Ahmad, I. CRISPR/Cas9—A Promising Therapeutic Tool to Cure Blindness: Current Scenario and Future Prospects. Int. J. Mol. Sci. 2022, 23, 11482. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, S.; Li, P.; Yao, K. Retinitis Pigmentosa: Progress in Molecular Pathology and Biotherapeutical Strategies. Int. J. Mol. Sci. 2022, 23, 4883. [Google Scholar] [CrossRef] [PubMed]
- Tomita, H.; Sugano, E.; Fukazawa, Y.; Isago, H.; Sugiyama, Y.; Hiroi, T.; Ishizuka, T.; Mushiake, H.; Kato, M.; Hirabayashi, M.; et al. Visual Properties of Transgenic Rats Harboring the Channelrhodopsin-2 Gene Regulated by the Thy-1.2 Promoter. PLoS ONE 2009, 4, e7679. [Google Scholar] [CrossRef] [PubMed]
- Simon, C.-J.; Sahel, J.-A.; Duebel, J.; Herlitze, S.; Dalkara, D. Opsins for Vision Restoration. Biochem. Biophys. Res. Commun. 2020, 527, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Deisseroth, K. Optogenetics: 10 Years of Microbial Opsins in Neuroscience. Nat. Neurosci. 2015, 18, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Nascimento-Dos-Santos, G.; Teixeira-Pinheiro, L.C.; da Silva-Júnior, A.J.; de Carvalho, L.R.P.; Mesentier-Louro, L.A.; Hauswirth, W.W.; Mendez-Otero, R.; Santiago, M.F.; Petrs-Silva, H. Effects of a Combinatorial Treatment with Gene and Cell Therapy on Retinal Ganglion Cell Survival and Axonal Outgrowth after Optic Nerve Injury. Gene Ther. 2020, 27, 27–39. [Google Scholar] [CrossRef]
- Barrett, J.M.; Berlinguer-Palmini, R.; Degenaar, P. Optogenetic Approaches to Retinal Prosthesis. Vis. Neurosci. 2014, 31, 345–354. [Google Scholar] [CrossRef]
- Li, G.; Wang, F.; Yang, W.; Yang, J.; Wang, Y.; Wang, W.; Liu, L. Development of an Image Biosensor Based on an Optogenetically Engineered Cell for Visual Prostheses. Nanoscale 2019, 11, 13213–13218. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Wan, S.; Su, S.; Huang, H.; Dou, G.; Sun, L. Electrode Materials for Brain–Machine Interface: A Review. InfoMat 2021, 3, 1174–1194. [Google Scholar] [CrossRef]
- Yang, J.-W.; Chen, C.-Y.; Yu, Z.-Y.; Chung, J.H.Y.; Liu, X.; Wu, C.-Y.; Chen, G.-Y. An Electroactive Hybrid Biointerface for Enhancing Neuronal Differentiation and Axonal Outgrowth on Bio-Subretinal Chip. Mater. Today Bio 2022, 14, 100253. [Google Scholar] [CrossRef] [PubMed]
- Niina, O.; Hirotaka, T.; Yoshiuki, K.; Toshiya, K.; Risato, K.; Tetsu, T.; Jari, H. Comparison of Electrode Materials for the Use of Retinal Prosthesis. Biomed. Mater. Eng. 2011, 21, 83–97. [Google Scholar] [CrossRef]
- Vetter, R.J.; Williams, J.C.; Hetke, J.F.; Nunamaker, E.A.; Kipke, D.R. Chronic Neural Recording Using Silicon-Substrate Microelectrode Arrays Implanted in Cerebral Cortex. IEEE Trans. Biomed. Eng. 2004, 51, 896–904. [Google Scholar] [CrossRef]
- George, P.M.; Lyckman, A.W.; LaVan, D.A.; Hegde, A.; Leung, Y.; Avasare, R.; Testa, C.; Alexander, P.M.; Langer, R.; Sur, M. Fabrication and Biocompatibility of Polypyrrole Implants Suitable for Neural Prosthetics. Biomaterials 2005, 26, 3511–3519. [Google Scholar] [CrossRef]
- Green, R.A.; Lovell, N.H.; Wallace, G.G.; Poole-Warren, L.A. Conducting Polymers for Neural Interfaces: Challenges in Developing an Effective Long-Term Implant. Biomaterials 2008, 29, 3393–3399. [Google Scholar] [CrossRef]
- Barriga-Rivera, A.; Bareket, L.; Goding, J.; Aregueta-Robles, U.A.; Suaning, G.J. Visual Prosthesis: Interfacing Stimulating Electrodes with Retinal Neurons to Restore Vision. Front. Neurosci. 2017, 11, 620. [Google Scholar] [CrossRef]
