Surface-Electromyography-Based Co-Contraction Index for Monitoring Upper Limb Improvements in Post-Stroke Rehabilitation: A Pilot Randomized Controlled Trial Secondary Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Rehabilitation Program
2.4. Outcome Measures
2.4.1. Clinical Assessment
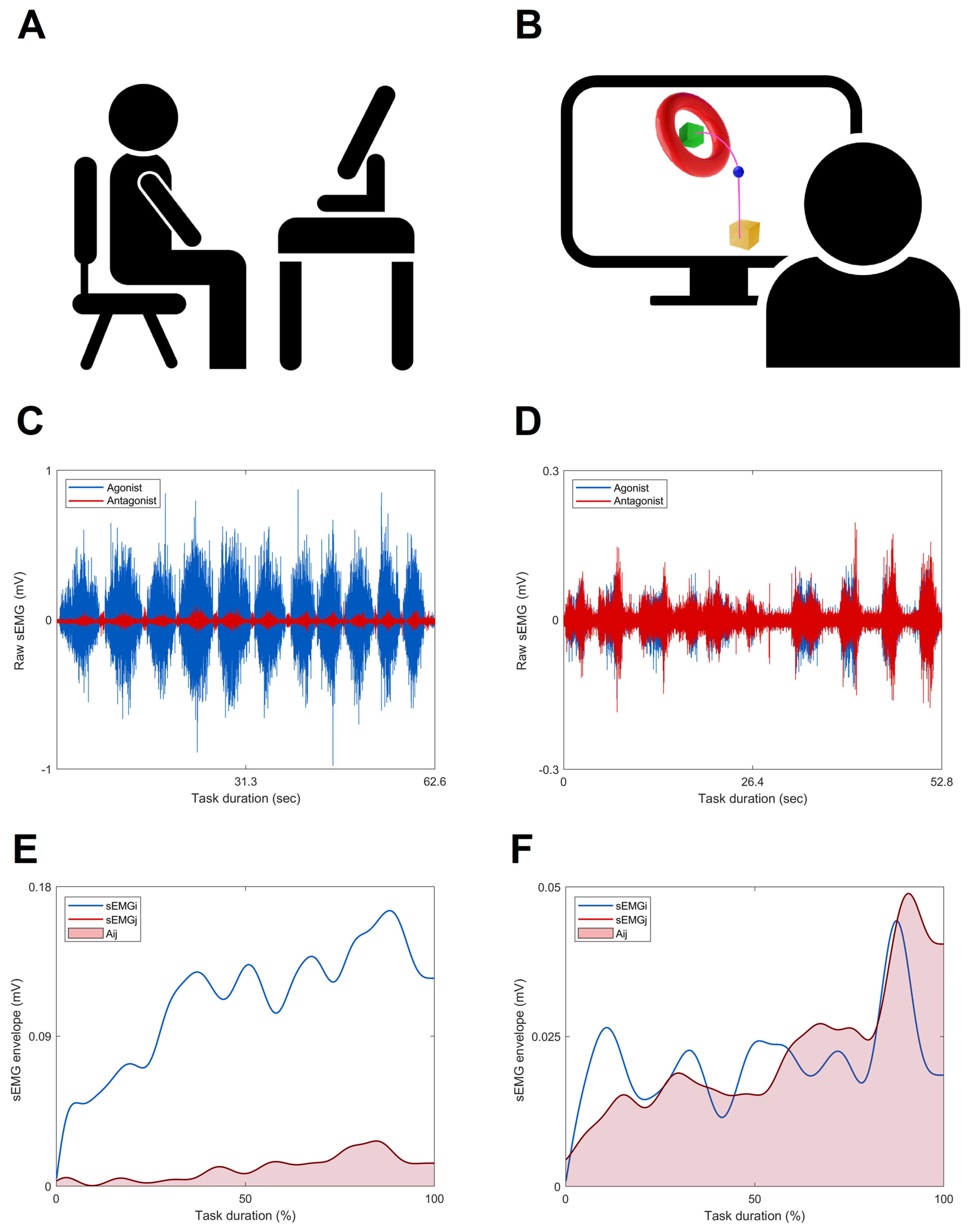
2.4.2. Instrumented Assessment
2.4.3. CCI Computation
2.4.4. Kinematic Variables Quantification
2.5. Statistics
2.5.1. Sample Size Estimation
2.5.2. Statistical Analyses
3. Results
3.1. Baseline Assessment
3.2. Comparison of the Instrumental Indices between Healthy Subjects and Persons Post-Stroke at T0
3.3. Treatment Effects
3.4. Correlation Analysis
4. Discussion
4.1. Comparison of the Instrumental Indices between Healthy Subjects and Persons Post-Stroke at T0
4.2. Treatment Effects
4.3. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carpinella, I.; Lencioni, T.; Bowman, T.; Bertoni, R.; Turolla, A.; Ferrarin, M.; Jonsdottir, J. Effects of Robot Therapy on Upper Body Kinematics and Arm Function in Persons Post Stroke: A Pilot Randomized Controlled Trial. J. Neuroeng. Rehabil. 2020, 17, 1–19. [Google Scholar] [CrossRef]
- Lencioni, T.; Fornia, L.; Bowman, T.; Marzegan, A.; Caronni, A.; Turolla, A.; Jonsdottir, J. A Randomized Controlled Trial on the Effects Induced by Robot-Assisted and Usual-Care Rehabilitation on Upper Limb Muscle Synergies in Post-Stroke Subjects. Sci. Rep. 2021, 11, 5323. [Google Scholar] [CrossRef] [PubMed]
- Hammond, M.C.; Fitts, S.S.; Kraft, G.H.; Nutter, P.B.; Trotter, M.J.; Robinson, L.M. Co-Contraction in the Hemiparetic Forearm: Quantitative EMG Evaluation. Arch. Phys. Med. Rehabil. 1988, 69, 348–351. [Google Scholar] [PubMed]
- Sheng, W.; Li, S.; Zhao, J.; Wang, Y.; Luo, Z.; Lo, W.L.A.; Ding, M.; Wang, C.; Li, L. Upper Limbs Muscle Co-Contraction Changes Correlated With the Impairment of the Corticospinal Tract in Stroke Survivors: Preliminary Evidence From Electromyography and Motor-Evoked Potential. Front. Neurosci. 2022, 16, 886909. [Google Scholar] [CrossRef] [PubMed]
- Kamper, D.; Rymer, W. Impairment of Voluntary Control of Finger Motion Following Stroke: Role of Inappropriate Muscle Coactivation. Muscle Nerve 2001, 24, 673–681. [Google Scholar] [CrossRef]
- Stoeckmann, T.M.; Sullivan, K.J.; Scheidt, R.A. Elastic, Viscous, and Mass Load Effects on Poststroke Muscle Recruitment and Co-Contraction During Reaching: A Pilot Study. Phys. Ther. 2009, 89, 665–678. [Google Scholar] [CrossRef]
- Silva, C.C.; Silva, A.; Sousa, A.; Pinheiro, A.R.; Bourlinova, C.; Silva, A.; Salazar, A.; Borges, C.; Crasto, C.; Correia, M.V.; et al. Co-Activation of Upper Limb Muscles during Reaching in Post-Stroke Subjects: An Analysis of the Contralesional and Ipsi-lesional Limbs. J. Electromyogr. Kinesiol. 2014, 24, 731–738. [Google Scholar] [CrossRef]
