Noninvasive Glucose Sensing In Vivo
Abstract
:1. Introduction
2. Background
2.1. Diabetes
2.2. Glucose Management
2.3. Biological Fluid
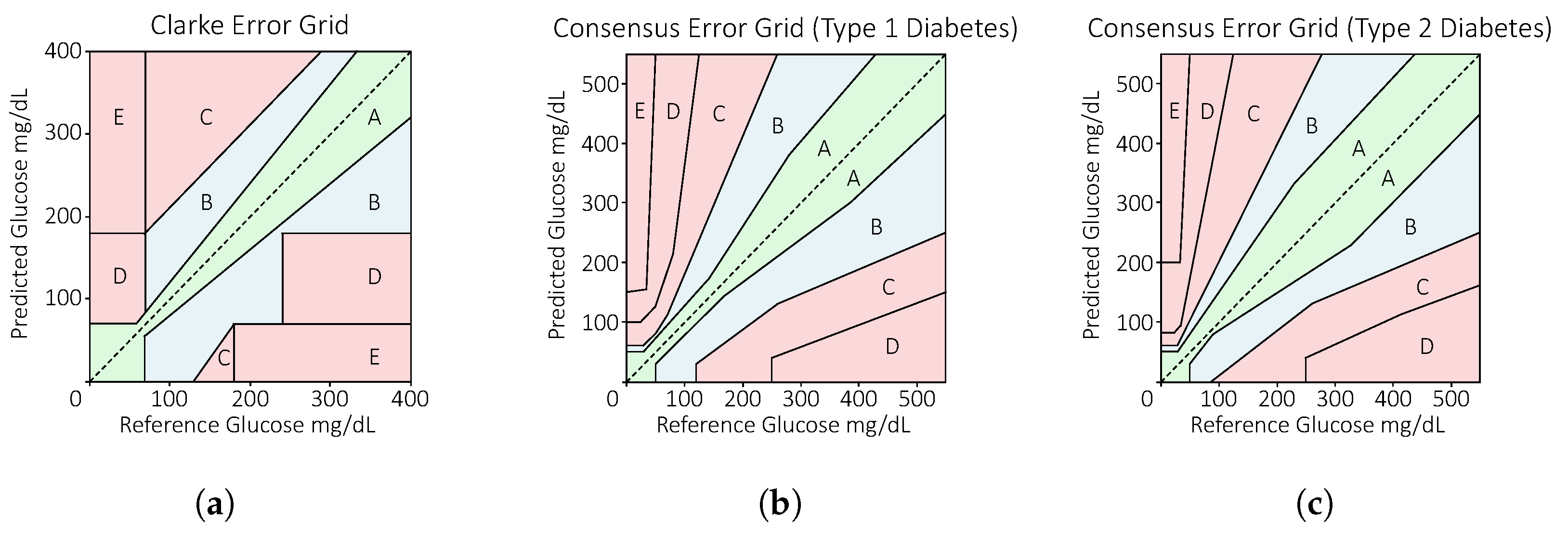
2.4. Evaluation Metrics
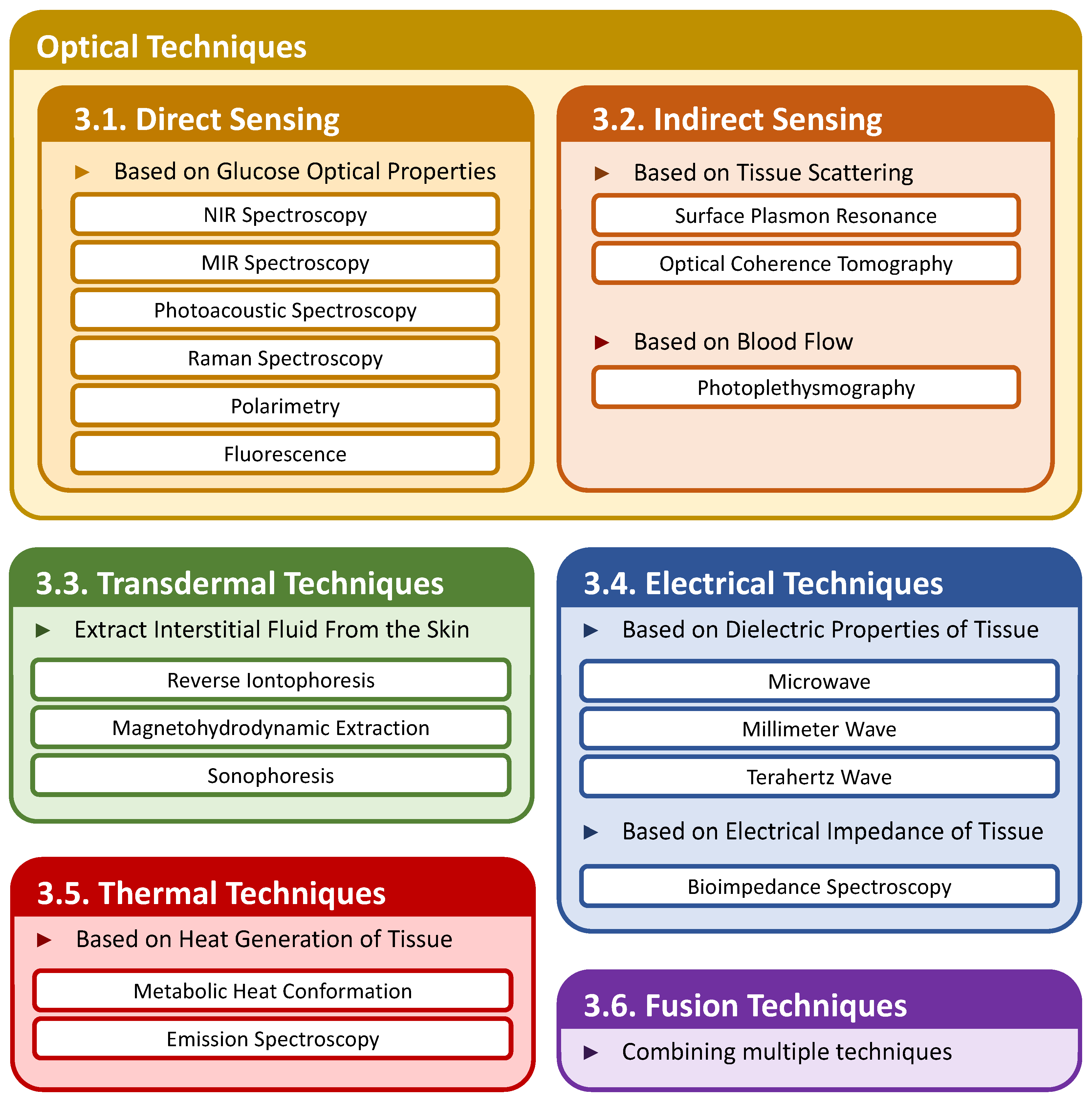
3. Noninvasive Sensing Techniques
3.1. Optical Techniques—Direct Sensing
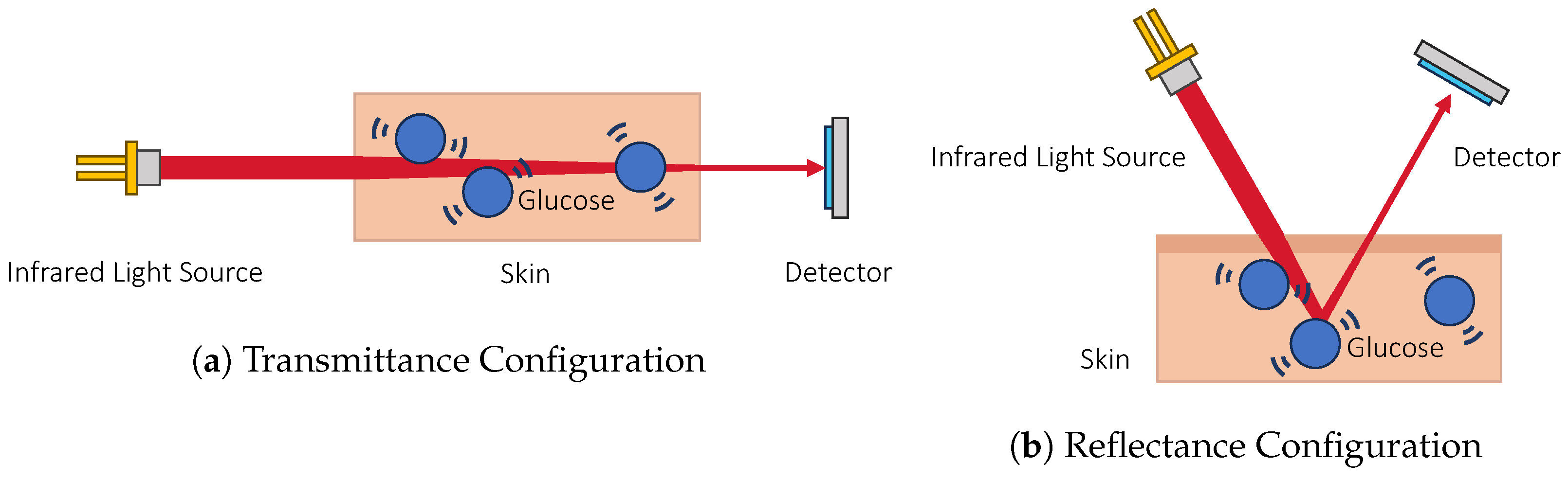
3.1.1. Infrared Absorption
3.1.2. Photoacoustic Spectroscopy
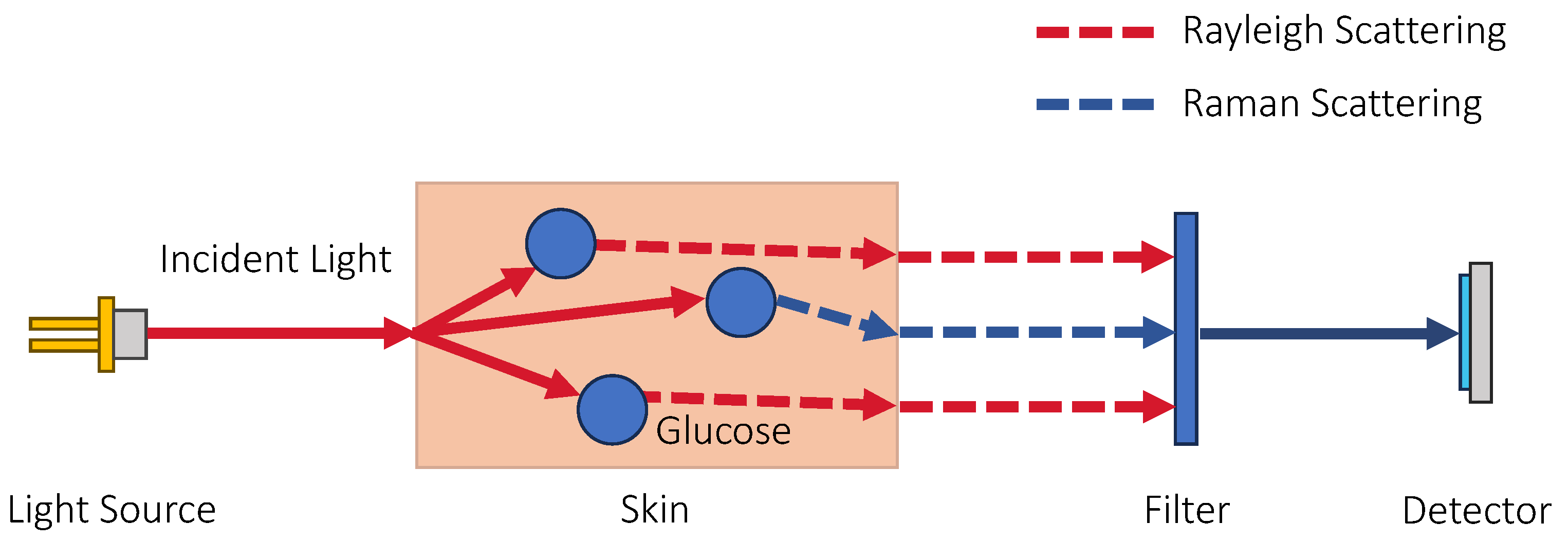
3.1.3. Raman Spectroscopy
3.1.4. Polarimetry
3.1.5. Fluorescence
3.1.6. Summary
3.2. Optical Techniques—Indirect Sensing
3.3. Transdermal Techniques
3.3.1. Reverse Iontophoresis
3.3.2. Magnetohydrodynamic Extraction
3.3.3. Sonophoresis
3.4. Electrical Technique
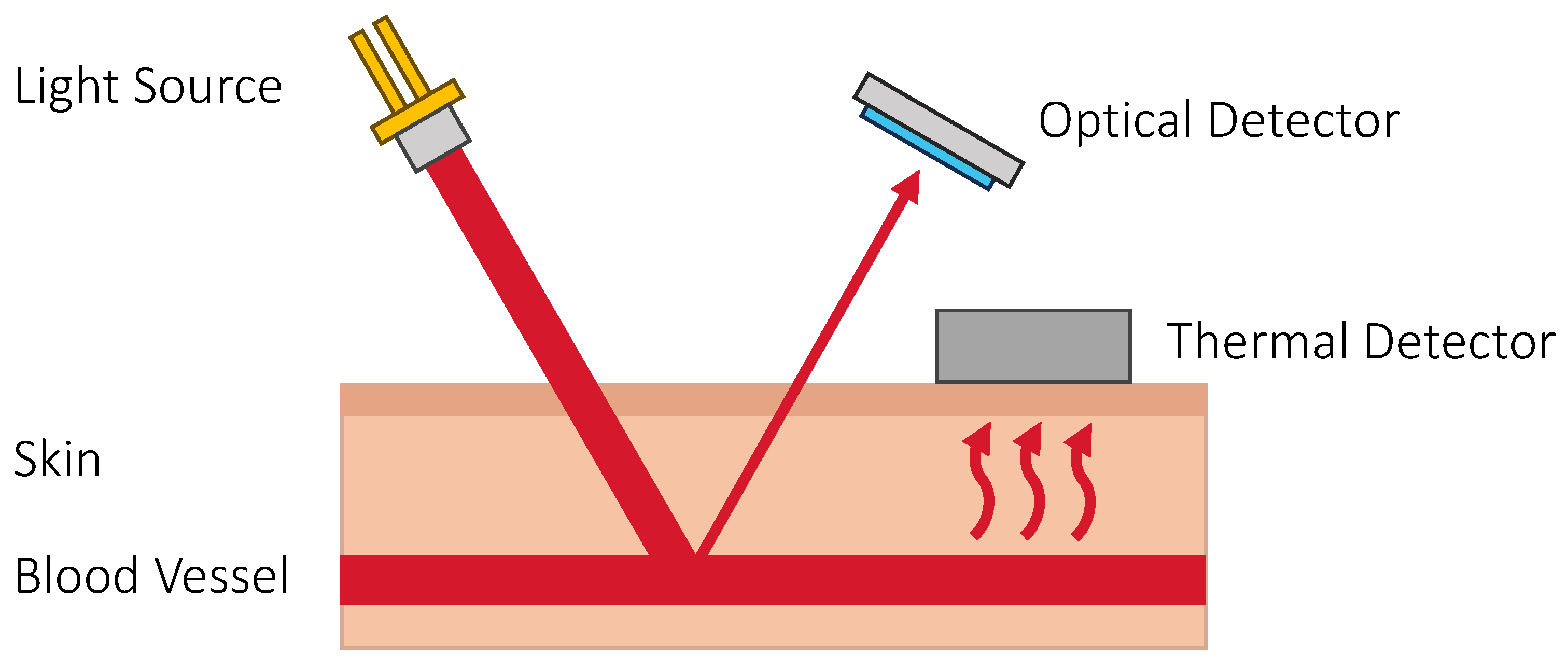
3.5. Thermal Techniques
3.6. Fusion Techniques
3.7. Summary
4. Current Barriers to Noninvasive Glucose Sensing
4.1. Confounding Factors
4.2. Selection of Sensing Location
4.3. Glucose Distribution
4.4. Model Generalization
4.5. Hardware Design
4.6. Acquisition of Ground Truth
4.7. Clinical Study
5. Potential Solution and Future Directions
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Centers for Disease Control and Prevention. National Diabetes Statistics Report. 2020. Available online: https://www.cdc.gov/diabetes/data/statistics-report/index.html (accessed on 7 February 2023).
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Das, S.R.; Hilliard, M.E.; Isaacs, D.; et al. 10. Cardiovascular Disease and Risk Management: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46, S158–S190. [Google Scholar] [CrossRef] [PubMed]
- DeSalvo, D.J.; Noor, N.; Xie, C.; Corathers, S.D.; Majidi, S.; McDonough, R.J.; Polsky, S.; Izquierdo, R.; Rioles, N.; Weinstock, R.; et al. Patient demographics and clinical outcomes among type 1 diabetes patients using continuous glucose monitors: Data from T1D Exchange real-world observational study. J. Diabetes Sci. Technol. 2021, 17, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Cengiz, E.; Tamborlane, W.V. A tale of two compartments: Interstitial versus blood glucose monitoring. Diabetes Technol. Ther. 2009, 11, S-11. [Google Scholar] [CrossRef] [PubMed]
- Thennadil, S.N.; Rennert, J.L.; Wenzel, B.J.; Hazen, K.H.; Ruchti, T.L.; Block, M.B. Comparison of glucose concentration in interstitial fluid, and capillary and venous blood during rapid changes in blood glucose levels. Diabetes Technol. Ther. 2001, 3, 357–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waghule, T.; Singhvi, G.; Dubey, S.K.; Pandey, M.M.; Gupta, G.; Singh, M.; Dua, K. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed. Pharmacother. 2019, 109, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.L. Real-time measurement of glucose concentration and average refractive index using a laser interferometer. Opt. Lasers Eng. 2008, 46, 666–670. [Google Scholar] [CrossRef]
- Mekonnen, B.K.; Yang, W.; Hsieh, T.H.; Liaw, S.K.; Yang, F.L. Accurate prediction of glucose concentration and identification of major contributing features from hardly distinguishable near-infrared spectroscopy. Biomed. Signal Process. Control 2020, 59, 101923. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Chen, S.; Yu, Y.L.; Wang, J.H. A miniaturized photoacoustic device with laptop readout for point-of-care testing of blood glucose. Talanta 2020, 209, 120527. [Google Scholar] [CrossRef]
- Aloraynan, A.; Rassel, S.; Xu, C.; Ban, D. A single wavelength mid-infrared photoacoustic spectroscopy for noninvasive glucose detection using machine learning. Biosensors 2022, 12, 166. [Google Scholar] [CrossRef] [PubMed]
- Kottmann, J.; Rey, J.M.; Luginbühl, J.; Reichmann, E.; Sigrist, M.W. Glucose sensing in human epidermis using mid-infrared photoacoustic detection. Biomed. Opt. Express 2012, 3, 667–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, T.P.; Shieh, H.L.; Ching, C.T.S.; Yao, Y.D.; Huang, S.H.; Liu, C.M.; Liu, W.H.; Chen, C.Y. Carbon nanotube composites for glucose biosensor incorporated with reverse iontophoresis function for noninvasive glucose monitoring. Int. J. Nanomed. 2010, 5, 343. [Google Scholar]
- Ching, C.T.S.; Sun, T.P.; Huang, S.H.; Shieh, H.L.; Chen, C.Y. A mediated glucose biosensor incorporated with reverse iontophoresis function for noninvasive glucose monitoring. Ann. Biomed. Eng. 2010, 38, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Olesberg, J.T.; Liu, L.; Zee, V.V.; Arnold, M.A. In vivo near-infrared spectroscopy of rat skin tissue with varying blood glucose levels. Anal. Chem. 2006, 78, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.A.; Liu, L.; Olesberg, J.T. Selectivity assessment of noninvasive glucose measurements based on analysis of multivariate calibration vectors. J. Diabetes Sci. Technol. 2007, 1, 454–462. [Google Scholar] [CrossRef] [Green Version]
- Shih, W.C.; Bechtel, K.L.; Rebec, M.V. Noninvasive glucose sensing by transcutaneous Raman spectroscopy. J. Biomed. Opt. 2015, 20, 051036. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.W.; Park, Y.S.; Chang, H.; Lee, W.; Singh, S.P.; Choi, W.; Galindo, L.H.; Dasari, R.R.; Nam, S.H.; Park, J.; et al. Direct observation of glucose fingerprint using in vivo Raman spectroscopy. Sci. Adv. 2020, 6, eaay5206. [Google Scholar] [CrossRef] [Green Version]
- Vashist, S.K. Non-invasive glucose monitoring technology in diabetes management: A review. Anal. Chim. Acta 2012, 750, 16–27. [Google Scholar] [CrossRef]
- Lin, T. Non-invasive glucose monitoring: A review of challenges and recent advances. Curr. Trends Biomed. Eng. Biosci. 2017, 6, 1–8. [Google Scholar] [CrossRef]
