1. Introduction
Miniaturized sensors are increasingly being deployed as key components of connected networks and smart systems, providing indispensable solutions to societal needs from healthcare to communications, infotainment, transportation, food processing, sustainable energy, or resource management. The worldwide demand is projected to increase to dozens of trillions by the end of the 2020s [
1]. Since 1979, when Terry et al. published the first paper on a miniaturized gel chromatography system [
2], micro electro-mechanical system (MEMS)-based sensors have increasingly been applied in chemical sensing, biotechnological, and life-science applications, expanding macroscopic total analysis systems (TAS) to micro TAS (μ TAS) [
3], micro reactors, and eventually coining the terms
Lab-on-Chip (LoC) and
cells on chip systems. Such systems generally combine miniaturized fluidic handling and sensing capabilities to exploit multiple benefits when compared to classical macroscopic systems. Key benefits include increased performance per volume, portability, availability, and reliability, along with faster analysis and response times, at reduced cost and minimized fluid volumes and consumption of analytes and reagents. Ongoing improvements of the above characteristics, often combined with implementation of more complex functionalities, demand constant improvements of underlying MEMS fabrication technologies [
4,
5,
6].
Many classical LoC-type systems are based on silicon for the availability of well-established patterning techniques, and/or on glass for its excellent chemical stability. Emerging polymer-based systems afford a range of advantages over the above-mentioned materials, including tunable materials properties (thermal, mechanical, optical, electrical) and surface functionalization. Many polymers can also directly be micro-/nanopatterned by lithographic or by mass replication techniques (e.g., embossing, molding, soft lithography approaches) as well as by precision machining and additive processes (e.g., 3D printing) [
4,
5,
6]. Such polymer-based systems, however, generally offer limited solvent compatibility. More importantly, they must often be integrated into multi-material sandwiches or composites (e.g., with metals, ceramics, biological material) for functional or auxiliary functions of the sensing or the fluidic handling sub-units. Examples of metal thin films are seeding layers and adhesion promoters, patterned electrodes, and membranes. Polymer-based microfluidics and sensing systems are therefore usually associated with a considerable range of fabrication processes and materials, offering both opportunities for advanced sensing applications and challenges for technical implementation. Several references [
7,
8] exemplify typical challenges of polymer-based multi-material composites in microfluidics applications.
The present study forms part of the tremendous recent interest in microfluidic platforms for chemical and biological reaction chemistry [
9]. In such systems, detection and imaging are required to spatially resolve chemical sensitivity and selectivity for the detection of chemical distributions in the device, e.g., during on-flow reactions. Sensing must therefore be rapid and non-invasive, and the devices should be reusable [
10]. Besides optical methods, vibrational spectroscopy using Fourier transform infrared spectroscopy (FTIR) has emerged as a technological approach that meets those requirements [
11]. Due to the strong absorption of IR radiation in aqueous media, as typically encountered in microfluidics, effective penetration lengths are limited to only a few micrometers. However, with common channel dimensions of tens to hundreds of micrometers as desirable in many applications, FTIR is hard or even prohibitive to implement, or else restricted to very shallow fluid channels, when using a standard transmission architecture where the IR radiation enters through an IR-transparent window, such as CaF
2, crosses through the fluid channel, exits the fluid cell through a second IR-transparent window, and the remaining signal is then detected [
12,
13,
14,
15].