- Latash, M.L. Muscle Coactivation: Definitions, Mechanisms, and Functions. J. Neurophysiol. 2018, 120, 88–104. [Google Scholar] [CrossRef]
- Li, G.; Shourijeh, M.S.; Ao, D.; Patten, C.; Fregly, B.J. How Well Do Commonly Used Co-Contraction Indices Approximate Lower Limb Joint Stiffness Trends During Gait for Individuals Post-Stroke? Front. Bioeng. Biotechnol. 2021, 8, 588908. [Google Scholar] [CrossRef]
- Busse, M.E.; Wiles, C.M.; Van Deursen, R.W.M. Co-Activation: Its Association with Weakness and Specific Neurological Pa-thology. J. Neuroeng. Rehabil. 2006, 3, 26. [Google Scholar] [CrossRef]
- Ranavolo, A.; Mari, S.; Conte, C.; Serrao, M.; Silvetti, A.; Iavicoli, S.; Draicchio, F. A New Muscle Co-Activation Index for Biomechanical Load Evaluation in Work Activities. Ergonomics 2015, 58, 966–979. [Google Scholar] [CrossRef]
- Souissi, H.; Zory, R.; Bredin, J.; Gerus, P. Comparison of Methodologies to Assess Muscle Co-Contraction during Gait. J. Biomech. 2017, 57, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Frost, G.; Dowling, J.; Dyson, K.; Bar-Or, O. Cocontraction in Three Age Groups of Children during Treadmill Locomotion. J. Electromyogr. Kinesiol. 1997, 7, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Wright, Z.A.; Rymer, W.Z.; Slutzky, M.W. Reducing Abnormal Muscle Coactivation After Stroke Using a Myoelec-tric-Computer Interface: A Pilot Study. Neurorehabil. Neural. Repair. 2014, 28, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Campanini, I.; Disselhorst-Klug, C.; Rymer, W.Z.; Merletti, R. Surface EMG in Clinical Assessment and Neurorehabilitation: Barriers Limiting Its Use. Front. Neurol. 2020, 11, 934. [Google Scholar] [CrossRef]
- Ginszt, M.; Zieliński, G. Novel Functional Indices of Masticatory Muscle Activity. J. Clin. Med. 2021, 10, 1440. [Google Scholar] [CrossRef]
- Munoz-Novoa, M.; Kristoffersen, M.B.; Sunnerhagen, K.S.; Naber, A.; Alt Murphy, M.; Ortiz-Catalan, M. Upper Limb Stroke Rehabilitation Using Surface Electromyography: A Systematic Review and Meta-Analysis. Front. Hum. Neurosci. 2022, 16, 897870. [Google Scholar] [CrossRef]
- McManus, L.; De Vito, G.; Lowery, M.M. Analysis and Biophysics of Surface EMG for Physiotherapists and Kinesiologists: Toward a Common Language With Rehabilitation Engineers. Front. Neurol. 2020, 11, 576729. [Google Scholar] [CrossRef]
- Soylu, A.R.; Arpinar-Avsar, P. Detection of Surface Electromyography Recording Time Interval without Muscle Fatigue Effect for Biceps Brachii Muscle during Maximum Voluntary Contraction. J. Electromyogr. Kinesiol. 2010, 20, 773–776. [Google Scholar] [CrossRef]
- Sbriccoli, P.; Bazzucchi, I.; Rosponi, A.; Bernardi, M.; De Vito, G.; Felici, F. Amplitude and Spectral Characteristics of Biceps Brachii SEMG Depend upon Speed of Isometric Force Generation. J. Electromyogr. Kinesiol. 2003, 13, 139–147. [Google Scholar] [CrossRef]