- Villena Gonzales, W.; Mobashsher, A.T.; Abbosh, A. The progress of glucose monitoring—A review of invasive to minimally and non-invasive techniques, devices and sensors. Sensors 2019, 19, 800. [Google Scholar] [CrossRef] [Green Version]
- Shokrekhodaei, M.; Quinones, S. Review of non-invasive glucose sensing techniques: Optical, electrical and breath acetone. Sensors 2020, 20, 1251. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46, S19–S40. [Google Scholar] [CrossRef]
- Hamman, R.F.; Bell, R.A.; Dabelea, D.; D’Agostino, R.B., Jr.; Dolan, L.; Imperatore, G.; Lawrence, J.M.; Linder, B.; Marcovina, S.M.; Mayer-Davis, E.J.; et al. The SEARCH for Diabetes in Youth study: Rationale, findings, and future directions. Diabetes Care 2014, 37, 3336–3344. [Google Scholar] [CrossRef] [Green Version]
- Mayer-Davis, E.J.; Lawrence, J.M.; Dabelea, D.; Divers, J.; Isom, S.; Dolan, L.; Imperatore, G.; Linder, B.; Marcovina, S.; Pettitt, D.J.; et al. Incidence trends of type 1 and type 2 diabetes among youths, 2002–2012. N. Engl. J. Med. 2017, 376, 1419–1429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Group, D.R. The Diabetes Control and Complications Trial (DCCT): Design and methodologic considerations for the feasibility phase. Diabetes 1986, 35, 530–545. [Google Scholar] [CrossRef]
- Control, D.; Group, C.T.R. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993, 329, 977–986. [Google Scholar]
- Diabetes Control and Complications Trial Research Group. Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. J. Pediatr. 1994, 125, 177–188. [Google Scholar] [CrossRef] [PubMed]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 5. Facilitating Positive Health Behaviors and Well-being to Improve Health Outcomes: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46, S68–S96. [Google Scholar] [CrossRef]
- Association, A.D. Children and adolescents: Standards of medical care in diabetes—2021. Diabetes Care 2021, 44, S180–S199. [Google Scholar] [CrossRef] [PubMed]
- Battelino, T.; Danne, T.; Bergenstal, R.M.; Amiel, S.A.; Beck, R.; Biester, T.; Bosi, E.; Buckingham, B.A.; Cefalu, W.T.; Close, K.L.; et al. Clinical targets for continuous glucose monitoring data interpretation: Recommendations from the international consensus on time in range. Diabetes Care 2019, 42, 1593–1603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forlenza, G.P.; Kushner, T.; Messer, L.H.; Wadwa, R.P.; Sankaranarayanan, S. Factory-calibrated continuous glucose monitoring: How and why it works, and the dangers of reuse beyond approved duration of wear. Diabetes Technol. Ther. 2019, 21, 222–229. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; Wang, J. Wearable non-invasive epidermal glucose sensors: A review. Talanta 2018, 177, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Roe, J.N.; Smoller, B.R. Bloodless glucose measurements. Crit. Rev. Ther. Drug Carr. Syst. 1998, 15, 199–241. [Google Scholar]
- Christiansen, M.; Bailey, T.; Watkins, E.; Liljenquist, D.; Price, D.; Nakamura, K.; Boock, R.; Peyser, T. A new-generation continuous glucose monitoring system: Improved accuracy and reliability compared with a previous-generation system. Diabetes Technol. Ther. 2013, 15, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Siegmund, T.; Heinemann, L.; Kolassa, R.; Thomas, A. Discrepancies between blood glucose and interstitial glucose—Technological artifacts or physiology: Implications for selection of the appropriate therapeutic target. J. Diabetes Sci. Technol. 2017, 11, 766–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iguchi, S.; Kudo, H.; Saito, T.; Ogawa, M.; Saito, H.; Otsuka, K.; Funakubo, A.; Mitsubayashi, K. A flexible and wearable biosensor for tear glucose measurement. Biomed. Microdevices 2007, 9, 603–609. [Google Scholar] [CrossRef]
- Chu, M.X.; Miyajima, K.; Takahashi, D.; Arakawa, T.; Sano, K.; Sawada, S.I.; Kudo, H.; Iwasaki, Y.; Akiyoshi, K.; Mochizuki, M.; et al. Soft contact lens biosensor for in situ monitoring of tear glucose as non-invasive blood sugar assessment. Talanta 2011, 83, 960–965. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.; Kim, S.Y.; Cheong, W.H.; Jang, J.; Park, Y.G.; Na, K.; Kim, Y.T.; Heo, J.H.; Lee, C.Y.; et al. Soft, smart contact lenses with integrations of wireless circuits, glucose sensors, and displays. Sci. Adv. 2018, 4, eaap9841. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.R.; Hung, C.C.; Chiu, H.Y.; Chang, P.H.; Li, B.R.; Cheng, S.J.; Yang, J.W.; Lin, S.F.; Chen, G.Y. Noninvasive glucose monitoring with a contact lens and smartphone. Sensors 2018, 18, 3208. [Google Scholar] [CrossRef] [Green Version]
- Elsherif, M.; Hassan, M.U.; Yetisen, A.K.; Butt, H. Wearable contact lens biosensors for continuous glucose monitoring using smartphones. ACS Nano 2018, 12, 5452–5462. [Google Scholar] [CrossRef]
- Deng, M.; Song, G.; Zhong, K.; Wang, Z.; Xia, X.; Tian, Y. Wearable fluorescent contact lenses for monitoring glucose via a smartphone. Sens. Actuators B Chem. 2022, 352, 131067. [Google Scholar] [CrossRef]
- Heikenfeld, J. Non-invasive analyte access and sensing through eccrine sweat: Challenges and outlook circa 2016. Electroanalysis 2016, 28, 1242–1249. [Google Scholar] [CrossRef]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 2016, 529, 509–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, A.; Kang, D.; Xue, Y.; Lee, S.; Pielak, R.M.; Kim, J.; Hwang, T.; Min, S.; Banks, A.; Bastien, P.; et al. A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Sci. Transl. Med. 2016, 8, 366ra165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín, A.; Kim, J.; Kurniawan, J.F.; Sempionatto, J.R.; Moreto, J.R.; Tang, G.; Campbell, A.S.; Shin, A.; Lee, M.Y.; Liu, X.; et al. Epidermal microfluidic electrochemical detection system: Enhanced sweat sampling and metabolite detection. ACS Sens. 2017, 2, 1860–1868. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Song, C.; Hong, Y.S.; Kim, M.; Cho, H.R.; Kang, T.; Shin, K.; Choi, S.H.; Hyeon, T.; Kim, D.H. Wearable/disposable sweat-based glucose monitoring device with multistage transdermal drug delivery module. Sci. Adv. 2017, 3, e1601314. [Google Scholar] [CrossRef] [Green Version]
