Illuminating Progress: The Contribution of Bioluminescence to Sustainable Development Goal 6—Clean Water and Sanitation—Of the United Nations 2030 Agenda
Abstract
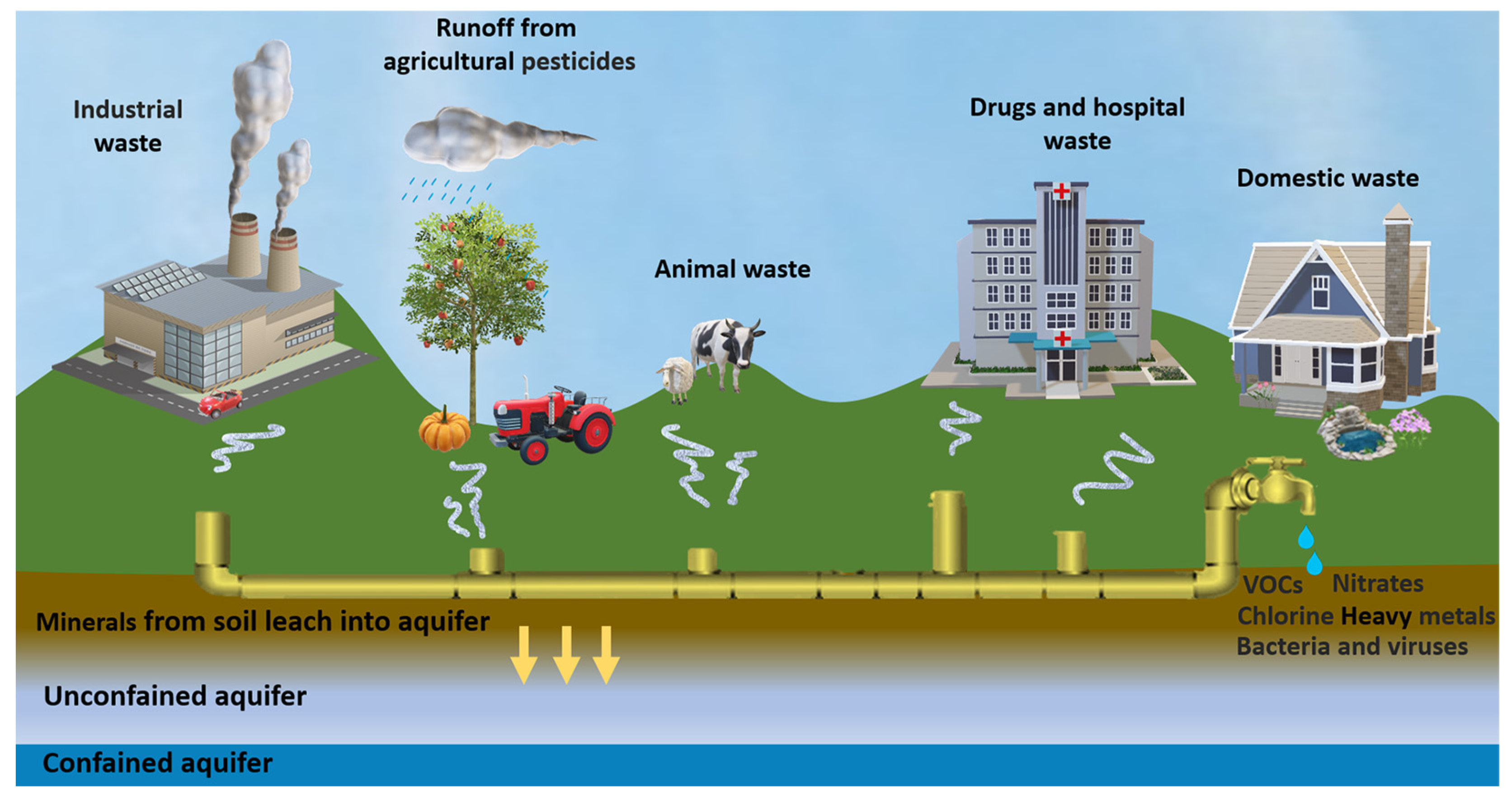
:1. Introduction
2. Bioluminescence Tools
3. BL Biosensors Applied to Water Quality Monitoring
3.1. BL Biosensors for Water Pollutants
3.2. BL Biosensors for Toxicity Evaluation

3.3. BL Tools for Monitoring Bioremediation Efficiency
4. Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Available online: https://sdgs.un.org/goals/goal6 (accessed on 1 August 2023).