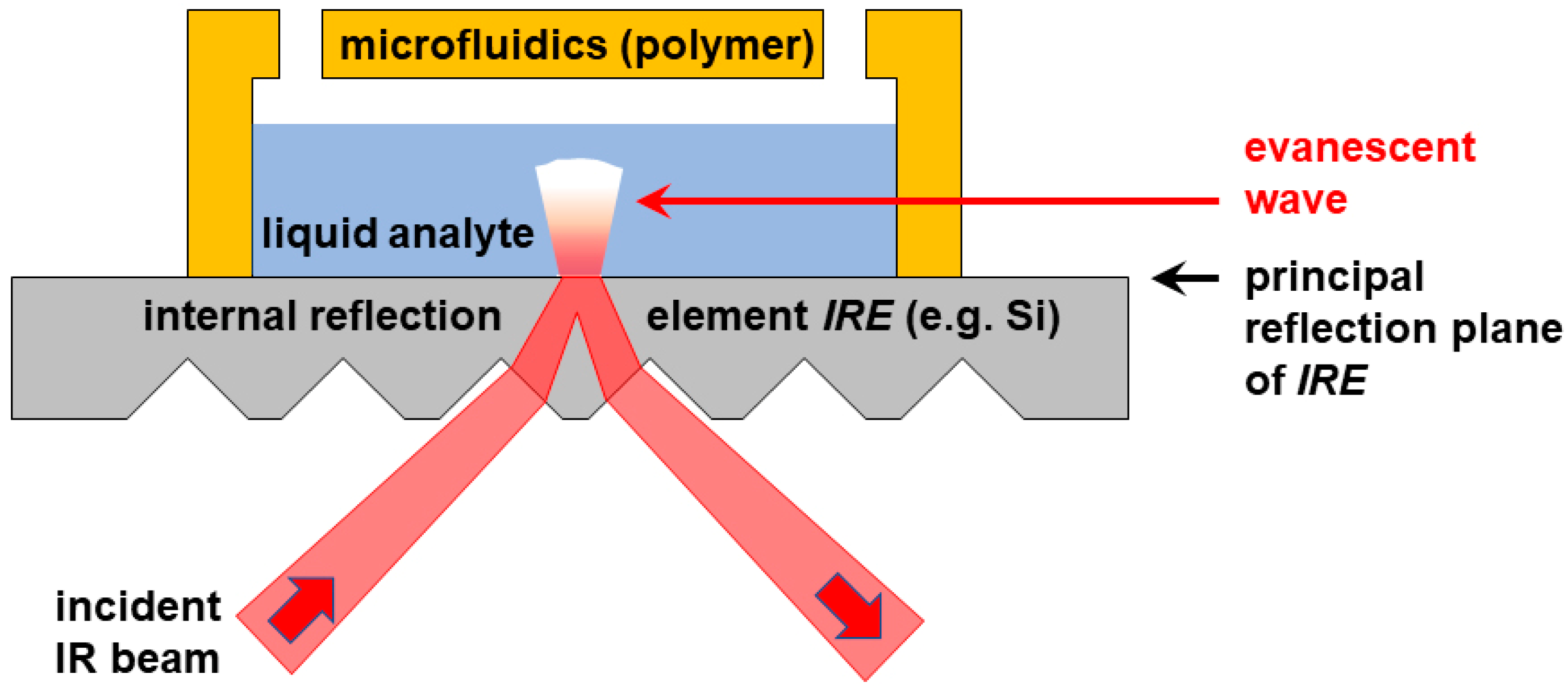
These limitations are circumvented when changing from a transmission to an attenuated total reflection (ATR) configuration as illustrated in
Figure 1. In ATR-FTIR, IR radiation is shone under an oblique angle onto an optically dense material, such as silicon, which serves as an
internal reflection element (IRE). In a prism- or grooved topography on the incident side, but a flat surface on the back plane of this IRE, radiation incident above the critical angle undergoes total internal reflection at the flat back plane (the
principal reflection plane). As a result, a spatially confined evanescent wave originates from the principal reflection plane of the IRE. This wave can be applied to analyze matter located within about a micrometer off that plane. An emerging conceptual approach is to apply ATR-FTIR in combination with microfluidics, using the flat IRE surface as the substrate for a microfluidic cell to detect and analyze the chemicals inside the fluid channel. This technique allows one to reliably interrogate the fluid close to the interface, regardless of the overall channel height. Due to the spatial confinement and short penetration length, this approach even allows for chemical imaging [
10,
16,
17,
18].
The main aim of the research presented is to develop, verify, and apply a sequence of microfabrication processes that allows one to build Lab-on-Chip (LoC) devices that enable
- (i)
manipulation of small sample volumes of liquid analytes (e.g., co-laminar flow, mixing);
- (ii)
initiation and control of electro-chemical reactions on flow (e.g., using electrodes in the fluidic setup);
- (iii)
single-point infrared (IR) spectroscopy to detect and analyze various chemicals on-flow; and
- (iv)
IR imaging of areas of interest along a fluidic setup.