- Bariya, M.; Nyein, H.Y.Y.; Javey, A. Wearable sweat sensors. Nat. Electron. 2018, 1, 160–171. [Google Scholar] [CrossRef]
- Cui, Y.; Duan, W.; Jin, Y.; Wo, F.; Xi, F.; Wu, J. Ratiometric fluorescent nanohybrid for noninvasive and visual monitoring of sweat glucose. ACS Sens. 2020, 5, 2096–2105. [Google Scholar] [CrossRef]
- Soni, A.; Jha, S.K. A paper strip based non-invasive glucose biosensor for salivary analysis. Biosens. Bioelectron. 2015, 67, 763–768. [Google Scholar] [CrossRef]
- Zhang, W.; Du, Y.; Wang, M.L. Noninvasive glucose monitoring using saliva nano-biosensor. Sens. Bio-Sens. Res. 2015, 4, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Arakawa, T.; Kuroki, Y.; Nitta, H.; Chouhan, P.; Toma, K.; Sawada, S.I.; Takeuchi, S.; Sekita, T.; Akiyoshi, K.; Minakuchi, S.; et al. Mouthguard biosensor with telemetry system for monitoring of saliva glucose: A novel cavitas sensor. Biosens. Bioelectron. 2016, 84, 106–111. [Google Scholar] [CrossRef] [Green Version]
- Diouf, A.; Bouchikhi, B.; El Bari, N. A nonenzymatic electrochemical glucose sensor based on molecularly imprinted polymer and its application in measuring saliva glucose. Mater. Sci. Eng. C 2019, 98, 1196–1209. [Google Scholar] [CrossRef]
- Hu, S.; Jiang, Y.; Wu, Y.; Guo, X.; Ying, Y.; Wen, Y.; Yang, H. Enzyme-free tandem reaction strategy for surface-enhanced Raman scattering detection of glucose by using the composite of Au nanoparticles and porphyrin-based metal–organic framework. ACS Appl. Mater. Interfaces 2020, 12, 55324–55330. [Google Scholar] [CrossRef]
- Geelhoed-Duijvestijn, P.; Vegelyte, D.; Kownacka, A.; Anton, N.; Joosse, M.; Wilson, C. Performance of the prototype NovioSense noninvasive biosensor for tear glucose in type 1 diabetes. J. Diabetes Sci. Technol. 2021, 15, 1320–1325. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Pratt, B.; Honikel, M.; Jenish, A.; Ramesh, B.; Alkhan, A.; La Belle, J.T. Toward the development of a glucose dehydrogenase-based saliva glucose sensor without the need for sample preparation. J. Diabetes Sci. Technol. 2018, 12, 83–89. [Google Scholar] [CrossRef] [Green Version]
- La Count, T.D.; Jajack, A.; Heikenfeld, J.; Kasting, G.B. Modeling glucose transport from systemic circulation to sweat. J. Pharm. Sci. 2019, 108, 364–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brothers, M.C.; DeBrosse, M.; Grigsby, C.C.; Naik, R.R.; Hussain, S.M.; Heikenfeld, J.; Kim, S.S. Achievements and challenges for real-time sensing of analytes in sweat within wearable platforms. Accounts Chem. Res. 2019, 52, 297–306. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Jeerapan, I.; Wang, J. Wearable chemical sensors: Present challenges and future prospects. ACS Sens. 2016, 1, 464–482. [Google Scholar] [CrossRef]
- Heikenfeld, J.; Jajack, A.; Feldman, B.; Granger, S.W.; Gaitonde, S.; Begtrup, G.; Katchman, B.A. Accessing analytes in biofluids for peripheral biochemical monitoring. Nat. Biotechnol. 2019, 37, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Johnston, L.; Wang, G.; Hu, K.; Qian, C.; Liu, G. Advances in biosensors for continuous glucose monitoring towards wearables. Front. Bioeng. Biotechnol. 2021, 9, 733810. [Google Scholar] [CrossRef]
- Vasconcelos, A.C.U.; Soares, M.S.M.; Almeida, P.C.; Soares, T.C. Comparative study of the concentration of salivary and blood glucose in type 2 diabetic patients. J. Oral Sci. 2010, 52, 293–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyein, H.Y.Y.; Bariya, M.; Kivimäki, L.; Uusitalo, S.; Liaw, T.S.; Jansson, E.; Ahn, C.H.; Hangasky, J.A.; Zhao, J.; Lin, Y.; et al. Regional and correlative sweat analysis using high-throughput microfluidic sensing patches toward decoding sweat. Sci. Adv. 2019, 5, eaaw9906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aihara, M.; Kubota, N.; Minami, T.; Shirakawa, R.; Sakurai, Y.; Hayashi, T.; Iwamoto, M.; Takamoto, I.; Kubota, T.; Suzuki, R.; et al. Association between tear and blood glucose concentrations: Random intercept model adjusted with confounders in tear samples negative for occult blood. J. Diabetes Investig. 2021, 12, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.K.; Potts, R.O.; Ackerman, N.R.; Fermi, S.J.; Tamada, J.A.; Chase, H.P. Correlation of fingerstick blood glucose measurements with GlucoWatch biographer glucose results in young subjects with type 1 diabetes. Diabetes Care 1999, 22, 1708–1714. [Google Scholar] [CrossRef] [PubMed]
- Clarke, W.L.; Cox, D.; Gonder-Frederick, L.A.; Carter, W.; Pohl, S.L. Evaluating clinical accuracy of systems for self-monitoring of blood glucose. Diabetes Care 1987, 10, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Parkes, J.L.; Slatin, S.L.; Pardo, S.; Ginsberg, B.H. A new consensus error grid to evaluate the clinical significance of inaccuracies in the measurement of blood glucose. Diabetes Care 2000, 23, 1143–1148. [Google Scholar] [CrossRef] [Green Version]
- Food, U.; Administration, D. Self-Monitoring Blood Glucose Test Systems for Over-the-Counter Use. 2020. Available online: https://www.regulations.gov/docket/FDA-2013-D-1446 (accessed on 9 January 2023).