- Deng, S.; Wang, C.; Hao Ngo, H.; Guo, W.; You, N.; Tang, H.; Yu, H.; Tang, L.; Han, J. Comparative review on microbial electrochemical technologies for resource recovery from wastewater towards circular economy and carbon neutrality. Bioresour. Technol. 2023, 376, 128906. [Google Scholar] [CrossRef] [PubMed]
- Progress on Household Drinking Water, Sanitation and Hygiene 2000–2020: Five Years into the SDGs; World Health Organization (WHO)/United Nations Children’s Fund (UNICEF) Joint Monitoring Programme for Water Supply, Sanitation and Hygiene (JMP): Geneva, Switzerland, 2021.
- European Commission. Directive 2000/60/EC of the European Parliament and of the Council Establishing a Framework for the Community Action in the Field of Water Policy; European Commission: Brussels, Belgium, 23 October 2000; Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A32000L0060 (accessed on 1 August 2023).
- European Commission. COUNCIL DIRECTIVE of 21 May 1991 Concerning Urban Waste Water Treatment. 1991. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:31991L0271&from=EN (accessed on 1 August 2023).
- Available online: https://www.epa.gov/regulatory-information-topic/regulatory-and-guidance-information-topic-water (accessed on 1 August 2023).
- Ghorbanpour, M.; Bhargava, P.; Varma, A.; Choudhary, D.K. Biogenic Nano-Particles and Their Use in Agro-Ecosystems; Springer: Singapore, 2020; ISBN 9789811529856. [Google Scholar]
- Kirchherr, J.; Reike, D.; Hekkert, M. Conceptualizing the circular economy: An analysis of 114 definitions. Resour. Conserv. Recycl. 2017, 127, 221–232. [Google Scholar] [CrossRef]
- Carus, M.; Dammer, L. The Circular Bioeconomy—Concepts, Opportunities, and Limitations. Ind. Biotechnol. 2018, 14, 83–91. [Google Scholar] [CrossRef]
- D’Odorico, P.; Davis, K.F.; Rosa, L.; Carr, J.A.; Chiarelli, D.; Dell’Angelo, J.; Gephart, J.; MacDonald, G.K.; Seekell, D.A.; Suweis, S.; et al. The Global Food-Energy-Water Nexus. Rev. Geophys. 2018, 56, 456–531. [Google Scholar] [CrossRef]
- Deviller, G.; Lundy, L.; Fatta-Kassinos, D. Recommendations to derive quality standards for chemical pollutants in reclaimed water intended for reuse in agricultural irrigation 124911. Chemosphere 2020, 240, 12491. [Google Scholar] [CrossRef]
- Koop, S.H.; Grison, C.; Eisenreich, S.J.; Hofman, J.; van Leeuwen, K. Integrated water resources management in cities in the world: Global solutions. Sustain. Cities Soc. 2022, 86, 104137. [Google Scholar] [CrossRef]
- Tazoe, H. Water quality monitoring. Anal. Sci. 2023, 39, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Nazeer, M.; Nichol, J.E. Development and application of a remote sensing-based Chlorophyll-a concentration prediction model for complex coastal waters of Hong Kong. J. Hydrol. 2016, 532, 80–89. [Google Scholar] [CrossRef]
- Kara, S.; Karadirek, I.E.; Muhammetoglu, A.; Muhammetoglu, H. Real time monitoring and control in water distribution systems for improving operational efficiency. Desalination Water Treat. 2016, 57, 11506–11519. [Google Scholar] [CrossRef]