- Ouyang, L.; Wei, B.; Kuo, C.; Pathak, S.; Farrell, B.; Martin, D.C. Enhanced PEDOT Adhesion on Solid Substrates with Electrografted P(EDOT-NH2). Sci. Adv. 2017, 3, e1600448. [Google Scholar] [CrossRef]
- Green, R.A.; Devillaine, F.; Dodds, C.; Matteucci, P.; Chen, S.; Byrnes-Preston, P.; Poole-Warren, L.A.; Lovell, N.H.; Suaning, G.J. Conducting Polymer Electrodes for Visual Prostheses. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 6769–6772. [Google Scholar]
- Keefer, E.W.; Botterman, B.R.; Romero, M.I.; Rossi, A.F.; Gross, G.W. Carbon Nanotube Coating Improves Neuronal Recordings. Nat. Nanotechnol. 2008, 3, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriou, C.G.; Zimmermann, J.B.; Kjeldsen, H.D.; David-Pur, M.; Hanein, Y.; Sernagor, E. Carbon Nanotube Electrodes for Retinal Implants: A Study of Structural and Functional Integration over Time. Biomaterials 2017, 112, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.; Kostarelos, K.; Prato, M. Making Carbon Nanotubes Biocompatible and Biodegradable. Chem. Commun. 2011, 47, 10182. [Google Scholar] [CrossRef]
- Shi, S.; Chen, F.; Ehlerding, E.B.; Cai, W. Surface Engineering of Graphene-Based Nanomaterials for Biomedical Applications. Bioconjugate Chem. 2014, 25, 1609–1619. [Google Scholar] [CrossRef]
- Hébert, C.; Masvidal-Codina, E.; Suarez-Perez, A.; Calia, A.B.; Piret, G.; Garcia-Cortadella, R.; Illa, X.; Del Corro Garcia, E.; De la Cruz Sanchez, J.M.; Casals, D.V.; et al. Flexible Graphene Solution-Gated Field-Effect Transistors: Efficient Transducers for Micro-Electrocorticography. Adv. Funct. Mater. 2018, 28, 1703976. [Google Scholar] [CrossRef]
- Qing, Q.; Pal, S.K.; Tian, B.; Duan, X.; Timko, B.P.; Cohen-Karni, T.; Murthy, V.N.; Lieber, C.M. Nanowire Transistor Arrays for Mapping Neural Circuits in Acute Brain Slices. Proc. Natl. Acad. Sci. USA 2010, 107, 1882–1887. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, T.; Zwang, T.J.; Hong, G.; Zhao, Y.; Viveros, R.D.; Fu, T.-M.; Gao, T.; Lieber, C.M. Bioinspired Neuron-like Electronics. Nat. Mater. 2019, 18, 510–517. [Google Scholar] [CrossRef]
- Yao, X.; Toslak, D.; Son, T.; Ma, J. Understanding the Relationship between Visual-Angle and Eye-Angle for Reliable Determination of the Field-of-View in Ultra-Wide Field Fundus Photography. Biomed. Opt. Express 2021, 12, 6651–6659. [Google Scholar] [CrossRef]
- Borda, E.; Ghezzi, D. Advances in Visual Prostheses: Engineering and Biological Challenges. Prog. Biomed. Eng. 2022, 4, 032003. [Google Scholar] [CrossRef]
- Chenais, N.A.L.; Airaghi Leccardi, M.J.I.; Ghezzi, D. Photovoltaic Retinal Prosthesis Restores High-Resolution Responses to Single-Pixel Stimulation in Blind Retinas. Commun. Mater. 2021, 2, 28. [Google Scholar] [CrossRef]
- Guo, F.; Yang, Y.; Gao, Y. Optimization of Visual Information Presentation for Visual Prosthesis. Int. J. Biomed. Imaging 2018, 2018, 3198342. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, M.; Wallis, T.S.A.; Bethge, M. DeepGaze II: Reading Fixations from Deep Features Trained on Object Recognition. arXiv 2016, arXiv:1610.01563. [Google Scholar]
- Godard, C.; Aodha, O.M.; Firman, M.; Brostow, G. Digging Into Self-Supervised Monocular Depth Estimation. In Proceedings of the 2019 IEEE/CVF International Conference on Computer Vision (ICCV), Seoul, Republic of Korea, 27 October–2 November 2019; pp. 3827–3837. [Google Scholar]