- Doyle, R.J.; Burgan, H.M. Use of ultraviolet absorption for determination of sugars. Anal. Biochem. 1966, 17, 171–174. [Google Scholar] [CrossRef]
- Geddes, C.D.; Lakowicz, J.R. Glucose Sensing; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007; Volume 11. [Google Scholar]
- Kottmann, J.; Rey, J.M.; Sigrist, M.W. Mid-Infrared photoacoustic detection of glucose in human skin: Towards non-invasive diagnostics. Sensors 2016, 16, 1663. [Google Scholar] [CrossRef] [Green Version]
- Tura, A.; Maran, A.; Pacini, G. Non-invasive glucose monitoring: Assessment of technologies and devices according to quantitative criteria. Diabetes Res. Clin. Pract. 2007, 77, 16–40. [Google Scholar] [CrossRef]
- Notingher, I.; Imhof, R.E. Mid-infrared in vivo depth-profiling of topical chemicals on skin. Ski. Res. Technol. 2004, 10, 113–121. [Google Scholar] [CrossRef]
- Duck, F.A. Physical Properties of Tissues: A Comprehensive Reference Book; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Chowdhury, M.K.; Srivastava, A.; Sharma, N.; Sharma, S. Challenges & countermeasures in optical noninvasive blood glucose detection. Int. J. Innov. Res. Sci. Eng. Technol. 2013, 2, 324–329. [Google Scholar]
- Maruo, K.; Yamada, Y. Near-infrared noninvasive blood glucose prediction without using multivariate analyses: Introduction of imaginary spectra due to scattering change in the skin. J. Biomed. Opt. 2015, 20, 047003. [Google Scholar] [CrossRef]
- Zhang, Z.; Cai, Z.; Han, G.; Sheng, W.; Liu, J. Estimation of glucose absorption spectrum at its optimum pathlength for every wavelength over a wide range. Spectrosc. Lett. 2016, 49, 588–595. [Google Scholar] [CrossRef]
- Chen, J.; Arnold, M.A.; Small, G.W. Comparison of combination and first overtone spectral regions for near-infrared calibration models for glucose and other biomolecules in aqueous solutions. Anal. Chem. 2004, 76, 5405–5413. [Google Scholar] [CrossRef] [PubMed]
- Ash, C.; Dubec, M.; Donne, K.; Bashford, T. Effect of wavelength and beam width on penetration in light-tissue interaction using computational methods. Lasers Med. Sci. 2017, 32, 1909–1918. [Google Scholar] [CrossRef]
- Srichan, C.; Srichan, W.; Danvirutai, P.; Ritsongmuang, C.; Sharma, A.; Anutrakulchai, S. Non-invasively accuracy enhanced blood glucose sensor using shallow dense neural networks with NIR monitoring and medical features. Sci. Rep. 2022, 12, 1769. [Google Scholar] [CrossRef] [PubMed]
- Malin, S.F.; Ruchti, T.L.; Blank, T.B.; Thennadil, S.N.; Monfre, S.L. Noninvasive prediction of glucose by near-infrared diffuse reflectance spectroscopy. Clin. Chem. 1999, 45, 1651–1658. [Google Scholar] [CrossRef]
- Maruo, K.; Tsurugi, M.; Chin, J.; Ota, T.; Arimoto, H.; Yamada, Y.; Tamura, M.; Ishii, M.; Ozaki, Y. Noninvasive blood glucose assay using a newly developed near-infrared system. IEEE J. Sel. Top. Quantum Electron. 2003, 9, 322–330. [Google Scholar] [CrossRef]
- Blank, T.B.; Ruchti, T.L.; Lorenz, A.D.; Monfre, S.L.; Makarewicz, M.; Mattu, M.; Hazen, K. Clinical results from a noninvasive blood glucose monitor. In Proceedings of the Optical Diagnostics and Sensing of Biological Fluids and Glucose and Cholesterol Monitoring II, SPIE, San Jose, CA, USA, 23–24 January 2002; Volume 4624, pp. 1–10. [Google Scholar]
- Burmeister, J.J.; Arnold, M.A. Evaluation of measurement sites for noninvasive blood glucose sensing with near-infrared transmission spectroscopy. Clin. Chem. 1999, 45, 1621–1627. [Google Scholar] [CrossRef]
- Lam, S.C.; Chung, J.W.; Fan, K.; Wong, T.K. Non-invasive blood glucose measurement by near infrared spectroscopy: Machine drift, time drift and physiological effect. Spectroscopy 2010, 24, 629–639. [Google Scholar] [CrossRef]
- Liakat, S.; Bors, K.A.; Xu, L.; Woods, C.M.; Doyle, J.; Gmachl, C.F. Noninvasive in vivo glucose sensing on human subjects using mid-infrared light. Biomed. Opt. Express 2014, 5, 2397–2404. [Google Scholar] [CrossRef]
- Haxha, S.; Jhoja, J. Optical based noninvasive glucose monitoring sensor prototype. IEEE Photonics J. 2016, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Kasahara, R.; Kino, S.; Soyama, S.; Matsuura, Y. Noninvasive glucose monitoring using mid-infrared absorption spectroscopy based on a few wavenumbers. Biomed. Opt. Express 2018, 9, 289–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.Y.; Zhou, Q.; Xu, G.; Wang, R.T.; Tai, E.G.; Xie, L.; Zhang, Q.; Guan, Y.; Huang, X. Non-invasive blood glucose measurement of 95% certainty by pressure regulated Mid-IR. Talanta 2019, 197, 211–217. [Google Scholar] [CrossRef]
- Han, G.; Chen, S.; Wang, X.; Wang, J.; Wang, H.; Zhao, Z. Noninvasive blood glucose sensing by near-infrared spectroscopy based on PLSR combines SAE deep neural network approach. Infrared Phys. Technol. 2021, 113, 103620. [Google Scholar] [CrossRef]
- Amir, O.; Weinstein, D.; Zilberman, S.; Less, M.; Perl-Treves, D.; Primack, H.; Weinstein, A.; Gabis, E.; Fikhte, B.; Karasik, A. Continuous noninvasive glucose monitoring technology based on “occlusion spectroscopy”. J. Diabetes Sci. Technol. 2007, 1, 463–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segman, Y. Device and method for noninvasive glucose assessment. J. Diabetes Sci. Technol. 2018, 12, 1159–1168. [Google Scholar] [CrossRef] [Green Version]
- Pfützner, A.; Strobl, S.; Demircik, F.; Redert, L.; Pfützner, J.; Pfützner, A.H.; Lier, A. Evaluation of a new noninvasive glucose monitoring device by means of standardized meal experiments. J. Diabetes Sci. Technol. 2018, 12, 1178–1183. [Google Scholar] [CrossRef]
- Beard, P. Biomedical photoacoustic imaging. Interface Focus 2011, 1, 602–631. [Google Scholar] [CrossRef]
- Tam, A.C. Applications of photoacoustic sensing techniques. Rev. Mod. Phys. 1986, 58, 381. [Google Scholar] [CrossRef]
- Yao, J.; Wang, L.V. Sensitivity of photoacoustic microscopy. Photoacoustics 2014, 2, 87–101. [Google Scholar] [CrossRef] [Green Version]
- Von Lilienfeld-Toal, H.; Weidenmüller, M.; Xhelaj, A.; Mäntele, W. A novel approach to non-invasive glucose measurement by mid-infrared spectroscopy: The combination of quantum cascade lasers (QCL) and photoacoustic detection. Vib. Spectrosc. 2005, 38, 209–215. [Google Scholar] [CrossRef]
- Pleitez, M.; von Lilienfeld-Toal, H.; Mäntele, W. Infrared spectroscopic analysis of human interstitial fluid in vitro and in vivo using FT-IR spectroscopy and pulsed quantum cascade lasers (QCL): Establishing a new approach to non invasive glucose measurement. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 85, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Pai, P.P.; Sanki, P.K.; De, A.; Banerjee, S. NIR photoacoustic spectroscopy for non-invasive glucose measurement. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; IEEE: Piscataway, NJ, USA, 2015; pp. 7978–7981. [Google Scholar]
- Pai, P.P.; Sanki, P.K.; Sahoo, S.K.; De, A.; Bhattacharya, S.; Banerjee, S. Cloud computing-based non-invasive glucose monitoring for diabetic care. IEEE Trans. Circuits Syst. I Regul. Pap. 2017, 65, 663–676. [Google Scholar] [CrossRef]
- Pleitez, M.A.; Lieblein, T.; Bauer, A.; Hertzberg, O.; von Lilienfeld-Toal, H.; Mäntele, W. Windowless ultrasound photoacoustic cell for in vivo mid-IR spectroscopy of human epidermis: Low interference by changes of air pressure, temperature, and humidity caused by skin contact opens the possibility for a non-invasive monitoring of glucose in the interstitial fluid. Rev. Sci. Instruments 2013, 84, 084901. [Google Scholar]
- Pleitez, M.A.; Lieblein, T.; Bauer, A.; Hertzberg, O.; von Lilienfeld-Toal, H.; Mäntele, W. In vivo noninvasive monitoring of glucose concentration in human epidermis by mid-infrared pulsed photoacoustic spectroscopy. Anal. Chem. 2013, 85, 1013–1020. [Google Scholar] [CrossRef]
- Bauer, A.; Hertzberg, O.; Küderle, A.; Strobel, D.; Pleitez, M.A.; Mäntele, W. IR-spectroscopy of skin in vivo: Optimal skin sites and properties for non-invasive glucose measurement by photoacoustic and photothermal spectroscopy. J. Biophotonics 2018, 11, e201600261. [Google Scholar] [CrossRef]
- Sim, J.Y.; Ahn, C.G.; Jeong, E.J.; Kim, B.K. In vivo microscopic photoacoustic spectroscopy for non-invasive glucose monitoring invulnerable to skin secretion products. Sci. Rep. 2018, 8, 1059. [Google Scholar] [CrossRef] [Green Version]