- Pearson, D.; Chakraborty, S.; Duda, B.; Li, B.; Weindorf, D.C.; Deb, S.; Brevik, E.; Ray, D. Water analysis via portable X-ray fluorescence spectrometry. J. Hydrol. 2017, 544, 172–179. [Google Scholar] [CrossRef]
- Hui, Y.; Huang, Z.; Alahi, M.E.E.; Nag, A.; Feng, S.; Mukhopadhyay, S.C. Recent Advancements in Electrochemical Biosensors for Monitoring the Water Quality. Biosensors 2022, 12, 551. [Google Scholar] [CrossRef] [PubMed]
- Malik, L.A.; Bashir, A.; Qureashi, A.; Pandith, A.H. Detection and removal of heavy metal ions: A review. Environ. Chem. Lett. 2019, 17, 1495–1521. [Google Scholar] [CrossRef]
- Gumpu, M.B.; Sethuraman, S.; Krishnan, U.M.; Rayappan, J.B.B. A review on detection of heavy metal ions in water—An electrochemical approach. Sens. Actuators B Chem. 2015, 213, 515–533. [Google Scholar] [CrossRef]
- Chen, Q.; Yao, Y.; Li, X.; Lu, J.; Zhou, J.; Huang, Z. Comparison of heavy metal removals from aqueous solutions by chemical precipitation and characteristics of precipitates. J. Water Process. Eng. 2018, 26, 289–300. [Google Scholar] [CrossRef]
- Kuznetsova, O.V.; Dushenko, N.V.; Timerbaev, A.R. How Feasible is Direct Determination of Rare Earth Elements in Seawater by ICP-MS? Anal. Sci. 2021, 37, 1633–1636. [Google Scholar] [CrossRef] [PubMed]
- Gałuszka, A.; Migaszewski, Z.; Namieśnik, J. The 12 principles of green analytical chemistry and the SIGNIFICANCE mnemonic of green analytical practices. TrAC Trends Anal. Chem. 2013, 50, 78–84. [Google Scholar] [CrossRef]
- Nowak, P.M.; Wietecha-Posłuszny, R.; Pawliszyn, J. White Analytical Chemistry: An approach to reconcile the principles of Green Analytical Chemistry and functionality. TrAC Trends Anal. Chem. 2021, 138, 116223. [Google Scholar] [CrossRef]
- Haddock, S.H.D.; Moline, M.A.; Case, J.F. Bioluminescence in the Sea. Annu. Rev. Mar. Sci. 2010, 2, 443–493. [Google Scholar] [CrossRef] [PubMed]
- Oba, Y.; Stevani, C.V.; Oliveira, A.G.; Tsarkova, A.S.; Chepurnykh, T.V.; Yampolsky, I.V. Selected Least Studied but not Forgotten Bioluminescent Systems. Photochem. Photobiol. 2017, 93, 405–415. [Google Scholar] [CrossRef]
- Iwano, S.; Obata, R.; Miura, C.; Kiyama, M.; Hama, K.; Nakamura, M.; Amano, Y.; Kojima, S.; Hirano, T.; Maki, S.; et al. Development of simple firefly luciferin analogs emitting blue, green, red, and near-infrared biological window light. Tetrahedron 2013, 69, 3847–3856. [Google Scholar] [CrossRef]
- Hall, M.P.; Unch, J.; Binkowski, B.F.; Valley, M.P.; Butler, B.L.; Wood, M.G.; Otto, P.; Zimmerman, K.; Vidugiris, G.; Machleidt, T.; et al. Engineered Luciferase Reporter from a Deep Sea Shrimp Utilizing a Novel Imidazopyrazinone Substrate. ACS Chem. Biol. 2012, 7, 1848–1857. [Google Scholar] [CrossRef] [PubMed]
- Branchini, B.R.; Behney, C.E.; Southworth, T.L.; Fontaine, D.M.; Gulick, A.M.; Vinyard, D.J.; Brudvig, G.W. Experimental Support for a Single Electron-Transfer Oxidation Mechanism in Firefly Bioluminescence. J. Am. Chem. Soc. 2015, 137, 7592–7595. [Google Scholar] [CrossRef] [PubMed]