- Han, N.; Srivastava, S.; Xu, A.; Klein, D.; Beyeler, M. Deep Learning–Based Scene Simplification for Bionic Vision. In Proceedings of the Augmented Humans Conference 2021, Rovaniemi, Finland, 22–24 February 2021; pp. 45–54. [Google Scholar]
- Wang, C.; Fang, C.; Zou, Y.; Yang, J.; Sawan, M. SpikeSEE: An Energy-Efficient Dynamic Scenes Processing Framework for Retinal Prostheses. Neural Netw. 2022, 164, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Jang, Y.J.; Kim, T.; Lee, B.C.; Lee, S.H.; Im, M. Electric Stimulation Elicits Heterogeneous Responses in ON but Not OFF Retinal Ganglion Cells to Transmit Rich Neural Information. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, D.; Simon, E.; Buc, G.; Deterre, M. In Vitro Reliability Testing and in Vivo Lifespan Estimation of Wireless Pixium Vision PRIMA Photovoltaic Subretinal Prostheses Suggest Prolonged Durability and Functionality in Clinical Practice. J. Neural Eng. 2020, 17, 035005. [Google Scholar] [CrossRef] [PubMed]
- Muqit, M.M.K.; Mer, Y.L.; Holz, F.G.; Sahel, J.A. Long-Term Observations of Macular Thickness after Subretinal Implantation of a Photovoltaic Prosthesis in Patients with Atrophic Age-Related Macular Degeneration. J. Neural Eng. 2022, 19, 055011. [Google Scholar] [CrossRef] [PubMed]
- Özmert, E.; Arslan, U. Retinal Prostheses and Artificial Vision. Turk. J. Ophthalmol. 2019, 49, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Weiland, J.D.; Cho, A.K.; Humayun, M.S. Retinal Prostheses: Current Clinical Results and Future Needs. Ophthalmology 2011, 118, 2227–2237. [Google Scholar] [CrossRef]
- Lagali, P.; Balya, D.; Awatramani, G.; Münch, T.; Kim, D.; Busskamp, V.; Cepko, C.; Roska, B. Light-Activated Channels Targeted to ON Bipolar Cells Restore Visual Function in Retinal Degeneration. Nat. Neurosci. 2008, 11, 667–675. [Google Scholar] [CrossRef]
- Wang, C.; Fang, C.; Zou, Y.; Yang, J.; Sawan, M. Artificial Intelligence Techniques for Retinal Prostheses: A Comprehensive Review and Future Direction. J. Neural Eng. 2023, 20, 36634357. [Google Scholar] [CrossRef]
- Hu, Z.; Beyeler, M. Explainable AI for Retinal Prostheses: Predicting Electrode Deactivation from Routine Clinical Measures. In Proceedings of the 2021 10th International IEEE/EMBS Conference on Neural Engineering (NER), Virtual Event, Italy, 4–6 May 2021; pp. 792–796. [Google Scholar]


| Prosthesis Type | Adverse Events | Resolution of Adverse Events | Visual Function and Outcomes | |
|---|---|---|---|---|
| Epiretinal | Argus II | Conjunctival erosions, hypotony, explantation, ocular inflammation, and retinal detachment. | Successfully treated or managed, except for hypotony and permanent retinal detachment. | Mixed visual function outcomes, self-report improvements in functional tasks. |
| IRIS 2 | Non-serious events: phlebitis, tack detachment, etc.; serious events: hypotony and persistent pain. | Successfully treated or managed. | Improved square localization, direction of motion detection, visual fields, and picture recognition with the device on. | |
| Subretinal | Alpha IMS and AMS | Alpha IMS: increased intraocular pressure, retinal detachment, and retinal fibrotic changes; newer Alpha AMS: surgical dehiscence, implant displacement, partial silicone oil tamponade loss, and pain. | Successfully treated or managed. | Improved light source perception but difficulties with localizing and motion detection tasks; mixed benefits for daily living activities. |