- Smith, E.; Dent, G. Modern Raman Spectroscopy: A Practical Approach; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Shao, J.; Lin, M.; Li, Y.; Li, X.; Liu, J.; Liang, J.; Yao, H. In vivo blood glucose quantification using Raman spectroscopy. PLoS ONE 2012, 7, e48127. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Zang, H.; Sun, H.; Jiao, X.; Wang, K.; Liu, T.C.Y.; Meng, Y. A noninvasive accurate measurement of blood glucose levels with raman spectroscopy of blood in microvessels. Molecules 2019, 24, 1500. [Google Scholar] [CrossRef] [Green Version]
- Pleus, S.; Schauer, S.; Jendrike, N.; Zschornack, E.; Link, M.; Hepp, K.D.; Haug, C.; Freckmann, G. Proof of concept for a new Raman-based prototype for noninvasive glucose monitoring. J. Diabetes Sci. Technol. 2021, 15, 11–18. [Google Scholar] [CrossRef]
- Enejder, A.M.; Scecina, T.G.; Oh, J.; Hunter, M.; Shih, W.; Sasic, S.; Horowitz, G.L.; Feld, M.S. Raman spectroscopy for noninvasive glucose measurements. J. Biomed. Opt. 2005, 10, 031114. [Google Scholar] [CrossRef] [PubMed]
- Lipson, J.; Bernhardt, J.; Block, U.; Freeman, W.R.; Hofmeister, R.; Hristakeva, M.; Lenosky, T.; McNamara, R.; Petrasek, D.; Veltkamp, D.; et al. Requirements for calibration in noninvasive glucose monitoring by Raman spectroscopy. J. Diabetes Sci. Technol. 2009, 3, 233–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundsgaard-Nielsen, S.M.; Pors, A.; Banke, S.O.; Henriksen, J.E.; Hepp, D.K.; Weber, A. Critical-depth Raman spectroscopy enables home-use non-invasive glucose monitoring. PLoS ONE 2018, 13, e0197134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanlon, E.; Manoharan, R.; Koo, T.; Shafer, K.; Motz, J.; Fitzmaurice, M.; Kramer, J.; Itzkan, I.; Dasari, R.; Feld, M. Prospects for in vivo Raman spectroscopy. Phys. Med. Biol. 2000, 45, R1. [Google Scholar] [CrossRef] [Green Version]
- Windholz, M. The Merck Index: An Encyclopedia of Chemicals and Drugs; Merck & Co.: Rahway, NJ, USA, 1976. [Google Scholar]
- Stefan-van Staden, R.I.; Mitrofan, G. Molecular enantiorecognition of l-glucose and d-glucose in whole blood samples. Chirality 2018, 30, 680–685. [Google Scholar] [CrossRef]
- Wood, M.F.; Ghosh, N.; Guo, X.; Vitkin, I.A. Towards noninvasive glucose sensing using polarization analysis of multiply scattered light. In Handbook of Optical Sensing of Glucose in Biological Fluids and Tissues; CRC Press: Boca Raton, FL, USA, 2008; Volume 12. [Google Scholar]
- Rabinovitch, B.; March, W.; Adams, R.L. Noninvasive glucose monitoring of the aqueous humor of the eye: Part I. Measurement of very small optical rotations. Diabetes Care 1982, 5, 254–258. [Google Scholar] [CrossRef]
- Coté, G.L.; Fox, M.D.; Northrop, R.B. Noninvasive optical polarimetric glucose sensing using a true phase measurement technique. IEEE Trans. Biomed. Eng. 1992, 39, 752–756. [Google Scholar] [CrossRef]
- Cameron, B.D.; Cote, G.L. Noninvasive glucose sensing utilizing a digital closed-loop polarimetric approach. IEEE Trans. Biomed. Eng. 1997, 44, 1221–1227. [Google Scholar] [CrossRef]
- Malik, B.H.; Coté, G.L. Real-time, closed-loop dual-wavelength optical polarimetry for glucose monitoring. J. Biomed. Opt. 2010, 15, 017002. [Google Scholar] [CrossRef]
- Phan, Q.H.; Lai, Y.R.; Xiao, W.Z.; Pham, T.T.; Lien, C.H. Surface plasmon resonance prism coupler for enhanced circular birefringence sensing and application to non-invasive glucose detection. Opt. Express 2020, 28, 24889–24899. [Google Scholar] [CrossRef]
- Baba, J.S.; Cameron, B.D.; Cote, G.L. Effect of temperature, pH, and corneal birefringence on polarimetric glucose monitoring in the eye. J. Biomed. Opt. 2002, 7, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Purvinis, G.; Cameron, B.D.; Altrogge, D.M. Noninvasive polarimetric-based glucose monitoring: An in vivo study. J. Diabetes Sci. Technol. 2011, 5, 380–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirnstill, C.W.; Malik, B.H.; Gresham, V.C.; Coté, G.L. In vivo glucose monitoring using dual-wavelength polarimetry to overcome corneal birefringence in the presence of motion. Diabetes Technol. Ther. 2012, 14, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Bai, D.; Prioleau, T.; Bui, N.; Vu, T.; Zhou, X. Noninvasive glucose monitoring using polarized light. In Proceedings of the Proceedings of the 18th Conference on Embedded Networked Sensor Systems, Virtual, 16–19 November 2020; pp. 544–557. [Google Scholar]
- Jacques, S.L.; Lee, K.; Ramella-Roman, J.C. Scattering of polarized light by biological tissues. In Proceedings of the Saratov Fall Meeting’99: Optical Technologies in Biophysics and Medicine, SPIE, Saratov, Russia, 5–8 October 1999; Volume 4001, pp. 14–28. [Google Scholar]
- Aslan, K.; Lakowicz, J.R.; Geddes, C.D. Nanogold-plasmon-resonance-based glucose sensing. Anal. Biochem. 2004, 330, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Ji, W.; Chu, S.; Qian, S.; Wang, F.; Masson, J.F.; Han, X.; Peng, W. Fiber-optic surface plasmon resonance glucose sensor enhanced with phenylboronic acid modified Au nanoparticles. Biosens. Bioelectron. 2018, 117, 637–643. [Google Scholar] [CrossRef]
- Maier, J.S.; Walker, S.A.; Fantini, S.; Franceschini, M.A.; Gratton, E. Possible correlation between blood glucose concentration and the reduced scattering coefficient of tissues in the near infrared. Opt. Lett. 1994, 19, 2062–2064. [Google Scholar] [CrossRef] [Green Version]
- Larin, K.V.; Motamedi, M.; Ashitkov, T.V.; Esenaliev, R.O. Specificity of noninvasive blood glucose sensing using optical coherence tomography technique: A pilot study. Phys. Med. Biol. 2003, 48, 1371. [Google Scholar] [CrossRef]
- Kinnunen, M.T.; Myllyla, R.A.; Vainio, S. Detecting glucose-induced changes in in vitro and in vivo experiments with optical coherence tomography. J. Biomed. Opt. 2008, 13, 021111. [Google Scholar] [CrossRef]
- Gabbay, R.A.; Sivarajah, S. Optical coherence tomography-based continuous noninvasive glucose monitoring in patients with diabetes. Diabetes Technol. Ther. 2008, 10, 188–193. [Google Scholar] [CrossRef]
- Kuranov, R.V.; Sapozhnikova, V.V.; Prough, D.S.; Cicenaite, I.; Esenaliev, R.O. In vivo study of glucose-induced changes in skin properties assessed with optical coherence tomography. Phys. Med. Biol. 2006, 51, 3885. [Google Scholar] [CrossRef]
- Esenaliev, R.O.; Larin, K.V.; Larina, I.V.; Motamedi, M. Noninvasive monitoring of glucose concentration with optical coherence tomography. Opt. Lett. 2001, 26, 992–994. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.L.; Lo, Y.L.; Liao, C.C.; Phan, Q.H. Noninvasive measurement of glucose concentration on human fingertip by optical coherence tomography. J. Biomed. Opt. 2018, 23, 047001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, E.Y.; Baik, J.; Kim, H.; Park, S.M.; Kim, C. Ultrasound-modulated optical glucose sensing using a 1645 nm laser. Sci. Rep. 2020, 10, 13361. [Google Scholar] [CrossRef] [PubMed]
- Monte-Moreno, E. Non-invasive estimate of blood glucose and blood pressure from a photoplethysmograph by means of machine learning techniques. Artif. Intell. Med. 2011, 53, 127–138. [Google Scholar] [CrossRef]
- Hossain, S.; Debnath, B.; Biswas, S.; Al-Hossain, M.J.; Anika, A.; Navid, S.K.Z. Estimation of Blood Glucose from PPG Signal Using Convolutional Neural Network. In Proceedings of the 2019 IEEE International Conference on Biomedical Engineering, Computer and Information Technology for Health (BECITHCON), Dhaka, Bangladesh, 28–30 November 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 53–58. [Google Scholar]
- Habbu, S.; Dale, M.; Ghongade, R. Estimation of blood glucose by non-invasive method using photoplethysmography. Sādhanā 2019, 44, 135. [Google Scholar] [CrossRef] [Green Version]
- Hina, A.; Saadeh, W. A noninvasive glucose monitoring SoC based on single wavelength photoplethysmography. IEEE Trans. Biomed. Circuits Syst. 2020, 14, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ling, B.W.K.; Tian, Z.; Ho, Y.W.; Teo, K.L. Joint empirical mode decomposition, exponential function estimation and L 1 norm approach for estimating mean value of photoplethysmogram and blood glucose level. IET Signal Process. 2020, 14, 652–665. [Google Scholar] [CrossRef]