- Baljinnyam, B.; Ronzetti, M.; Simeonov, A. Advances in luminescence-based technologies for drug discovery. Expert Opin. Drug Discov. 2022, 18, 25–35. [Google Scholar] [CrossRef]
- Roda, A.; Guardigli, M.; Michelini, E.; Mirasoli, M.; Pasini, P. Peer Reviewed: Analytical Bioluminescence and Chemiluminescence. Anal. Chem. 2003, 75, 462 A–470 A. [Google Scholar] [CrossRef]
- Ando, Y.; Niwa, K.; Yamada, N.; Enomoto, T.; Irie, T.; Kubota, H.; Ohmiya, Y.; Akiyama, H. Firefly bioluminescence quantum yield and colour change by pH-sensitive green emission. Nat. Photonics 2008, 2, 44–47. [Google Scholar] [CrossRef]
- Branchini, B.R.; Southworth, T.L.; Fontaine, D.M.; Davis, A.L.; Behney, C.E.; Murtiashaw, M.H. A Photinus pyralis and Luciola italica Chimeric Firefly Luciferase Produces Enhanced Bioluminescence. Biochemistry 2014, 53, 6287–6289. [Google Scholar] [CrossRef]
- Santangelo, M.F.; Libertino, S.; Turner, A.P.F.; Filippini, D.; Mak, W.C. Integrating printed microfluidics with silicon photomultipliers for miniaturised and highly sensitive ATP bioluminescence detection. Biosens. Bioelectron. 2018, 99, 464–470. [Google Scholar] [CrossRef]
- Calabretta, M.M.; Álvarez-Diduk, R.; Michelini, E.; Roda, A.; Merkoçi, A. Nano-lantern on paper for smartphone-based ATP detection. Biosens. Bioelectron. 2019, 150, 111902. [Google Scholar] [CrossRef]
- Martínez-Pérez-Cejuela, H.; Calabretta, M.M.; Bocci, V.; D’elia, M.; Michelini, E. Super-Stable Metal–Organic Framework (MOF)/Luciferase Paper-Sensing Platform for Rapid ATP Detection. Biosensors 2023, 13, 451. [Google Scholar] [CrossRef]
- Bashmakova, E.E.; Krasitskaya, V.V.; Kudryavtsev, A.N.; Grigorenko, V.G.; Frank, L.A. Hybrid Minimal Core Streptavidin-Obelin as a Versatile Reporter for Bioluminescence-based Bioassay. Photochem. Photobiol. 2016, 93, 548–552. [Google Scholar] [CrossRef]
- Michelini, E.; Calabretta, M.M.; Cevenini, L.; Lopreside, A.; Southworth, T.; Fontaine, D.M.; Simoni, P.; Branchini, B.R.; Roda, A. Smartphone-based multicolor bioluminescent 3D spheroid biosensors for monitoring inflammatory activity. Biosens. Bioelectron. 2019, 123, 269–277. [Google Scholar] [CrossRef]
- Williams, S.J.; Prescher, J.A. Building Biological Flashlights: Orthogonal Luciferases and Luciferins for in Vivo Imaging. Accounts Chem. Res. 2019, 52, 3039–3050. [Google Scholar] [CrossRef]
- Scott, D.; Dikici, E.; Ensor, M.; Daunert, S. Bioluminescence and Its Impact on Bioanalysis. Annu. Rev. Anal. Chem. 2011, 4, 297–319. [Google Scholar] [CrossRef] [PubMed]
- Lopreside, A.; Montali, L.; Wang, B.; Tassoni, A.; Ferri, M.; Calabretta, M.M.; Michelini, E. Orthogonal paper biosensor for Mercury(II) combining bioluminescence and colorimetric smartphone detection. Biosens. Bioelectron. 2021, 194, 113569. [Google Scholar] [CrossRef]
- Ramanathan, S.; Shi, W.; Rosen, B.P.; Daunert, S. Sensing Antimonite and Arsenite at the Subattomole Level with Genetically Engineered Bioluminescent Bacteria. Anal. Chem. 1997, 69, 3380–3384. [Google Scholar] [CrossRef]