- Naeije, M.; McCarroll, R.S.; Weijs, W.A. Electromyographic Activity of the Human Masticatory Muscles during Submaximal Clenching in the Inter-Cuspal Position. J. Oral. Rehabil. 1989, 16, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Feldner, H.A.; Howell, D.; Kelly, V.E.; McCoy, S.W.; Steele, K.M. “Look, Your Muscles Are Firing!”: A Qualitative Study of Clinician Perspectives on the Use of Surface Electromyography in Neurorehabilitation. Arch. Phys. Med. Rehabil. 2019, 100, 663–675. [Google Scholar] [CrossRef] [PubMed]
- Merletti, R.; Campanini, I.; Rymer, W.Z.; Disselhorst-Klug, C. Editorial: Surface Electromyography: Barriers Limiting Widespread Use of SEMG in Clinical Assessment and Neurorehabilitation. Front. Neurol. 2021, 12, 642257. [Google Scholar] [CrossRef] [PubMed]
- Charafeddine, J.; Pradon, D.; Chevallier, S.; Alfayad, S.; Khalil, M. Neuromotor Strategy of Gait Rehabilitation for Lower-Limb Spasticity. In Proceedings of the Fifth International Conference on Advances in Biomedical Engineering (ICABME), Tripoli, Lebanon, 17–19 October 2019; pp. 1–4. [Google Scholar]
- Hu, X.; Tong, K.Y.; Song, R.; Tsang, V.S.; Leung, P.O.; Li, L. Variation of Muscle Coactivation Patterns in Chronic Stroke During Robot-Assisted Elbow Training. Arch. Phys. Med. Rehabil. 2007, 88, 1022–1029. [Google Scholar] [CrossRef]
- Qian, Q.; Hu, X.; Lai, Q.; Ng, S.C.; Zheng, Y.; Poon, W. Early Stroke Rehabilitation of the Upper Limb Assisted with an Elec-tromyography-Driven Neuromuscular Electrical Stimulation-Robotic Arm. Front. Neurol. 2017, 8, 447. [Google Scholar] [CrossRef] [PubMed]
- Casadio, M.; Sanguineti, V.; Morasso, P.G.; Arrichiello, V. Braccio Di Ferro: A New Haptic Workstation for Neuromotor Rehabilitation. Technol. Health. Care. 2006, 14, 123–142. [Google Scholar] [CrossRef] [PubMed]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of Recommendations for SEMG Sensors and Sensor Placement Procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Blanc, Y.; Dimanico, U. Electrode Placement in Surface Electromyography (SEMG) “Minimal Crosstalk Area” (MCA). Open. Rehabil. J. 2010, 3, 110–126. [Google Scholar] [CrossRef]
- Morris, A.D.; Kemp, G.J.; Lees, A.; Frostick, S.P. A Study of the Reproducibility of Three Different Normalisation Methods in Intramuscular Dual Fine Wire Electromyography of the Shoulder. J. Electromyogr. Kinesiol. 1998, 8, 317–322. [Google Scholar] [CrossRef]
- Rinaldi, M.; D’Anna, C.; Schmid, M.; Conforto, S. Assessing the Influence of SNR and Pre-Processing Filter Bandwidth on the Extraction of Different Muscle Co-Activation Indexes from Surface EMG Data. J. Electromyogr. Kinesiol. 2018, 43, 184–192. [Google Scholar] [CrossRef]