- Gupta, S.S.; Kwon, T.H.; Hossain, S.; Kim, K.D. Towards non-invasive blood glucose measurement using machine learning: An all-purpose PPG system design. Biomed. Signal Process. Control 2021, 68, 102706. [Google Scholar] [CrossRef]
- Castaneda, D.; Esparza, A.; Ghamari, M.; Soltanpur, C.; Nazeran, H. A review on wearable photoplethysmography sensors and their potential future applications in health care. Int. J. Biosens. Bioelectron. 2018, 4, 195. [Google Scholar]
- Merino, V.; Kalia, Y.N.; Guy, R.H. Transdermal therapy and diagnosis by iontophoresis. Trends Biotechnol. 1997, 15, 288–290. [Google Scholar] [CrossRef]
- Green, P.G. Iontophoretic delivery of peptide drugs. J. Control. Release 1996, 41, 33–48. [Google Scholar] [CrossRef]
- Burnette, R.R.; Ongpipattanakul, B. Characterization of the permselective properties of excised human skin during iontophoresis. J. Pharm. Sci. 1987, 76, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Potts, R.O.; Tamada, J.A.; Tierney, M.J. Glucose monitoring by reverse iontophoresis. Diabetes/Metab. Res. Rev. 2002, 18, S49–S53. [Google Scholar] [CrossRef] [PubMed]
- Tierney, M.J.; Tamada, J.A.; Potts, R.O.; Eastman, R.C.; Pitzer, K.; Ackerman, N.R.; Fermi, S.J. The GlucoWatch® biographer: A frequent, automatic and noninvasive glucose monitor. Ann. Med. 2000, 32, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Tierney, M.; Tamada, J.; Potts, R.; Jovanovic, L.; Garg, S.; Cygnus Research Team. Clinical evaluation of the GlucoWatch® biographer: A continual, non-invasive glucose monitor for patients with diabetes. Biosens. Bioelectron. 2001, 16, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Pitzer, K.R.; Desai, S.; Dunn, T.; Jayalakshmi, S.E.; Kennedy, J.; Tamada, J.A.; Potts, R.O. Detection of hypoglycemia with the GlucoWatch biographer. Clin. Diabetol. 2001, 2, 307–314. [Google Scholar] [CrossRef] [Green Version]
- McCormick, C.; Heath, D.; Connolly, P. Towards blood free measurement of glucose and potassium in humans using reverse iontophoresis. Sensors Actuators B Chem. 2012, 166, 593–600. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Jia, W.; Yardımcı, C.; Wang, X.; Ramirez, J.; Wang, J. Tattoo-based noninvasive glucose monitoring: A proof-of-concept study. Anal. Chem. 2015, 87, 394–398. [Google Scholar] [CrossRef]
- De la Paz, E.; Barfidokht, A.; Rios, S.; Brown, C.; Chao, E.; Wang, J. Extended Noninvasive Glucose Monitoring in the Interstitial Fluid Using an Epidermal Biosensing Patch. Anal. Chem. 2021, 93, 12767–12775. [Google Scholar] [CrossRef]
- Cai, S.; Xu, C.; Jiang, D.; Yuan, M.; Zhang, Q.; Li, Z.; Wang, Y. Air-permeable electrode for highly sensitive and noninvasive glucose monitoring enabled by graphene fiber fabrics. Nano Energy 2022, 93, 106904. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, J.; Guo, Y.; Lv, T.; Chen, Z.; Li, N.; Cao, S.; Chen, B.; Chen, T. Integration of interstitial fluid extraction and glucose detection in one device for wearable non-invasive blood glucose sensors. Biosens. Bioelectron. 2021, 179, 113078. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Jiang, D.; Ge, Y.; Huang, L.; Xiao, Y.; Ren, X.; Liu, X.; Zhang, Q.; Wang, Y. A PEDOT: PSS conductive hydrogel incorporated with Prussian blue nanoparticles for wearable and noninvasive monitoring of glucose. Chem. Eng. J. 2022, 431, 134109. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, S.; Zhang, S.; Li, Y.; Qu, Z.; Chen, Y.; Lu, B.; Wang, X.; Feng, X. Skin-like biosensor system via electrochemical channels for noninvasive blood glucose monitoring. Sci. Adv. 2017, 3, e1701629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, T.; Li, H.; Zhang, N.; Jiang, X.; Yu, X.; Yang, Q.; Jin, Z.; Meng, H.; Chang, L. Highly integrated watch for noninvasive continual glucose monitoring. Microsystems Nanoeng. 2022, 8, 25. [Google Scholar] [CrossRef]
- Hakala, T.A.; García Pérez, A.; Wardale, M.; Ruuth, I.A.; Vänskä, R.T.; Nurminen, T.A.; Kemp, E.; Boeva, Z.A.; Alakoskela, J.M.; Pettersson-Fernholm, K.; et al. Sampling of fluid through skin with magnetohydrodynamics for noninvasive glucose monitoring. Sci. Rep. 2021, 11, 7609. [Google Scholar] [CrossRef]
- Kemp, E.; Palomäki, T.; Ruuth, I.A.; Boeva, Z.A.; Nurminen, T.A.; Vänskä, R.T.; Zschaechner, L.K.; Pérez, A.G.; Hakala, T.A.; Wardale, M.; et al. Influence of enzyme immobilization and skin-sensor interface on non-invasive glucose determination from interstitial fluid obtained by magnetohydrodynamic extraction. Biosens. Bioelectron. 2022, 206, 114123. [Google Scholar] [CrossRef]
- Hakala, T.A.; Zschaechner, L.K.; Vänskä, R.T.; Nurminen, T.A.; Wardale, M.; Morina, J.; Boeva, Z.A.; Saukkonen, R.; Alakoskela, J.M.; Pettersson-Fernholm, K.; et al. Pilot study in human healthy volunteers on the use of magnetohydrodynamics in needle-free continuous glucose monitoring. Sci. Rep. 2022, 12, 18318. [Google Scholar] [CrossRef]
- Meidan, V.; Michniak-Kohn, B.B. Ultrasound-based Technology for skin barrier permeabilization. In Handbook of Non-Invasive Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2010; pp. 119–133. [Google Scholar]
- Kost, J.; Mitragotri, S.; Gabbay, R.A.; Pishko, M.; Langer, R. Transdermal monitoring of glucose and other analytes using ultrasound. Nat. Med. 2000, 6, 347–350. [Google Scholar] [CrossRef]
- Lee, S.; Nayak, V.; Dodds, J.; Pishko, M.; Smith, N.B. Glucose measurements with sensors and ultrasound. Ultrasound Med. Biol. 2005, 31, 971–977. [Google Scholar] [CrossRef]
- Buford, R.J.; Green, E.C.; McClung, M.J. A microwave frequency sensor for non-invasive blood-glucose measurement. In Proceedings of the 2008 IEEE Sensors Applications Symposium, Atlanta, GA, USA, 12–14 February 2008; IEEE: Piscataway, NJ, USA, 2008; pp. 4–7. [Google Scholar]
- Hofmann, M.; Fischer, G.; Weigel, R.; Kissinger, D. Microwave-based noninvasive concentration measurements for biomedical applications. IEEE Trans. Microw. Theory Tech. 2013, 61, 2195–2204. [Google Scholar] [CrossRef]
- Omer, A.E.; Shaker, G.; Safavi-Naeini, S.; Kokabi, H.; Alquié, G.; Deshours, F.; Shubair, R.M. Low-cost portable microwave sensor for non-invasive monitoring of blood glucose level: Novel design utilizing a four-cell CSRR hexagonal configuration. Sci. Rep. 2020, 10, 15200. [Google Scholar] [CrossRef] [PubMed]
- Baghelani, M.; Abbasi, Z.; Daneshmand, M.; Light, P.E. Non-invasive continuous-time glucose monitoring system using a chipless printable sensor based on split ring microwave resonators. Sci. Rep. 2020, 10, 12980. [Google Scholar] [CrossRef] [PubMed]
- Kiani, S.; Rezaei, P.; Fakhr, M. Dual-frequency microwave resonant sensor to detect noninvasive glucose-level changes through the fingertip. IEEE Trans. Instrum. Meas. 2021, 70, 1–8. [Google Scholar] [CrossRef]
- Obaid, S.M.; Elwi, T.A.; Ilyas, M. Fractal Minkowski-shaped resonator for noninvasive biomedical measurements: Blood glucose test. Prog. Electromagn. Res. C 2021, 107, 143–156. [Google Scholar] [CrossRef]
- Siegel, P.H.; Tang, A.; Virbila, G.; Kim, Y.; Chang, M.F.; Pikov, V. Compact non-invasive millimeter-wave glucose sensor. In Proceedings of the 2015 40th International Conference on Infrared, Millimeter, and Terahertz waves (IRMMW-THz), Hong Kong, China, 23–28 August 2015; IEEE: Piscataway, NJ, USA, 2015; pp. 1–3. [Google Scholar]
- Siegel, P.H.; Dai, W.; Kloner, R.A.; Csete, M.; Pikov, V. First millimeter-wave animal in vivo measurements of L-Glucose and D-Glucose: Further steps towards a non-invasive glucometer. In Proceedings of the 2016 41st International Conference on Infrared, Millimeter, and Terahertz waves (IRMMW-THz), Copenhagen, Denmark, 25–30 September 2016; IEEE: Piscataway, NJ, USA, 2016; pp. 1–3. [Google Scholar]
- Omer, A.E.; Shaker, G.; Safavi-Naeini, S.; Murray, K.; Hughson, R. Glucose levels detection using mm-wave radar. IEEE Sens. Lett. 2018, 2, 1–4. [Google Scholar] [CrossRef]
- Omer, A.E.; Safavi-Naeini, S.; Hughson, R.; Shaker, G. Blood glucose level monitoring using an FMCW millimeter-wave radar sensor. Remote. Sens. 2020, 12, 385. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, J.; Herrling, T.; Groth, N. Detection of free radicals in skin: A review of the literature and new developments. Curr. Probl. Dermatol. 2001, 29, 1–17. [Google Scholar]
