State-of-the-Art on Brain-Computer Interface Technology
Abstract
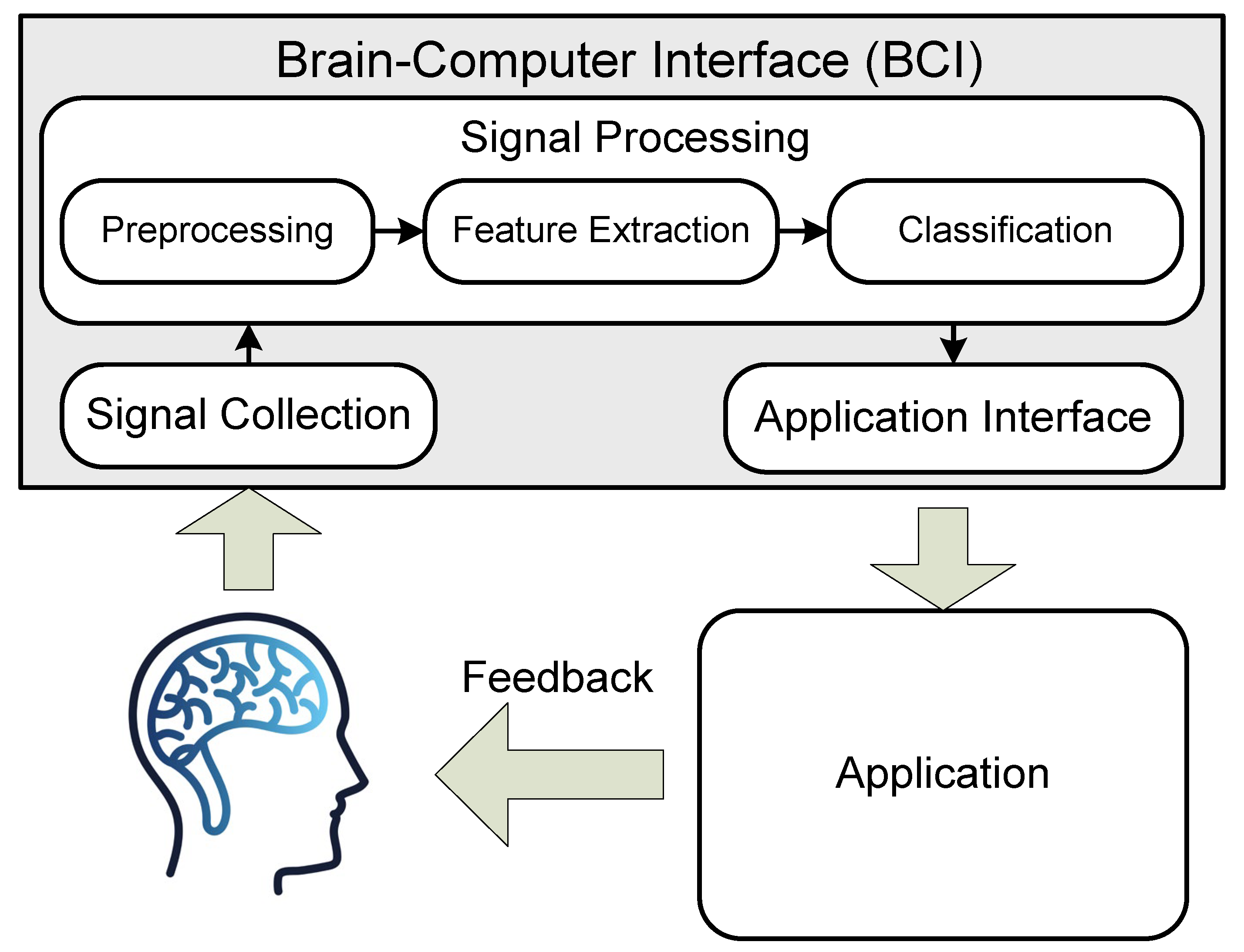
:1. Introduction
2. Platforms
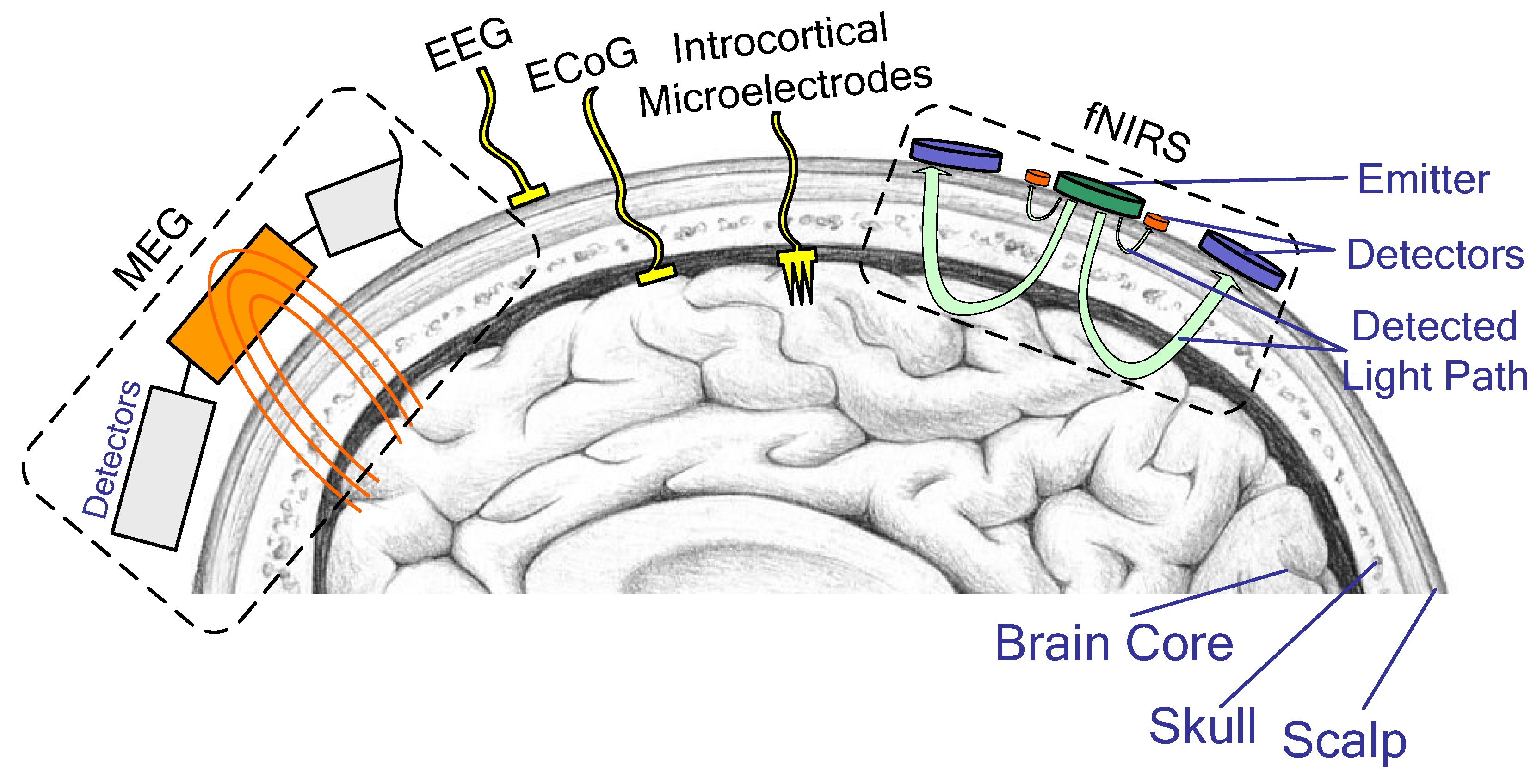
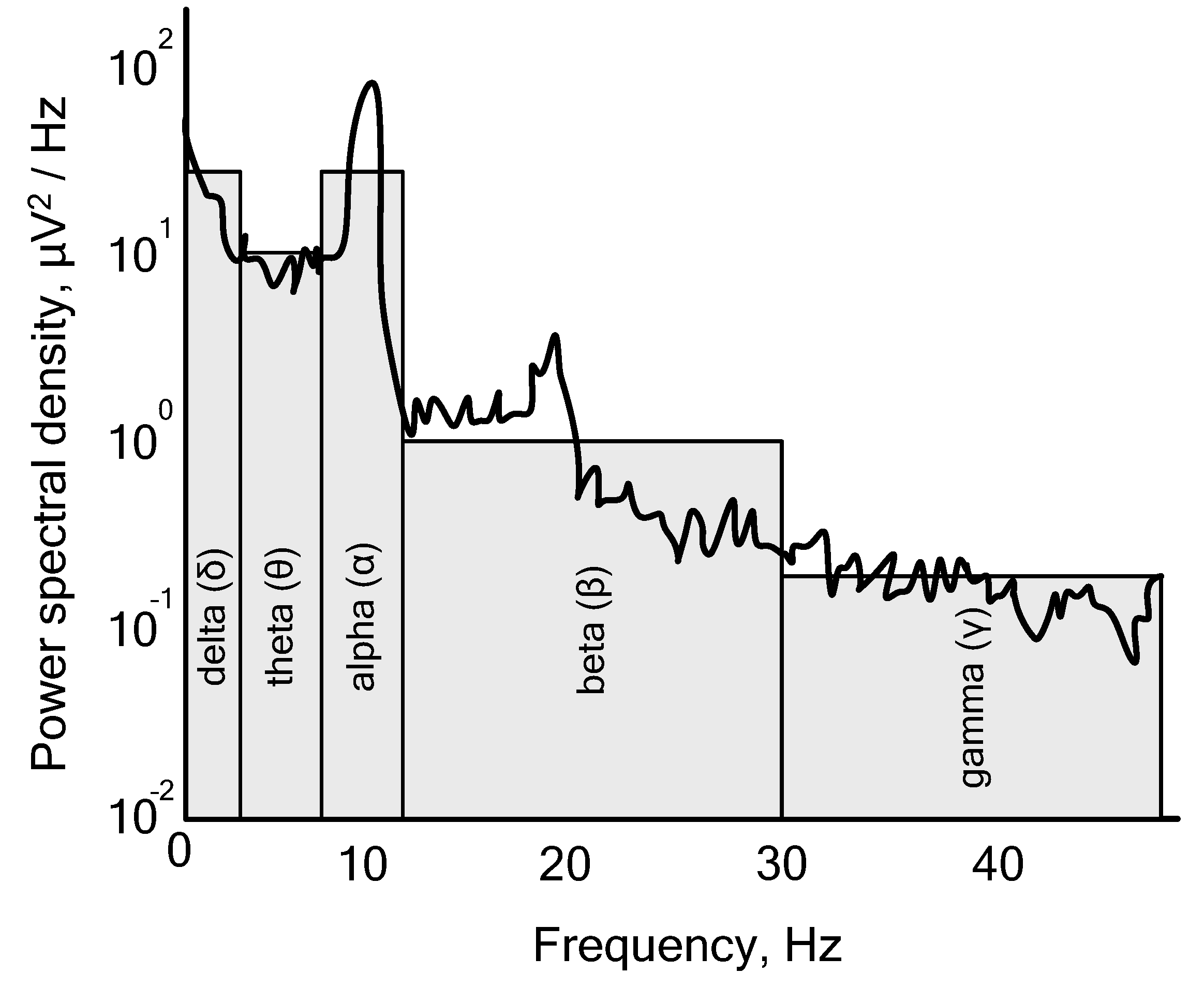
2.1. EEG Platform
2.2. Other Platforms
2.3. BCI Platforms Comparison
- -
- Signal quality: EEG signals are highly sensitive to noise and artifacts, so it is important to ensure that the signal quality is optimal for BCI applications;
- -
- Feature extraction: the ability to accurately extract meaningful information from raw EEG data is a key issue in BCI research as this determines how effective the system will be at recognizing user intentions and commands;
- -
- Classification accuracy: designing efficient algorithms for classifying EEG signals into different categories (e.g., left vs. right hand movement) is an important issue in BCI research as it determines how well the system can recognize user commands or intentions.
- -
- User interface design: designing user interfaces that are intuitive and easy to use is an important issue in EEG-based BCIs as it can determine how easily users can interact with the system;
- -
- Adaptability: developing algorithms that can adapt to individual users’ brain activity and recognize subtle changes in EEG patterns is an important research topic for creating robust BCI systems;
- -
- System reliability: ensuring reliable performance of a BCI system over long periods of time with minimal calibration or setup requirements is an important challenge in EEG-based BCIs due to the dynamic nature of brain activity and its variability across users and sessions.
- -
- Signal quality: fNIRS signals are relatively weak and affected by noise, making it difficult to accurately detect changes in brain activity;
- -
- Spatial resolution: the spatial resolution of fNIRS is limited due to the limited number of sources and detectors, which may lead to incorrect interpretations of the data;
- -
- Temporal resolution: fNIRS has a relatively slow response time compared with other BCI modalities such as EEG or MEG, meaning that more complex cognitive tasks may not be suitable for this technology;
- -
- Cost: while fNIRS systems are becoming increasingly affordable, they remain significantly more expensive than EEG or MEG systems and require specialized training in their use and interpretation of results;
- -
- Safety: fNIRS systems operate by sending light into the head, which could potentially lead to eye damage if not used correctly.
- -
- Good signal-to-noise ratio: MEG signals are relatively strong and easy to detect reliably, making them beneficial for BCI applications;
- -
- High cost of equipment: the cost of equipment necessary for MEG is high, limiting its practicality in many settings;
- -