| PRIMA | Choroidal hemorrhage, subretinal hemorrhage, device displacement, and increased intraocular pressure due to medication non-adherence. | Successfully treated or managed. | Improved eccentric natural acuity and accurate identification of bar orientation. | |
| Suprachoroidal | Fewer adverse outcomes compared to other types of implants; Non-serious events: pain, swelling, conjunctival injection, and local inflammation; one case of increased ocular pressure. | Successfully treated or managed. | Facilitated daily activities such as washing dishes, folding and organizing laundry, and identifying doorways and people in non-crowded spaces; difficulties remained in tasks such as identifying food on a plate; improved square localization and motion discrimination with the device on. | |
| Treatment Modality | Description | Advantages | Limitations |
|---|---|---|---|
| Retinal Prostheses | This technology works by artificially stimulating the retinal nerve cells to mimic the function of lost or damaged photoreceptors. | Can restore some vision in patients with advanced retinal diseases, such as retinitis pigmentosa. | Safety and efficacy are still being evaluated. High cost may limit widespread adoption. |
| Cell-Based Therapies | Therapies involve the use of stem cells (pluripotent stem cells, bone marrow stem cells, and retinal progenitor cells) to replace or restore dysfunctional cells in the retina. | Potential to delay disease progression and restore vision loss, and can provide trophic support to remaining photoreceptors. | Potential risk of immune rejection. |
| Gene-Based Therapies | Therapies involve the use of viral and non-viral vectors and CRISPR-cas9 gene editing to correct genetic mutations causing retinal diseases. | Targets the root cause of the disease, potentially restoring vision. | Limited by the variety of gene mutations, so long-term outcomes and safety still require further investigation. |
| Optogenetics | This technique involves introducing photosensitive proteins to the degenerated retina to restore function and provide photosensitivity to remaining retinal cells. | Can restore photosensitivity to non-light-sensitive cells. | Requires further research on the structure, transport modes, dynamics, and optical properties of photosensitive proteins. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, K.Y.; Mina, M.; Sahyoun, J.-Y.; Kalevar, A.; Tran, S.D. Retinal Prostheses: Engineering and Clinical Perspectives for Vision Restoration. Sensors 2023, 23, 5782. https://doi.org/10.3390/s23135782
Wu KY, Mina M, Sahyoun J-Y, Kalevar A, Tran SD. Retinal Prostheses: Engineering and Clinical Perspectives for Vision Restoration. Sensors. 2023; 23(13):5782. https://doi.org/10.3390/s23135782
Chicago/Turabian StyleWu, Kevin Y., Mina Mina, Jean-Yves Sahyoun, Ananda Kalevar, and Simon D. Tran. 2023. "Retinal Prostheses: Engineering and Clinical Perspectives for Vision Restoration" Sensors 23, no. 13: 5782. https://doi.org/10.3390/s23135782
APA StyleWu, K. Y., Mina, M., Sahyoun, J.-Y., Kalevar, A., & Tran, S. D. (2023). Retinal Prostheses: Engineering and Clinical Perspectives for Vision Restoration. Sensors, 23(13), 5782. https://doi.org/10.3390/s23135782