- Abdalla, S.; Al-Ameer, S.; Al-Magaishi, S. Electrical properties with relaxation through human blood. Biomicrofluidics 2010, 4, 034101. [Google Scholar] [CrossRef] [Green Version]
- Karacolak, T.; Moreland, E.C.; Topsakal, E. Cole–cole model for glucose-dependent dielectric properties of blood plasma for continuous glucose monitoring. Microw. Opt. Technol. Lett. 2013, 55, 1160–1164. [Google Scholar] [CrossRef]
- Choi, H.; Naylon, J.; Luzio, S.; Beutler, J.; Birchall, J.; Martin, C.; Porch, A. Design and in vitro interference test of microwave noninvasive blood glucose monitoring sensor. IEEE Trans. Microw. Theory Tech. 2015, 63, 3016–3025. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.; Luzio, S.; Beutler, J.; Porch, A. Microwave noninvasive blood glucose monitoring sensor: Penetration depth and sensitivity analysis. In Proceedings of the 2018 IEEE International Microwave Biomedical Conference (IMBioC), Philadelphia, PA, USA, 14–15 June 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 52–54. [Google Scholar]
- Yang, J.; Qi, L.; Li, B.; Wu, L.; Shi, D.; Uqaili, J.A.; Tao, X. A terahertz metamaterial sensor used for distinguishing glucose concentration. Results Phys. 2021, 26, 104332. [Google Scholar] [CrossRef]
- Chen, H.; Chen, X.; Ma, S.; Wu, X.; Yang, W.; Zhang, W.; Li, X. Quantify glucose level in freshly diabetic’s blood by terahertz time-domain spectroscopy. J. Infrared Millim. Terahertz Waves 2018, 39, 399–408. [Google Scholar] [CrossRef] [Green Version]
- Lykina, A.A.; Nazarov, M.M.; Konnikova, M.R.; Mustafin, I.A.; Vaks, V.L.; Anfertev, V.A.; Domracheva, E.G.; Chernyaeva, M.B.; Kistenev, Y.V.; Vrazhnov, D.A.; et al. Terahertz spectroscopy of diabetic and non-diabetic human blood plasma pellets. J. Biomed. Opt. 2021, 26, 043006. [Google Scholar] [CrossRef] [PubMed]
- Smolyanskaya, O.; Chernomyrdin, N.; Konovko, A.; Zaytsev, K.; Ozheredov, I.; Cherkasova, O.; Nazarov, M.; Guillet, J.P.; Kozlov, S.; Kistenev, Y.V.; et al. Terahertz biophotonics as a tool for studies of dielectric and spectral properties of biological tissues and liquids. Prog. Quantum Electron. 2018, 62, 1–77. [Google Scholar] [CrossRef]
- Cherkasova, O.; Nazarov, M.; Konnikova, M.; Shkurinov, A. THz spectroscopy of bound water in glucose: Direct measurements from crystalline to dissolved state. J. Infrared Millim. Terahertz Waves 2020, 41, 1057–1068. [Google Scholar] [CrossRef]
- Kamat, D.; Bagul, D.; Patil, P. Blood glucose measurement using bioimpedance technique. Adv. Electron. 2014, 2014, 406257. [Google Scholar] [CrossRef]
- Li, J.; Igbe, T.; Liu, Y.; Nie, Z.; Qin, W.; Wang, L.; Hao, Y. An approach for noninvasive blood glucose monitoring based on bioimpedance difference considering blood volume pulsation. IEEE Access 2018, 6, 51119–51129. [Google Scholar] [CrossRef]
- Pedro, B.G.; Marcôndes, D.W.C.; Bertemes-Filho, P. Analytical model for blood glucose detection using electrical impedance spectroscopy. Sensors 2020, 20, 6928. [Google Scholar] [CrossRef]
- Cho, O.K.; Kim, Y.O.; Mitsumaki, H.; Kuwa, K. Noninvasive measurement of glucose by metabolic heat conformation method. Clin. Chem. 2004, 50, 1894–1898. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, J.M.; Liang, Y.B.; Chen, H.B.; Yin, S.M.; Chen, Z.C. Non-invasive blood glucose detection system based on conservation of energy method. Physiol. Meas. 2017, 38, 325. [Google Scholar] [CrossRef]
- Kenny, G.P.; Sigal, R.J.; McGinn, R. Body temperature regulation in diabetes. Temperature 2016, 3, 119–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitazaki, T.; Morimoto, Y.; Yamashita, S.; Anabuki, D.; Tahara, S.; Nishiyama, A.; Wada, K.; Ishimaru, I. Glucose emission spectra through mid-infrared passive spectroscopic imaging of the wrist for non-invasive glucose sensing. Sci. Rep. 2022, 12, 20558. [Google Scholar] [CrossRef] [PubMed]
- Harman-Boehm, I.; Gal, A.; Raykhman, A.M.; Zahn, J.D.; Naidis, E.; Mayzel, Y. Noninvasive glucose monitoring: A novel approach. J. Diabetes Sci. Technol. 2009, 3, 253–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahartan, K.; Horman, K.; Gal, A.; Drexler, A.; Mayzel, Y.; Lin, T. Assessing the performance of a noninvasive glucose monitor in people with type 2 diabetes with different demographic profiles. J. Diabetes Res. 2017, 2017, 4393497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, T.; Mayzel, Y.; Bahartan, K. The accuracy of a non-invasive glucose monitoring device does not depend on clinical characteristics of people with type 2 diabetes mellitus. J. Drug Assess. 2018, 7, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyström, J.; Lindholm-Sethson, B.; Stenberg, L.; Ollmar, S.; Eriksson, J.W.; Geladi, P. Combined near-infrared spectroscopy and multifrequency bio-impedance investigation of skin alterations in diabetes patients based on multivariate analyses. Med. Biol. Eng. Comput. 2003, 41, 324–329. [Google Scholar] [CrossRef]
- Buscemi, S.; Blunda, G.; Maneri, R.; Verga, S. Bioelectrical characteristics of type 1 and type 2 diabetic subjects with reference to body water compartments. Acta Diabetol. 1998, 35, 220–223. [Google Scholar] [CrossRef]
- Fouad, M.M.; Mahmoud, D.Y.; Abd El Ghany, M.A. Joint NIR-BIS Based Non-Invasive Glucose Monitoring System. In Proceedings of the 2018 30th International Conference on Microelectronics (ICM), Sousse, Tunisia, 16–19 December 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 88–91. [Google Scholar]
- Lister, T.; Wright, P.A.; Chappell, P.H. Optical properties of human skin. J. Biomed. Opt. 2012, 17, 090901. [Google Scholar] [CrossRef] [Green Version]
- Groenendaal, W.; Schmidt, K.; Von Basum, G.; Van Riel, N.; Hilbers, P. Modeling glucose and water dynamics in human skin. Diabetes Technol. Ther. 2008, 10, 283–293. [Google Scholar] [CrossRef]
- Sternberg, F.; Meyerhoff, C.; Mennel, F.; Mayer, H.; Bischof, F.; Pfeiffer, E.F. Does fall in tissue glucose precede fall in blood glucose? Diabetologia 1996, 39, 609–612. [Google Scholar] [CrossRef]
- Braverman, I.M. The cutaneous microcirculation. J. Investig. Dermatol. Symp. Proc. 2000, 5, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Sandby-Møller, J.; Poulsen, T.; Wulf, H.C. Epidermal thickness at different body sites: Relationship to age, gender, pigmentation, blood content, skin type and smoking habits. Acta Derm. Venereol. 2003, 83, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lei, D.; Swindell, W.R.; Xia, W.; Weng, S.; Fu, J.; Worthen, C.A.; Okubo, T.; Johnston, A.; Gudjonsson, J.E.; et al. Age-associated increase in skin fibroblast–derived prostaglandin E2 contributes to reduced collagen levels in elderly human skin. J. Investig. Dermatol. 2015, 135, 2181–2188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimberly, M.M.; Vesper, H.W.; Caudill, S.P.; Ethridge, S.F.; Archibold, E.; Porter, K.H.; Myers, G.L. Variability among five over-the-counter blood glucose monitors. Clin. Chim. Acta 2006, 364, 292–297. [Google Scholar] [CrossRef]
- Olansky, L.; Kennedy, L. Finger-stick glucose monitoring: Issues of accuracy and specificity. Diabetes Care 2010, 33, 948–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovatchev, B.P.; Patek, S.D.; Ortiz, E.A.; Breton, M.D. Assessing sensor accuracy for non-adjunct use of continuous glucose monitoring. Diabetes Technol. Ther. 2015, 17, 177–186. [Google Scholar] [CrossRef]
- Bodenlenz, M.; Schaupp, L.A.; Druml, T.; Sommer, R.; Wutte, A.; Schaller, H.C.; Sinner, F.; Wach, P.; Pieber, T.R. Measurement of interstitial insulin in human adipose and muscle tissue under moderate hyperinsulinemia by means of direct interstitial access. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E296–E300. [Google Scholar] [CrossRef] [Green Version]
- International Electrotechnical Commission. IEC 60825-1:2014; Safety Of Laser Products—Part 1: Equipment Classification And Requirements. International Standard, International Electrotechnical Commission: Geneva, Switzerland, 2014.
- Laser Institute of America. ANSI Z136.1:2014; American National Standard for Safe Use of Lasers. American National Standard, Laser Institute of America: Orlando, FL, USA, 2014.




