Detection of Defective Lettuce Seedlings Grown in an Indoor Environment under Different Lighting Conditions Using Deep Learning Algorithms
Abstract
:1. Introduction
2. Related Works
3. Materials and Methods
3.1. Plant Material and Cultivation Conditions
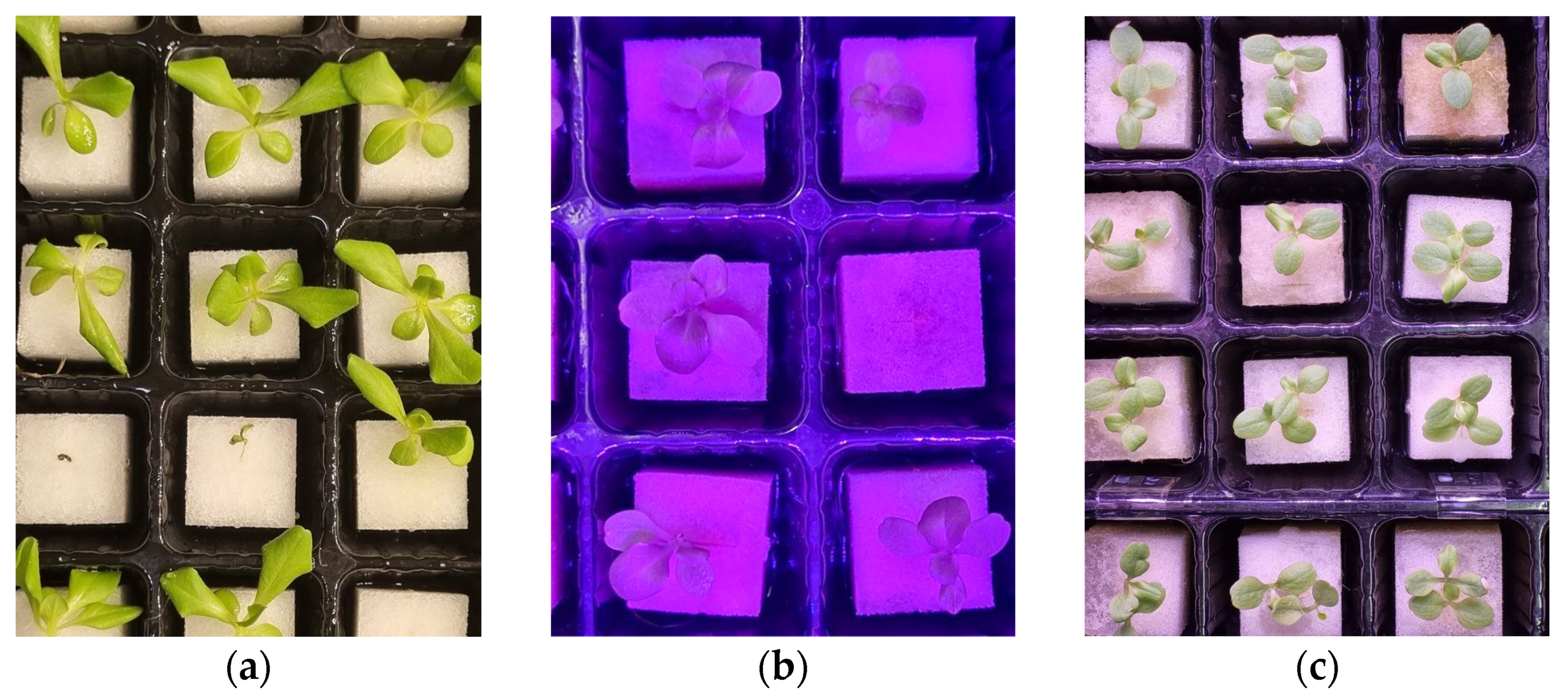
3.2. Acquisition of Lettuce Seedling Images
3.3. Dataset Preparations
3.3.1. Data Augmentation
3.3.2. Data Annotation
3.4. Deep Learning Detection System
3.4.1. CenterNet
3.4.2. YOLOv5 and YOLOv7
3.4.3. Faster R-CNN
3.5. Model Performance Evaluation
3.6. Training Environment Details
4. Results and Analysis
4.1. Model Training and Testing
4.1.1. Model Training Results
4.1.2. Model Testing Results
4.2. Detection Results under Different Lighting Conditions
5. Discussion
5.1. Analysis and Comparison of Different Target Detection Algorithms
5.2. Analysis of the Influence of Different Indoor Lighting Conditions on the Detection Results
5.3. Study Limitations and Recommendations for Improvement
6. Conclusions
- Defective seedlings under a different range of indoor lighting conditions were detected using deep learning algorithms;
- The detection accuracies of CenterNet, YOLOv5, YOLOv7, and faster RCNN was 82.8%, 96.5%, 97.2%, and 88.6%, respectively;
- In terms of detection under white lights, YOLOv7 had the highest accuracy of 95.8%. YOLOv5 had the highest accuracy (99.0%) under red/blue lights. YOLOv7 also achieved maximum accuracy of 98.8% under red/blue/white lights;
- The YOLOv7 model had the best overall results, with an mAP of 97.2%, and has the potential to be employed for automated sorting systems.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Artificial intelligence |
| CNN | Convolutional neural network |
| DL | Deep learning |
| SOTA | State-of-the-art |
| SSD | Single-shot multibox detector |
| YOLO | You only look once |
| NMS | Non-maximum suppression |
| RPN | Region proposal network |
| RoI | Region of interest |
| TP | True positive |
| TN | True negative |
| FP | False positive |
| FN | False negative |
| MOT | Multiple object tracking |
| MHT | Multiple hypothesis tracking |
| KCF | Kernelized correlation filter |
| SORT | Simple online and real-time tracking |
References
- Kozai, T.; Niu, G.; Takagaki, M. Plant Factory: An Indoor Vertical Farming System for Efficient Quality Food Production; Academic Press: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Ahmed, H.A.; Yu-Xin, T.; Qi-Chang, Y. Optimal control of environmental conditions affecting lettuce plant growth in a controlled environment with artificial lighting: A review. S. Afr. J. Bot. 2019, 130, 75–89. [Google Scholar] [CrossRef]
- Kozai, T.; Kubota, C.; Chun, C.; Afreen, F.; Ohyama, K. Necessity and Concept of the Closed Transplant Production System. In Transplant Production in the 21st Century; Springer: Dordrecht, The Netherlands, 2000; pp. 3–19. [Google Scholar] [CrossRef]