- Limited spatial resolution: the spatial resolution of MEG is limited compared to other imaging technologies such as EEG, making it difficult to accurately map brain activity patterns with a single scan;
- -
- Long acquisition times: the data acquisition times for MEG can be quite long, making it difficult to measure dynamic processes such as those involved in motor control tasks used in BCI systems;
- -
- Head motion artifacts: head motion artifacts can significantly interfere with the accuracy of the recorded signal and lead to false positives or negatives, which could confuse the user’s experience with the system or even cause harm if medical decisions were made based on incorrect information from an artifactually contaminated signal.
- -
- Signal acquisition: ECoG signals have relatively low amplitudes and might contain a certain degree of noise; therefore, reliable signal acquisition is essential for successful BCI applications;
- -
- Data interpretation: properly interpreting the data collected from ECoG recordings can be challenging due to the complexity of neural activity as well as the need to distinguish between different types of brain activity (e.g., motor vs. non-motor);
- -
- Safety concerns: since ECoG involves implanting electrodes directly onto the surface of the brain, there are potential safety risks that must be taken into consideration when designing an ECoG-based BCI system;
- -
- Ethical considerations: the ethical implications associated with using invasive technology such as ECoG must also be considered when developing a BCI system for clinical use or research purposes.
3. Classical Paradigms in BCI Systems
4. BCI Signal Processing Techniques
4.1. ICA Use in BCI Systems
- -
- The number of independent components must not exceed the number of electrodes used in recording EEG signals;
- -
- Neuronal and artifact sources are considered to be linearly mixed yet independent from each other;
- -
- A negligible signal propagation delay is assumed between brain sources and electrodes.
4.2. Wavelet Transformations and Autoregressive Modeling in BCI
4.3. SVM in BCI Systems
4.4. HMM’s for BCI
- The evaluation problem can be stated as follows: Given an HMM with transition probabilities aij and bjk, determine the probability that a particular sequence of visible states (VT) was generated by this model.
- Decoding problem. Given an HMM and a set of observations (VT), we need to determine the most probable sequence of hidden states ωT that result in these observations.
- The learning problem. Given an enlarged structure of the model with a specified number of states and visible states but without knowledge of transition probabilities aij and bjk, learning can be performed by determining the most plausible model from a training sample of visible states.