- Ripp, S.; Daumer, K.A.; McKnight, T.; Levine, L.H.; Garland, J.L.; Simpson, M.L.; Sayler, G.S. Bioluminescent bioreporter integrated-circuit sensing of microbial volatile organic compounds. J. Ind. Microbiol. Biotechnol. 2003, 30, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Turner, K.; Xu, S.; Pasini, P.; Deo, S.; Bachas, L.; Daunert, S. Hydroxylated Polychlorinated Biphenyl Detection Based on a Genetically Engineered Bioluminescent Whole-Cell Sensing System. Anal. Chem. 2007, 79, 5740–5745. [Google Scholar] [CrossRef] [PubMed]
- Branchini, B.R.; Southworth, T.L.; Fontaine, D.M.; Kohrt, D.; Welcome, F.S.; Florentine, C.M.; Henricks, E.R.; DeBartolo, D.B.; Michelini, E.; Cevenini, L.; et al. Red-emitting chimeric firefly luciferase for in vivo imaging in low ATP cellular environments. Anal. Biochem. 2017, 534, 36–39. [Google Scholar] [CrossRef]
- Natashin, P.V.; Burakova, L.P.; Kovaleva, M.I.; Shevtsov, M.B.; Dmitrieva, D.A.; Eremeeva, E.V.; Markova, S.V.; Mishin, A.V.; Borshchevskiy, V.I.; Vysotski, E.S. The Role of Tyr-His-Trp Triad and Water Molecule Near the N1-Atom of 2-Hydroperoxycoelenterazine in Bioluminescence of Hydromedusan Photoproteins: Structural and Mutagenesis Study. Int. J. Mol. Sci. 2023, 24, 6869. [Google Scholar] [CrossRef]
- Markova, S.V.; Larionova, M.D.; Vysotski, E.S. Shining Light on the Secreted Luciferases of Marine Copepods: Current Knowledge and Applications. Photochem. Photobiol. 2019, 95, 705–721. [Google Scholar] [CrossRef]
- Calabretta, M.M.; Gregucci, D.; Martínez-Pérez-Cejuela, H.; Michelini, E. A Luciferase Mutant with Improved Brightness and Stability for Whole-Cell Bioluminescent Biosensors and In Vitro Biosensing. Biosensors 2022, 12, 742. [Google Scholar] [CrossRef]
- White, P.J.; Squirrell, D.J.; Arnaud, P.; Lowe, C.R.; Murray, J.A.H. Improved thermostability of the North American firefly luciferase: Saturation mutagenesis at position 354. Biochem. J. 1996, 319, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Yeh, A.H.-W.; Norn, C.; Kipnis, Y.; Tischer, D.; Pellock, S.J.; Evans, D.; Ma, P.; Lee, G.R.; Zhang, J.Z.; Anishchenko, I.; et al. De novo design of luciferases using deep learning. Nature 2023, 614, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, J.; Wang, P.; Zhang, J.; Guo, W. D-Luciferin Analogues: A Multicolor Toolbox for Bioluminescence Imaging. Angew. Chem. Int. Ed. Engl. 2012, 51, 8428–8430. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, G.; Kitada, N.; Furuta, T.; Hirano, T.; Maki, S.A.; Kim, S.-B. S-Series Coelenterazine-Driven Combinatorial Bioluminescence Imaging Systems for Mammalian Cells. Int. J. Mol. Sci. 2023, 24, 1420. [Google Scholar] [CrossRef]
- Qin, J.; Wang, W.; Gao, L.; Yao, S.Q. Emerging biosensing and transducing techniques for potential applications in point-of-care diagnostics. Chem. Sci. 2022, 13, 2857–2876. [Google Scholar] [CrossRef]
- Robert, K.W.; Parris, T.M.; Leiserowitz, A.A. What is Sustainable Development? Goals, Indicators, Values, and Practice. Environ. Sci. Policy Sustain. Dev. 2005, 47, 8–21. [Google Scholar] [CrossRef]
- United Nations. The 17 GOALS|Sustainable Development. Available online: https://sdgs.un.org/goals (accessed on 1 August 2023).