The fabrication processes should
- (a)
be repeatable and configurable;
- (b)
allow one to handle non-aggressive chemicals compatible with polymeric materials (e.g., in tubing, connectors, fluidic cell);
- (c)
allow for fluidic structures that are optically transparent (for simplified alignment to electrodes and the FTIR patterns on the back side, see
Figure 1) and that can be quickly and cost-efficiently replicated, i.e., polymer-based; and
- (d)
allow for integration with electrochemical reaction and FTIR sensing elements (e.g., aligned and reliable bonding to/hermetic sealing with metal electrodes and silicon internal reflection elements).
Key outcomes achieved in the work presented include the following individual contributions: A microfabrication process sequence was developed that applies direct-write UV lithography to pattern a UV mask which is then used in soft-contact UV lithography to pattern a negative-tone dry resist (SUEX) as a primary microstructure which subsequently serves as a master mold for the replication of fluidic structures in a silicone elastomer, polydimethylsiloxane (PDMS). Bulk micromachining (potassium hydroxide—KOH etch) is applied to pattern the grooves into the silicon-based internal reflection elements (IREs). The back side of these IREs can be patterned with electrodes and microfluidics structures: A lift-off process is applied to pattern gold (Au) electrodes on the silicon/silicon nitride substrates, and a combination of surface activation in oxygen reactive ion etching (RIE), adhesion promotion using 3-aminopropyl-triethoxysilane (APTES), and a UV-curable glue (NOA 73) are used to ensure proper adhesion of the PDMS structure to the substrate. Alignment between electrodes and PDMS prior to bonding is achieved using a custom-designed active vacuum positioning and alignment setup. Demonstrator devices were designed and fabricated to demonstrate electrochemical reactions in the electrodes, which also ensured that nitride-coated silicon wafers sufficiently minimize leak currents otherwise observed across a bare silicon substrate. Moreover, 10 millimolar ferricyanide (potassium hexacyanoferrate (III), [Fe(CN)6]3−) and 10 millimolar ferrocyanide (potassium hexacyanoferrate (II), [Fe(CN)6]4−), both in 0.1 molar potassium perchlorate (KClO4), were co-flown and analyzed using the built-in electrodes for cyclic voltammograms (CVs). Single-point FTIR experiments were conducted using co-flowing water (H2O) and heavy water (D2O) to demonstrate detection of the formation of the proton/deuterium exchange product HOD.
All targeted individual goals have been reached, and approaches chosen are compatible to allow for a single integrated chip with all the properties and functionalities described above. This final step is work in progress, but has not been completed to date due to the substantial interruptions of the sudden and shocking death of the primary author, Noah Atkinson.
2. Materials and Methods: Micro Fabrication Process Development
Development and application of micro fabrication processes described in this paper were performed at the
Synchrotron Laboratory for Micro and Nano Devices (
SyLMAND) [
19], Canadian Light Source (CLS), on the campus of the University of Saskatchewan, Saskatoon, Canada.
Processes described in this section are generally adapted from the literature or our own previous research to fit the overall processing requirements of this research and to reflect equipment capabilities and restrictions at SyLMAND. While the overall processing sequence is custom designed and unique, the individual process steps described in this section generally have not been specifically optimized for this research.
Figure 2 provides an overview of the individual blocks of processes. Many of them can be used modularly, e.g., to include ATR-FTIR capabilities or electrochemical functionality where needed for the specific application. Omission of any of these modules does not compromise the overall fabrication concept. Core processes are briefly described in this section for completeness.
2.1. Direct-Write UV Lithography and UV Mask Fabrication
The proposed integrated chip requires (i) back-side patterning of a silicon wafer with parallel grooves for ATR-FTIR analysis, (ii) front-side patterning of a metal thin film to serve as electrodes in electrochemical processes, and (iii) separate patterning of a polymer resist structure to be later used as a master mold, facilitating replication of the fluidic structures to be aligned and bonded to the silicon wafer which was previously patterned in steps (i) and (ii).