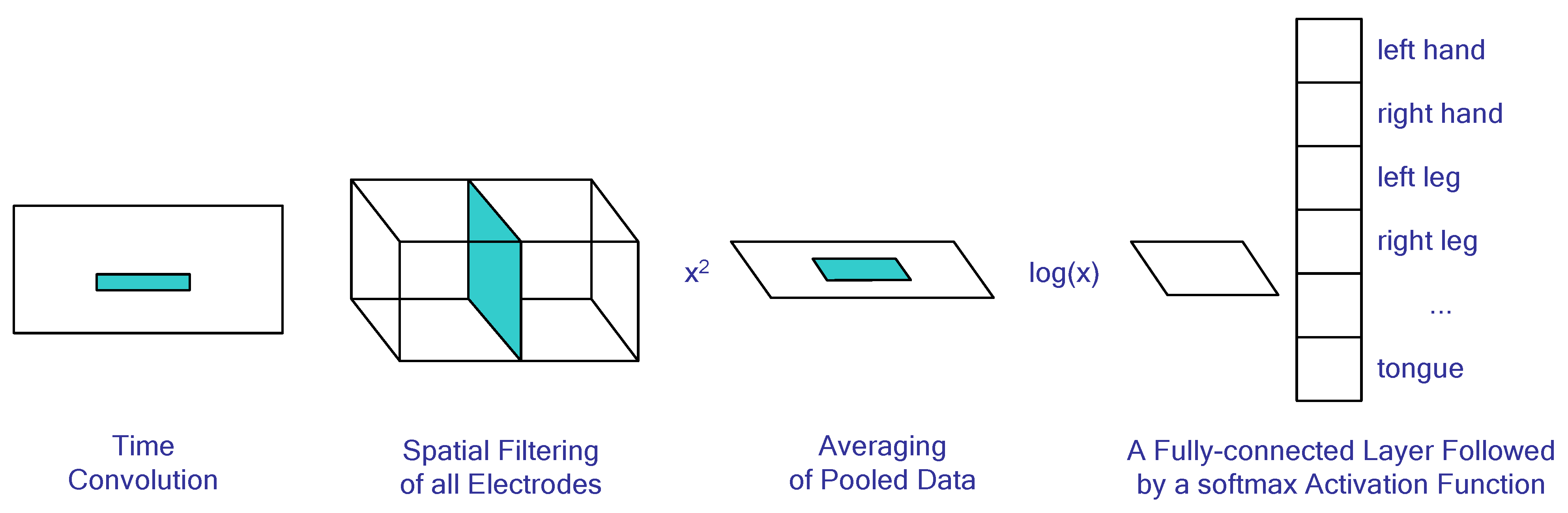
4.5. Neural Network Algorithms for BCI Systems
4.6. Genetic Algorithms and Particle Swarm Optimization in BCI
- -
- PSO is simpler than other optimizers since it does not require costly derivatives or linear algebra operations;
- -
- PSO can be used with any type of problem formulation, such as discrete, continuous, constrained, or unconstrained optimization problems;
- -
- PSO can find solutions faster compared to traditional algorithms because it uses parallel computing techniques that allow multiple particles to explore the search space simultaneously and cooperatively;
- -
- The algorithm is easy to implement due to its simple structure and few parameters to adjust during its execution process.
- -
- It does not require an initial guess from the user and thus can be useful in cases where one may not know what kind of solution they are looking for.
- -
- The results obtained by using this method depend on the choice of parameters such as inertia weight, cognition factor, social factor, etc., so if these values are set too high or too low, then the result will also suffer accordingly.
- -
- It may take more time than other methods since many iterations need to be done until a good solution is found;
- -
- Some features may remain unexplored due to a lack of exploration strategies implemented in some versions of PSO algorithms, resulting in sub-optimal solutions being returned instead of optimal ones.
4.7. BCI Datasets and Benchmarking
- -
- BCI Competition IV Dataset 2a: This dataset consists of EEG and EOG recordings from nine subjects performing motor imagery tasks such as left/right hand or foot movement, imagining a circle or a line, and other more complex movements;
- -
- The BCI Competition IV Dataset 2b: This dataset consists of EEG recordings from nine subjects performing motor imagery tasks while a visual cue was presented at different time points during the task;
- -
- The BCI Competition IV Dataset 3: This dataset consists of MEG recordings from two subjects performing motor imagery tasks such as wrist movement in different directions;
- -
- The BCI Competition IV Dataset 4: This dataset contains ECoG recordings from three subjects performing motor imagery tasks such as finger movement acquired with a data glove;
- -
- The OpenMIIR Dataset [112]: This dataset includes EEG recordings from 20 healthy volunteers who were asked to imagine either moving their hands, feet, tongue, or eyes in order to control a virtual avatar on screen by using their thoughts alone;
- -
4.8. Noise and Environmental Disturbances Impact on BCI Systems
5. Applications
5.1. Neuroprosthetics
5.2. Communication
5.3. Gaming
5.4. Education
5.5. Mental Health
5.6. Sleep Medicine
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mridha, M.F.; Das, S.C.; Kabir, M.M.; Lima, A.A.; Islam, M.R.; Watanobe, Y. Brain-Computer Interface: Advancement and Challenges. Sensors 2021, 21, 5746. [Google Scholar] [CrossRef]
- Mora-Cortes, A.; Manyakov, N.V.; Chumerin, N.; Van Hulle, M.M. Language Model Applications to Spelling with Brain-Computer Interfaces. Sensors 2014, 14, 5967–5993. [Google Scholar] [CrossRef] [Green Version]
- Belwafi, K.; Gannouni, S.; Aboalsamh, H. Embedded Brain Computer Interface: State-of-the-Art in Research. Sensors 2021, 21, 4293. [Google Scholar] [CrossRef] [PubMed]
- Värbu, K.; Muhammad, N.; Muhammad, Y. Past, Present, and Future of EEG-Based BCI Applications. Sensors 2022, 22, 3331. [Google Scholar] [CrossRef] [PubMed]
- Siribunyaphat, N.; Punsawad, Y. Brain–Computer Interface Based on Steady-State Visual Evoked Potential Using Quick-Response Code Pattern for Wheelchair Control. Sensors 2023, 23, 2069. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Oniga, S. Classification of Motor Imagery EEG Signals Based on Data Augmentation and Convolutional Neural Networks. Sensors 2023, 23, 1932. [Google Scholar] [CrossRef] [PubMed]
- Tsiamalou, A.; Dardiotis, E.; Paterakis, K.; Fotakopoulos, G.; Liampas, I.; Sgantzos, M.; Siokas, V.; Brotis, A.G. EEG in Neurorehabilitation: A Bibliometric Analysis and Content Review. Neurol. Int. 2022, 14, 1046–1061. [Google Scholar] [CrossRef]
- Saichoo, T.; Boonbrahm, P.; Punsawad, Y. Investigating User Proficiency of Motor Imagery for EEG-Based BCI System to Control Simulated Wheelchair. Sensors 2022, 22, 9788. [Google Scholar] [CrossRef]
- Abdullah; Faye, I.; Islam, M.R. EEG Channel Selection Techniques in Motor Imagery Applications: A Review and New Perspectives. Bioengineering 2022, 9, 726. [Google Scholar] [CrossRef]
- Gannouni, S.; Belwafi, K.; Al-Sulmi, M.R.; Al-Farhood, M.D.; Al-Obaid, O.A.; Al-Awadh, A.M.; Aboalsamh, H.; Belghith, A. A Brain Controlled Command-Line Interface to Enhance the Accessibility of Severe Motor Disabled People to Personnel Computer. Brain Sci. 2022, 12, 926. [Google Scholar] [CrossRef]
- Asanza, V.; Peláez, E.; Loayza, F.; Lorente-Leyva, L.L.; Peluffo-Ordóñez, D.H. Identification of Lower-Limb Motor Tasks via Brain–Computer Interfaces: A Topical Overview. Sensors 2022, 22, 2028. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Mishra, S.; Gupta, S.; Padmanabhan, P.; Jia, L.; Colin, T.K.A.; Tsai, Y.T.; Kejia, T.; Sankarapillai, P.; Mohan, A.; et al. Functional Mapping of the Brain for Brain–Computer Interfacing: A Review. Electronics 2023, 12, 604. [Google Scholar] [CrossRef]
- He, Z.; Li, Z.; Yang, F.; Wang, L.; Li, J.; Zhou, C.; Pan, J. Advances in Multimodal Emotion Recognition Based on Brain–Computer Interfaces. Brain Sci. 2020, 10, 687. [Google Scholar] [CrossRef] [PubMed]
- Orban, M.; Elsamanty, M.; Guo, K.; Zhang, S.; Yang, H. A Review of Brain Activity and EEG-Based Brain–Computer Interfaces for Rehabilitation Application. Bioengineering 2022, 9, 768. [Google Scholar] [CrossRef]
- Park, J.; Park, J.; Shin, D.; Choi, Y. A BCI Based Alerting System for Attention Recovery of UAV Operators. Sensors 2021, 21, 2447. [Google Scholar] [CrossRef]
- Yang, L.; Van Hulle, M.M. Real-Time Navigation in Google Street View® Using a Motor Imagery-Based BCI. Sensors 2023, 23, 1704. [Google Scholar] [CrossRef]
- Amprimo, G.; Rechichi, I.; Ferraris, C.; Olmo, G. Measuring Brain Activation Patterns from Raw Single-Channel EEG during Exergaming: A Pilot Study. Electronics 2023, 12, 623. [Google Scholar] [CrossRef]
- Glavas, K.; Prapas, G.; Tzimourta, K.D.; Giannakeas, N.; Tsipouras, M.G. Evaluation of the User Adaptation in a BCI Game Environment. Appl. Sci. 2022, 12, 12722. [Google Scholar] [CrossRef]
- Chang, D.; Xiang, Y.; Zhao, J.; Qian, Y.; Li, F. Exploration of Brain-Computer Interaction for Supporting Children’s Attention Training: A Multimodal Design Based on Attention Network and Gamification Design. Int. J. Environ. Res. Public Health 2022, 19, 15046. [Google Scholar] [CrossRef]
- Knierim, M.T.; Bleichner, M.G.; Reali, P. A Systematic Comparison of High-End and Low-Cost EEG Amplifiers for Concealed, Around-the-Ear EEG Recordings. Sensors 2023, 23, 4559. [Google Scholar] [CrossRef]