- Nagano, S.; Moriyuki, S.; Wakamori, K.; Mineno, H.; Fukuda, H. Leaf-Movement-Based Growth Prediction Model Using Optical Flow Analysis and Machine Learning in Plant Factory. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, Y.; Yang, Y.; Guo, R.; Yang, J.; Yue, J.; Wang, Y. A high-precision detection method of hydroponic lettuce seedlings status based on improved Faster RCNN. Comput. Electron. Agric. 2021, 182, 106054. [Google Scholar] [CrossRef]
- Vatistas, C.; Avgoustaki, D.D.; Bartzanas, T. A Systematic Literature Review on Controlled-Environment Agriculture: How Vertical Farms and Greenhouses Can Influence the Sustainability and Footprint of Urban Microclimate with Local Food Production. Atmosphere 2022, 13, 1258. [Google Scholar] [CrossRef]
- Syed, T.N.; Jizhan, L.; Xin, Z.; Shengyi, Z.; Yan, Y.; Mohamed, S.H.A.; Lakhiar, I.A. Seedling-lump integrated non-destructive monitoring for automatic transplanting with Intel RealSense depth camera. Artif. Intell. Agric. 2019, 3, 18–32. [Google Scholar] [CrossRef]
- Ouzounis, T.; Rosenqvist, E.; Ottosen, C.-O. Spectral Effects of Artificial Light on Plant Physiology and Secondary Metabolism: A Review. Hortscience 2015, 50, 1128–1135. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.K.L.; Cho, K.M.; Lee, H.Y.; Cho, D.Y.; Lee, G.O.; Jang, S.N.; Lee, Y.; Kim, D.; Son, K.-H. Effects of White LED Lighting with Specific Shorter Blue and/or Green Wavelength on the Growth and Quality of Two Lettuce Cultivars in a Vertical Farming System. Agronomy 2021, 11, 2111. [Google Scholar] [CrossRef]
- Rabara, R.C.; Behrman, G.; Timbol, T.; Rushton, P.J. Effect of Spectral Quality of Monochromatic LED Lights on the Growth of Artichoke Seedlings. Front. Plant Sci. 2017, 8, 190. [Google Scholar] [CrossRef] [Green Version]
- Pennisi, G.; Orsini, F.; Blasioli, S.; Cellini, A.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Stanghellini, C.; et al. Resource use efficiency of indoor lettuce (Lactuca sativa L.) cultivation as affected by red:blue ratio provided by LED lighting. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Sabzalian, M.R.; Heydarizadeh, P.; Zahedi, M.; Boroomand, A.; Agharokh, M.; Sahba, M.R.; Schoefs, B. High performance of vegetables, flowers, and medicinal plants in a red-blue LED incubator for indoor plant production. Agron. Sustain. Dev. 2014, 34, 879–886. [Google Scholar] [CrossRef] [Green Version]
- Boyce, P.R. Review: The Impact of Light in Buildings on Human Health. Indoor Built Environ. 2010, 19, 8–20. [Google Scholar] [CrossRef]
- Tong, J.H.; Li, J.B.; Jiang, H.Y. Machine vision techniques for the evaluation of seedling quality based on leaf area. Biosyst. Eng. 2013, 115, 369–379. [Google Scholar] [CrossRef]
- Jin, X.; Yuan, Y.; Ji, J.; Zhao, K.; Li, M.; Chen, K. Design and implementation of anti-leakage planting system for transplanting machine based on fuzzy information. Comput. Electron. Agric. 2020, 169, 105204. [Google Scholar] [CrossRef]