- Rosa, M.C.N.; Marques, A.; Demain, S.; Metcalf, C.D. Lower Limb Co-Contraction during Walking in Subjects with Stroke: A Systematic Review. J. Electromyogr. Kinesiol. 2014, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gulde, P.; Hermsdörfer, J. Smoothness Metrics in Complex Movement Tasks. Front. Neurol. 2018, 9, 615. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.A.; Willén, C.; Sunnerhagen, K.S. Kinematic Variables Quantifying Upper-Extremity Performance after Stroke during Reaching and Drinking from a Glass. Neurorehabil. Neural. Repair. 2011, 25, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Casadio, M.; Giannoni, P.; Morasso, P.; Sanguineti, V. A Proof of Concept Study for the Integration of Robot Therapy with Physiotherapy in the Treatment of Stroke Patients. Clin. Rehabil. 2009, 23, 217–228. [Google Scholar] [CrossRef]
- Wu, C.Y.; Huang, P.C.; Chen, Y.T.; Lin, K.C.; Yang, H.W. Effects of Mirror Therapy on Motor and Sensory Recovery in Chronic Stroke: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2013, 94, 1023–1030. [Google Scholar] [CrossRef]
- Dobkin, B.H. Progressive Staging of Pilot Studies to Improve Phase III Trials for Motor Interventions. Neurorehabil. Neural. Repair. 2009, 23, 197–206. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis. Curr. Dir. Psychol. Sci. 1992, 1, 98–101. [Google Scholar] [CrossRef]
- Hobart, J.C.; Cano, S.J.; Zajicek, J.P.; Thompson, A.J. Rating Scales as Outcome Measures for Clinical Trials in Neurology: Problems, Solutions, and Recommendations. Lancet. Neurology. 2007, 6, 1094–1105. [Google Scholar] [CrossRef]
- Mehrholz, J.; Pohl, M.; Platz, T.; Kugler, J.; Elsner, B. Electromechanical and Robot-assisted Arm Training for Improving Ac-tivities of Daily Living, Arm Function, and Arm Muscle Strength after Stroke. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef]
- Dursun, E.; Dursun, N.; Ural, C.E.; Çakci, A. Glenohumeral Joint Subluxation and Reflex Sympathetic Dystrophy in Hemiplegic Patients. Arch. Phys. Med. Rehabil. 2000, 81, 944–946. [Google Scholar] [CrossRef]
- Wang, X.; Fu, Y.; Ye, B.; Babineau, J.; Ding, Y.; Mihailidis, A. Technology-Based Compensation Assessment and Detection of Upper Extremity Activities of Stroke Survivors: Systematic Review. J. Med. Internet. Res. 2022, 24, e34307. [Google Scholar] [CrossRef]
- Roby-Brami, A.; Jarrassé, N.; Parry, R. Impairment and Compensation in Dexterous Upper-Limb Function After Stroke. From the Direct Consequences of Pyramidal Tract Lesions to Behavioral Involvement of Both Upper-Limbs in Daily Activities. Front. Hum. Neurosci. 2021, 15, 662006. [Google Scholar] [CrossRef]
- Longatelli, V.; Torricelli, D.; Tornero, J.; Pedrochi, A.; Molteni, F.; Pons, J.L.; Gandolla, M. A Unified Scheme for the Bench-marking of Upper Limb Functions in Neurological Disorders. J. Neuroeng. Rehabil. 2022, 19, 102. [Google Scholar] [CrossRef] [PubMed]