Consequently, for each chip, three independent patterning tasks were performed using ultraviolet (UV) lithography. All required chromium masks were designed and fabricated in-house. Primary patterning was achieved using a direct-write UV lithography laser writer (DWL 66+, Heidelberg Instruments, Heidelberg, Germany). The system can pattern up to 6” large substrates, providing up to 250 mW of laser power at a wavelength of 355 nm. The applied optics allow for a nominal minimum feature size of about 3 µm at a working distance of 10 mm (referred to as write mode III). Since all critical dimensions in the work reported are substantially larger, this is an adequate patterning approach. Patterning details have previously been optimized on a comparable machine [
20]. The overall fabrication sequence is comparable to established Cr masking processes [
4,
5,
6]. Briefly, commercial UV mask blanks (soda-lime glass, precoated with approximately 100 nm of chromium and 0.5 μm of an AZ-1500-series positive-tone photoresist; Nanofilm, Westlake Village, CA, USA) were exposed using the UV writer. Exposure dose values for the laser-based UV writer are not defined in standard UV lithography units (energy per area), but rather in percent of the maximum nominal laser power incident for a fixed time increment onto each pixel. These values are floating, without direct means for absolute measurements. Nominally, 80 mW was applied. Dip (puddle) development was performed in standard AZ400K developer at room temperature, for 60 s. The resulting primary resist pattern served as a processing mask during local etching of the unprotected Cr areas on the mask for 3 min in a nitric acid-based chromium etchant (Transene 1020, Transene Inc., Danvers, MA, USA). This completed defining the geometry of the Cr UV mask absorbers. In a final stripping process with acetone, isopropanol, and water, the resist etch mask was removed in acetone.
2.2. Soft-Contact UV Lithography for Dry Resist Mold Patterning
The final fluidic devices are replicated in PDMS using a polymer-based, 100 μm deep master mold. These molds were lithographically patterned on 4” silicon wafers. An SU-8-like, negative-tone epoxy-based resist [
21] was applied to pattern the fluidic structures. However, to avoid wet chemical processing, a 100 μm thick sheet of chemically comparable dry resist (SUEX, DJ Microlaminates Inc., Sudbury, MA, USA) was attached directly to the silicon substrate. At 70 °C, the SUEX sheet was laminated [
22] to the substrate, using an RSL-382S Laminator (RS Royal Sovereign, Rockleigh, NJ, USA) with fixed feed velocity. Any bubbles potentially trapped in the interface were removed using a post-lamination bake (PLB) at 85 °C for 5 min. UV exposure was performed in soft-contact shadow projection mode using the UV masks described in
Section 2.1 (iii) and a mercury vapor lamp-based light house (Oriel Instruments, Stratford, CT, USA) with a low-pass filter and an exposure dose of 1000 mJ/cm
2 at 365 nm wavelength. After a post-exposure bake (PEB) at 85 °C for 30 min in a convection oven (Binder Vacuum Drying Oven, Binder GmbH, Tuttlingen, Germany), the resist was dip-developed in propylene glycol methyl ether acetate (PGMEA) for 10 min at room temperature, followed by a rinse in isopropyl alcohol (IPA) for 5 min. Drying in the convection oven at 100 °C for 10 min was followed by a hard bake at 150 °C for 30 min.
2.3. Bulk Micromachining for Patterning of Grooves in the Internal Reflection Elements (IREs)
One side of the chip must be structured with grooves or a prism to allow the incident IR beam to penetrate into the silicon and reach the principal reflection plane (see
Figure 1). Three options were applied and tested to obtain this topography:
Classically, a macroscopic single prism 60° face-angled crystal (FAC) made of silicon (e.g., PIKE Technologies, Madison, WI, USA) has been used. This approach offers simplicity in the overall optical setup, but it does substantially limit positions at which the fluidic setup on the opposite side can be analyzed. Furthermore, macroscopic silicon IREs may involve long beam travel through the silicon material. Associated attenuation of radiation effectively causes a cut-off of IR light at wavenumbers of approximately 1500 1/cm and less (i.e., wavelengths of approximately 6.7 μm and above). This spectral range would, however, be of substratial interest as this “fingerprint region” of the IR spectrum contains many characteristic peaks in IR spectroscopy. By reducing the size of the IRE, eventually applying micromachined IREs, the fingerprint region becomes accessible.
Secondly, such micro-grooved silicon IREs can commercially be obtained, e.g., as substrates of 9 mm by 11 mm size with 35° face angle and approximately 700 μm pitch (Universal ATR wafers, IRUBIS GmbH, München, Germany) [
17]. These combine micro-scale ATR capabilities with the ability to analyze at a large variety of locations. However, geometry, angles, overall chip size, and micro structure quality are not adjustable to experimental needs.