- Jung, W.; Han, J.; Choi, J.-W.; Ahn, C.H. Point-of-care testing (POCT) diagnostic systems using microfluidic lab-on-a-chip technologies. Microelectron. Eng. 2015, 132, 46–57. [Google Scholar] [CrossRef]
- Long, F.; Zhu, A.; Shi, H. Recent Advances in Optical Biosensors for Environmental Monitoring and Early Warning. Sensors 2013, 13, 13928–13948. [Google Scholar] [CrossRef]
- Kuswandi, B.; Nuriman; Huskens, J.; Verboom, W. Optical sensing systems for microfluidic devices: A review. Anal. Chim. Acta 2007, 601, 141–155. [Google Scholar] [CrossRef]
- Chen, Y.; Xianyu, Y.; Wu, J.; Dong, M.; Zheng, W.; Sun, J.; Jiang, X. Double-Enzymes-Mediated Bioluminescent Sensor for Quantitative and Ultrasensitive Point-of-Care Testing. Anal. Chem. 2017, 89, 5422–5427. [Google Scholar] [CrossRef] [PubMed]
- Roda, A.; Mirasoli, M.; Michelini, E.; Di Fusco, M.; Zangheri, M.; Cevenini, L.; Roda, B.; Simoni, P. Progress in chemical luminescence-based biosensors: A critical review. Biosens. Bioelectron. 2016, 76, 164–179. [Google Scholar] [CrossRef] [PubMed]
- Denisov, I.; Lukyanenko, K.; Yakimov, A.; Kukhtevich, I.; Esimbekova, E.; Belobrov, P. Disposable luciferase-based microfluidic chip for rapid assay of water pollution. Luminescence 2018, 33, 1054–1061. [Google Scholar] [CrossRef]
- Esimbekova, E.N.; Kalyabina, V.P.; Kopylova, K.V.; Torgashina, I.G.; Kratasyuk, V.A. Design of bioluminescent biosensors for assessing contamination of complex matrices. Talanta 2021, 233, 122509. [Google Scholar] [CrossRef]
- Esimbekova, E.N.; Torgashina, I.G.; Kalyabina, V.P.; Kratasyuk, V.A. Enzymatic Biotesting: Scientific Basis and Application. Contemp. Probl. Ecol. 2021, 14, 290–304. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, Y.; Zhao, Y.; Zhang, X.; Ren, H. Highly Sensitive and Selective Detection of Inorganic Phosphates in the Water Environment by Biosensors Based on Bioluminescence Resonance Energy Transfer. Anal. Chem. 2023, 95, 4904–4913. [Google Scholar] [CrossRef] [PubMed]
- Muthulakshmi, L.; Mohan, S.; Tatarchuk, T. Microplastics in water: Types, detection, and removal strategies. Environ. Sci. Pollut. Res. 2023, 30, 84933–84948. [Google Scholar] [CrossRef]
- Manivannan, B.; Eltzov, E.; Borisover, M. Submicron polymer particles may mask the presence of toxicants in wastewater effluents probed by reporter gene containing bacteria. Sci. Rep. 2021, 11, 7424. [Google Scholar] [CrossRef]
- Bopp, S.; Franco, A.; Cusinato, A.; Kephalopoulos, S.; Ceridono, M. Information Platform for Chemical Monitoring IPCHEM 2020—Update on the State of Play of IPCHEM; European Commission: Ispra, Italy, 2020; JRC123154. [Google Scholar]
- Cevenini, L.; Lopreside, A.; Calabretta, M.M.; D’Elia, M.; Simoni, P.; Michelini, E.; Roda, A. A novel bioluminescent NanoLuc yeast-estrogen screen biosensor (nanoYES) with a compact wireless camera for effect-based detection of endocrine-disrupting chemicals. Anal. Bioanal. Chem. 2017, 410, 1237–1246. [Google Scholar] [CrossRef]
- Bazin, I.; Bin Seo, H.; Suehs, C.M.; Ramuz, M.; De Waard, M.; Gu, M.B. Profiling the biological effects of wastewater samples via bioluminescent bacterial biosensors combined with estrogenic assays. Environ. Sci. Pollut. Res. 2017, 24, 33–41. [Google Scholar] [CrossRef]