- Chae, J.; Yang, G.; Park, B.K.; Labatia, I. Muscle Weakness and Cocontraction in Upper Limb Hemiparesis: Relationship to Motor Impairment and Physical Disability. Neurorehabil. Neural. Repair. 2002, 16, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Chalard, A.; Amarantini, D.; Tisseyre, J.; Marque, P.; Tallet, J.; Gasq, D. Spastic Co-Contraction, Rather than Spasticity, Is Associated with Impaired Active Function in Adults with Acquired Brain Injury: A Pilot Study. J. Rehabil. Med. 2019, 51, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, A.; Kanzler, C.M.; Lambercy, O.; Luft, A.R.; Veerbeek, J.M. Systematic Review on Kinematic Assessments of Upper Limb Movements after Stroke. Stroke 2019, 50, 718–727. [Google Scholar] [CrossRef] [PubMed]



| Variable | UCG (N = 17) Median (1st–3rd) | RG (N = 17) Median (1st–3rd) | p-Value | |
|---|---|---|---|---|
| Age (years) | 59.0 (46.0–70.0) | 67.0 (58.0–72.0) | 0.20 | |
| Time since stroke (months) | 5.8 (1.9–91.4) | 7.8 (1.4–13.9) | 0.55 | |
| FM-UE | 21.0 (12.0–46.5) | 33.0 (16.0–50.5) | 0.22 | |
| Number | Number | |||
| Sex | 0.73 | |||
| Female | 8 | 9 | ||
| Male | 9 | 8 | ||
| Stroke type | 1.00 | |||
| Ischemic | 11 | 11 | ||
| Hemorrhagic | 6 | 6 | ||
| Paretic side | 0.49 | |||
| Right | 6 | 8 | ||
| Left | 11 | 9 | ||
| Chronicity (>3 months) | 1.00 | |||
| Chronic | 10 | 10 | ||
| Sub-acute | 7 | 7 | ||
| FM-UE CS | |||
|---|---|---|---|
| Correlation Coefficient | p-Value | ||
| CCI CS | Anterior/Posterior deltoids | 0.04 | 0.83 |
| Triceps/Biceps | 0.03 | 0.85 | |
| Pronator/Supinator | –0.09 | 0.63 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bandini, V.; Carpinella, I.; Marzegan, A.; Jonsdottir, J.; Frigo, C.A.; Avanzino, L.; Pelosin, E.; Ferrarin, M.; Lencioni, T. Surface-Electromyography-Based Co-Contraction Index for Monitoring Upper Limb Improvements in Post-Stroke Rehabilitation: A Pilot Randomized Controlled Trial Secondary Analysis. Sensors 2023, 23, 7320. https://doi.org/10.3390/s23177320
Bandini V, Carpinella I, Marzegan A, Jonsdottir J, Frigo CA, Avanzino L, Pelosin E, Ferrarin M, Lencioni T. Surface-Electromyography-Based Co-Contraction Index for Monitoring Upper Limb Improvements in Post-Stroke Rehabilitation: A Pilot Randomized Controlled Trial Secondary Analysis. Sensors. 2023; 23(17):7320. https://doi.org/10.3390/s23177320
Chicago/Turabian StyleBandini, Virginia, Ilaria Carpinella, Alberto Marzegan, Johanna Jonsdottir, Carlo Albino Frigo, Laura Avanzino, Elisa Pelosin, Maurizio Ferrarin, and Tiziana Lencioni. 2023. "Surface-Electromyography-Based Co-Contraction Index for Monitoring Upper Limb Improvements in Post-Stroke Rehabilitation: A Pilot Randomized Controlled Trial Secondary Analysis" Sensors 23, no. 17: 7320. https://doi.org/10.3390/s23177320