- Hughes, D.; Salathé, M. An Open Access Repository of Images on Plant Health to Enable the Development of Mobile Disease Diagnostics. arXiv 2015, arXiv:1511.08060. [Google Scholar]
- Hassan, A.; Islam, S.; Hasan, M.; Shorif, S.B.; Habib, T.; Uddin, M.S. Medicinal Plant Recognition from Leaf Images Using Deep Learning; Springer: Singapore, 2022; pp. 137–154. [Google Scholar] [CrossRef]
- Parico, A.I.B.; Ahamed, T. Real Time Pear Fruit Detection and Counting Using YOLOv4 Models and Deep SORT. Sensors 2021, 21, 4803. [Google Scholar] [CrossRef]
- Lu, S.; Song, Z.; Chen, W.; Qian, T.; Zhang, Y.; Chen, M.; Li, G. Counting Dense Leaves under Natural Environments via an Improved Deep-Learning-Based Object Detection Algorithm. Agriculture 2021, 11, 1003. [Google Scholar] [CrossRef]
- Abeyrathna, R.M.R.D.; Nakaguchi, V.M.; Minn, A.; Ahamed, T. Recognition and Counting of Apples in a Dynamic State Using a 3D Camera and Deep Learning Algorithms for Robotic Harvesting Systems. Sensors 2023, 23, 3810. [Google Scholar] [CrossRef]
- Jin, X.; Che, J.; Chen, Y. Weed Identification Using Deep Learning and Image Processing in Vegetable Plantation. IEEE Access 2021, 9, 10940–10950. [Google Scholar] [CrossRef]
- Jin, X.; Sun, Y.; Che, J.; Bagavathiannan, M.; Yu, J.; Chen, Y. A novel deep learning-based method for detection of weeds in vegetables. Pest Manag. Sci. 2022, 78, 1861–1869. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J. Tomato Anomalies Detection in Greenhouse Scenarios Based on YOLO-Dense. Front. Plant Sci. 2021, 12. [Google Scholar] [CrossRef]
- Rashid, J.; Khan, I.; Ali, G.; Almotiri, S.H.; AlGhamdi, M.A.; Masood, K. Multi-Level Deep Learning Model for Potato Leaf Disease Recognition. Electronics 2021, 10, 2064. [Google Scholar] [CrossRef]
- Li, Y.; Sun, S.; Zhang, C.; Yang, G.; Ye, Q. One-Stage Disease Detection Method for Maize Leaf Based on Multi-Scale Feature Fusion. Appl. Sci. 2022, 12, 7960. [Google Scholar] [CrossRef]
- Hamidon, M.H.; Ahamed, T. Detection of Tip-Burn Stress on Lettuce Grown in an Indoor Environment Using Deep Learning Algorithms. Sensors 2022, 22, 7251. [Google Scholar] [CrossRef]
- Zhang, P.; Li, D. EPSA-YOLO-V5s: A novel method for detecting the survival rate of rapeseed in a plant factory based on multiple guarantee mechanisms. Comput. Electron. Agric. 2022, 193, 106714. [Google Scholar] [CrossRef]
- Samiei, S.; Rasti, P.; Vu, J.L.; Buitink, J.; Rousseau, D. Deep learning-based detection of seedling development. Plant Methods 2020, 16, 1–11. [Google Scholar] [CrossRef]
- Jiao, L.C.; Zhang, F.; Liu, F.; Yang, S.Y.; Li, L.L.; Feng, Z.X.; Qu, R. A Survey of Deep Learning-Based Object Detection. IEEE Access 2019, 7, 128837–128868. [Google Scholar] [CrossRef]
- Luvizon, D.C.; Tabia, H.; Picard, D. SSP-Net: Scalable sequential pyramid networks for real-Time 3D human pose regression. Pattern Recognit. 2023, 142. [Google Scholar] [CrossRef]