- Melamed, S.; Naftaly, S.; Belkin, S. Improved detection of antibiotic compounds by bacterial reporter strains achieved by manipulations of membrane permeability and efflux capacity. Appl. Microbiol. Biotechnol. 2014, 98, 2267–2277. [Google Scholar] [CrossRef] [PubMed]
- Bhuvaneshwari, M.; Eltzov, E.; Veltman, B.; Shapiro, O.; Sadhasivam, G.; Borisover, M. Toxicity of chlorinated and ozonated wastewater effluents probed by genetically modified bioluminescent bacteria and cyanobacteria Spirulina sp. Water Res. 2019, 164, 114910. [Google Scholar] [CrossRef] [PubMed]
- Mirjani, M.; Soleimani, M.; Salari, V. Toxicity assessment of total petroleum hydrocarbons in aquatic environments using the bioluminescent bacterium Aliivibrio fischeri. Ecotoxicol. Environ. Saf. 2020, 207, 111554. [Google Scholar] [CrossRef] [PubMed]
- Baumstark-Khan, C.; Rabbow, E.; Rettberg, P.; Horneck, G. The combined bacterial Lux-Fluoro test for the detection and quantification of genotoxic and cytotoxic agents in surface water: Results from the “Technical Workshop on Genotoxicity Biosensing”. Aquat. Toxicol. 2007, 85, 209–218. [Google Scholar] [CrossRef]
- Lovinskaya, A.; Kolumbayeva, S.; Begimbetova, D.; Suvorova, M.; Bekmagambetova, N.; Abilev, S. Toxic and genotoxic activity of river waters of the Kazakhstan. Acta Ecol. Sin. 2021, 41, 499–511. [Google Scholar] [CrossRef]
- Roda, A.; Cevenini, L.; Borg, S.; Michelini, E.; Calabretta, M.M.; Schüler, D. Bioengineered bioluminescent magnetotactic bacteria as a powerful tool for chip-based whole-cell biosensors. Lab Chip 2013, 13, 4881–4889. [Google Scholar] [CrossRef]
- Abilev, S.K.; Igonina, E.V.; Sviridova, D.A.; Smirnova, S.V. Bacterial Lux Biosensors in Genotoxicological Studies. Biosensors 2023, 13, 511. [Google Scholar] [CrossRef]
- Calabretta, M.M.; Gregucci, D.; Guarnieri, T.; Bonini, M.; Neri, E.; Zangheri, M.; Michelini, E. Bioluminescence Sensing in 3D Spherical Microtissues for Multiple Bioactivity Analysis of Environmental Samples. Sensors 2022, 22, 4568. [Google Scholar] [CrossRef]
- Jouanneau, S.; Durand-Thouand, M.-J.; Thouand, G. Design of a toxicity biosensor based on Aliivibrio fischeri entrapped in a disposable card. Environ. Sci. Pollut. Res. 2015, 23, 4340–4345. [Google Scholar] [CrossRef]
- Tsai, H.-F.; Tsai, Y.-C.; Yagur-Kroll, S.; Palevsky, N.; Belkin, S.; Cheng, J.-Y. Water pollutant monitoring by a whole cell array through lens-free detection on CCD. Lab Chip 2015, 15, 1472–1480. [Google Scholar] [CrossRef]
- Ma, J.; Harpaz, D.; Liu, Y.; Eltzov, E. Smartphone-Based Whole-Cell Biosensor Platform Utilizing an Immobilization Approach on a Filter Membrane Disk for the Monitoring of Water Toxicants. Sensors 2020, 20, 5486. [Google Scholar] [CrossRef]
- Wethasinghe, C.; Yuen, S.T.S.; Kaluarachchi, J.J.; Hughes, R. Uncertainty in biokinetic parameters on bioremediation: Health risks and economic implications. Environ. Int. 2006, 32, 312–323. [Google Scholar] [CrossRef]
- Marín, J.A.; Moreno, J.L.; Hernández, T.; García, C. Bioremediation by Composting of Heavy Oil Refinery Sludge in Semiarid Conditions. Biodegradation 2006, 17, 251–261. [Google Scholar] [CrossRef]
- Płaza, G.; Nałęcz-Jawecki, G.; Ulfig, K.; Brigmon, R.L. The application of bioassays as indicators of petroleum-contaminated soil remediation. Chemosphere 2005, 59, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, B.; Donaldson, J.D.; Grimes, S.M. Contaminated and polluted land: A general review of decontamination management and control. J. Chem. Technol. Biotechnol. 1994, 60, 227–240. [Google Scholar] [CrossRef]