These drawbacks can be overcome when bulk micromachining is performed in-house. Silicon wafers coated with 100 nm of silicon nitride on either side and additionally with a thin layer of AZ resist (see above) on one side are applied in this process. A UV mask as described in
Section 2.1 (i) is used to pattern the resist layer, which ultimately opens up a processing window with bare silicon nitride. This is selectively etched using a Buffered Oxide Etch (BOE), a mixture of 6% hydrofluoric acid (HF) with ammonium fluoride (NH
4F) as the buffering agent (J.T.Baker BOE 7:1, Avantor/VWR, Edmonton, AB, Canada). The etch is performed at room temperature for 4 h. After local removal of the Si
3N
4 thin film, the bulk of the silicon can be etched through these processing windows using potassium hydroxide (KOH) at 70 °C for about 10 h. This approach allows one to custom-design IREs to perfectly match the microfluidics on the opposite side of the substrate. First demonstrators have been successfully fabricated. However, reliable adhesion of the masking layers, simplification of the process without the need for fluorine etch chemistry, and increasing the process stability and quality are in progress (full manuscript with processing details in preparation).
2.4. Lift-off Process for Patterning of Gold Electrodes
After bulk micromachining one side of an internal reflection element (IRE), separately prepared polymeric microfluidics (see below) will eventually become attached to the opposite side of the IRE, i.e., to the principal reflection plane (see
Figure 1). In addition to the components depicted in
Figure 1, complete LoC chips as defined in
Section 1 also have metal electrodes to allow for electrochemical functionality. These electrodes must be aligned relative to the microfluidic structures and are patterned onto the principal reflection plane, underneath the microfluidics.
In a standard processing sequence, the metal thin film would first be deposited onto the entire substrate, followed by lithographic patterning of resist which serves as a processing mask to locally protect the metal underneath from removal during an etch step. This concept requires good adhesion of both, resist and metal, to avoid unwanted removal of metal underneath the processing mask, or delamination of the metal thin film, during often-harsh processes required to etch the metal layer. These requirements can be eased if the alternative, a lift-off process [
4,
5], is applied, where the order of lithography and metal deposition steps is reversed. As depicted in
Figure 3, first, a resist structure is patterned. These structures require the opposite tone relative to the standard process as they define the areas in which final metal structures, e.g., electrodes, are not supposed to be. Unlike in classical lithography, the resist sidewalls should not be as vertical as possible, but rather offer a back cut such that in the subsequent flood metallization process, only the top sides of the bare substrate and the resist are coated, but not the resist sidewalls. This enables sufficient sideways resist attack in the subsequent wet chemical resist removal (stripping) process, which also lifts off the unwanted metal parts residing on top of the resist. In this process, adhesion between resist and metal is less critical, and the metal layer itself does not need to be etched. The resist back cut can be promoted by elaborate lithographic process optimizations or by applying custom-made lift-off resists that tend to form “mushroom-like” profiles.
The electrodes applied in this research were fabricated using a lift-off process. First, hexamethyldisilazane (HMDS) was applied to the substrate as an adhesion promotor. Then, 1 mL of an MCC primer (20% HMDS, 80% PM acetate, MicroChem/Kayaku Advanced Materials, Westborough, MA, USA) was pipetted to the substrate surface, allowed to react for 10 s, and then spin coated to dry at 3000 rpm for 1 min. A dedicated negative-tone lift-off resist (ma-N 1407, micro resist technology, Berlin, Germany/Kayaku Advanced Materials, Westborough, MA, USA) was spin coated at 3000 rpm for 30 s to a nominal thickness of 0.7 μm and soft baked at 100 °C for 60 s prior to UV exposure to 350 mJ/cm
2 at 365 nm wavelength, using the UV light house described above in soft-contact exposure mode, together with a mask fabricated in-house; see
Section 2.1 (ii). Post-exposure bake (PEB) was performed at 100 °C for 10 min prior to 40 s of room temperature dip development in a tetramethylammonium hydroxide (TMAH)-based developer, ma-D 533, from the same vendor.
The patterned wafer is then flood metallized by room temperature magnetron sputtering (PVD 75 Sputtering System, Kurt J. Lesker, Concord, ON, Canada). First, an approximately 5 nm thick chromium layer serves as an adhesion promoter to silicon, followed by typically 30 nm of gold as the electrode conductor. Cr sputtering was performed for 2.5 min at 100 W with a base pressure of 3 × 10−6 torr and a process pressure of 5 × 10−3 torr Ar. Au sputtering was performed for 6 min at 90 W and the same pressure. Resist stripping and lift-off using the wet chemical remover mr-Rem from the same vendor takes approximately 1 h.