- Ferracuti, F.; Iarlori, S.; Mansour, Z.; Monteriù, A.; Porcaro, C. Comparing between Different Sets of Preprocessing, Classifiers, and Channels Selection Techniques to Optimise Motor Imagery Pattern Classification System from EEG Pattern Recognition. Brain Sci. 2022, 12, 57. [Google Scholar] [CrossRef] [PubMed]
- Baradaran, F.; Farzan, A.; Danishvar, S.; Sheykhivand, S. Customized 2D CNN Model for the Automatic Emotion Recognition Based on EEG Signals. Electronics 2023, 12, 2232. [Google Scholar] [CrossRef]
- Wang, Y.; Song, C.; Zhang, T.; Yao, Z.; Chang, Z.; Wang, D. Feature Extraction of Motor Imagery EEG via Discrete Wavelet Transform and Generalized Maximum Fuzzy Membership Difference Entropy: A Comparative Study. Electronics 2023, 12, 2207. [Google Scholar] [CrossRef]
- Ortega-Rodríguez, J.; Gómez-González, J.F.; Pereda, E. Selection of the Minimum Number of EEG Sensors to Guarantee Biometric Identification of Individuals. Sensors 2023, 23, 4239. [Google Scholar] [CrossRef]
- Cardona-Álvarez, Y.N.; Álvarez-Meza, A.M.; Cárdenas-Peña, D.A.; Castaño-Duque, G.A.; Castellanos-Dominguez, G. A Novel OpenBCI Framework for EEG-Based Neurophysiological Experiments. Sensors 2023, 23, 3763. [Google Scholar] [CrossRef]
- Saibene, A.; Caglioni, M.; Corchs, S.; Gasparini, F. EEG-Based BCIs on Motor Imagery Paradigm Using Wearable Technologies: A Systematic Review. Sensors 2023, 23, 2798. [Google Scholar] [CrossRef]
- Ali, M.U.; Kim, K.S.; Kallu, K.D.; Zafar, A.; Lee, S.W. OptEF-BCI: An Optimization-Based Hybrid EEG and fNIRS–Brain Computer Interface. Bioengineering 2023, 10, 608. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.; Hussain, S.J.; Ali, M.U.; Lee, S.W. Metaheuristic Optimization-Based Feature Selection for Imagery and Arithmetic Tasks: An fNIRS Study. Sensors 2023, 23, 3714. [Google Scholar] [CrossRef]
- Erdoğan, S.B.; Yükselen, G. Four-Class Classification of Neuropsychiatric Disorders by Use of Functional Near-Infrared Spectroscopy Derived Biomarkers. Sensors 2022, 22, 5407. [Google Scholar] [CrossRef]
- Varandas, R.; Lima, R.; Bermúdez I Badia, S.; Silva, H.; Gamboa, H. Automatic Cognitive Fatigue Detection Using Wearable fNIRS and Machine Learning. Sensors 2022, 22, 4010. [Google Scholar] [CrossRef]
- Zapała, D.; Augustynowicz, P.; Tokovarov, M. Recognition of Attentional States in VR Environment: An fNIRS Study. Sensors 2022, 22, 3133. [Google Scholar] [CrossRef] [PubMed]
- Gulraiz, A.; Naseer, N.; Nazeer, H.; Khan, M.J.; Khan, R.A.; Shahbaz Khan, U. LASSO Homotopy-Based Sparse Representation Classification for fNIRS-BCI. Sensors 2022, 22, 2575. [Google Scholar] [CrossRef] [PubMed]
- Hamid, H.; Naseer, N.; Nazeer, H.; Khan, M.J.; Khan, R.A.; Shahbaz Khan, U. Analyzing Classification Performance of fNIRS-BCI for Gait Rehabilitation Using Deep Neural Networks. Sensors 2022, 22, 1932. [Google Scholar] [CrossRef]
- McClay, W. A Magnetoencephalographic/Encephalographic (MEG/EEG) Brain-Computer Interface Driver for Interactive iOS Mobile Videogame Applications Utilizing the Hadoop Ecosystem, MongoDB, and Cassandra NoSQL Databases. Diseases 2018, 6, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reichert, C.; Dürschmid, S.; Kruse, R.; Hinrichs, H. An Efficient Decoder for the Recognition of Event-Related Potentials in High-Density MEG Recordings. Computers 2016, 5, 5. [Google Scholar] [CrossRef] [Green Version]
- Dash, D.; Ferrari, P.; Dutta, S.; Wang, J. NeuroVAD: Real-Time Voice Activity Detection from Non-Invasive Neuromagnetic Signals. Sensors 2020, 20, 2248. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.; Rong, F.; Miao, Y.; Sun, Y.; Dong, G.; Li, H.; Li, J.; Wang, Y.; Leng, J. Representation Learning for Motor Imagery Recognition with Deep Neural Network. Electronics 2021, 10, 112. [Google Scholar] [CrossRef]
- Shokoueinejad, M.; Park, D.-W.; Jung, Y.H.; Brodnick, S.K.; Novello, J.; Dingle, A.; Swanson, K.I.; Baek, D.-H.; Suminski, A.J.; Lake, W.B.; et al. Progress in the Field of Micro-Electrocorticography. Micromachines 2019, 10, 62. [Google Scholar] [CrossRef] [Green Version]
- Tasnim, N.; Ajam, A.; Ramos, R.; Koripalli, M.K.; Chennamsetti, M.; Choi, Y. Handcrafted Electrocorticography Electrodes for a Rodent Behavioral Model. Technologies 2016, 4, 23. [Google Scholar] [CrossRef] [Green Version]
- Wolpaw, J.R.; McFarland, D.J.; Neat, G.W.; Forneris, C.A. An EEG-based brain-computer interface for cursor control. Electroencephalogr. Clin. Neurophysiol. 1991, 78, 252–259. [Google Scholar] [CrossRef]
- Nagel, S.; Spüler, M. Modelling the brain response to arbitrary visual stimulation patterns for a flexible high-speed Brain-Computer Interface. PLoS ONE 2018, 13, e0206107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuvaraj, R.; Thagavel, P.; Thomas, J.; Fogarty, J.; Ali, F. Comprehensive Analysis of Feature Extraction Methods for Emotion Recognition from Multichannel EEG Recordings. Sensors 2023, 23, 915. [Google Scholar] [CrossRef] [PubMed]
- Damalerio, R.B.; Lim, R.; Gao, Y.; Zhang, T.-T.; Cheng, M.-Y. Development of Low-Contact-Impedance Dry Electrodes for Electroencephalogram Signal Acquisition. Sensors 2023, 23, 4453. [Google Scholar] [CrossRef] [PubMed]
- Shivaraja, T.R.; Remli, R.; Kamal, N.; Wan Zaidi, W.A.; Chellappan, K. Assessment of a 16-Channel Ambulatory Dry Electrode EEG for Remote Monitoring. Sensors 2023, 23, 3654. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, L.; Zhang, Z.; Yang, H.; Zhang, Y.; Wu, J. The Feature, Performance, and Prospect of Advanced Electrodes for Electroencephalogram. Biosensors 2023, 13, 101. [Google Scholar] [CrossRef]
- Liang, H.; Liu, R. A New Generic Single-Channel Ear-EEG Recording Platform. Proceedings 2022, 81, 41. [Google Scholar] [CrossRef]
- Yuan, H.; Li, Y.; Yang, J.; Li, H.; Yang, Q.; Guo, C.; Zhu, S.; Shu, X. State of the Art of Non-Invasive Electrode Materials for Brain–Computer Interface. Micromachines 2021, 12, 1521. [Google Scholar] [CrossRef]
- Mwata-Velu, T.; Niyonsaba-Sebigunda, E.; Avina-Cervantes, J.G.; Ruiz-Pinales, J.; Velu-A-Gulenga, N.; Alonso-Ramírez, A.A. Motor Imagery Multi-Tasks Classification for BCIs Using the NVIDIA Jetson TX2 Board and the EEGNet Network. Sensors 2023, 23, 4164. [Google Scholar] [CrossRef]
- Al-Ayyad, M.; Owida, H.A.; De Fazio, R.; Al-Naami, B.; Visconti, P. Electromyography Monitoring Systems in Rehabilitation: A Review of Clinical Applications, Wearable Devices and Signal Acquisition Methodologies. Electronics 2023, 12, 1520. [Google Scholar] [CrossRef]
- Moontaha, S.; Schumann, F.E.F.; Arnrich, B. Online Learning for Wearable EEG-Based Emotion Classification. Sensors 2023, 23, 2387. [Google Scholar] [CrossRef]
- Mascia, A.; Collu, R.; Spanu, A.; Fraschini, M.; Barbaro, M.; Cosseddu, P. Wearable System Based on Ultra-Thin Parylene C Tattoo Electrodes for EEG Recording. Sensors 2023, 23, 766. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Fu, C.; Shu, F.; Yu, H.; Chen, C.; Chen, W. The Effect of Coupled Electroencephalography Signals in Electrooculography Signals on Sleep Staging Based on Deep Learning Methods. Bioengineering 2023, 10, 573. [Google Scholar] [CrossRef] [PubMed]