- Brar, S.K.; Verma, M.; Surampalli, R.Y.; Misra, K.; Tyagi, R.D.; Meunier, N.; Blais, J.F. Bioremediation of Hazardous Wastes—A Review. Pract. Period. Hazard. Toxic Radioact. Waste Manag. 2006, 10, 59–72. [Google Scholar] [CrossRef]
- Girotti, S.; Ferri, E.N.; Fumo, M.G.; Maiolini, E. Monitoring of environmental pollutants by bioluminescent bacteria. Anal. Chim. Acta 2008, 608, 2–29. [Google Scholar] [CrossRef]
- Girotti, S.; Maiolini, E.; Bolelli, L.; Ferri, E.; Piccolo, M.; Camanzi, L.; Pompei, A. Bioremediation of hydrocarbons contaminated waters and soils: Monitoring by luminescent bacteria test. Int. J. Environ. Anal. Chem. 2011, 91, 900–909. [Google Scholar] [CrossRef]
- Sajayan, A.; Seghal Kiran, G.; Priyadharshini, S.; Poulose, N.; Selvin, J. Revealing the ability of a novel polysaccharide bioflocculant in bioremediation of heavy metals sensed in a Vibrio bioluminescence reporter assay. Environ. Pollut. 2017, 228, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Sutar, S.S.; Patil, P.J.; Tamboli, A.S.; Patil, D.N.; Apine, O.A.; Jadhav, J.P. Biodegradation and detoxification of malachite green by a newly isolated bioluminescent bacterium Photobacterium leiognathi strain MS under RSM optimized culture conditions. Biocatal. Agric. Biotechnol. 2019, 20, 101183. [Google Scholar] [CrossRef]
- Mansouri, A.; Cregut, M.; Jouanneau, S.; Thouand, G.; Durand, M.-J. Evaluation of Biomonitoring Strategies to Assess Performance of a Bioremediation Bioprocess. Sustainability 2022, 14, 10932. [Google Scholar] [CrossRef]
- Ceccanti, B.; Masciandaro, G.; Garcia, C.; Macci, C.; Doni, S. Soil Bioremediation: Combination of Earthworms and Compost for the Ecological Remediation of a Hydrocarbon Polluted Soil. Water Air Soil Pollut. 2006, 177, 383–397. [Google Scholar] [CrossRef]
- Watthaisong, P.; Kamutira, P.; Kesornpun, C.; Pongsupasa, V.; Phonbuppha, J.; Tinikul, R.; Maenpuen, S.; Wongnate, T.; Nishihara, R.; Ohmiya, Y.; et al. Luciferin Synthesis and Pesticide Detection by Luminescence Enzymatic Cascades. Angew. Chem. Int. Ed. 2022, 61, e202116908. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gregucci, D.; Nazir, F.; Calabretta, M.M.; Michelini, E. Illuminating Progress: The Contribution of Bioluminescence to Sustainable Development Goal 6—Clean Water and Sanitation—Of the United Nations 2030 Agenda. Sensors 2023, 23, 7244. https://doi.org/10.3390/s23167244
Gregucci D, Nazir F, Calabretta MM, Michelini E. Illuminating Progress: The Contribution of Bioluminescence to Sustainable Development Goal 6—Clean Water and Sanitation—Of the United Nations 2030 Agenda. Sensors. 2023; 23(16):7244. https://doi.org/10.3390/s23167244
Chicago/Turabian StyleGregucci, Denise, Faisal Nazir, Maria Maddalena Calabretta, and Elisa Michelini. 2023. "Illuminating Progress: The Contribution of Bioluminescence to Sustainable Development Goal 6—Clean Water and Sanitation—Of the United Nations 2030 Agenda" Sensors 23, no. 16: 7244. https://doi.org/10.3390/s23167244
APA StyleGregucci, D., Nazir, F., Calabretta, M. M., & Michelini, E. (2023). Illuminating Progress: The Contribution of Bioluminescence to Sustainable Development Goal 6—Clean Water and Sanitation—Of the United Nations 2030 Agenda. Sensors, 23(16), 7244. https://doi.org/10.3390/s23167244