| Biological Fluid | Lag Time | Glucose | Advantages | Disadvantages | Maturity |
|---|---|---|---|---|---|
| Tears [54,63] | ∼15 min | ∼2% of blood |
|
| Low |
| Sweat [62] | ∼10 min | ∼2% of blood |
|
| Moderate |
| Saliva [61] | ∼15 min | ∼1% of blood |
|
| High |
| ISF [4,5] | ∼8–10 min | similar to blood |
|
| High |
| ISF (RI) [64] | ∼15–20 min | ∼1% of ISF |
|
| High |
| Technique | Signal-to-Noise Ratio | Penetration Depth | Affected by Scattering | Cost |
|---|---|---|---|---|
| Near-Infrared Spectroscopy | Low | Moderate | Moderate | Low |
| Mid-Infrared Spectroscopy | Moderate | Low | Low | Moderate |
| Photoacoustic Spectroscopy | NIR—Low MIR—Moderate | NIR—Moderate MIR—Low | None | High |
| Raman Spectroscopy | High | NIR—Moderate MIR—Low | None | High |
| Polarimetry | Low | Low | High | Low |
| Fluorescence | High | None | None | Low |
| Ref. | Year | Technique | Wavelength nm | Location | Clinical Study | Study Result | ||
|---|---|---|---|---|---|---|---|---|
| N | w/ Diabetes | w/o Diabetes | ||||||
| [80] | 1999 | NIR Spectroscopy | 1050–2450 | Forearm | 7 | Yes | No | MARD of 3 participants: 9.1%, 17.6%, 3.6% |
| [83] | 1999 | NIR Spectroscopy | 630 | Multiple | 19 | n/a | n/a | Tongue is most reliable for glucose sensing |
| [82] | 2002 | NIR Spectroscopy | 1050–2450 | Forearm | 9 | Yes | No | MARD: 20.6%; Zone A: 63.5%; Zone B: 34.9% |
| [81] | 2003 | NIR Spectroscopy | Unspecified | Forearm | 1 | Yes | No | r: 0.928; standard error of prediction: 32.2 mg/dL |
| [108] | 2005 | Raman Spectroscopy | 830 | Forearm | 17 | No | Yes | MARD: 7.8% ± 1.8%; R: 0.83 ± 0.10 |
| [96] | 2005 | Photoacoustic Spectroscopy | 9259, 9381 | Forearm | 1 | No | Yes | A positive correlation is observed |
| [90] | 2007 | Occlusion Spectroscopy | 10 Unspecified | Finger | 23 | Yes | No | MARD: 17.2%; Zone A: 69.7%; Zone B: 25.7% |
| [109] | 2009 | Raman Spectroscopy | 670, 827, 829 | Forearm | 30 | Yes | No | Zone A: 53%; Zone B: 39%; Mean absolute difference: 38 mg/dL |
| [84] | 2010 | NIR Spectroscopy | 905–1701 | Finger | 36 | No | Yes | r: 0.48; RMSE: 1.34 mmol/l; Zone A + B: 100% |
| [97] | 2012 | Photoacoustic Spectroscopy | 9225, 9488 | Palm | 2 | No | Yes | r: 0.7. Recommends using 6–10 IR wavelengths |
| [101] | 2013 | Photoacoustic Spectroscopy | 8197–10,000 | Hypothenar | 2 | Yes | Yes | MAD: 11 mg/dL (without diabetes) and 15 mg/dL (T1D) |
| [100] | 2013 | Photoacoustic Spectroscopy | 8032–10,000 | Hypothenar | 1 | No | Yes | A windowless PA cell design is proposed and verified |
| [85] | 2014 | MIR Spectroscopy | 8000–10,000 | Palm | 3 | No | Yes | Zone A: 84% |
| [98] | 2015 | Photoacoustic Spectroscopy | 905 | Palm | 30 | No | Yes | MARD: 9.61% ± 10.55%. Zone A: 87.24%; Zone B: 12.76% |
| [86] | 2016 | NIR Spectroscopy | 940 | Finger | 5 | No | Yes | Zone A + B: 100% |
| [70] | 2016 | Photoacoustic Spectroscopy | 8475, 9259 | Finger | n/a | n/a | n/a | R = 0.8, uncertainty of ±30 mg/dL at 90% confidence level |
| [99] | 2017 | Photoacoustic Spectroscopy | 905, 1550 | Forefinger | 24 | No | Yes | MARD: 8.84%; Zone A: 92.86%; Zone B: 7.14% |
| [91] | 2018 | NIR Spectroscopy | 625, 740, 850, 940 | Finger | 19 | n/a | n/a | Result of 3 Studies: MARD: 17.9%, 14.9%, 17.1%; Zone A + B: 100%, 100%, 98.8% (consensus) |
| [92] | 2018 | NIR Spectroscopy | 625, 740, 850, 940 | Finger | 36 | Yes | Yes | MARD: 14.4%; Zone A: 96.6%; Zone B: 3.4% (consensus) |
| [87] | 2018 | MIR Spectroscopy | 1050, 1070, 1100 | Finger | 6 | No | Yes | r: 0.36; Zone A + B: 100% |
| [110] | 2018 | Raman Spectroscopy | 830 | Thenar | 35 | Yes | No | MARD: 25.8%; Zone A + B: 93% (consensus) |
| [102] | 2018 | Photoacoustic Spectroscopy | 8032–9852 | Multiple | 5 | Yes | Yes | MAD 16 ± 7 mg/dL. Thumb is most suitable for glucose sensing |
| [103] | 2018 | Photoacoustic Spectroscopy | 8065–10,526 | Finger | 2 | Yes | Yes | MARD: 14.4% ± 10.5%; Zone A: 70%; Zone B: 30% |
| [88] | 2019 | MIR Spectroscopy | 6250–12,500 | Finger | 6 | No | Yes | 95% certainty and 100% comparability with firm finger pressure |
| [106] | 2019 | Raman Spectroscopy | 785 | Nailfold | 12 | No | Yes | RMSE = 0.27 mmol/L; R = 0.98; Zone A + B: 100% |
| [123] | 2020 | Polarimetry | 450, 520, 658 | Palm | 50 | Yes | Yes | MARD: 10.0%; Zone A: 89%; Zone B: 11%; r: 0.91; p = |
| [89] | 2021 | NIR Spectroscopy | 1050, 1219, 1314, 1409, 1550, 1609 | Finger | 19 | No | Yes | r: 0.92, Zone A: 97.96% |
| [107] | 2021 | Raman Spectroscopy | 830 | Thenar | 15 | Yes | No | MARD: 26.3% ± 10.8%; Zone A + B: 93.6% |
| [79] | 2022 | NIR Spectroscopy | 850, 950, 1150 | Finger | 635 | Yes | Yes | Zone A: 100.0% |
| Types of Techniques | Advantages | Disadvantages |
|---|---|---|
| Optical (Direct) |
|
|
| Optical (Indirect) |
|
|
| Transdermal |
|
|
| Electrical |
|
|
| Thermal |
|
|
| Fusion |
|
|
| Ref. | Year | Clinical Study | Study Result | ||
|---|---|---|---|---|---|
| N | w/ Diabetes | w/o Diabetes | |||
| NIR Spectroscopy | |||||
| [82] | 2002 | 9 | Yes | No | MARD: 20.6%; Zone A: 63.5%; Zone B: 34.9% |
| [84] | 2010 | 36 | No | Yes | r: 0.48; RMSE: 1.34 mmol/l; Zone A + B: 100.0% |
| [86] | 2016 | 5 | No | Yes | Zone A + B: 100.0% |
| [92] | 2018 | 36 | Yes | Yes | MARD: 14.4%; Zone A: 96.6%; Zone B: 3.4% (consensus) |
| [89] | 2021 | 19 | No | Yes | r: 0.92, Zone A: 97.96% |
| [79] | 2022 | 635 | Yes | Yes | Zone A: 100.0% |
| MIR Spectroscopy | |||||
| [85] | 2014 | 3 | No | Yes | Zone A: 84.0% |
| [87] | 2018 | 6 | No | Yes | r: 0.36; Zone A + B: 100.0% |
| Occlusion Spectroscopy | |||||
| [90] | 2007 | 23 | Yes | No | MARD: 17.2%; Zone A: 69.7%; Zone B: 25.7% |
| Photoacoustic Spectroscopy | |||||
| [98] | 2015 | 30 | No | Yes | MARD: 9.61% ± 10.55%. Zone A: 87.24%; Zone B: 12.76% |
| [99] | 2017 | 24 | No | Yes | MARD: 8.84%; Zone A: 92.86%; Zone B: 7.14% |
| [102] | 2018 | 5 | Yes | Yes | MAD: 16 ± 7 mg/dL. |
| Raman Spectroscopy | |||||
| [108] | 2005 | 17 | No | Yes | MARD: 7.8% ± 1.8%; R: 0.83 ± 0.10 |
| [109] | 2009 | 30 | Yes | No | MAD: 38 mg/dL; Zone A: 53.0%; Zone B: 39.0% |
| [110] | 2018 | 35 | Yes | No | MARD: 25.8%; Zone A + B: 93.0% (consensus) |
| [106] | 2019 | 12 | No | Yes | RMSEP = 0.27 mmol/L; R = 0.98; Zone A + B: 100.0% |
| [107] | 2021 | 15 | Yes | No | MARD: 26.3% ± 10.8%; Zone A + B: 93.6% |
| Polarimetry | |||||
| [123] | 2020 | 50 | Yes | Yes | MARD: 10.0%; Zone A: 89.0%; Zone B: 11.0%; r: 0.91 |
| Photoplethysmography | |||||
| [136] | 2019 | 30 | Yes | Yes | r: 0.95 |
| [137] | 2019 | 611 | Yes | Yes | Zone A: 80.6%; Zone B: 17.4% |
| [138] | 2020 | 200 | Yes | Yes | MARD: 7.62% |
| [139] | 2020 | 8 | Yes | Yes | r: 0.858; Zone A: 74.29%; Zone B: 25.71% |
| [140] | 2021 | 26 | n/a | n/a | Zone A: 96.15%; Zone B: 3.85% |
| Reverse Iontophoresis | |||||
| [147] | 2001 | 231 | Yes | Yes | MARD: 19.0%; r: 0.85; Zone A + B: 95.3% |
| [156] | 2022 | 23 | Yes | Yes | Zone A: 46.99%; Zone B: 37.35% |
| Metabolic Heat Conformation | |||||
| [186] | 2004 | 10 | Yes | Yes | r: 0.91 |
| [187] | 2017 | 31 | Yes | Yes | r: 0.89; Zone A + B: 94.4% |
| Fusion Techniques | |||||
| [192] | 2018 | 114 | Yes | No | MARD: 22.7%; Zone A + B: 98.0% |
| [195] | 2018 | 5 | Yes | No | MAD: 3.794 mg/dL; r: 0.92; Zone A: 100.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leung, H.M.C.; Forlenza, G.P.; Prioleau, T.O.; Zhou, X. Noninvasive Glucose Sensing In Vivo. Sensors 2023, 23, 7057. https://doi.org/10.3390/s23167057
Leung HMC, Forlenza GP, Prioleau TO, Zhou X. Noninvasive Glucose Sensing In Vivo. Sensors. 2023; 23(16):7057. https://doi.org/10.3390/s23167057
Chicago/Turabian StyleLeung, Ho Man Colman, Gregory P. Forlenza, Temiloluwa O. Prioleau, and Xia Zhou. 2023. "Noninvasive Glucose Sensing In Vivo" Sensors 23, no. 16: 7057. https://doi.org/10.3390/s23167057
APA StyleLeung, H. M. C., Forlenza, G. P., Prioleau, T. O., & Zhou, X. (2023). Noninvasive Glucose Sensing In Vivo. Sensors, 23(16), 7057. https://doi.org/10.3390/s23167057