- Jakubowitz, E.; Feist, T.; Obermeier, A.; Gempfer, C.; Hurschler, C.; Windhagen, H.; Laves, M.-H. Early Predictability of Grasping Movements by Neurofunctional Representations: A Feasibility Study. Appl. Sci. 2023, 13, 5728. [Google Scholar] [CrossRef]
- de Brito Guerra, T.C.; Nóbrega, T.; Morya, E.; de Martins, A.M.; de Sousa, V.A., Jr. Electroencephalography Signal Analysis for Human Activities Classification: A Solution Based on Machine Learning and Motor Imagery. Sensors 2023, 23, 4277. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.; Van der Donck, S.; Moerkerke, M.; Dlhosova, T.; Vettori, S.; Dzhelyova, M.; van Winkel, R.; Alaerts, K.; Boets, B. Frequency-Tagging EEG of Superimposed Social and Non-Social Visual Stimulation Streams Provides No Support for Social Salience Enhancement after Intranasal Oxytocin Administration. Brain Sci. 2022, 12, 1224. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Kim, M.-J.; Yum, M.-S.; Jeong, D.-H. Deep Convolutional Gated Recurrent Unit Combined with Attention Mechanism to Classify Pre-Ictal from Interictal EEG with Minimized Number of Channels. J. Pers. Med. 2022, 12, 763. [Google Scholar] [CrossRef]
- Ehiabhi, J.; Wang, H. A Systematic Review of Machine Learning Models in Mental Health Analysis Based on Multi-Channel Multi-Modal Biometric Signals. BioMedInformatics 2023, 3, 193–219. [Google Scholar] [CrossRef]
- Abdel-Hamid, L. An Efficient Machine Learning-Based Emotional Valence Recognition Approach Towards Wearable EEG. Sensors 2023, 23, 1255. [Google Scholar] [CrossRef]
- Doborjeh, M.; Liu, X.; Doborjeh, Z.; Shen, Y.; Searchfield, G.; Sanders, P.; Wang, G.Y.; Sumich, A.; Yan, W.Q. Prediction of Tinnitus Treatment Outcomes Based on EEG Sensors and TFI Score Using Deep Learning. Sensors 2023, 23, 902. [Google Scholar] [CrossRef]
- Donisi, L.; Cesarelli, G.; Pisani, N.; Ponsiglione, A.M.; Ricciardi, C.; Capodaglio, E. Wearable Sensors and Artificial Intelligence for Physical Ergonomics: A Systematic Review of Literature. Diagnostics 2022, 12, 3048. [Google Scholar] [CrossRef]
- AL-Quraishi, M.S.; Elamvazuthi, I.; Tang, T.B.; Al-Qurishi, M.; Adil, S.H.; Ebrahim, M. Bimodal Data Fusion of Simultaneous Measurements of EEG and fNIRS during Lower Limb Movements. Brain Sci. 2021, 11, 713. [Google Scholar] [CrossRef] [PubMed]
- Rampp, S.; Kaltenhäuser, M.; Müller-Voggel, N.; Doerfler, A.; Kasper, B.S.; Hamer, H.M.; Brandner, S.; Buchfelder, M. MEG Node Degree for Focus Localization: Comparison with Invasive EEG. Biomedicines 2023, 11, 438. [Google Scholar] [CrossRef] [PubMed]
- Fred, A.L.; Kumar, S.N.; Kumar Haridhas, A.; Ghosh, S.; Purushothaman Bhuvana, H.; Sim, W.K.J.; Vimalan, V.; Givo, F.A.S.; Jousmäki, V.; Padmanabhan, P.; et al. A Brief Introduction to Magnetoencephalography (MEG) and Its Clinical Applications. Brain Sci. 2022, 12, 788. [Google Scholar] [CrossRef] [PubMed]
- Morales Chacón, L.M.; González González, J.; Ríos Castillo, M.; Berrillo Batista, S.; Batista García-Ramo, K.; Santos Santos, A.; Quintanal Cordero, N.; Zaldívar Bermúdez, M.; Garbey Fernández, R.; Estupiñan Díaz, B.; et al. Surgical Outcome in Extratemporal Epilepsies Based on Multimodal Pre-Surgical Evaluation and Sequential Intraoperative Electrocorticography. Behav. Sci. 2021, 11, 30. [Google Scholar] [CrossRef]
- Seo, J.-H.; Tsuda, I.; Lee, Y.J.; Ikeda, A.; Matsuhashi, M.; Matsumoto, R.; Kikuchi, T.; Kang, H. Pattern Recognition in Epileptic EEG Signals via Dynamic Mode Decomposition. Mathematics 2020, 8, 481. [Google Scholar] [CrossRef] [Green Version]
- Allison, B.Z.; Kübler, A.; Jin, J. 30+ years of P300 brain–computer interfaces. Psychophysiology 2020, 57, e13569. [Google Scholar] [CrossRef]
- Nakanishi, M.; Wang, Y.; Wang, Y.T.; Jung, T.P. A comparison study of canonical correlation analysis based methods for detecting steady-state visual evoked potentials. PLoS ONE 2015, 10, e0140703. [Google Scholar] [CrossRef] [Green Version]
- Thomas, E.; Dyson, M.; Clerc, M. An analysis of performance evaluation for motor-imagery based BCI. J. Neural Eng. 2013, 10, 031001. [Google Scholar] [CrossRef]
- Wang, H.; Yan, F.; Xu, T.; Yin, H.; Chen, P.; Yue, H.; Chen, C.; Zhang, H.; Xu, L.; He, Y.; et al. Brain-controlled wheelchair review: From wet electrode to dry electrode, from single modal to hybrid modal, from synchronous to asynchronous. IEEE Access 2021, 9, 55920–55938. [Google Scholar] [CrossRef]
- Nooh, A.A.; Yunus, J.; Daud, S.M. A review of asynchronous electroencephalogram-based brain computer interface systems. Int. Conf. Biomed. Eng. Technol. IPCBEE 2011, 11, 55–59. [Google Scholar]
- Zhou, Y.; He, S.; Huang, Q.; Li, Y. A hybrid asynchronous brain-computer interface combining SSVEP and EOG signals. IEEE Trans. Biomed. Eng. 2020, 67, 2881–2892. [Google Scholar] [CrossRef] [PubMed]
- Delorme, A.; Sejnowski, T.; Makeig, S. Enhanced detection of artifacts in EEG data using higher-order statistics and independent component analysis. NeuroImage 2006, 34, 1443–1449. [Google Scholar] [CrossRef] [Green Version]
- Mannan, M.M.N.; Kamran, M.A.; Jeong, M.Y. Identification and Removal of Physiological Artifacts from Electroencephalogram Signals: A Review. IEEE Access 2018, 6, 30630–30652. [Google Scholar] [CrossRef]
- Urigüen, J.A.; Garcia-Zapirain, B. EEG artifact removal—Stateof-the-art and guidelines. J. Neural Eng. 2015, 12, 031001. [Google Scholar] [CrossRef]
- Abdullah, A.A.; Zhang, C.Z.; Abdullah, A.; Lian, S. Automatic Extraction System for Common Artifacts in EEG Signals Based on Evolutionary Stone’s BSS Algorithm. Math. Probl. Eng. 2014, 2014, 324750. [Google Scholar] [CrossRef] [Green Version]
- Urigüen, J.A.; García-Zapirain, B.; Artieda, J.; Iriarte, J.; Valencia, M. Comparison of background EEG activity of different groups of patients with idiopathic epilepsy using Shannon spectral entropy and cluster-based permutation statistical testing. PLoS ONE 2017, 12, e0184044. [Google Scholar] [CrossRef] [Green Version]
- Roy, V.; Shukla, S.; Shukla, P.K.; Rawat, P. Gaussian Elimination-Based Novel Canonical Correlation Analysis Method for EEG Motion Artifact Removal. J. Healthc. Eng. 2017, 2017, 9674712. [Google Scholar] [CrossRef] [Green Version]
- Picton, T.W.; Van Roon, P.; Armilio, M.L.; Berg, P.; Ille, N.; Scherg, M. The correction of ocular artifacts: A topographic perspective. Clin. Neurophysiol. 2000, 111, 53–65. [Google Scholar] [CrossRef]
- Klados, M.A.; Papadelis, C.; Bamidis, P.D.; Braun, C. REG-ICA: A hybrid methodology combining blind source separation and regression techniques for the rejection of ocular artifacts. Biomed. Signal Process. Control 2011, 6, 291–300. [Google Scholar] [CrossRef]
- Liu, W.; Park, I.; Wang, Y.; Principe, J.C. Extended kernel recursive least squares algorithm. IEEE Trans. Signal Process. 2009, 57, 3801–3814. [Google Scholar] [CrossRef]