- Ren, S.; He, K.; Girshick, R.; Sun, J. Faster R-CNN: Towards real-time object detection with region proposal networks. IEEE Trans. Pattern Anal. Mach. Intell. 2017, 39, 1137–1149. [Google Scholar] [CrossRef] [Green Version]
- He, K.; Gkioxari, G.; Dollár, P.; Girshick, R. Mask R-CNN. In Proceedings of the 2017 IEEE International Conference on Computer Vision (ICCV), Venice, Italy, 22–29 October 2017; pp. 2961–2969. [Google Scholar] [CrossRef]
- Duan, K.; Bai, S.; Xie, L.; Qi, H.; Huang, Q.; Tian, Q. Centernet: Keypoint triplets for object detection. In Proceedings of the IEEE/CVF International Conference on Computer Vision 2019, Seoul, Republic of Korea, 27 October–2 November 2019. [Google Scholar]
- Liu, W.; Anguelov, D.; Erhan, D.; Szegedy, C.; Reed, S.; Fu, C.Y.; Berg, A.C. SSD: Single shot multibox detector. In Proceedings of the European Conference on Computer Vision, Amsterdam, The Netherlands, 11–14 October 2016. [Google Scholar] [CrossRef] [Green Version]
- Redmon, J.; Divvala, S.; Girshick, R.; Farhadi, A. You Only Look Once: Unified, Real-Time Object Detection. In Proceedings of the 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Las Vegas, NV, USA, 27–30 June 2016; pp. 779–788. [Google Scholar] [CrossRef] [Green Version]
- Quan, L.; Feng, H.; Lv, Y.; Wang, Q.; Zhang, C.; Liu, J.; Yuan, Z. Maize seedling detection under different growth stages and complex field environments based on an improved Faster R-CNN. Biosyst. Eng. 2019, 184, 1–23. [Google Scholar] [CrossRef]
- Oh, S.; Chang, A.; Ashapure, A.; Jung, J.; Dube, N.; Maeda, M.; Gonzalez, D.; Landivar, J. Plant Counting of Cotton from UAS Imagery Using Deep Learning-Based Object Detection Framework. Remote Sens. 2020, 12, 2981. [Google Scholar] [CrossRef]
- Fang, L.; Wu, Y.; Li, Y.; Guo, H.; Zhang, H.; Wang, X.; Xi, R.; Hou, J. Ginger Seeding Detection and Shoot Orientation Discrimination Using an Improved YOLOv4-LITE Network. Agronomy 2021, 11, 2328. [Google Scholar] [CrossRef]
- Liu, S.; Jin, Y.; Ruan, Z.; Ma, Z.; Gao, R.; Su, Z. Real-Time Detection of Seedling Maize Weeds in Sustainable Agriculture. Sustainability 2022, 14, 15088. [Google Scholar] [CrossRef]
- Tan, C.; Li, C.; He, D.; Song, H. Towards real-time tracking and counting of seedlings with a one-stage detector and optical flow. Comput. Electron. Agric. 2022, 193, 106683. [Google Scholar] [CrossRef]
- Fraiwan, M.; Faouri, E.; Khasawneh, N. Classification of Corn Diseases from Leaf Images Using Deep Transfer Learning. Plants 2022, 11, 2668. [Google Scholar] [CrossRef]
- Muneer, S.; Kim, E.J.; Park, J.S.; Lee, J.H. Influence of Green, Red and Blue Light Emitting Diodes on Multiprotein Complex Proteins and Photosynthetic Activity under Different Light Intensities in Lettuce Leaves (Lactuca sativa L.). Int. J. Mol. Sci. 2014, 15, 4657–4670. [Google Scholar] [CrossRef] [Green Version]