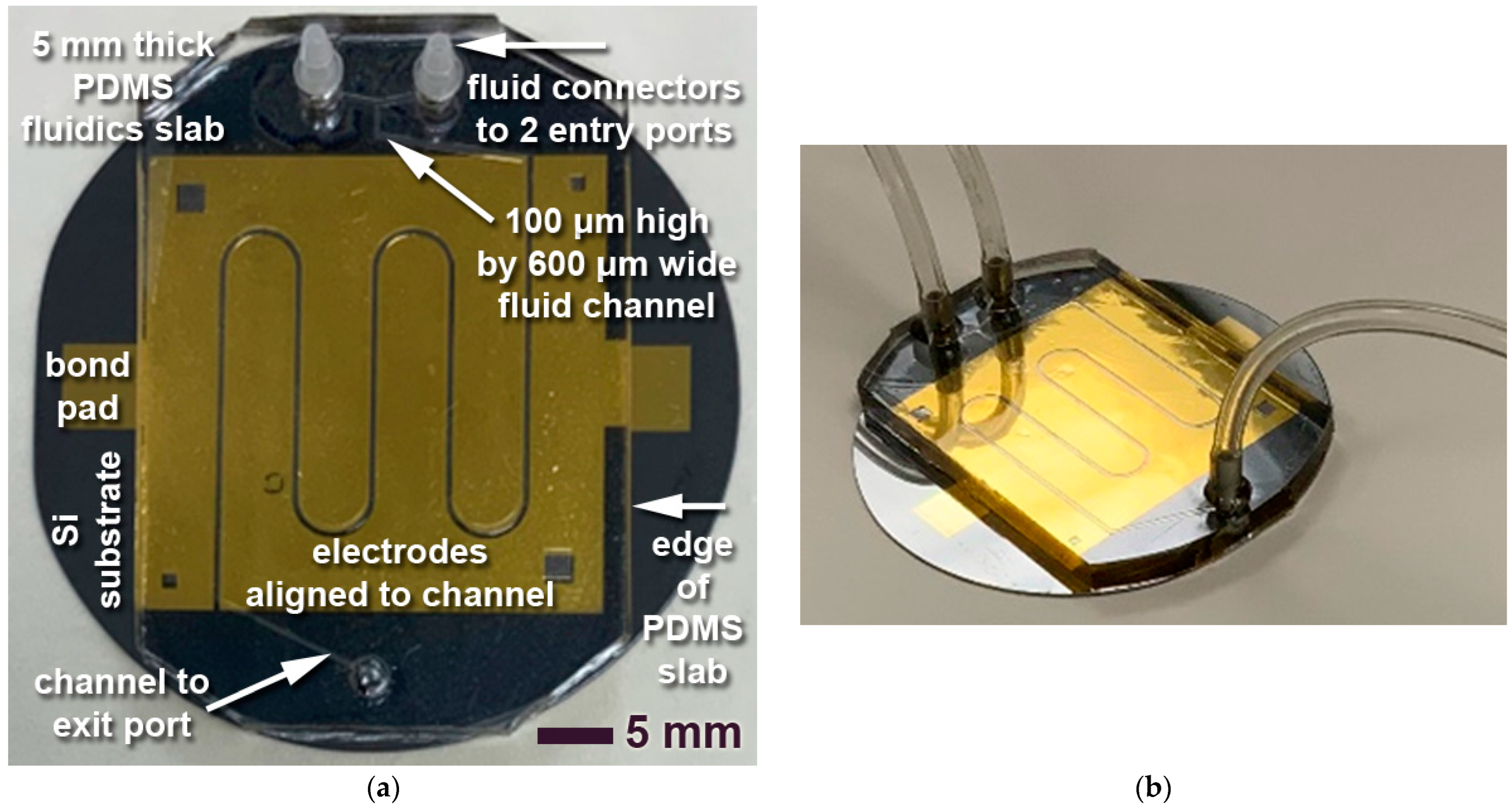
Figure 4 illustrates patterned electrodes in a sample layout for snake channels on a 2” test wafer. Gold, unlike the silicon substrate, is not sufficiently IR transparent. While other electrically conductive materials, such as indium zinc oxide (IZO), are optically and IR transparent, gold has the advantage of much higher electrical conductivity, optical opaqueness facilitating optical alignment to the back side and/or the fluidics, better compatibility with established processes, and much better stability in wet chemistry and water. Gold therefore continues to be applied as the electrode material of choice, even though it fundamentally prohibits ATR-FTIR analyses. To allow for such spectroscopic measurements while still enabling electrochemical capabilities, the electrodes must be carefully designed and located. In this case, the electrodes are aligned along the future fluidic channel, always slightly (100 μm) reaching into the channel for electrical contact with the fluid, while leaving the bulk of the fluid area IR transparent. This requires alignment between the electrodes and the subsequently added fluidics layer, described in
Section 3.