- Mannan, M.M.; Jeong, M.Y.; Kamran, M.A. Hybrid ICA-Regression: Automatic Identification and Removal of Ocular Artifacts from Electroencephalographic Signals. Front. Hum. Neurosci. 2016, 10, 193. [Google Scholar] [CrossRef]
- Vapnik, V.N. An overview of statistical learning theory. IEEE Transact. Neural Netw. 1999, 10, 988–999. [Google Scholar] [CrossRef] [Green Version]
- Lotte, F.; Congedo, M.; Lecuyer, A.; Lamarche, F.; Arnaldi, B. Review of classification algorithms for EEG based brain computer interfaces. J. Neural Eng. 2007, 4, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Rabiner, L.R. A tutorial on hidden Markov models and selected applications in speech recognition. Proc. IEEE 1989, 77, 257–286. [Google Scholar] [CrossRef] [Green Version]
- Cerný, V. Thermodynamical approach to the traveling salesman problem: An efficient simulation algorithm. J. Optim. Theory Appl. 1985, 45, 41–51. [Google Scholar] [CrossRef]
- Obermeier, B.; Guger, C.; Neuper, C.; Pfurtscheller, G. Hidden Markov models for online classification of single trial EEG data. Pattern Recognit. Lett. 2001, 22, 1299–1309. [Google Scholar] [CrossRef]
- Cincotti, F.; Scipione, A.; Tiniperi, A.; Mattia, D.; Marciani, A.; Millan, J.; Salinari, S.; Bianchi, L.; Bablioni, F. Comparison of different feature classifiers for brain computer interfaces. In Proceedings of the First International IEEE EMBS Conference on Neural Engineering, 2003. Conference Proceedings, Capri, Italy, 20–22 March 2023; pp. 645–647. [Google Scholar]
- Pekša, J. Autonomous Data-Driven Integration Algorithm. In Proceedings of the 2020 4th International Conference on Cloud and Big Data Computing, ICCBDC ’20, Liverpool, UK, 26–28 August 2020; ACM: New York, NY, USA, 2020; pp. 63–67. [Google Scholar] [CrossRef]
- Abiodun, O.I.; Jantan, A.; Omolara, A.E.; Dada, K.V.; Umar, A.M.; Linus, O.U.; Arshad, H.; Kazaure, A.A.; Gana, U.; Kiru, M.U. Comprehensive review of artificial neural network applications to pattern recognition. IEEE Access 2019, 7, 158820–158846. [Google Scholar] [CrossRef]
- Zagirnyak, M.; Prus, V. Use of neuronets in problems of forecasting the reliability of electric machines with a high degree of mean time between failures. Prz. Elektrotechniczny (Electr. Rev.) 2016, 92, 132–135. [Google Scholar] [CrossRef] [Green Version]
- Ko, W.; Jeon, E.; Jeong, S.; Suk, H.I. Multi-scale neural network for EEG representation learning in BCI. IEEE Comput. Intell. Mag. 2021, 16, 31–45. [Google Scholar] [CrossRef]
- LeCun, Y.; Boser, B.; Denker, J.S.; Henderson, D.; Howard, R.E.; Hubbard, W.; Jackel, L.D. Backpropagation Applied to Handwritten Zip Code Recognition. Neural Comput. 1989, 1, 541–551. [Google Scholar] [CrossRef]
- Schirrmeister, R.T.; Springenberg, J.T.; Fiederer, L.D.J.; Glasstetter, M.; Eggensperger, K.; Tangermann, M.; Hutter, F.; Burgard, W.; Ball, T. Deep learning with convolutional neural networks for EEG decoding and visualization. Hum. Brain Mapp. 2017, 38, 5391–5420. [Google Scholar] [CrossRef] [Green Version]
- Christou, V.; Miltiadous, A.; Tsoulos, I.; Karvounis, E.; Tzimourta, K.D.; Tsipouras, M.G.; Anastasopoulos, N.; Tzallas, A.T.; Giannakeas, N. Evaluating the Window Size’s Role in Automatic EEG Epilepsy Detection. Sensors 2022, 22, 9233. [Google Scholar] [CrossRef] [PubMed]
- Cerasa, A.; Tartarisco, G.; Bruschetta, R.; Ciancarelli, I.; Morone, G.; Calabrò, R.S.; Pioggia, G.; Tonin, P.; Iosa, M. Predicting Outcome in Patients with Brain Injury: Differences between Machine Learning versus Conventional Statistics. Biomedicines 2022, 10, 2267. [Google Scholar] [CrossRef] [PubMed]
- Naebi, A.; Feng, Z.; Hosseinpour, F.; Abdollahi, G. Dimension Reduction Using New Bond Graph Algorithm and Deep Learning Pooling on EEG Signals for BCI. Appl. Sci. 2021, 11, 8761. [Google Scholar] [CrossRef]
- Łysiak, A.; Paszkiel, S. A Method to Obtain Parameters of One-Column Jansen–Rit Model Using Genetic Algorithm and Spectral Characteristics. Appl. Sci. 2021, 11, 677. [Google Scholar] [CrossRef]
- Z-Flores, E.; Trujillo, L.; Legrand, P.; Faïta-Aïnseba, F. EEG Feature Extraction Using Genetic Programming for the Classification of Mental States. Algorithms 2020, 13, 221. [Google Scholar] [CrossRef]
- Hag, A.; Handayani, D.; Altalhi, M.; Pillai, T.; Mantoro, T.; Kit, M.H.; Al-Shargie, F. Enhancing EEG-Based Mental Stress State Recognition Using an Improved Hybrid Feature Selection Algorithm. Sensors 2021, 21, 8370. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Si, J.; Wu, S.; Li, W.; Liu, H.; Chen, J.; He, Q.; Zhang, Y. Improvement of the Classification Accuracy of Steady-State Visual Evoked Potential-Based Brain-Computer Interfaces by Combining L1-MCCA with SVM. Appl. Sci. 2021, 11, 11453. [Google Scholar] [CrossRef]
- Li, Z.; Qiu, L.; Li, R.; He, Z.; Xiao, J.; Liang, Y.; Wang, F.; Pan, J. Enhancing BCI-Based Emotion Recognition Using an Improved Particle Swarm Optimization for Feature Selection. Sensors 2020, 20, 3028. [Google Scholar] [CrossRef]
- Majidov, I.; Whangbo, T. Efficient Classification of Motor Imagery Electroencephalography Signals Using Deep Learning Methods. Sensors 2019, 19, 1736. [Google Scholar] [CrossRef] [Green Version]
- Reñosa, C.R.M.; Bandala, A.A.; Vicerra, R.R.P. Classification of Confusion Level Using EEG Data and Artificial Neural Networks. In Proceedings of the 2019 IEEE 11th International Conference on Humanoid, Nanotechnology, Information Technology, Communication and Control, Environment, and Management (HNICEM), Laoag, Philippines, 29 November–1 December 2019; pp. 1–6. [Google Scholar] [CrossRef]
- Sareen, E.; Singh, L.; Varkey, B.; Achary, K.; Gupta, E. EEG dataset of individuals with intellectual and developmental disorder and healthy controls under rest and music stimuli. Data Brief 2020, 30, 10548. [Google Scholar] [CrossRef] [PubMed]
- Malete, T.N.; Moruti, K.; Thapelo, T.S.; Jamisola, R.S. EEG-based Control of a 3D Game Using 14-channel Emotiv Epoc+. In Proceedings of the 2019 IEEE International Conference on Cybernetics and Intelligent Systems (CIS) and IEEE Conference on Robotics, Automation and Mechatronics (RAM), Bangkok, Thailand, 18–20 November 2019; pp. 463–468. [Google Scholar] [CrossRef]
- Peterson, V.; Galván, C.; Hernández, H.; Saavedra, M.P.; Spies, R. A motor imagery vs. rest dataset with low-cost consumer grade EEG hardware. Data Brief 2022, 42, 108225. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Choi, Y.-S. A convolution neural networks scheme for classification of motor imagery EEG based on wavelet time-frequecy image. In Proceedings of the 2018 International Conference on Information Networking (ICOIN), Chiang Mai, Thailand, 10–12 January 2018. [Google Scholar] [CrossRef]
- Goldberger, A.L.; Amaral, L.A.; Glass, L.; Hausdorff, J.M.; Ivanov, P.C.; Mark, R.G.; Mietus, J.E.; Moody, G.B.; Peng, C.-K.; Stanley, H.E. PhysioNet: Components of a new research resource for complex physiologic signals. Circulation 2000, 101, E215–E220. [Google Scholar] [CrossRef] [Green Version]