- Matysiak, B.; Kaniszewski, S.; Dyśko, J.; Kowalczyk, W.; Kowalski, A.; Grzegorzewska, M. The Impact of LED Light Spectrum on the Growth, Morphological Traits, and Nutritional Status of ‘Elizium’ Romaine Lettuce Grown in an Indoor Controlled Environment. Agriculture 2021, 11, 1133. [Google Scholar] [CrossRef]
- Susan, S.; Kumar, A. The balancing trick: Optimized sampling of imbalanced datasets—A brief survey of the recent State of the Art. Eng. Rep. 2020, 3, e12298. [Google Scholar] [CrossRef]
- Luo, W.; Xing, J.; Milan, A.; Zhang, X.; Liu, W.; Zhao, X.; Kim, T.-K. Multiple Object Tracking: A Literature Review. arXiv 2014, arXiv:1409.7618. [Google Scholar]
- Kim, C.; Li, F.; Ciptadi, A.; Rehg, J.M. Multiple hypothesis tracking revisited. In Proceedings of the IEEE International Conference on Computer Vision (ICCV), Santiago, Chile, 7–13 December 2015. [Google Scholar]
- Henriques, J.F.; Caseiro, R.; Martins, P.; Batista, J. High-Speed Tracking with Kernelized Correlation Filters. IEEE Trans. Pattern Anal. Mach. Intell. 2014, 37, 583–596. [Google Scholar] [CrossRef] [Green Version]
- Wojke, N.; Bewley, A.; Paulus, D. Simple online and realtime tracking with a deep association metric. In Proceedings of the 2017 IEEE International Conference on Image Processing (ICIP), Beijing, China, 17–20 September 2017; pp. 3645–3649. [Google Scholar]



















| Model | Input Size | Epoch | Batch | |
|---|---|---|---|---|
| CenterNet | ctdet_pascal_dla_512 | 512 × 512 | 50 | - |
| YOLOv5 | CSP-Darknet53 | 512 × 512 | 50 | - |
| YOLOv7 | Extended-ELAN | 512 × 512 | 50 | - |
| Faster R-CNN | VGG-16 | 512 × 512 | - | 10,000 |
| Model | Precision | Recall | mAP @0.5 |
|---|---|---|---|
| CenterNet | - | 0.850 | 0.812 |
| YOLOv5 | 0.936 | 0.990 | 0.973 |
| YOLOv7 | 0.937 | 0.989 | 0.966 |
| Faster R-CNN | 0.853 | 1.000 | 0.982 |
| Model | Precision | Recall | mAP @0.5 | Model Size |
|---|---|---|---|---|
| CenterNet | - | 0.880 | 0.828 | 75.1 MB |
| YOLOv5 | 0.936 | 0.980 | 0.965 | 10.2 MB |
| YOLOv7 | 0.939 | 0.965 | 0.972 | 11.7 MB |
| Faster R-CNN | 0.850 | 0.996 | 0.886 | 1.2 GB |
| Model | White | Red/Blue | Red/Blue/White |
|---|---|---|---|
| CenterNet | 0.817 | 0.826 | 0.883 |
| YOLOv5 | 0.949 | 0.990 | 0.980 |
| YOLOv7 | 0.958 | 0.985 | 0.988 |
| Faster R-CNN | 0.866 | 0.981 | 0.912 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamidon, M.H.; Ahamed, T. Detection of Defective Lettuce Seedlings Grown in an Indoor Environment under Different Lighting Conditions Using Deep Learning Algorithms. Sensors 2023, 23, 5790. https://doi.org/10.3390/s23135790
Hamidon MH, Ahamed T. Detection of Defective Lettuce Seedlings Grown in an Indoor Environment under Different Lighting Conditions Using Deep Learning Algorithms. Sensors. 2023; 23(13):5790. https://doi.org/10.3390/s23135790
Chicago/Turabian StyleHamidon, Munirah Hayati, and Tofael Ahamed. 2023. "Detection of Defective Lettuce Seedlings Grown in an Indoor Environment under Different Lighting Conditions Using Deep Learning Algorithms" Sensors 23, no. 13: 5790. https://doi.org/10.3390/s23135790