2.5. Replication of Polymer Fluid Channels into PDMS
The last individual component required for the LoC chip is the fluidic cell itself. Sidewalls and cover (lid) are replicated as a single component from the master mold described in
Section 2.2, while the bottom layer is formed by the principal reflection plane of the silicon or silicon–silicon nitride IRE and eventually Cr/Au thin film electrodes. Microfabrication by replication greatly decreases fabrication timelines and costs, compared to individual lithographic processing. Microreplication is primarily conducted in polymeric materials. Most of them are also optically transparent, allowing, as an added advantage for microfluidic devices, the inspection of the mass transport inside the fluidic cell as well as optical alignment during device fabrication. Commonly used MEMS replication materials for micro hot embossing or injection molding are thermoplasts, which deliver solid and rigid micro components but require high temperature and/or pressure during fabrication, associated with expensive specialty equipment, and preferably very costly, metal-based mold masters [
4]. In this work, an alternative class of polymers, elastomers, is applied in the replication process, which is soft, chemically quite resistant, transparent, and easy to work with. Polydimethylsiloxane (PDMS) is a silicone-based elastomer which is increasingly applied in fluidic MEMS for its compatibility with less-demanding molds [
23] such as the SUEX-silicon-based one described above. The elastomer prepolymer is poured into the mold and cured to a soft and easily deformable structure. Besides the many advantages, using PDMS in microfluidics is also associated with a variety of technological and applications challenges: PDMS cannot be used with non-polar solvents, such as alkanes (e.g., hexane), aromatics (e.g., benzene), or acetic acid, as diffusion of the solvent into the PDMS matrix would result in massive swelling. For the applications anticipated in this research, this does not constitute a significant drawback, but extended future applications might require revisiting the fluidic material for that reason. Untreated PDMS is quite hydrophobic. Initial tests in our lab delivered contact angles with water of about 90°. This results in wetting issues with, e.g., glues applied in fluidic integration. Detailed process development to overcome this concern is described in
Section 3. PDMS further noticeably shrinks upon curing, typically between 1% and 3% [
24]. This constitutes a challenge when designing the proper fluidics dimensions for subsequent alignment to prepatterned layers. Considerations to overcome this challenge are discussed in
Section 3 as well. Finally, the soft nature of PDMS, advantageous as it allows one to conformally follow a potentially prepatterned substrate surface, at the same time can prove to be a technical challenge when it is insufficiently rigid during alignment and tends to droop during positioning. This aspect can be optimized when adjusting the mixing ratio of base elastomer-to-curing agent (hardener), as it substantially impacts the final material stiffness.
The PDMS grade applied in this research is Sylgard 184 (Sigma-Aldrich, Oakville, ON, Canada). It was thoroughly mixed with an electric stirrer in a base elastomer-to-curing agent (hardener) ratio of 10:1. The mixture was then briefly degassed to approximately 0.1 bar to remove most of the potential bubbles. In a subsequent pouring and overcasting step, the slurry was poured into the master mold with the negative-tone layout of the 100 μm thick SUEX structures, defining channels of typically 600 μm width, along with reservoirs for entry and exit ports. The overall thickness was typically 5 mm, offering sufficient overall structural rigidity and doubling as the fluid channel surface cover as well. The samples were cured at 60 °C for 2 h (see more details and discussion in
Section 3.1.2) and cooled overnight before demolding and cutting the outside walls to roughly the desired overall dimensions.