- Blankertz, B.; Muller, K.-R.; Krusienski, D.; Schalk, G.; Wolpaw, J.; Schlogl, A.; Pfurtscheller, G.; Millan, J.; Schroder, M.; Birbaumer, N. The BCI competition III: Validating alternative approaches to actual BCI problems. IEEE Trans. Neural Syst. Rehabil. Eng. 2006, 14, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Tangermann, M.; Müller, K.-R.; Aertsen, A.; Birbaumer, N.; Braun, C.; Brunner, C.; Leeb, R.; Mehring, C.; Miller, K.J.; Müller-Putz, G.R.; et al. Review of the BCI competition IV. Front. Neurosci. 2012, 6, 55. [Google Scholar] [CrossRef] [Green Version]
- Hajipour Sardouie, S.; Shamsollahi, M.B. Selection of efficient features for discrimination of hand movements from MEG using a BCI competition IV data set. Front. Neurosci. 2012, 6, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stober, S.; Avital, S.; Owen, A.M.; Grahn, J.A. Towards Music Imagery Information Retrieval: Introducing the OpenMIIR Dataset of EEG Recordings from Music Perception and Imagination. ISMIR 2015, 763–769. [Google Scholar]
- Altuwaijri, G.A.; Muhammad, G.; Altaheri, H.; Alsulaiman, M. A Multi-Branch Convolutional Neural Network with Squeeze-and-Excitation Attention Blocks for EEG-Based Motor Imagery Signals Classification. Diagnostics 2022, 12, 995. [Google Scholar] [CrossRef]
- Altuwaijri, G.A.; Muhammad, G. A Multibranch of Convolutional Neural Network Models for Electroencephalogram-Based Motor Imagery Classification. Biosensors 2022, 12, 22. [Google Scholar] [CrossRef]
- Hafeez, T.; Umar Saeed, S.M.; Arsalan, A.; Anwar, S.M.; Ashraf, M.U.; Alsubhi, K. EEG in game user analysis: A framework for expertise classification during gameplay. PLoS ONE 2021, 16, e0246913. [Google Scholar] [CrossRef]
- Bano, K.S.; Bhuyan, P.; Ray, A. EEG-Based Brain Computer Interface for Emotion Recognition. In Proceedings of the 2022 5th International Conference on Computational Intelligence and Networks (CINE), Bhubaneswar, India, 1–3 December 2022; pp. 1–6. [Google Scholar] [CrossRef]
- Luján, M.Á.; Jimeno, M.V.; Mateo Sotos, J.; Ricarte, J.J.; Borja, A.L. A Survey on EEG Signal Processing Techniques and Machine Learning: Applications to the Neurofeedback of Autobiographical Memory Deficits in Schizophrenia. Electronics 2021, 10, 3037. [Google Scholar] [CrossRef]
- Niedermeyer, E.; da Silva, F.L. (Eds.) Electroencephalography: Basic Principles, Clinical Applications, and Related Fields; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005. [Google Scholar]
- Cajigas, I.; Davis, K.C.; Meschede-Krasa, B.; Prins, N.W.; Gallo, S.; Naeem, J.A.; Palermo, A.; Wilson, A.; Guerra, S.; Parks, B.A.; et al. Implantable brain–computer interface for neuroprosthetic-enabled volitional hand grasp restoration in spinal cord injury. Brain Commun. 2021, 3, fcab248. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Lin, D.; Sohn, W.J.; McCrimmon, C.M.; Wang, P.T.; Nenadic, Z.; Do, A.H. BCI-Based Neuroprostheses and Physiotherapies for Stroke Motor Rehabilitation. In Reinkensmeyer, Neurorehabilitation Technology; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Sanna, A.; Manuri, F.; Fiorenza, J.; De Pace, F. BARI: An Affordable Brain-Augmented Reality Interface to Support Human–Robot Collaboration in Assembly Tasks. Information 2022, 13, 460. [Google Scholar] [CrossRef]
- Shieh, C.-P.; Yang, S.-H.; Liu, Y.-S.; Kuo, Y.-T.; Lo, Y.-C.; Kuo, C.-H.; Chen, Y.-Y. Simultaneously Spatiospectral Pattern Learning and Contaminated Trial Pruning for Electroencephalography-Based Brain Computer Interface. Symmetry 2020, 12, 1387. [Google Scholar] [CrossRef]
- Xu, B.; Li, W.; He, X.; Wei, Z.; Zhang, D.; Wu, C.; Song, A. Motor Imagery Based Continuous Teleoperation Robot Control with Tactile Feedback. Electronics 2020, 9, 174. [Google Scholar] [CrossRef] [Green Version]
- Tayeb, Z.; Fedjaev, J.; Ghaboosi, N.; Richter, C.; Everding, L.; Qu, X.; Wu, Y.; Cheng, G.; Conradt, J. Validating Deep Neural Networks for Online Decoding of Motor Imagery Movements from EEG Signals. Sensors 2019, 19, 210. [Google Scholar] [CrossRef] [Green Version]
- Edelman, B.J.; Meng, J.; Suma, D.; Zurn, C.; Nagarajan, E.; Baxter, B.S.; Cline, C.C.; He, B.J.S.R. Noninvasive neuroimaging enhances continuous neural tracking for robotic device control. Sci. Robot. 2019, 4, eaaw6844. [Google Scholar] [CrossRef]
- Wu, S.-J.; Nicolaou, N.; Bogdan, M. Consciousness Detection in a Complete Locked-in Syndrome Patient through Multiscale Approach Analysis. Entropy 2020, 22, 1411. [Google Scholar] [CrossRef]
- Powers, J.C.; Bieliaieva, K.; Wu, S.; Nam, C.S. The Human Factors and Ergonomics of P300-Based Brain-Computer Interfaces. Brain Sci. 2015, 5, 318–356. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Li, W.; Liu, D.; Zhang, K.; Miao, M.; Xu, G.; Song, A. Continuous Hybrid BCI Control for Robotic Arm Using Noninvasive Electroencephalogram, Computer Vision, and Eye Tracking. Mathematics 2022, 10, 618. [Google Scholar] [CrossRef]
- Dumitrescu, C.; Costea, I.-M.; Semenescu, A. Using Brain-Computer Interface to Control a Virtual Drone Using Non-Invasive Motor Imagery and Machine Learning. Appl. Sci. 2021, 11, 11876. [Google Scholar] [CrossRef]
- Shah, U.; Alzubaidi, M.; Mohsen, F.; Abd-Alrazaq, A.; Alam, T.; Househ, M. The Role of Artificial Intelligence in Decoding Speech from EEG Signals: A Scoping Review. Sensors 2022, 22, 6975. [Google Scholar] [CrossRef]
- Ron-Angevin, R.; Fernández-Rodríguez, Á.; Dupont, C.; Maigrot, J.; Meunier, J.; Tavard, H.; Lespinet-Najib, V.; André, J.-M. Comparison of Two Paradigms Based on Stimulation with Images in a Spelling Brain–Computer Interface. Sensors 2023, 23, 1304. [Google Scholar] [CrossRef]
- Akram, F.; Alwakeel, A.; Alwakeel, M.; Hijji, M.; Masud, U. A Symbols Based BCI Paradigm for Intelligent Home Control Using P300 Event-Related Potentials. Sensors 2022, 22, 10000. [Google Scholar] [CrossRef]
- Velasco-Álvarez, F.; Fernández-Rodríguez, Á.; Vizcaíno-Martín, F.-J.; Díaz-Estrella, A.; Ron-Angevin, R. Brain–Computer Interface (BCI) Control of a Virtual Assistant in a Smartphone to Manage Messaging Applications. Sensors 2021, 21, 3716. [Google Scholar] [CrossRef] [PubMed]
- Mannan, M.M.N.; Kamran, M.A.; Kang, S.; Choi, H.S.; Jeong, M.Y. A Hybrid Speller Design Using Eye Tracking and SSVEP Brain–Computer Interface. Sensors 2020, 20, 891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anumanchipalli, G.K.; Chartier, J.; Chang, E.F. Speech synthesis from neural decoding of spoken sentences. Nature 2019, 568, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Willett, F.R.; Avansino, D.T.; Hochberg, L.R.; Henderson, J.M.; Shenoy, K.V. High-performance brain-to-text communication via handwriting. Nature 2021, 593, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Cabañero-Gómez, L.; Hervas, R.; Bravo, J.; Rodriguez-Benitez, L. Computational EEG Analysis Techniques When Playing Video Games: A Systematic Review. Proceedings 2018, 2, 483. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.; Lim, H.; Kim, J.W.; Kang, Y.J.; Ku, J. Brain Computer Interface-Based Action Observation Game Enhances Mu Suppression in Patients with Stroke. Electronics 2019, 8, 1466. [Google Scholar] [CrossRef] [Green Version]
- Paszkiel, S.; Rojek, R.; Lei, N.; Castro, M.A. A Pilot Study of Game Design in the Unity Environment as an Example of the Use of Neurogaming on the Basis of Brain–Computer Interface Technology to Improve Concentration. NeuroSci 2021, 2, 109–119. [Google Scholar] [CrossRef]
- Cattan, G.; Mendoza, C.; Andreev, A.; Congedo, M. Recommendations for Integrating a P300-Based Brain Computer Interface in Virtual Reality Environments for Gaming. Computers 2018, 7, 34. [Google Scholar] [CrossRef] [Green Version]
- Ahn, M.; Lee, M.; Choi, J.; Jun, S.C. A Review of Brain-Computer Interface Games and an Opinion Survey from Researchers, Developers and Users. Sensors 2014, 14, 14601–14633. [Google Scholar] [CrossRef] [Green Version]
- Sung, Y.; Cho, K.; Um, K. A Development Architecture for Serious Games Using BCI (Brain Computer Interface) Sensors. Sensors 2012, 12, 15671–15688. [Google Scholar] [CrossRef] [Green Version]
- Kovyazina, M.S.; Varako, N.A.; Lyukmanov, R.K.; Asiatskaya, G.A.; Suponeva, N.A.; Trofimova, A.K. Neurofeedback in the Rehabilitation of Patients with Motor Disorders after Stroke. Hum. Physiol. 2019, 45, 444–451. [Google Scholar] [CrossRef]
- TajDini, M.; Sokolov, V.; Kuzminykh, I.; Shiaeles, S.; Ghita, B. Wireless Sensors for Brain Activity—A Survey. Electronics 2020, 9, 2092. [Google Scholar] [CrossRef]
- Serrano-Barroso, A.; Siugzdaite, R.; Guerrero-Cubero, J.; Molina-Cantero, A.J.; Gomez-Gonzalez, I.M.; Lopez, J.C.; Vargas, J.P. Detecting Attention Levels in ADHD Children with a Video Game and the Measurement of Brain Activity with a Single-Channel BCI Headset. Sensors 2021, 21, 3221. [Google Scholar] [CrossRef]
- Bulat, M.; Karpman, A.; Samokhina, A.; Panov, A. Playing a P300-based BCI VR game leads to changes in cognitive functions of healthy adults. bioRxiv 2020, 2020–2025. [Google Scholar]
- Kohli, V.; Tripathi, U.; Chamola, V.; Rout, B.K.; Kanhere, S.S. A review on Virtual Reality and Augmented Reality use-cases of Brain Computer Interface based applications for smart cities. Microprocess. Microsyst. 2022, 88, 104392. [Google Scholar] [CrossRef]
- Al-Nafjan, A.; Aldayel, M. Predict Students’ Attention in Online Learning Using EEG Data. Sustainability 2022, 14, 6553. [Google Scholar] [CrossRef]
- Rácz, M.; Noboa, E.; Détár, B.; Nemes, Á.; Galambos, P.; Szűcs, L.; Márton, G.; Eigner, G.; Haidegger, T. PlatypOUs—A Mobile Robot Platform and Demonstration Tool Supporting STEM Education. Sensors 2022, 22, 2284. [Google Scholar] [CrossRef] [PubMed]
- Balderas, D.; Ponce, P.; Lopez-Bernal, D.; Molina, A. Education 4.0: Teaching the Basis of Motor Imagery Classification Algorithms for Brain-Computer Interfaces. Future Internet 2021, 13, 202. [Google Scholar] [CrossRef]
- Burgos, D. Motor Imagery Experiment Using BCI: An Educational Technology Approach. In Radical Solutions and Learning Analytics; Springer: Cham, Swizterland, 2020; pp. 81–98. [Google Scholar]
- Teo, S.H.; Poh, X.W.; Lee, T.S.; Guan, C.; Cheung, Y.B.; Fung, D.S.; Zhang, H.H.; Chin, Z.Y.; Wang, C.C.; Sung, M.; et al. Brain-computer interface based attention and social cognition training programme for children with ASD and co-occurring ADHD: A feasibility trial. Res. Autism Spectr. Disord. 2021, 89, 101882. [Google Scholar] [CrossRef]
- Hadjiaros, M.; Neokleous, K.; Shimi, A.; Avraamides, M.N.; Pattichis, C.S. Virtual Reality Cognitive Gaming Based on Brain Computer Interfacing: A Narrative Review. IEEE Access 2023, 11, 18399–18416. [Google Scholar] [CrossRef]
- Ramírez-Moreno, M.A.; Carrillo-Tijerina, P.; Candela-Leal, M.O.; Alanis-Espinosa, M.; Tudón-Martínez, J.C.; Roman-Flores, A.; Ramírez-Mendoza, R.A.; Lozoya-Santos, J.D.J. Evaluation of a Fast Test Based on Biometric Signals to Assess Mental Fatigue at the Workplace—A Pilot Study. Int. J. Environ. Res. Public Health 2021, 18, 11891. [Google Scholar] [CrossRef]
- Lim, C.G.; Soh, C.P.; Lim, S.S.Y.; Fung, D.S.S.; Guan, C.; Lee, T.-S. Home-based brain–computer interface attention training program for attention deficit hyperactivity disorder: A feasibility trial. Child Adolesc. Psychiatry Ment. Health 2023, 17, 15. [Google Scholar] [CrossRef]
- Jia, Z.; Cai, X.; Jiao, Z. Multi-Modal Physiological Signals Based Squeeze-and-Excitation Network with Domain Adversarial Learning for Sleep Staging. IEEE Sens. J. 2022, 22, 3464–3471. [Google Scholar] [CrossRef]
- Chen, T.; Huang, H.; Pan, J.; Li, Y. An EEG-based brain-computer interface for automatic sleep stage classification. In Proceedings of the 13th IEEE Conference on Industrial Electronics and Applications (ICIEA), Wuhan, China, 31 May–2 June 2018; pp. 1988–1991. [Google Scholar] [CrossRef]
- Abenna, S.; Nahid, M.; Bouyghf, H. Sleep Stages Detection Based BCI: A Novel Single-Channel EEG Classification Based on Optimized Bandpass Filter. In Advanced Technologies for Humanity. ICATH 2021. Lecture Notes on Data Engineering and Communications Technologies; Springer: Cham, Switzerland, 2022; Volume 110. [Google Scholar] [CrossRef]
- Jia, Z.; Lin, Y.; Wang, J.; Ning, X.; He, Y.; Zhou, R.; Zhou, Y.; Li-wei, H.L. Multi-view spatial-temporal graph convolutional networks with domain generalization for sleep stage classification. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 1977–1986. [Google Scholar] [CrossRef]
- Eldele, E.; Chen, Z.; Liu, C.; Wu, M.; Kwoh, C.K.; Li, X.; Guan, C. An attention-based deep learning approach for sleep stage classification with single-channel EEG. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 809–818. [Google Scholar] [CrossRef]
- Michielli, N.; Acharya, U.R.; Molinari, F. Cascaded LSTM recurrent neural network for automated sleep stage classification using single-channel EEG signals. Comput. Biol. Med. 2019, 106, 71–81. [Google Scholar] [CrossRef]
- Santaji, S.; Desai, V. Analysis of EEG signal to classify sleep stages using machine learning. Sleep Vigil 2020, 4, 145–152. [Google Scholar] [CrossRef]
| Platform | Pros | Cons |
|---|---|---|
| EEG |
|
|
| fNIRS |
|
|
| MEG |
|
|
| ECoG |
|
|
| Paradigm | Pros | Cons |
|---|---|---|
| P300 |
|
|
| SSVEP |
|
|
| MI |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peksa, J.; Mamchur, D. State-of-the-Art on Brain-Computer Interface Technology. Sensors 2023, 23, 6001. https://doi.org/10.3390/s23136001
Peksa J, Mamchur D. State-of-the-Art on Brain-Computer Interface Technology. Sensors. 2023; 23(13):6001. https://doi.org/10.3390/s23136001
Chicago/Turabian StylePeksa, Janis, and Dmytro Mamchur. 2023. "State-of-the-Art on Brain-Computer Interface Technology" Sensors 23, no. 13: 6001. https://doi.org/10.3390/s23136001
APA StylePeksa, J., & Mamchur, D. (2023). State-of-the-Art on Brain-Computer Interface Technology. Sensors, 23(13), 6001. https://doi.org/10.3390/s23136001