4. Conclusions
Polymer-based Lab-on-Chip (LoC) devices often comprise micro- or nanopatterned multi-material composites, offering opportunities for advanced sensing applications due to the wide selection of materials. However, these devices also impose challenges to develop and optimize a growing range of microfabrication processes for technical implementation. This manuscript describes the process development to microfabricate LoC devices applicable in microfluidics combined with infrared vibrational spectroscopy and microscopy as well as in electrochemistry. Successful application of demonstrator devices in attenuated total reflection Fourier transform infrared spectroscopy (ATR-FTIR) and electrochemistry-based sensing and detection is demonstrated.
Polymers, silicon, and metals are patterned in a modular fabrication sequence. Patterning of the fluidic structures is based on ultraviolet (UV) lithography and replication. Direct-write laser lithography is applied to pattern UV masks subsequently applied in soft-contact UV lithography in SUEX dry resist to create a master mold on a silicon substrate. This tool then facilitates replication in polydimethylsiloxane (PDMS) elastomers as the final fluidic structure. Fluidic channels of 100 μm height and 600 μm width in 5 mm thick PDMS were fabricated on 2” and 4” demonstrators.
A silicon wafer, for some demonstrators in this study coated with 100 nm silicon nitride, doubles as an ATR-FTIR-active internal reflection element (IRE) patterned with grooves or prisms on one side, and as a flat substrate for the LoC fluidics on the opposite side. In one process variation, the Si wafer is patterned by bulk micromachining in-house, while in alternative variations, externally purchased micro-grooved wafers or macroscopic face-angled silicon crystals (FACs) can be applied. The opposite, flat surface of the silicon substrate serves as the principal reflection plane in ATR-FTIR, emitting the evanescent wave applied to interrogate the analytes in solution close to the interface between silicon substrate and PDMS fluidics. For electrochemical functionality, sputtered thin film electrodes of 5 nm chromium and 30 nm gold are structured by a lift-off process on the un-patterned flat side of the substrate. For sufficient electrical isolation between the electrodes in electrochemical experiments, the conductivity of the silicon surface must be reduced, which was achieved using a silicon wafer coated with 100 nm silicon nitride (SiN) prior to electrode patterning. Between the electrodes, the SiN thin film is later selectively and locally removed by hydrofluoric acid (HF) to improve ATR-FTIR performance.
Finally, the patterned PDMS slab is bonded to the wafer/gold electrode sandwich. Reliable adhesion between these bonding partners constitutes a challenge, which has been overcome by a combination of PDMS surface activation with oxygen plasma in a reactive ion etcher, immediately followed by functionalization with 3-aminopropyl-triethoxysilane (APTES). Resilient bonding of this primed surface to the substrate wafer is achieved using a UV-curable glue (NOA 73).
On-flow chemical and biological reaction chemistry is compatible with the proposed fabrication concept. In this case, the gold electrodes must be in contact with the fluid of interest within the fluidic cell. The electrodes are, however, opaque in the infrared wavelength range and could therefore prohibit simultaneous electrochemical and ATR-FTIR measurements. Precisely limiting the protrusion of the electrodes into the channel while ensuring that the bulk of the channel is free of gold is a conceptual precondition. This requires adjusted layouts of the fluidics and electrode layers, as well as proper alignment between them. A custom-designed active vacuum positioning and alignment setup was developed. It incorporates manual multi-axes positioners, a stereo microscope, and a custom-design 3D-printed vacuum paddle ring. This allows one to apply a vacuum seal between the paddle and the PDMS slab for controlled, precise positioning relative to the prepatterned substrate. After this alignment, the bond partners are brought into contact by lowering the PDMS slab onto the substrate, and a permanent bond is created by UV curing of the adhesive. Alignment results obtained prove the concept.
The demonstrated fabrication sequence is modular. For instance, patterning of micro-grooved silicon IREs can be part of the described process or be performed externally. Likewise, electrodes for electrochemical functionality can be added if needed for the application. The fabrication sequence allows one to implement all these aspects, or just those required for the specific sensing task. Demonstrators fabricated for this manuscript to prove the fabrication capability and measurement suitability concepts incorporated the functionality required for the individual devices. We are currently working on a completely integrated device.
Electrochemistry on-chip functionality was demonstrated by cyclic voltammetry (CV) of redox reactions involving a commonly studied redox couple, ferri/ferrocyandide, in different oxidation states. Further, ATR-FTIR measurements of laminar co-flows of H2O and D2O established the local concentration distribution of each species along with the formation of the proton/deuterium exchange product HOD. This demonstrated the FTIR capabilities of the microfabrication concept.